Abstract
Listeria monocytogenes is the causative agent of the foodborne disease listeriosis. During infection, L. monocytogenes produces an array of non-coding RNAs, including the multicopy sRNA LhrC. These five, nearly identical sRNAs are highly induced in response to cell envelope stress and target the virulence adhesin lapB at the post-transcriptional level. Here, we demonstrate that LhrC controls expression of additional genes encoding cell envelope-associated proteins with virulence function. Using transcriptomics and proteomics, we identified a set of genes affected by LhrC in response to cell envelope stress. Three targets were significantly down-regulated by LhrC at both the RNA and protein level: lmo2349, tcsA and oppA. All three genes encode membrane-associated proteins: A putative substrate binding protein of an amino acid ABC transporter (Lmo2349); the CD4+ T cell-stimulating antigen TcsA, and the oligopeptide binding protein OppA, of which the latter 2 are required for full virulence of L. monocytogenes. For OppA, we show that LhrC acts by direct base paring to the ribosome binding site of the oppA mRNA, leading to an impediment of its translation and a decreased mRNA level. The sRNA-mRNA interaction depends on 2 of 3 CU-rich regions in LhrC allowing binding of 2 oppA mRNAs to a single LhrC molecule. Finally, we found that LhrC contributes to infection in macrophage-like cells. These findings demonstrate a central role for LhrC in controlling the level of OppA and other virulence-associated cell envelope proteins in response to cell envelope stress.
Keywords: cell envelope stress, Listeria monocytogenes, proteomics, sRNA, transcriptomics
Introduction
Small non-coding RNAs (sRNAs) are known as regulators of bacterial gene expression controlling important physiological processes such as stress responses and virulence.1-5 In recent years, genome-wide surveys revealed the presence of a high number of sRNAs in a variety of bacterial species, including the Gram-positive, facultative intracellular pathogen Listeria monocytogenes. 6-12 So far, more than 200 sRNAs have been identified in L. monocytogenes, many of which are specifically induced under infection-relevant conditions such as in whole human blood and during infection of macrophage cells, suggesting an important role for sRNAs in virulence.9,10,12 Indeed, several sRNAs have been shown to contribute to the pathogenesis of L. monocytogenes in various models of infection.9,13,14
Bacterial sRNAs may act by several mechanisms, however the majority have been shown to act at the post-transcriptional level by base pairing to target mRNAs, affecting translation and/or mRNA stability either positively or negatively.1,2 The most studied examples of base pairing sRNAs, referred to as trans-encoded, are transcribed from intergenic regions far from their targets with which they share only limited complementarity. Trans-encoded sRNAs are often transcribed under specific conditions and regulate a multitude of genes. Recent studies on the function of sRNAs in L. monocytogenes revealed examples of trans-encoded sRNAs acting to induce or repress the expression of secreted or cell envelope-associated proteins implicated in virulence. The trans-encoded sRNA Rli27 positively affects the level of the cell wall surface protein Lmo0514 by base pairing with the 5′-untranslated region (UTR) of a long lmo0514 transcript variant during intracellular infection.15 Binding of Rli27 to its target mRNA is predicted to increase the accessibility of the ribosome binding site (RBS) by preventing the formation of an inhibitory secondary structure in the 5′-UTR, resulting in an increase in translation. The sRNA LhrA is another example of a trans-encoded sRNA with regulatory functions in L. monocytogenes.16 LhrA controls the expression of multiple targets including the cell wall surface protein Lmo0880 and the secreted chitinase ChiA.17 LhrA acts by direct binding to the RBS of target mRNAs thereby interfering with ribosome recruitment. As for most trans-encoded sRNAs in Gram-negative bacteria, LhrA depends on the RNA chaperone Hfq in terms of stability and base pairing to its target mRNAs.6,16,17 Finally, the multicopy sRNA LhrC down-regulates the level of the cell wall anchored virulence adhesin LapB.18 Three CU-rich motifs in LhrC target the expression of lapB at the post-transcriptional level by base pairing to the RBS of the target mRNA. Although LhrC was originally identified as an Hfq-binding sRNA,6 it does not require Hfq for stability or base pairing with its target mRNA. Disturbance of the cell envelope integrity was identified as the actual signal for LhrC induction, which strictly depends on the 2-component system LisRK.18 Collectively, the functional studies performed so far suggest a link between sRNA-mediated control and cell envelope-related functions in L. monocytogenes.
In this study, we employed 2 experimental strategies to identify additional target genes controlled by the multicopy sRNA LhrC. In both surveys, we found that LhrC negatively controls the expression of lmo2349 encoding a putative substrate binding protein of an amino acid ABC transporter; tcsA encoding a CD4+ T cell-stimulating antigen, and oppA encoding an oligopeptide-binding protein. For lmo2349, the regulatory effect of LhrC occurs at the level of transcription, whereas expression of tcsA and oppA is controlled by LhrC at the post-transcriptional level. Detailed analyses of the molecular mechanism by which LhrC affects expression of oppA showed that 2 CU-rich regions in LhrC are crucial for direct binding to the RBS of the oppA mRNA. Furthermore, a mutant strain lacking all 5 copies of lhrC is impaired in infection of macrophage-like cells.
Results
Novel LhrC target genes revealed by transcriptomics and proteomics analyses
To identify novel target genes for LhrC, we compared the transcriptomes of the L. monocytogenes LO28 wild type strain and a ΔlhrC1-5 mutant by microarray analysis. To induce cell envelope stress and hence the expression of LhrC1-5, cultures were exposed to a subinhibitory concentration of cefuroxime (4 µg/ml) for 30 min. Under these conditions, 11 genes distributed across 6 different operons were significantly up-regulated in ΔlhrC1-5 in comparison to wild type cells (≥1.5 fold; p < 0.05) whereas no genes were significantly downregulated (Table 1; Table S1A). Of the 11 genes found to be upregulated in the absence of LhrC1-5, 6 genes were predicted to encode cell envelope-associated proteins. As a control, we compared the transcriptomes of non-stressed cultures of the wild type strain and ΔlhrC1-5 (Table S1B). No genes were found to be differentially regulated in the non-stressed cultures (≥1.5 fold; p < 0.05). The validity of the microarray data was supported by the presence of known cell envelope stress responsive genes, such as lmo2210, lmo1690 and lmo1518, among the genes highly induced in the wild type strain exposed to cell envelope stress, relative to the non-stressed wild type strain (Table S1C).19 The microarray data set was validated by RT-qPCR analysis of 10 selected genes (Table S2).
Table 1.
Putative LhrC targets identified by transcriptomics and proteomics. Locus tags and if available gene symbols of 13 putative LhrC targets are given. Gene descriptions were extracted from ncbi. Ratios for RNA abundance and protein abundance in ΔlhrC1-5 vs. wild type are based on the average of 3 independent experiments with a cutoff ratio of 1.5 and 2, respectively. RNA ratios that showed a confidence less than 95% (n.s. for not significant) or did not make the 1.5 cutoff appear in italics. Protein ratios in italics were below the cutoff of 2 or could not be identified in all 3 replicates. The superscript gives the number of replicates a protein ratio could be calculated for. Gene ontology annotations originate from UniProtKB, or if stated so from the KEGG database. n.d. = not detected
| locus tag / gene symbol | gene description (ncbi) | Micro array [RT-qPCR] | proteomics | GO function (UniProtKB) |
|---|---|---|---|---|
| lmo0227 | tRNA-dihydrouridine synthase | 0.88 | 0.32 | tRNA processing, oxidation-reduction process, flavin adenine dinucleotide binding |
| lmo0947 | hypothetical transport protein | 3.98 | 1.002 | transmembrane transport |
| lmo0948 | transcriptional regulator | 2.40 | 0.452 | DNA binding, transcription regulation |
| lmo1388 / tcsA | CD4+ T cell-stimulating antigen, lipoprotein | 1.50 | 2.63 | plasma membrane, lipid anchor |
| lmo1584 | hypothetical protein | 1.51 | 1.532 | |
| lmo1585 | peptidase | 1.53 n.s. | 2.84 | serine-type endopeptidase activity, proteolysis |
| lmo1983 / ilvD | dihydroxy-acid dehydratase | 1.77 | 7.552 | iron-sulfur cluster binding, branched-chain amino acid biosynthetic process |
| lmo1986 / ilvC | ketol-acid reductoisomerase | 1.62 | 3.331 | ketol-acid reductoisomerase activity, branched-chain amino acid biosynthetic process |
| lmo2196 / oppA | peptide ABC transporter substrate-binding protein | 1.56 | 2.94 | oligopeptide transport system substrate-binding protein, ABC transporter (KEGG) |
| lmo2347 | amino acid ABC transporter permease | 1.67 | n.d. | transporter activity |
| lmo2348 | amino acid ABC transporter permease | 1.59 | n.d. | transporter activity |
| lmo2349 | amino acid ABC transporter substrate-binding protein | 1.59 | 4.21 | L-cystine transport system substrate-binding protein, ABC transporter (KEGG) |
| lmo2351 | FMN reductase | 1.61 | 7.152 | alkanesulfonate catabolic process, oxidation-reduction process |
As a complementary approach, we carried out a comprehensive mass spectrometry (MS)-based proteome analysis of wild type vs. ΔlhrC1-5 cells exposed to a subinhibitory concentration of cefuroxime for one hour. Protein extracts were enriched in their membrane protein fraction, separated via 1D-SDS-PAGE and analyzed by a LC-MS/MS. In total 1587 proteins, of which 442 are predicted to possess membrane-spanning domains,20 were identified on the basis of at least 2 peptides (Table S3). Relative protein quantification of these proteins was performed making use of the emPAI algorithm.21 The variance of the label-free quantitative dataset was fairly high across the 3 biological replicates (Table S3). For this reason we applied a conservative filter and considered proteins as being differentially expressed in the wild type and the LhrC1-5 deficient mutant when their amount deviated by a factor of at least 2 in all 3 biological replicates. This criterion was met by only 5 proteins – 4 being higher abundant in ΔlhrC1-5 (TcsA, Lmo1585, OppA, Lmo2349) and one protein being down-regulated in the mutant (Lmo0227) (Table 1). Three of the 4 up-regulated proteins are known to be located at the cell envelope and the fourth (Lmo1585) is a predicted peptidase with homology to Escherichia coli's signal peptide peptidase (SppA), thus, probably also located at the cell membrane. As LhrC is induced during cell envelope stress, the cellular location of these 4 putative LhrC targets appears most expedient.
When comparing the results from the transcriptomic and proteomics analysis, 3 genes were found to be upregulated at both the RNA- and protein level in the ΔlhrC1-5 mutant relative to the wild type: lmo2349, tcsA and oppA (Table 1). Lmo2349 codes for a putative substrate binding protein of an amino acid ABC transporter; tcsA (lmo1388) for a CD4+ T cell-stimulating antigen,22 and oppA (lmo2196) for an oligopeptide-binding protein.23 These putative target genes were selected for further investigation.
Investigation of the regulatory effect of LhrC on 3 putative target genes
Lmo2349 is the third gene in an operon of 9 genes (lmo2351-lmo2343) according to the L. monocytogenes operon structure.12 In the microarray study, several genes belonging to this operon were found to be significantly up-regulated in the lhrC1-5 mutant strain relative to the wild type (lmo2351, lmo2349, lmo2348, and lmo2347). Also in the proteomics approach proteins of this operon could be detected additionally to Lmo2349, but not across all 3 biological replicates or not always in wild type and in ΔlhrC1-5 (Table S3). Since the proteomics approach revealed that several members of the operon were upregulated in the mutant, it could be hypothesized that LhrC1-5 might affect the expression of the lmo2351-2343 operon at the level of transcription. To investigate this, we constructed a transcriptional fusion of the promoter region upstream of the first gene in the operon, lmo2351, to the reporter gene lacZ in the vector pTCV-lac.24 Curiously, after one hour of cefuroxime stress β-galactosidase activity was significantly higher in ΔlhrC1-5 carrying the lmo2351-lacZ fusion plasmid relative to wild type (Fig. 1A). Under non-stress conditions, β-galactosidase activity of the wild type and mutant strain was comparable. This result demonstrates that LhrC1-5 regulate this operon at the level of transcription, most likely in an indirect manner by controlling the level and/or activity of an unknown transcriptional regulator of the lmo2351-2343 operon. The mechanism underlying the regulatory effect of LhrC on lmo2351-2343 was not analyzed any further in the present study.
Figure 1.
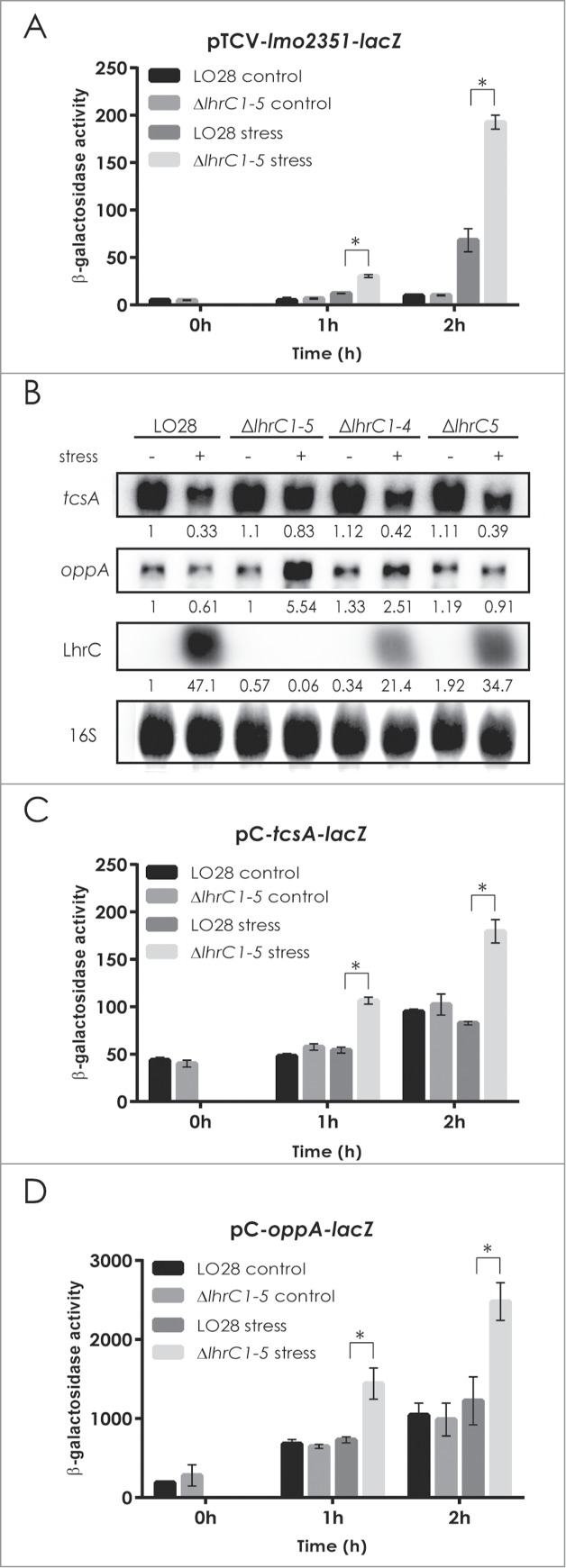
LhrC-mediated down-regulation of potential target genes. β-galactosidase activities were assessed in wild type and ΔlhrC1-5 strains carrying a transcriptional reporter gene fusion of the lmo2351 promoter to lacZ in pTCV-lac (A), or translational reporter gene fusions of tcsA (C) and oppA (D) to lacZ in the vector pCK. β-galactosidase activity of wild type and mutant cells was measured at the indicated time-points under non-stress conditions (control samples) and after exposure to 4 µg/ml cefuroxime (stress). Results of the β-galactosidase assays are the average of 3 biological replicates each conducted in technical duplicates. After 1 and 2 hours of stress, a significant difference between the mutant and wild type cells was observed for all 3 reporter gene fusions tested (asterisk: P < 0.005). (B) Northern blot analyses of tcsA mRNA, oppA mRNA and LhrC. Samples were taken from LO28 wild type, ΔlhrC1-5, ΔlhrC1-4 and ΔlhrC5 cultures exposed to 1 hour of cefuroxime stress (+) as well as from non-stressed cultures (−). Northern blots were probed for tcsA mRNA, oppA mRNA, LhrC and 16S (loading control). Relative levels of tcsA mRNA, oppA mRNA and LhrC (normalized to 16S) are shown below each lane.
According to the operon structure of L. monocytogenes, the tcsA gene (lmo1388) is transcribed monocistronically.12 The oppA gene (lmo2196) corresponds to the first gene of the oligopeptide permease operon (lmo2196-lmo2192) but is predominantly transcribed alone.12,23 To investigate the regulatory effect of LhrC1-5 on tcsA and oppA, we made use of northern blot analysis to compare transcript levels in the wild type and 3 mutant strains lacking one (ΔlhrC5), 4 (ΔlhrC1-4) or all 5 LhrC-encoding genes (ΔlhrC1-5), under non-stress conditions and after one hour of cell envelope stress (Fig. 1B). In wild type, ΔlhrC1-4 and ΔlhrC5 cells, we observed a decreased level of tcsA mRNA in response to cefuroxime exposure, whereas in ΔlhrC1-5 cells, no major change was seen. For oppA, a minor decrease was seen in wild type cells in response to cefuroxime stress, whereas in ΔlhrC1-5 cells a 5-fold increase in the level of oppA mRNA was observed. In ΔlhrC1-4 and ΔlhrC5 cells, a down-regulation of oppA was seen in response to cefuroxime exposure. Collectively, RNA gel blot analysis showed that upon cell envelope stress, induction of the LhrC homologues results in downregulation of tcsA and abrogated induction of oppA expression.
To further analyze the effect of LhrC1-5 on tcsA and oppA, we chose a reporter gene fusion strategy that allows detection of regulatory effects at the post-transcriptional level. First, the 5′-ends of the tcsA and oppA transcripts were mapped by primer extension analysis to position −133 and −41, respectively, relative to the translation start site (+1) (Figure S1). Then, sequences encoding the 5′-ends of the tcsA or oppA transcripts, and additional 53 or 32 bp of the tcsA or oppA coding regions, respectively, were fused downstream of a moderate promoter which is not affected by LhrC1-5. These sequences were inserted in-frame to lacZ in vector pCK-lac18 to give the translational reporter plasmids pC-tcsA-lacZ and pC-oppA-lacZ. Results of the β-galactosidase assays for both plasmid constructs in wild type or ΔlhrC1-5 are presented in Figure 1C, D. One hour after the onset of cefuroxime stress, the β-galactosidase activity was clearly higher in ΔlhrC1-5 relative to wild type cells, whereas under non-stress conditions no difference in activity was observed. Thus, the 5′-ends of the tcsA and oppA transcripts contain important information for mediating the regulatory effect of LhrC1-5.
LhrC binds to the Shine Dalgarno region of oppA mRNA
Based on the results so far, we hypothesized that LhrC1-5 control the expression of tcsA and oppA at the post-transcriptional level by direct binding to the mRNAs. To explore this, we performed an in vitro binding experiment using 5′-end labeled LhrC4 (Fig. 2). The gel shift assay showed that LhrC4 is indeed capable of interacting with tcsA and oppA mRNA. The RNA chaperone Hfq is known to bind LhrC, however, the addition of Hfq to the binding reaction did not stimulate sRNA-mRNA complex formation (Fig. 2).
Figure 2.
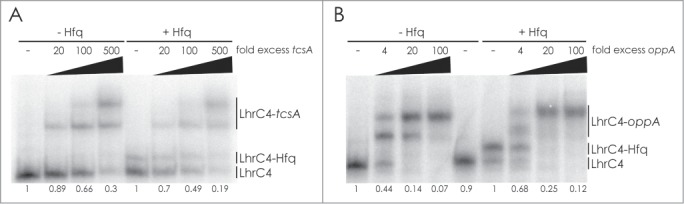
Gel mobility shift assay of sRNA-mRNA interaction and the role of Hfq. Labeled LhrC4 was shifted with increasing concentrations of tcsA RNA (A) or oppA RNA (B) in the absence or presence of 10 µg/ml Hfq. “Fold excess” refers to the amount of mRNA added to each sample, relative to the amount of labeled LhrC4. The fraction of unbound LhrC is shown below each lane.
A direct binding of a sRNA to the RBS of a target mRNA is likely to cause repression of translation. To investigate this possibility, we searched for putative LhrC1-5 binding sites in tcsA and oppA mRNAs using the webserver IntaRNA.25 In both cases, interactions involve the CU-rich single-stranded region or the terminator loop in LhrC1-5, with LhrC4 forming the most stable complexes (Figs. S2 and S3). For tcsA, the putative LhrC1-5 binding sites were located far upstream from the translation start site (from −87 to −96, Fig. S2), whereas for oppA, LhrC1-5 were predicted to interact with the AG-rich Shine Dalgarno (SD) region (from −10 to −19 for binding of LhrC1-3 and LhrC5, and from −10 to −21 for binding of LhrC4, see Fig. S3). Based on the location of the predicted interaction site, oppA was selected for further analyses.
In order to validate the presence of a LhrC1-5 binding site in the SD region, we performed a structure probing experiment on 5′-end labeled oppA RNA using RNase T1 (cleaves at single-stranded G residues), RNase V1 (cleaves double-stranded and stacked residues), lead(II) acetate treatment (cleaves at single-stranded residues) and RNase S1 (cleaves at single-stranded residues). Upon addition of unlabeled LhrC4, the AG-rich SD region was clearly protected from cleavage by RNase T1 (G12 to G14) and RNase S1 (from −16 to −23) (Fig. 3A). Furthermore, an increased cleavage was observed by RNase V1 at G-13, G-14 and A-20 relative to translation start site (+1).
Figure 3.
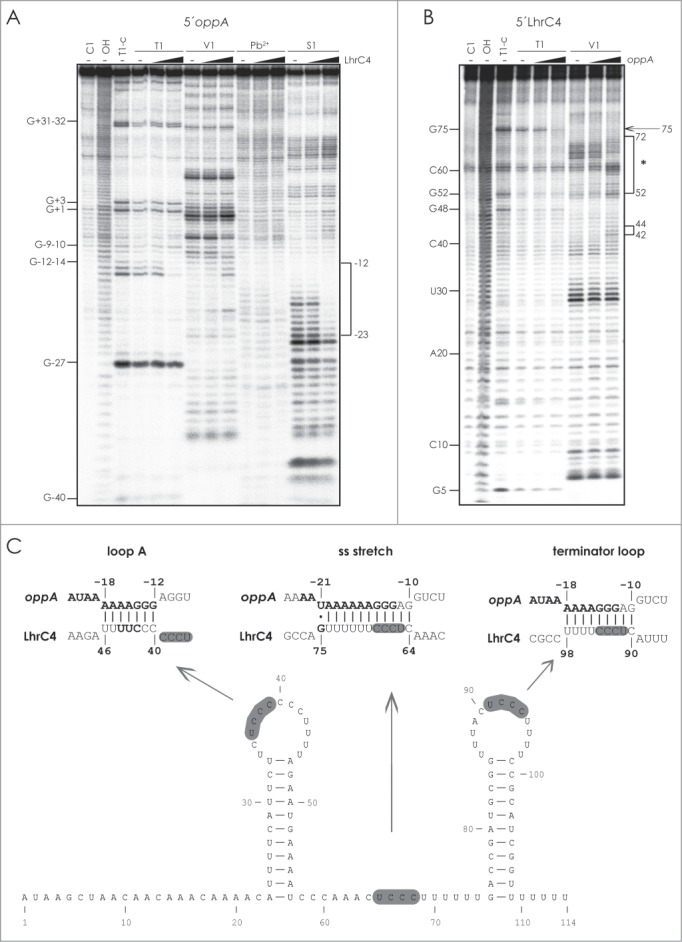
Probing of the interaction between LhrC and oppA mRNA. (A) 5′-end labeled oppA RNA was partially digested with RNase T1, RNase V1, Pb2+, or RNase S1 in the absence (−) or presence of 4 or 250 fold excess of LhrC4. As a control, untreated oppA RNA was separated (C1, lane 1). Alkaline (OH) and RNase T1 (T1-C) ladders are shown in lane 2 and 3, respectively. The bracket marks the oppA sequence for which an altered cleavage pattern was observed upon addition of LhrC4 (from −23 to −12 relative to the translation start site of oppA). (B) 5′-end labeled LhrC4 was digested with RNase T1 and RNase V1 in the absence (−) or presence of 4 or 250 fold excess of oppA RNA. In the presence of oppA RNA, an altered cleavage pattern was observed at position G75 (marked by an arrow) and in the regions ranging from G52 to U72 (upper bracket, marked by an asterisk) and C42-C44 (lower bracket). (C) Predicted interactions between the SD region of oppA mRNA and the CU-rich regions of loop A (left), the single stranded stretch (center), and the terminator loop of LhrC4 (right), respectively. The sequence in oppA mRNA, for which an altered cleavage pattern was observed upon addition of LhrC4, is shown in bold. In LhrC4, residues C42-C44 (left) and G75 (center) are marked in bold. Conserved UCCC motifs in LhrC4 are shown in gray.
Two CU-rich motifs in LhrC are important for binding to oppA mRNA
Even though either the single-stranded stretch or the terminator loop in LhrC1-5 were predicted to interact with the AG-rich SD region of oppA mRNA, a total of 3 CU-rich regions in each of the different LhrC copies are in fact likely to interact with this specific SD sequence. Matching interactions between these 3 regions in LhrC4 and oppA mRNA are shown in Figure 3C. To investigate this idea, we first performed structure probing experiments using 5′-end labeled LhrC4 (Fig. 3B). In general, no major structural changes were observed upon the addition of unlabeled oppA RNA, however, we noted a partial increase in cleavage by RNase V1 of residues C42 to C44 residing in loop A in addition to a structural rearrangement involving the lower 3′-side of stem A and the single-stranded region (from G52 to U72) (Fig. 3B). Furthermore, protection of G75 from cleavage by RNase T1 was observed in the presence of 250 fold excess of oppA RNA (Fig. 3B). These findings were in accordance with the predicted interactions between the oppA mRNA and the CU-rich regions of loop A and single-stranded stretch (Fig. 3C).
To further address the importance of the CU-rich regions of LhrC4 for binding to oppA mRNA, and in particular the CU-rich region of the terminator loop which was not covered by the structure probing experiment, we performed gel shift assays. Several different LhrC4 mutants originally designed for testing the interaction of LhrC4 to lapB mRNA18 were employed for an initial screening of loop A, the single-stranded region and the terminator loop, respectively (Fig. 4). When all 3 binding regions in LhrC4 were mutated (LhrC4_mut_7), there was no detectable binding to oppA RNA. Mutation of the single-stranded region (LhrC4_mut_2) and terminator loop (LhrC4_mut_3) alone resulted in decreased binding, whereas mutation of loop A alone (LhrC4_mut_4) did not reduce any of the interaction ability of the 2 RNAs. Interestingly, mutating both the single-stranded region and the terminator loop (LhrC4_mut_5) completely abolished the binding to oppA RNA. In conclusion, the single-stranded region and the terminator loop are capable of mediating the binding to oppA RNA, whereas loop A is not important for the interaction.
Figure 4.
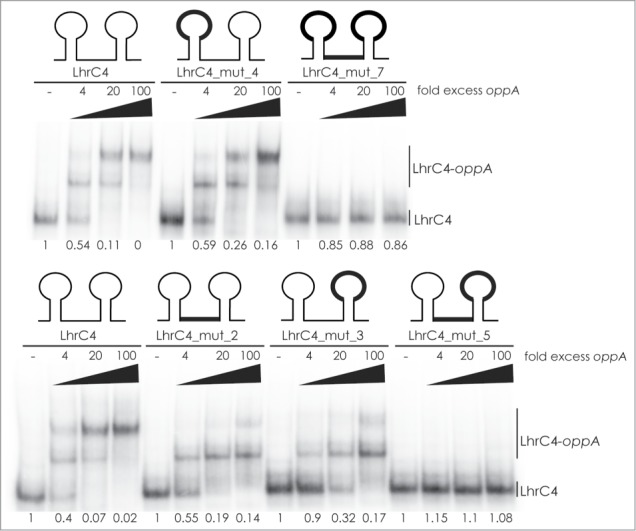
Mutational screening of loop A, single-stranded stretch, and terminator loop in LhrC. Labeled LhrC4 and several mutant derivatives were tested for their ability to bind oppA RNA (4, 20 and 100 fold excess relative to labeled LhrC4). In the LhrC4 sketches, the mutated regions are indicated in black (thick line). LhrC4: wild type LhrC4. LhrC4_mut_4: mutation in loop A. LhrC4_mut_7: mutation in loop A, single-stranded stretch, and terminator loop. LhrC4_mut_2: mutation in single-stranded stretch. LhrC4_mut_3: mutation in terminator loop. LhrC_mut_5: mutation in single-stranded stretch and terminator loop. The fraction of unbound LhrC4 is shown below each lane.
To better explore the interaction between LhrC4 and oppA mRNA, we performed a gel shift assay using 5′-end labeled LhrC4 and 2 variants of unlabeled oppA mRNA: A wild type version and a mutant version containing substitutions of 3 nucleotides in the SD region (oppA_MUT) (Fig. 5A, B). The gel shift experiment showed that binding of LhrC4 to the mutant oppA RNA is significantly reduced in comparison to wild type, clearly supporting the idea of LhrC4 binding to the SD region (Fig. 5C—E, left). Next, we constructed minimal mutations in LhrC4 in which 3 nucleotides in the UCCC motifs of the single-stranded region or terminator loop were mutated (Fig. 5A, B). The mutations were designed to avoid changes in the secondary structure of LhrC4 and to match the minimal oppA mutant (oppA_MUT). The minimal mutations in LhrC4_ssMUT and LhrC4_terMUT significantly reduced the interaction with wild type oppA RNA (Fig. 5C and 5D, right). We also tested whether the LhrC4 mutants were capable of interacting with the complementary oppA_MUT variant. Intriguingly, LhrC4_ssMUT partially restored the interaction with oppA_MUT (Fig. 5C) demonstrating a direct interaction between the single-stranded region of LhrC4 and the SD region of oppA RNA. For LhrC_terMUT, only a minor increase in binding to oppA_MUT was observed compared to wild type LhrC4 (Fig. 5D, right). The shift was characterized by smeared bands suggesting the formation of a rather unstable complex between LhrC_terMUT and oppA_MUT possibly due to a disturbed secondary or tertiary structure of LhrC_terMUT. Finally, we tested the binding of a double mutant, LhrC_combiMUT, containing the substitutions of both LhrC4_ssMUT and LhrC4_terMUT, to oppA RNA (Fig. 5E, right). No binding was detected between LhrC4_combiMUT and wild type oppA RNA, whereas a strong interaction between LhrC4_combiMUT and oppA_MUT could be observed. Collectively, these results demonstrate that 2 of 3 CU-rich regions in LhrC4 are implicated in binding of LhrC4 to the SD region of oppA mRNA.
Figure 5.
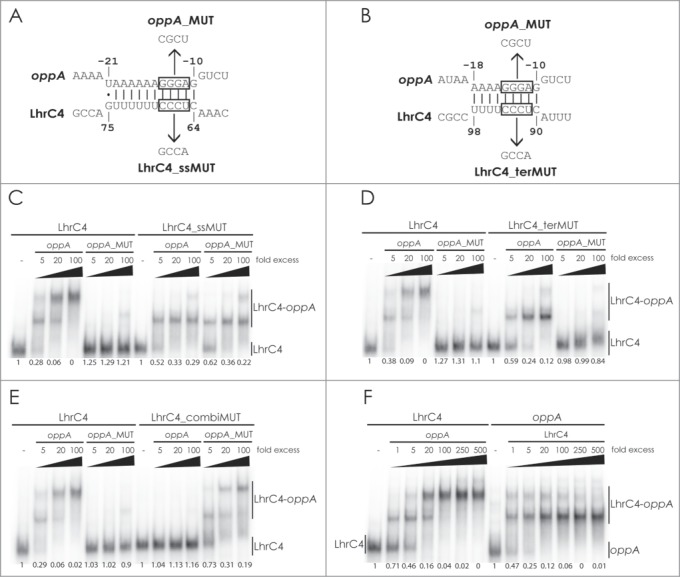
Gel mobility shift assay of the interaction between LhrC and oppA mRNA. Deduced binding of the CU-rich region of the single-stranded stretch (A) or terminator loop (B) of LhrC4 to oppA mRNA. The UCCC motifs in LhrC4 and the complementary GGGA sequence in oppA mRNA are boxed. Sequences of the minimal mutant variants oppA_MUT, LhrC4_ssMUT and LhrC4_terMUT are shown. The double mutant LhrC4_combiMUT contains the substitutions of both LhrC4_ssMUT and LhrC4_terMUT. For gel mobility shift assays, labeled wild type LhrC4 and labeled mutant variants LhrC4_ssMUT (C), LhrC4_terMUT (D) or LhrC4_combiMUT (E) were incubated with increasing concentrations of unlabeled wild type oppA RNA or the mutant variant oppA_MUT. (F) Equal amounts (40 pmol) of labeled LhrC4 (left) or labeled oppA RNA (right) were incubated with increasing concentrations of unlabeled oppA RNA or LhrC4, respectively. The fraction of unbound LhrC4 or oppA RNA is shown below each lane.
In the gel shift assays presented so far, 2 shifted bands appeared upon the addition of increasing levels of oppA RNA to 5′-end labeled LhrC4. Furthermore, mutating one of the CU-rich regions still allowed the formation of a single shifted band. Since two CU-rich regions of LhrC4 can mediate the interaction with oppA mRNA, we hypothesized that the 2 shifted bands might correspond to one and 2 oppA RNA molecules bound to a single LhrC4 molecule, respectively. To further investigate this, we performed gel shift assays using 0.04 pmol 5′-end labeled oppA RNA, adding increasing amounts of unlabeled LhrC4 (Fig. 5F, right). For comparison, samples containing 0.04 pmol 5′-end labeled LhrC4 and increasing amounts of unlabeled oppA RNA were loaded on the same gel (Fig. 5F, left). At a molar ratio of 1:1, approximately half of the 5′-end labeled oppA RNA reside in the first or second shifted bands, whereas the rest remains unbound. When the fold excess of unlabeled LhrC4 increases, the second shifted band disappears and oppA RNA ends up in the first shifted band only. This can be explained by the increasing probability of one LhrC4 molecule binding to only a single oppA molecule with accumulating levels of LhrC4. In the gel shift using 5′-end labeled LhrC4 at a molar ratio of 1:1 with oppA RNA, approximately one third of the sRNA is shifted, mostly to the first shifted band, whereas the residual sRNA remains unbound. With increasing amount of unlabeled oppA RNA, the 5′-end labeled LhrC4 ends up in the second, more slowly migrating band. The explanation for this pattern is that each LhrC4 molecule becomes saturated with 2 oppA molecules when the oppA levels are increasing. Collectively, these results clearly support a model of 2 oppA mRNAs binding to a single LhrC4 sRNA.
LhrC and LisR contribute to infection of J774.A1 mouse macrophage cells
Several of the genes affected by LhrC1-5 encode virulence-associated factors. Furthermore, LhrC1-5 are induced under infection-relevant conditions, such as in whole human blood and during infection of macrophage cells.9,12 These observations prompted us to determine if LhrC1-5 contribute to intracellular survival and multiplication in the macrophage-like murine J774.A1 cell line. Knowing that the 2-component system LisRK contributes to infection in mice26,27 and is essential for the induction of LhrC1-5,18 the ΔlisR strain was included as a control. For this assay, J774.A1 cells were infected with equal numbers of wild type, ΔlhrC1-5 and ΔlisR cells (MOI: 1) for one hour before the addition of gentamicin. The number of intracellular bacteria was determined 2, 4 and 6 hours after the onset of gentamicin treatment. As shown in Figure 6, the ΔlhrC1-5 and ΔlisR cells were impaired in their ability to infect J774.A1 in comparison to the wild type.
Figure 6.
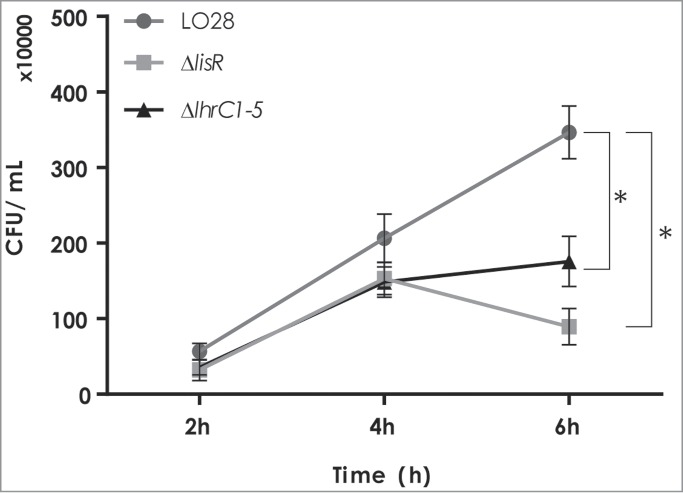
Infection of J774.A1 mouse macrophage cells with L. monocytogenes LO28 wild type, ΔlisR, and ΔlhrC1-5 mutant strains. The J774.A1 cells were infected with the wild type, ΔlisR, and ΔlhrC1-5 mutant strains at a MOI of 1. At 2, 4 and 6 hours after the onset of gentamicin treatment, cells were lysed and plated onto BHI agar plates for CFU counts. Results of the infection assay are the average of 3 biological replicates each conducted in technical duplicates. After 6 hours, a significant difference between wild type and mutant strains was observed (asterisk: P < 0.005).
Discussion
Studies on sRNA-mediated control in bacteria continue to reveal examples of how sRNAs contribute to bacterial adaptation to environmental stresses.1,2,5 In Gram-negative enteric bacteria, the σE-regulated sRNAs MicA, RyhB, and MicL are part of the cell envelope stress response acting to down-regulate the synthesis of all major outer membrane proteins, including porins and the lipoprotein Lpp, when envelope homeostasis is perturbed.28-35 In the Gram-positive pathogen L. monocytogenes, 4 2-component systems, including LisRK, orchestrate the cell envelope stress response by inducing the expression of proteins involved in stress management and cell wall synthesis.19,36-40 In parallel, LisRK also induces the production of LhrC, a multicopy sRNA that acts as a repressor of the cell envelope-associated adhesin LapB in response to cell envelope stress.18 LhrC is known to be highly induced during infection-relevant conditions such as in whole human blood which contains both cellular and non-cellular components participating in host immunity to bacterial pathogens.12 Since surface exposed proteins are recognized by the immune system their down-regulation by LhrC may be viewed as an attempt by L. monocytogenes to evade detection by immune cells. LhrC is also known to be highly induced during infection of macrophage cells9 suggesting a role for LhrC-mediated control in the intracellular environment. In support of this, we showed that a mutant lacking all 5 copies of LhrC was impaired in infection of macrophage-like cells. This adds LhrC to a growing list of sRNAs known to modulate the virulence of L. monocytogenes. Of these, Rli55 was recently shown to act by sequestering the regulatory protein EutV controlling the expression of the eut genes whose products enable ethanolamine utilization.13 Additionally, the sRNA RliB was shown to be a cas-less CRISPR RNA requiring endogenously encoded PNPase enzyme for processing and activity, however the exact function of the RliB-CRISPRs in L. monocytogenes is unknown.41 Other sRNAs contributing to L. monocytogenes virulence include Rli31, Rli33-1, Rli38, Rli50, and Rli60, but their function and mechanism of action remain to be examined.9,12,14 In this study, we show that several cell envelope-associated proteins with virulence function are downregulated in response to cell envelope stress by the multicopy sRNA LhrC. A combination of transcriptomics and proteomics proved to be very well suited for the finding of novel targets of this sRNA. The three targets representing the overlap of the hit lists of both approaches could be confirmed in follow up experiments to be indeed regulated by LhrC, one at the transcriptional level (lmo2349) and tcsA and oppA at the post-transcriptional level via a direct binding to LhrC. The lipoprotein TcsA was first identified as a CD4+ T cell stimulating antigen.22 However, its function in L. monocytogenes is unknown. OppA is involved in transport of oligopeptides and is required for growth of L. monocytogenes at low temperature.23 The significance of both genes for the virulence of L. monocytogenes23,42 is reinforced by the fact that both are also upregulated by the key virulence regulator PrfA.42 Following the lapB gene, now tcsA and oppA expand the LhrC regulon by 2 additional cell envelope-associated proteins with virulence function and turn LhrC into a sRNA functioning as a fine-tuner with respect to virulence gene expression.
Samples for the transcriptomics analysis were taken after 30 min of stress to avoid the detection of indirect effects. However, for the previously identified target of LhrC, lapB, only a small effect could be observed after 30 min, possibly due to a delayed induction of LhrC upon cell envelope stress.18 Differences between wild type and mutant are rather small in the transcriptomics study even for subsequently confirmed targets. Therefore, it cannot be ruled out that LhrC regulated genes that would turn up in transcriptomics analysis, when cells are harvested at later time points, was overlooked. In the proteomics study, samples were collected after 60 min of cefuroxime stress, since regulatory effects on proteome level are generally expected to occur time-delayed to RNA regulation. Because the transcriptomics approach was based on an array featuring all annotated genes, one can assume that essentially all RNAs present in a sample have been detected. In contrast, a detection of all protein species in a cell still represents an analytical challenge entailing a missed detection of possible LhrC targets. For instance LapB, known to be regulated by LhrC, could only be detected in mutant cells in the first replicate of the proteomics study. We therefore anticipate more LhrC targets among the proteins that were found in only one or 2 of the replicates but showed different amounts in wild type vs. mutant (Table S3).
Each of the 5 LhrC molecules contains 3 UCCC motifs located in 2 loops and the single-stranded stretch between the 2 stem loops. All three regions in LhrC4 are capable of binding the SD region of lapB mRNA.18 In addition, an extended LhrC4-lapB mRNA duplex is formed involving an opening of stem loop A and base pairing of the 5′-side of stem A to lapB mRNA.18 Interestingly, loop A did not contribute to the interaction between LhrC4 and oppA mRNA. Mutations in loop A did not alter the binding of LhrC4 to oppA mRNA and no major structural changes in stem loop A were observed. In line with this, the UCCC motif in loop A was not predicted to interact with the SD region (Fig. 3C, left). Instead, interaction between LhrC4 and the SD region of oppA mRNA strictly relies on the UCCC motifs in the single-stranded region and the terminator loop (illustrated in Fig. 7). These findings demonstrate that different binding schemes are followed for the interaction of LhrC4 with lapB and oppA, respectively. The UCCC motif is present in several trans-encoded sRNAs in Gram-positive bacteria, including the Rsa family members and RNAIII in Staphylococcus aureus.43-45 These conserved motifs are located in accessible regions and contribute to the initial base pairing between the sRNA and its target mRNAs. The mode of action of LhrC on lapB and oppA clearly support the view that UCCC motifs are specific to sRNAs acting to recognize the AG-rich SD regions of mRNAs resulting in repression of translation. With respect to UCCC motifs, LhrC is outstanding, being present in 5 copies, each carrying the specific motif at 3 sites. Strikingly, we could show that LhrC4 employs 2 of its 3 UCCC motifs to bind 2 copies of the oppA mRNA (Fig. 7). Different flanking regions adjacent to the UCCC motifs provide LhrC with a high degree of flexibility in terms of binding to SD regions of various target mRNAs. Having multiple binding sites for identical or even different target mRNAs in a multicopy sRNA may permit a very sensitive reaction acting to turn a small input signal into a large output response. According to intensities on the microarray and emPAI values of the proteomics study, oppA represents a highly abundant mRNA and protein, respectively. LhrC is not detectable under control conditions, but first produced with the onset of stress. This means nascent LhrC molecules will meet an excess of oppA mRNAs in this moment. The ability to bind more than one oppA mRNA molecule would accelerate the effect of LhrC. Future experiments have to be performed in order to address if simultaneous binding of several target mRNAs to a single LhrC molecule occurs in vivo. This involves studies of mutant variants of all 5 LhrC copies, addressing the in vivo importance of all possible interactions of LhrC1-5 with their targets, including tcsA. Since LhrC is predicted to bind far upstream from the RBS of tcsA, the regulatory effect of LhrC on tcsA may represent a new class of a long-ranging sRNA in L. monocytogenes. As proposed for the sRNA IstR-1 in Escherichia coli, an antisense RNA may inhibit translation of its target mRNA by binding to an upstream ribosome loading or “standby” site.46,47 In this model of regulation, IstR-1 competes with ribosomes by base pairing to a ribosome standby site located approximately 100 nucleotides upstream from the tisB RBS.
Figure 7.
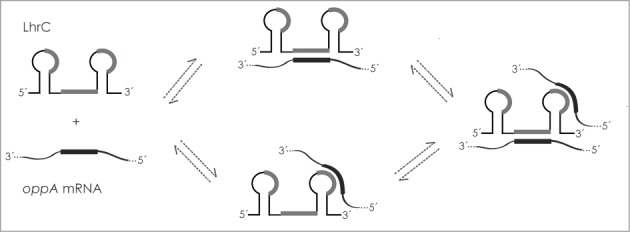
Model of LhrC-oppA mRNA interaction. Two CU-rich sequences in the single-stranded region and the terminator loop of LhrC are capable of binding the AG-rich SD region of oppA mRNA. When increasing the level of oppA mRNA relative to LhrC, one LhrC molecule may bind 2 oppA mRNA molecules simultaneously. The CU-rich sequence in loop A does not contribute to the binding. CU-rich sequences in LhrC predicted to interact with oppA mRNA, as shown in Figure 3C, are marked in gray. The binding region in oppA mRNA is marked in black.
Collectively, future studies on the action of LhrC on additional targets are likely to reveal more binding schemes for the sRNA interacting with SD sequences, and additionally alternative mechanisms by which this multicopy sRNA may exert its regulatory effect are anticipated. In this regard, a role of Hfq for the interaction of LhrC with a still unknown target might be found which would eventually answer the purpose of LhrC's Hfq binding capability.
Experimental Procedures
Bacterial strains and growth conditions
The wild type strain used in this study was L. monocytogenes serotype 1/2c strain LO28.48 The isogenic mutant derivatives ΔlhrC1-5, ΔlhrC1-4, ΔlhrC5, and ΔlisR were constructed in previous work.18,40 L. monocytogenes was routinely grown at 37°C with shaking in brain heart infusion broth (BHI, Oxoid). When appropriate, cultures were supplemented with kanamycin (50 µg/ml) or cefuroxime (4 µg/ml). For cloning of plasmid vectors Escherichia coli TOP10 cells (Invitrogen) were grown at 37°C with shaking in Luria-Bertani broth supplemented with 50 µg/ml kanamycin.
Plasmid constructions and β-galactosidase assays
To examine the influence of LhrC1-5 on transcription of lmo2351, we used the promoter-less, lacZ transcriptional fusion vector pTCV-lac.24 The promoter region of lmo2351 not including the SD sequence was acquired by PCR from chromosomal DNA using the primers EcoRI_F_lmo2351_promotor and BamHI_R2_lmo2351_promoter (Table S4). The fragment was digested with EcoRI and BamHI and ligated into pTCV-lac.
To monitor post-transcriptional effects on potential LhrC target genes, the vector pCK-lac was used.16 pCK is a derivate of pTCV-lac containing a promoter-less lacZ gene without a start codon and SD sequence allowing the construction of in-frame fusions to lacZ. In-frame translational lacZ fusions of tcsA and oppA, preceded by a moderate core promoter, were constructed in pCK-lac as previously described18 with a few modifications: DNA fragments containing the moderate lhrA core promoter,17 as well as the region ranging from the transcriptional start site into the first codons of the gene of interest, were acquired by PCR. The PCR-products were digested with EcoRI and BamHI and ligated into pCK-lac. All primers and additional information are listed in Supplemental Table 4. β-galactosidase assay was carried out as described previously.49
RNA isolation and purification
Total RNA was routinely extracted as previously described with a few modifications.18 In short, bacterial cells were harvested by centrifugation for 3 min at 5000g at 4°C and snap-freezed in liquid nitrogen. Cells were interrupted by the FastPrep instrument (Bio101, Thermo Scientific Corporation) and total RNA was extracted using TRIzol Reagent (Ambion) as previously described.16 The integrity, concentration and purity of the RNA were confirmed by agarose gel electrophoresis and NanoDrop 2000.
Primer extensions
The primer extension experiments were performed as previously described. 49 32P-labeled primers used for detection of tcsA and oppA transcription start sites are listed in Supplemental Table 4.
Northern blotting
Northern blotting analysis was performed as previously described with few modifications.50 Briefly, 20 µg of total RNA was separated on a formaldehyde agarose gel for 4 hours prior to capillary blotting on a Hybond-N membrane (GE-healthcare). The membrane was hybridized with 32P-labeled DNA probes (Megaprime DNA labeling system, Amersham, GE-healthcare). The primers used for PCR amplification of the DNA probes are listed in Supplemental Table 4. For LhrC, a 32P-endlabeled single-stranded probe was employed (Table S4).
Microarray analysis
Triplicate overnight cultures of L. monocytogenes LO28 wild type cells and ΔlhrC1-5 cells were diluted to a start OD600 of 0.02 in fresh liquid medium and grown to an OD600 of 0.35 (mid-exponential phase). Each culture was split and 4 µg/ml of cefuroxime was added to one of the samples. The samples were incubated for 30 min at 37°C with shaking (190 rpm). 10 ml of each sample were treated with 2 volumes of RNA protect Bacteria Reagent (Quiagen) as recommended by manufacturer. The samples were vortexed and incubated at room temperature for 5 min followed by centrifugation 10 min (5000g). Pellets were stored at −80°C and RNA was extracted by the TRIzol protocol (described above).
DNase treatment was performed using 20 units of DNase 1 recombinant RNase-free (Roche) in the presence of 200 units RNasin Plus (Promega). Subsequently, RNA was purified by one phenol-chloroform extraction and one chloroform extraction followed by RNA precipitation. The RNA was re-suspended in RNase free water (Ambion) and stored at −80°C. RNA was quantified using a NanoDrop 2000 and the quality was assessed by the Agilent 2100 Bioanalyzer utilizing the RNA 6000 LabChip kit.
The commercially available Roche NimbleGen high density single 385K array (catalog number A4287-00-01, Roche NimbleGen, Inc.., Madison, WI) was used for the microarray analysis. It was designed with 60-mer probes targeting 2846 L. monocytogenes EGD-e open reading frames.
Expression data were normalized by Robust Multi-array Analysis (RMA). A modified t-test was applied across gene expression results of the 3 biological replicates using the Benjamini-Hochberg correction to control the False Discovery Rate. Significant differentially expressed genes (95% confidence) with a cut-off value of 1.5 for up- and down-regulated genes were accepted.
The complete microarray data set is available at the NCBI Gene Expression Omnibus (GEO) database under accession number GSE68996.
Reverse transcriptase-quantitative polymerase chain reaction (RT-qPCR)
Fifty µg of total RNA was DNase-treated according to the manufacturer (Roche) in the presence of 80 units of RNasin Plus (Promega). From each samples 3 µg of RNA was used for cDNA synthesis using the Maxima First Strand cDNA Synthesis Kit (Fermentas) in the presence of 20 units RNasin Plus (Promega). RT-qPCR was performed using SYBR Green PCR Master Mix (Fermentas) and specific primer sets for the genes of interest (Table S4). The primer sets were generated by the online designer tool Sigma OligoArchitect and were designed to target approximately 100 base pair long amplicons. The samples were run on a MX3000 quantitative PCR thermocycler (Stratagene) with an initial step at 95°C for 10 min, 40 cycles of 15 s at 95°C, 30 s at 60°C and 30 s at 72°C. Data were analyzed by the use of the Relative Expression Software Tool - Multiple Condition Solver REST-MCS version 2.51 The tpi and rpoB genes served as reference genes. RNA from 2 independent biological experiments was analyzed in technical duplicates.
Preparation of protein extracts and 1D-SDS-PAGE
Cells were grown in 1 l of BHI up to an OD600 of 0.3, stressed with 4 µg/ml cefuroxime for 1 h and harvested by centrifugation (8000 × g) for 10 min at 4°C. Cell pellets were washed with ice-cold TBS buffer (50 mM Tris, 150 mM NaCl, pH 8.0) with protease inhibitor (cOmplete ULTRA Tablets, Mini, Roche) and a second time with TBS with protease inhibitor without EDTA (cOmplete ULTRA Tablets, Mini, EDTA-free, Roche). Cells were resuspended in lysis buffer (20 mM Tris-HCl, 10 mM MgCl2, 1mM CaCl2 pH 7.5) containing protease inhibitor without EDTA and cracked in a FastPrep cell disrupter (Bio101, Thermo Scientific Corporation). DNase I (200 u/ml sample; grade II from bovine pancreas, Roche) and RNase (10 u/mL sample; crude mixture of RNases from bovine pancreas, Roche) were added to the lysate which was incubated 20 min at 37°C to allow for nucleolytic digestion. Cell debris and glass beads were removed by centrifugation (8000 × g, 4 °C, 10 min). Protein concentration of the supernatant was determined by a BCA assay (Pierce BCA Protein Assay Kit, Life technologies). The crude protein extract was ultracentrifuged (100,000 × g, 1 hour, 4°C). The pellet containing the crude membrane fraction was homogenized in 2 ml of ice-cold high salt buffer (20 mM Tris-HCl, 1 M NaCl, protease inhibitor, pH 7.5) using a hand homogenizer (VWR PTFE Tissue Grinder, 2 ml capacity) and filled up to 6 ml with high salt buffer. The sample was incubated for 1 hour at 4°C on a rotary shaker before the membrane fraction was pelleted again by ultracentrifugation. The pellet was solubilized in 2 ml of ice-cold carbonate buffer using the homogenizer, filled up to 6 ml with carbonate buffer (100 mM Na2CO3, 100 mM NaCl, pH 11), incubated for 1 hour at 4°C on an overhead shaker and again ultracentrifuged. The purified membrane pellet was solubilized in 500 µl 50 mM Tris-HCl, 8 M urea, 1% CHAPS, pH 7.5 and the concentration of proteins determined. Twenty µg of proteins were separated via 1D-SDS-PAGE (NuPAGE Novex 4-12% Bis-Tris Midi Gels, Life Technologies) at 150 V. Proteins were visualized by Coomassie staining. A full gel lane was cut into 10 pieces and proteins prepared for LC-MS/MS analysis as described before.52
LC-MS/MS analysis and data evaluation
Peptide mixtures resulting from protein in-gel digestion were separated by C18 nano reversed-phase chromatography and analyzed in an Orbitrap XL mass spectrometer as described before.53 Raw MS data were searched against a Listeria monocytogenes EGD-e database using Mascot version 2.3.02 (Matrix Science Ltd.). In the database search one missed cleavage of trypsin was allowed, cysteine was considered statically carbamidomethylated, methionine variably oxidized and asparagine and glutamine possibly deamidated. Tolerance of mass deviation was 10 ppm for the peptide precursor and 0.8 Da for fragment ions. The significance threshold was adjusted to P < 0.01 to obtain a false discovery rate of less than 2% on the peptide level as estimated from decoy database searching. Results of the 10 data files (originating from the 10 gel slices) belonging to one protein sample were merged by Mascot which also provided an emPAI value21 indicating the abundance of every identified protein (Table S5). In order to find differences in protein abundance between wild type and mutant, single emPAI values were normalized by the sum of emPAI values of all proteins in the specific sample. Subsequently, the ratio of emPAI mutant / emPAI wild type was formed for each protein (only possible if protein was identified with 2 peptides in wild type and in ΔlhrC1-5). The entire analysis was carried out in 3 biological replicates (Table S3).
Bioinformatics prediction of sRNA-mRNA interaction
The IntaRNA software25,54 was used for predicting interactions between LhrC1-5 and target mRNAs. Full length sequences of sRNAs and targets were employed. The predicted interaction between LhrC1-5 and tcsA mRNA is shown in Supplemental Figure 2. The predicted interaction between LhrC1-5 and oppA mRNA is shown in Supplemental Figure 3.
Electrophoretic mobility shift assays (EMSA)
Templates for in vitro transcription carried a T7 RNA polymerase binding site at their 5′-end and were generated by PCR. The primers used are listed in Supplemental Table 4. In vitro transcription, RNA purification, dephosphorylation and labeling was performed as described previously.18 EMSAs were done according to Nielsen et al.16 In brief, 40 fmol 5′-end labeled RNA was incubated with excess of non-labeled RNA in the presence of non-specific tRNA for 1 hour at 37°C followed by 10 min on ice. For EMSAs where the role Hfq was studied, purified Hfq protein6 was added to a final concentration of 10 µM to the indicated samples and all of them were incubated for 20 min at 37°C followed by 10 min on ice. Samples were separated on a 5% non-denaturing polyacrylamide gel at 4°C.
In vitro structure probing
5′-end labeled RNAs were prepared as described for EMSA experiments. The assay was performed as previously described18 with some deviations: T1 control sample was incubated with 0.2 U of T1 RNase (Ambion). Structure probing RNA interactions were incubated for 1 hour at 37°C before treating the samples with the indicated cleaving agent: 0.2 U T1 RNase for 5 min, 0.02 U V1 RNase (Ambion) for 2 min, 25 mM lead(II) acetate (Fluka) for 1 min and 20 U S1 nuclease (Promega) for 1 min. S1 nuclease samples were incubated in the S1 nuclease buffer (Promega). Samples were mixed with 2 × loading buffer type II (Ambion) and 5 µl of each sample was separated on an 8% denaturing polyacrylamide gel.
Intracellular infection assay
J774.A1 murine macrophage-like cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Invitrogen). The day before the infection assay J774.A1 cells were seeded out in 24-well tissue culture plates to obtain monolayers (˜1×105 cell per well). Cells were incubated for 24 hours at 37°C in the presence of 5% CO2.
Bacteria colonies from freshly streaked colonies of LO28 wild type, LO28ΔlisR and LO28ΔlhrC1-5 were inoculated overnight in 5 ml cultures at 37°C. The overnight cultures were diluted in fresh BHI to an OD600 of 0.02 and grown at 37°C with shaking to an OD600 of 1.0 (cell concentrations: 2 × 108 CFU/ml for all strains). Bacterial cells were washed in phosphate-buffered saline (PBS) and added to the cell monolayers at a multiplicity of infection (MOI) of 1 per eukaryotic cell. After one hour of incubation, each infected monolayer was washed with PBS. The infected monolayers were overlaid with cell culture medium containing 50 μg/ml gentamicin to kill extracellular bacteria and the plates were incubated for 2, 4 and 6 hours in a humidified incubator (5% CO2).
After incubation the monolayers were washed twice with PBS and eukaryotic cells were lysed by adding 1 ml of PBS + 1% Triton X-100 to each well. Bacterial cells were plated in appropriate dilutions on BHI agar plates and CFU were determined after 24 hours of incubation at 37°C. The CFU of PBS washed bacteria added to the cell monolayers was determined as well. Each experiment was carried out in technical duplicates and repeated 3 times. A two-tailed t-test was applied for statistical analysis (p<0.05).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Eva Maria Sternkopf Lillebæk for excellent technical assistance.
Funding
This work was supported by The German Research Foundation [Si 1678/1–1 to S.S.]; The Danish Council for Independent Research │Natural Sciences [12–124735 to B.H.K.]; The Lundbeck Foundation [R100-A9235 to B.H.K.]; and The Novo Nordisk Foundation [to B.H.K.]. J. J. was supported by Umeå University, the Swedish Research Council grants K2011-56X-15144-08-6 and 621-2012-2451 and an ERC starting grant no 260764 – RNAntibiotics.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1.Waters LS, Storz G. Regulatory RNAs in bacteria. Cell 2009; 136:615-28; PMID:19239884; http://dx.doi.org/ 10.1016/j.cell.2009.01.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Storz G, Vogel J, Wassarman KM. Regulation by small RNAs in bacteria: Expanding frontiers. Mol Cell 2011; 43:880-91; PMID:21925377; http://dx.doi.org/ 10.1016/j.molcel.2011.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gripenland J, Netterling S, Loh E, Tiensuu T, Toledo-Arana A, Johansson J. RNAs: regulators of bacterial virulence. Nat Rev Microbiol 2010; 8:857-66; PMID:21079634; http://dx.doi.org/ 10.1038/nrmicro2457 [DOI] [PubMed] [Google Scholar]
- 4.Papenfort K, Vogel J. Regulatory RNA in bacterial pathogens. Cell Host Microbe 2010; 8:116-27; PMID:20638647; http://dx.doi.org/ 10.1016/j.chom.2010.06.008 [DOI] [PubMed] [Google Scholar]
- 5.Papenfort K, Vogel J. Small RNA functions in carbon metabolism and virulence of enteric pathogens. Front Cell Infect Microbiol 2014; 4:91; PMID:25077072; http://dx.doi.org/ 10.3389/fcimb.2014.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christiansen JK, Nielsen JS, Ebersbach T, Valentin-Hansen P, Sogaard-Andersen L, Kallipolitis BH. Identification of small Hfq-binding RNAs in Listeria monocytogenes. RNA 2006; 12:1383-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nielsen JS, Olsen AS, Bonde M, Valentin-Hansen P, Kallipolitis BH. Identification of a sigma B-dependent small noncoding RNA in Listeria monocytogenes. J Bacteriol 2008; 190:6264-70; PMID:18621897; http://dx.doi.org/ 10.1128/JB.00740-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mandin P, Repoila F, Vergassola M, Geissmann T, Cossart P. Identification of new noncoding RNAs in Listeria monocytogenes and prediction of mRNA targets. Nucleic Acids Res 2007; 35:962-74; PMID:17259222; http://dx.doi.org/ 10.1093/nar/gkl1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mraheil MA, Billion A, Mohamed W, Mukherjee K, Kuenne C, Pischimarov J, Krawitz C, Retey J, Hartsch T, Chakraborty T, et al.. The intracellular sRNA transcriptome of Listeria monocytogenes during growth in macrophages. Nucleic Acids Res 2011; 39:4235-48; PMID:21278422; http://dx.doi.org/ 10.1093/nar/gkr033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Behrens S, Widder S, Mannala GK, Qing X, Madhugiri R, Kefer N, Abu Mraheil M, Rattei T, Hain T. Ultra deep sequencing of Listeria monocytogenes sRNA transcriptome revealed new antisense RNAs. PloS One 2014; 9:e83979; PMID:24498259; http://dx.doi.org/ 10.1371/journal.pone.0083979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliver HF, Orsi RH, Ponnala L, Keich U, Wang W, Sun Q, Cartinhour SW, Filiatrault MJ, Wiedmann M, Boor KJ. Deep RNA sequencing of L. monocytogenes reveals overlapping and extensive stationary phase and sigma B-dependent transcriptomes, including multiple highly transcribed noncoding RNAs. BMC Genomics 2009; 10:641; PMID:20042087; http://dx.doi.org/ 10.1186/1471-2164-10-641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toledo-Arana A, Dussurget O, Nikitas G, Sesto N, Guet-Revillet H, Balestrino D, Loh E, Gripenland J, Tiensuu T, Vaitkevicius K, et al. The Listeria transcriptional landscape from saprophytism to virulence. Nature 2009; 459:950-6; PMID:19448609; http://dx.doi.org/ 10.1038/nature08080 [DOI] [PubMed] [Google Scholar]
- 13.Mellin JR, Koutero M, Dar D, Nahori MA, Sorek R, Cossart P. Riboswitches. Sequestration of a two-component response regulator by a riboswitch-regulated noncoding RNA. Science 2014; 345:940-3; PMID:25146292; http://dx.doi.org/ 10.1126/science.1255083 [DOI] [PubMed] [Google Scholar]
- 14.Peng YL, Meng QL, Qiao J, Xie K, Chen C, Liu TL, Hu ZX, Ma Y, Cai XP, Chen CF. The roles of noncoding RNA Rli60 in regulating the virulence of Listeria monocytogenes. J Microbiol Immunol Infect 2014; http://dx.doi.org/ 10.1016/j.jmii.2014.08.017 [DOI] [PubMed] [Google Scholar]
- 15.Quereda JJ, Ortega AD, Pucciarelli MG, Garcia-Del Portillo F. The Listeria Small RNA Rli27 Regulates a Cell Wall Protein inside Eukaryotic Cells by Targeting a Long 5′-UTR Variant. PLoS Genet 2014; 10:e1004765; PMID:25356775; http://dx.doi.org/ 10.1371/journal.pgen.1004765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nielsen JS, Lei LK, Ebersbach T, Olsen AS, Klitgaard JK, Valentin-Hansen P, Kallipolitis BH. Defining a role for Hfq in Gram-positive bacteria: evidence for Hfq-dependent antisense regulation in Listeria monocytogenes. Nucleic Acids Res 2010; 38:907-19; PMID:19942685; http://dx.doi.org/ 10.1093/nar/gkp1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nielsen JS, Larsen MH, Lillebaek EM, Bergholz TM, Christiansen MH, Boor KJ, Wiedmann M, Kallipolitis BH. A small RNA controls expression of the chitinase ChiA in Listeria monocytogenes. PloS One 2011; 6:e19019; PMID:21533114; http://dx.doi.org/ 10.1371/journal.pone.0019019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sievers S, Sternkopf Lillebaek EM, Jacobsen K, Lund A, Mollerup MS, Nielsen PK, Kallipolitis BH. A multicopy sRNA of Listeria monocytogenes regulates expression of the virulence adhesin LapB. Nucleic Acids Res 2014; 42:9383-98; PMID:25034691; http://dx.doi.org/ 10.1093/nar/gku630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nielsen PK, Andersen AZ, Mols M, van der Veen S, Abee T, Kallipolitis BH. Genome-wide transcriptional profiling of the cell envelope stress response and the role of LisRK and CesRK in Listeria monocytogenes. Microbiology 2012; 158:963-74; PMID:22282521; http://dx.doi.org/ 10.1099/mic.0.055467-0 [DOI] [PubMed] [Google Scholar]
- 20.Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol 2001; 305:567-80; PMID:11152613; http://dx.doi.org/ 10.1006/jmbi.2000.4315 [DOI] [PubMed] [Google Scholar]
- 21.Ishihama Y, Oda Y, Tabata T, Sato T, Nagasu T, Rappsilber J, Mann M. Exponentially modified protein abundance index (emPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol Cell Proteomics 2005; 4:1265-72; PMID:15958392; http://dx.doi.org/ 10.1074/mcp.M500061-MCP200 [DOI] [PubMed] [Google Scholar]
- 22.Sanderson S, Campbell DJ, Shastri N. Identification of a CD4+ T cell-stimulating antigen of pathogenic bacteria by expression cloning. J Exp Med 1995; 182:1751-7; PMID:7500019; http://dx.doi.org/ 10.1084/jem.182.6.1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borezee E, Pellegrini E, Berche P. OppA of Listeria monocytogenes, an oligopeptide-binding protein required for bacterial growth at low temperature and involved in intracellular survival. Infect Immun 2000; 68:7069-77; PMID:11083832; http://dx.doi.org/ 10.1128/IAI.68.12.7069-7077.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poyart C, Trieu-Cuot P. A broad-host-range mobilizable shuttle vector for the construction of transcriptional fusions to beta-galactosidase in gram-positive bacteria. FEMS Microbiol Lett 1997; 156:193-8; PMID:9513264; http://dx.doi.org/ 10.1016/S0378-1097(97)00423-0 [DOI] [PubMed] [Google Scholar]
- 25.Busch A, Richter AS, Backofen R. IntaRNA: efficient prediction of bacterial sRNA targets incorporating target site accessibility and seed regions. Bioinformatics 2008; 24:2849-56; PMID:18940824; http://dx.doi.org/ 10.1093/bioinformatics/btn544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cotter PD, Emerson N, Gahan CG, Hill C. Identification and disruption of lisRK, a genetic locus encoding a two-component signal transduction system involved in stress tolerance and virulence in Listeria monocytogenes. J Bacteriol 1999; 181:6840-3; PMID:10542190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kallipolitis BH, Ingmer H. Listeria monocytogenes response regulators important for stress tolerance and pathogenesis. FEMS Microbiol Lett 2001; 204:111-5; PMID:11682188; http://dx.doi.org/ 10.1111/j.1574-6968.2001.tb10872.x [DOI] [PubMed] [Google Scholar]
- 28.Guo MS, Updegrove TB, Gogol EB, Shabalina SA, Gross CA, Storz G. MicL, a new sigmaE-dependent sRNA, combats envelope stress by repressing synthesis of Lpp, the major outer membrane lipoprotein. Genes Dev 2014; 28:1620-34; PMID:25030700; http://dx.doi.org/ 10.1101/gad.243485.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johansen J, Rasmussen AA, Overgaard M, Valentin-Hansen P. Conserved small non-coding RNAs that belong to the sigmaE regulon: role in down-regulation of outer membrane proteins. J Mol Biol 2006; 364:1-8; PMID:17007876; http://dx.doi.org/ 10.1016/j.jmb.2006.09.004 [DOI] [PubMed] [Google Scholar]
- 30.Papenfort K, Pfeiffer V, Mika F, Lucchini S, Hinton JC, Vogel J. SigmaE-dependent small RNAs of Salmonella respond to membrane stress by accelerating global omp mRNA decay. Mol Microbiol 2006; 62:1674-88; PMID:17427289; http://dx.doi.org/ 10.1111/j.1365-2958.2006.05524.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papenfort K, Bouvier M, Mika F, Sharma CM, Vogel J. Evidence for an autonomous 5′ target recognition domain in an Hfq-associated small RNA. Proc Natl Acad Sci USA 2010; 107:20435-40; PMID:21059903; http://dx.doi.org/ 10.1073/pnas.1009784107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rasmussen AA, Eriksen M, Gilany K, Udesen C, Franch T, Petersen C, Valentin-Hansen P. Regulation of ompA mRNA stability: the role of a small regulatory RNA in growth phase-dependent control. Mol Microbiol 2005; 58:1421-9; PMID:16313626; http://dx.doi.org/ 10.1111/j.1365-2958.2005.04911.x [DOI] [PubMed] [Google Scholar]
- 33.Thompson KM, Rhodius VA, Gottesman S. SigmaE regulates and is regulated by a small RNA in Escherichia coli. J Bacteriol 2007; 189:4243-56; PMID:17416652; http://dx.doi.org/ 10.1128/JB.00020-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Udekwu KI, Wagner EG. Sigma E controls biogenesis of the antisense RNA MicA. Nucleic Acids Res 2007; 35:1279-88; PMID:17267407; http://dx.doi.org/ 10.1093/nar/gkl1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Udekwu KI, Darfeuille F, Vogel J, Reimegard J, Holmqvist E, Wagner EG. Hfq-dependent regulation of OmpA synthesis is mediated by an antisense RNA. Genes Dev 2005; 19:2355-66; PMID:16204185; http://dx.doi.org/ 10.1101/gad.354405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cotter PD, Guinane CM, Hill C. The LisRK signal transduction system determines the sensitivity of Listeria monocytogenes to nisin and cephalosporins. Antimicrob Agents Chemother 2002; 46:2784-90; PMID:12183229; http://dx.doi.org/ 10.1128/AAC.46.9.2784-2790.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fritsch F, Mauder N, Williams T, Weiser J, Oberle M, Beier D. The cell envelope stress response mediated by the LiaFSRLm three-component system of Listeria monocytogenes is controlled via the phosphatase activity of the bifunctional histidine kinase LiaSLm. Microbiology 2011; 157:373-86; PMID:21030435; http://dx.doi.org/ 10.1099/mic.0.044776-0 [DOI] [PubMed] [Google Scholar]
- 38.Mandin P, Fsihi H, Dussurget O, Vergassola M, Milohanic E, Toledo-Arana A, Lasa I, Johansson J, Cossart P. VirR, a response regulator critical for Listeria monocytogenes virulence. Mol Microbiol 2005; 57:1367-80; PMID:16102006; http://dx.doi.org/ 10.1111/j.1365-2958.2005.04776.x [DOI] [PubMed] [Google Scholar]
- 39.Stack HM, Sleator RD, Bowers M, Hill C, Gahan CG. Role for HtrA in stress induction and virulence potential in Listeria monocytogenes. Appl Environ Microbiol 2005; 71:4241-7; PMID:16085809; http://dx.doi.org/ 10.1128/AEM.71.8.4241-4247.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gottschalk S, Bygebjerg-Hove I, Bonde M, Nielsen PK, Nguyen TH, Gravesen A, Kallipolitis BH. The two-component system CesRK controls the transcriptional induction of cell envelope-related genes in Listeria monocytogenes in response to cell wall-acting antibiotics. J Bacteriol 2008; 190:4772-6; PMID:18456805; http://dx.doi.org/ 10.1128/JB.00015-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sesto N, Touchon M, Andrade JM, Kondo J, Rocha EP, Arraiano CM, Archambaud C, Westhof E, Romby P, Cossart P. A PNPase dependent CRISPR System in Listeria. PLoS Genetics 2014; 10:e1004065; PMID:24415952; http://dx.doi.org/ 10.1371/journal.pgen.1004065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Port GC, Freitag NE. Identification of novel Listeria monocytogenes secreted virulence factors following mutational activation of the central virulence regulator, PrfA. Infect Immun 2007; 75:5886-97; PMID:17938228; http://dx.doi.org/ 10.1128/IAI.00845-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chabelskaya S, Bordeau V, Felden B. Dual RNA regulatory control of a Staphylococcus aureus virulence factor. Nucleic Acids Res 2014; 42:4847-58; PMID:24510101; http://dx.doi.org/ 10.1093/nar/gku119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geissmann T, Chevalier C, Cros MJ, Boisset S, Fechter P, Noirot C, Schrenzel J, Francois P, Vandenesch F, Gaspin C, et al.. A search for small noncoding RNAs in Staphylococcus aureus reveals a conserved sequence motif for regulation. Nucleic Acids Res 2009; 37:7239-57; PMID:19786493; http://dx.doi.org/ 10.1093/nar/gkp668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boisset S, Geissmann T, Huntzinger E, Fechter P, Bendridi N, Possedko M, Chevalier C, Helfer AC, Benito Y, Jacquier A, et al. Staphylococcus aureus RNAIII coordinately represses the synthesis of virulence factors and the transcription regulator Rot by an antisense mechanism. Genes Dev 2007; 21:1353-66; PMID:17545468; http://dx.doi.org/ 10.1101/gad.423507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Darfeuille F, Unoson C, Vogel J, Wagner EG. An antisense RNA inhibits translation by competing with standby ribosomes. Mol Cell 2007; 26:381-92; PMID:17499044; http://dx.doi.org/ 10.1016/j.molcel.2007.04.003 [DOI] [PubMed] [Google Scholar]
- 47.Unoson C, Wagner EG. Dealing with stable structures at ribosome binding sites: bacterial translation and ribosome standby. RNA Biol 2007; 4:113-7; PMID:18094628; http://dx.doi.org/ 10.4161/rna.4.3.5350 [DOI] [PubMed] [Google Scholar]
- 48.Vazquez-Boland JA, Kocks C, Dramsi S, Ohayon H, Geoffroy C, Mengaud J, Cossart P. Nucleotide sequence of the lecithinase operon of Listeria monocytogenes and possible role of lecithinase in cell-to-cell spread. Infect Immun 1992; 60:219-30; PMID:1309513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Christiansen JK, Larsen MH, Ingmer H, Sogaard-Andersen L, Kallipolitis BH. The RNA-binding protein Hfq of Listeria monocytogenes: role in stress tolerance and virulence. J Bacteriology 2004; 186:3355-62; http://dx.doi.org/ 10.1128/JB.186.11.3355-3362.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sheehan B, Klarsfeld A, Msadek T, Cossart P. Differential activation of virulence gene expression by PrfA, the Listeria monocytogenes virulence regulator. J Bacteriol 1995; 177:6469-76; PMID:7592422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 2002; 30:e36; PMID:11972351; http://dx.doi.org/ 10.1093/nar/30.9.e36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem 1996; 68:850-8; PMID:8779443; http://dx.doi.org/ 10.1021/ac950914h [DOI] [PubMed] [Google Scholar]
- 53.Sanchez-Perez R, Belmonte FS, Borch J, Dicenta F, Moller BL, Jorgensen K. Prunasin hydrolases during fruit development in sweet and bitter almonds. Plant Physiol 2012; 158:1916-32; PMID:22353576; http://dx.doi.org/ 10.1104/pp.111.192021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wright PR, Georg J, Mann M, Sorescu DA, Richter AS, Lott S, Kleinkauf R, Hess WR, Backofen R. CopraRNA and IntaRNA: predicting small RNA targets, networks and interaction domains. Nucleic Acids Res 2014; 42:W119-23; PMID:24838564; http://dx.doi.org/ 10.1093/nar/gku359 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


