Abstract
Objective
Drawing on theories of bidirectional influence between relationship partners (Butler, 2011; Diamond & Aspinwall, 2003), the authors applied dyadic analytic methods to test convergence in cortisol patterns over time in newlywed couples.
Methods
Previous studies of bidirectional influence in couples’ cortisol levels (Liu, Rovine, Klein, & Almeida, 2013, Papp, Pendry, Simon, & Adam, 2013; Saxbe & Repetti, 2010) found significant covariation in couples’ daily cortisol levels over several days, but no studies have tested whether cortisol response similarity increases over time using a longitudinal design. In the present study, 183 opposite-sex couples (366 participants) engaged in a conflict discussion in a laboratory visit about 6 months after their marriage, and again about 2 years into the marriage. At each visit, spouses provided saliva samples that indexed cortisol levels before, during, and after the discussion. This multi-measure procedure enabled modeling of spouses’ cortisol trajectories around the conflict discussion.
Results
Findings showed significant convergence in couples’ cortisol trajectories across the early years of marriage; couples showed significantly greater similarity in cortisol trajectories around the conflict discussion as their relationship matured. Cohabitation length predicted stronger convergence in cortisol slopes prior to the conflict discussion. Couples’ relationship dissatisfaction was associated with a greater degree of convergence in spouses’ acute cortisol levels during the conflict discussion.
Conclusions
Findings suggest that spouses increasingly shape each other’s cortisol responses as their relationship matures. Findings also indicated that increased similarity in acute cortisol levels during conflict may be associated with poorer relationship functioning.
Keywords: dyadic processes, hypothalamic-pituitary-adrenal (HPA) axis, convergence, dyadic data analysis, psychophysiology
Numerous studies have shown that marital relationships contribute to health and health risk (e.g., Jaremka, Glaser, Malarkey, & Kiecolt-Glaser, 2013; Kiecolt-Glaser & Newton, 2001; Smith, Uchino, Berg, & Florsheim, 2012; Uchino et al., 2013). Those in healthy marriages have better physical and mental health than those who never marry or who have conflictual marriages (Holt-Lunstad, Smith, & Layton, 2010; Huston & Melz, 2004). At the same time, studies indicate that interpersonal stressors, such as partner psychopathology, can have a profound negative effect on the other partner’s mental health (e.g., Benazon & Coyne, 2000). The marital context is thus highly relevant for individual psychology and health.
A review of health similarity in long-married couples has shown that those who are married are more similar to one another than to the general population across multiple physical and mental health outcomes, from cardiovascular health to depressive symptomatology (Meyler, Stimpson, & Peek, 2007). Recent research on the hormone cortisol, which is released in response to stress, has also found significant linkages between romantic partners. Several studies of daily fluctuations in cortisol have found that romantic relationship partners exhibit significant covariation in their physiological responses to stress (Liu et al., 2013; Papp et al., 2013; Saxbe & Repetti, 2010). Short-term longitudinal designs, however, make it difficult to determine whether the degree of cortisol covariation observed in romantic partners changes over time or remains static. The present study addresses this issue by examining whether the degree of similarity in spouses’ cortisol responses changes in the first two years of marriage. In the current work, we use the term similarity to refer to spousal similarity at a single point in time. We use convergence to refer to the hypothesized process in which spouses become more similar in their cortisol responses over time.1
Relevance of Examining Couples’ Cortisol Patterns
The hypothalamic-pituitary-adrenal (HPA) axis is an important part of the body’s system for reacting to and regulating stress, among other functions. In response to stressors, the pituitary gland sends a signal to the adrenal glands to release cortisol into the bloodstream. Dysregulated cortisol patterns have been linked to a variety of health outcomes (Miller, Chen, & Zhou, 2007; Phillips, Ginty, & Hughes, 2013), including depression (e.g., Grynderup et al., 2013), breast cancer severity (e.g., Abercrombie et al., 2004), inflammation (e.g., DeSantis et al., 2012), wound healing (e.g., Gouin, Kiecolt-Glaser, Malarkey, & Glaser, 2008), and cardiovascular risk (e.g., Roy, Kirschbaum, & Steptoe, 2001).
Several studies of romantic relationships indicate that romantic partners’ HPA functioning is associated with aspects of the relationship such as quality, conflict, and attachment (e.g., Beck, Pietromonaco, DeBuse, Powers, & Sayer, 2013; Diamond, 2001; Kiecolt-Glaser, Bane, Glaser, & Malarkey, 2003; Laurent, Powers, & Granger, 2013; Powers, Pietromonaco, Gunlicks, & Sayer, 2006; for reviews, see Pietromonaco, DeBuse, & Powers, 2013 and Pietromonaco, Uchino, & Dunkel Schetter, 2013). Higher stress within marriage has been related to higher waking cortisol and a flatter slope in the expected natural decline in cortisol over the day, indicating greater health risk (Barnett, Steptoe, & Gareis, 2005). Although some studies have found such associations for only one spouse (e.g., Rodriguez & Margolin, 2013, Saxbe, Repetti, & Nishina, 2008), multiple studies have shown a link between marital quality and HPA activity.
Recent studies provide evidence not only that relationship processes may affect individuals’ HPA activity, but also that spouses may respond to stressors in a similar way. A few studies have examined spousal cortisol covariation (Liu et al., 2013; Papp et al., 2013; Saxbe & Repetti, 2010) using daily diary (i.e., short-term intensive longitudinal) methods. One study (Saxbe & Repetti, 2010) examined cortisol covariation in 30 married couples by sampling salivary cortisol four times per day over three days, generating 12 cortisol samples per spouse. They regressed each spouses’ cortisol value on the other spouses’ value at each measurement (controlling for time of day effects) and found that spouses’ cortisol levels significantly covaried with one other. Another study also used intensive longitudinal methods (seven saliva samples each day over two similar weekdays), and found evidence of spousal covariation in 47 opposite-sex couples (Papp et al., 2013). A third study found significant covariation in diurnal cortisol responses in 19 opposite-sex couples in multiple samples collected across four days (Liu et al., 2013). These studies also identified potential moderators of cortisol covariation. Cortisol similarity was greater when couples were in physical proximity (Papp et al. 2013; Saxbe & Repetti, 2010). Also, less marital satisfaction and greater marital distress were associated with a larger degree of covariation in spouses’ cortisol levels (Liu et al., 2013; Saxbe & Repetti, 2010).
These three studies (Liu et al., 2013; Papp et al., 2013; Saxbe & Repetti, 2010) have provided the first direct evidence that spouses show a correspondence in cortisol responding, covarying with one another in daily life. All of these prior studies, however, have utilized intensive short-term longitudinal designs, which capture similarity in spouses’ cortisol levels at one time period in their relationship. It is unknown whether significant covariation reflects static spousal similarity in cortisol responses, or instead whether it implies that spouses’ cortisol patterns become increasingly similar as their relationship matures.
Health Relevance of Spousal Convergence in Cortisol Response Patterns
Cortisol response patterns have been identified as one pathway through which relationship processes may affect later emotional and physical health outcomes (Pietromonaco et al., 2013). The literature has highlighted one important way in which spouses may influence each other’s physiological responses, through cortisol covariation in everyday life (Saxbe & Repetti, 2010). One potential consequence of repeated mutual influence is that spouses become more similar to one another over time, a process termed convergence. Although spouses may influence each other’s physiological processes in other ways that do not involve convergence, spouses’ convergence in other domains such as emotion and personality highlights the importance of considering convergence in examining the longitudinal consequences of HPA linkages as well. If spouses show convergence in cortisol patterns over the longer term, this provides evidence for the notion that relationship factors become increasingly influential on individual’s physiological processes over time, which cumulatively may have an impact on downstream health outcomes. Finding convergence in HPA response patterns would also provide longitudinal support for theories positing that mutual influence within romantic relationships may shape HPA functioning (Butler, 2011; Diamond & Aspinwall, 2003). In the present work, we test the idea that similarity between spouses is a dynamic, rather than static, characteristic (Gonzaga, Campos, & Bradbury, 2007). We hypothesize that spouses will become more similar in their stress responding over time, either via mutual influence or as a result of their shared experiences. Through repeated interactions and shared experiences (which we refer to mutual influence), spouses and family members shape one another’s emotional and physiological regulation patterns (Repetti, Wang, & Saxbe, 2011).
It is important to note, however, that convergence in HPA responses is not inherently beneficial or harmful for health. Instead, it is a relationship property within a dyad that can help to predict the degree to which dyad members mutually influence one another, for better or for worse (Saxbe & Repetti, 2010). Although no studies have examined longitudinal convergence in HPA activity, spousal convergence in other domains has been implicated in both adaptive and maladaptive processes. Studies have found that romantic partners who became more similar in their emotional responding had better relationship outcomes (Anderson, Keltner, & John, 2003), and personality convergence between partners predicted greater emotional convergence, which in turn predicted higher relationship satisfaction (Gonzaga et al., 2007). When one partner is depressed or under great strain, however, studies have found evidence of “contagion” of these negative qualities to the other partner (Benazon & Coyne, 2000; Joiner & Katz, 1999; Thompson & Bolger, 1999). Convergence processes, therefore, occur in both health-promoting as well as maladaptive processes within romantic relationships. Within marriages, partners are uniquely placed to affect one another’s physical and mental health: by buffering against negative health outcomes or by augmenting health risk, depending on their relationship functioning and their shared environment.
Thus, convergence in spouses’ HPA activity does not necessarily carry negative or positive consequences; rather, convergence would indicate only that spouses are implicated in each other’s HPA function in a chronic way. for example, spousal influence may be beneficial if spouses help each other regulate cortisol responses; however, if this influence leads to dysregulated patterns of cortisol response, then spouses may experience greater health risks. Prior work finding greater spousal cortisol covariation only among distressed couples (Liu et al., 2013) and in low-satisfied couples (Saxbe & Repetti, 2010) provides some evidence that spousal covariation is associated with poorer relationship functioning, which is known to be associated with a multitude of physical health problems (Berkman, Glass, Brissette, & Seeman, 2000; Holt-Lunstad et al., 2010). On the other hand, physical proximity also has been associated with greater cortisol covariation (Papp et al., 2013), and physical intimacy has been shown to lessen somatic symptoms in daily life (Stadler, Snyder, Horn, Shrout, & Bolger, 2012). Thus, conflicting evidence exists regarding whether the phenomenon of cortisol covariation is protective or harmful.
Thus, covariation in cortisol has been linked with benign (physical proximity) and harmful (relationship dissatisfaction and distress) contexts. One reason for the conflicting findings may be that in studies of daily fluctuation in cortisol it is unknown whether fluctuations are due to responses to social stress or to other factors (e.g., exercise). By design, the contexts in which spousal cortisol covariation occurred in daily diary studies were not controlled, so it is difficult to ascertain whether covariation corresponded with healthy or unhealthy behaviors. We posit that cortisol convergence may be a marker of unhealthy processes in some contexts but not others. An examination of spousal similarity in response to a known, shared stressor (e.g., a conflict discussion), will further understanding of the phenomenon of spousal similarity in cortisol responses, because it is a relationship context in which couples with more adaptive versus less adaptive or maladaptive relationships can be compared. If we find significantly greater convergence in dissatisfied couples during the conflict stressor, we will be one step further toward identifying specific contexts in which correspondence in spousal cortisol responses is maladaptive.
The Present Study
The present study extends prior research on spousal covariation in HPA activity in three key ways. First, it examines spousal similarity in cortisol patterns over a considerably longer time period than assessed in prior work (i.e., over about 18 months versus a period of days in prior studies), which allows for testing potential shifts in cortisol pattern similarity that may take some time to develop. Second, our study examines couples’ cortisol reactivity in responding to a relationship stressor, while prior studies have examined covariation in cortisol level fluctuations in daily life but not around particular stressors. As prior work has recommended (Miller, Chen, & Zhou, 2007; Powers et al., 2006), our study will distinguish cortisol reactivity, in this study the trajectory of cortisol change around a stressor, from acute cortisol levels during conflict discussion. We examine whether spouses’ showed increasingly similar trajectories of cortisol change, as well as similar cortisol levels, in response to trying to resolve a conflict in their relationship. The conflict discussion task used in this study is a quasi-experimental manipulation that provides some measure of experimental control (i.e. allows us to model a stress response) but also more closely resembles couples’ interactions in their daily lives than, for example, standard laboratory stressors such as the Trier stress test (Kirschbaum, Pirke, & Hellhammer, 1993). Social stressor tasks have been shown to elicit larger cortisol responses than many other laboratory stressors (Dickerson & Kemeny, 2004). Third, we applied these questions to a sample of couples at a relationship transition point (i.e., in the first 2 years after marriage) associated with higher mutual influence. Processes of mutual influence may be especially apparent in certain developmental stages of relationships, such as the beginning of married life (Rehman, Gollan, & Mortimer, 2008). In addition, both behavioral and physiological patterns observed in early marriage have been associated with relationship functioning and dissolution over a decade later (Huston, Niehaus, & Smith, 2001; Huston, Caughlin, Houts, Smith, & George, 2001; Kiecolt-Glaser et al., 2003).
We hypothesized that spouses would become more similar in their cortisol patterns in response to the conflict stressor over the early years of marriage, indicating convergence in HPA stress response. As found in prior work in this area (Papp et al., 2013), we hypothesized that those who cohabited longer would have greater convergence in cortisol response patterns (or trajectories) due to having spent more time in physical proximity. Finally, as this was a conflict stressor, we hypothesized that spouses who were less satisfied in their relationship would become more convergent in their cortisol responses during the conflict than other couples. This hypothesis is in line with prior work finding greater cortisol covariation in couples with lower relationship satisfaction (Saxbe & Repetti, 2010) and another finding significant covariation only in distressed couples (Liu et al., 2013).
Method
Participants
Marriage license records filed in western Massachusetts were used to identify recently married couples, and they were recruited via phone and mail invitations to participate in the study. Eligible couples were in their first marriage, had no children, and were between 18–50 years old. Couples were also required to be within the first 7 months of their marriage and not expecting a child at the time of the first lab visit. A total of 225 couples completed their first lab visit. Of these, 183 couples (366 individuals) returned for their second lab visit. Of the 42 couples who did not return, 13 refused to continue with the study, 8 had divorced, 3 could not be reached after multiple attempts to contact them, and 18 couples who were too busy or had moved away completed questionnaires online but could not attend the lab visit. Since the primary question in this study related to longitudinal changes in cortisol responses, only couples who completed the second lab visit were retained in the present sample. Comparisons to those excluded showed that participants in the present sample were slightly older (Mdiff = 1.63 years for husbands, Mdiff = 1.69 years for wives). Wives in the subsample also had significantly higher education than those who did not complete the second lab visit. There were no significant differences in marital satisfaction levels or cohabitation length of those who completed the second visit and those who did not. Cortisol response similarity also did not significantly differ between the two groups, and men and women in our subsample did not have significantly more extreme cortisol levels than those excluded from analyses (see Table S1 in online supplement).
For participants in the subsample (N =183 couples) at the first lab visit: (1) average age was 27.98 (SD = 4.77) for wives and 29.36 (SD = 5.25) for husbands, (2) most held a bachelor’s degree (51% for wives, 45% for husbands) or higher graduate degree (34% of wives, 20% of husbands), and (3) most identified as White (92% of wives, 96% of husbands). Average relationship length from the time couples began dating was 5.13 years (SD = 3.07). A majority of couples (85%) reported living together before they married, M =2.79 years, SD = 2.31.
Study Design and Procedures
Data for the present study are drawn from a larger study of biopsychosocial factors that influence health in newlywed couples. The current study uses data from a first lab visit (T1), conducted within the first 7 months of marriage, and a second lab visit (T2) conducted about 15–18 months later. Lab visits took place during the late afternoon to early evening when cortisol levels tend to be more stable than at other points in the day (Cone, Low, Elmquist, & Cameron, 2002; Dorn, Lucke, Loucks, & Berga, 2007), and lasted about 3 hours.
Spouses completed questionnaires about themselves and their relationship, including a form in which they identified three topics of unresolved conflict in their relationship. A research assistant examined these forms and selected the topic that both partners listed with the highest combined intensity rating. If there were no matched areas of conflict, the research assistant flipped a coin to determine which spouses’ topic would be discussed and selected his or her topic with the highest intensity rating. Couples were then seated in our lab “living room” and received a card on which the selected conflict topic was written. The experimenter asked them to begin talking about the issue for 15 minutes and instructed them to try to resolve the area of disagreement. The procedure was adapted from a standard conflict interaction task (Gottman, 1979) that has been used in numerous studies in the relationships literature, including Powers et al. (2006). Spouses provided saliva samples before, during, and after the conflict discussion to capture changes in salivary cortisol as a result of the conflict stressor. Participants were compensated $50 each for the first visit, and $70 each for the second lab visit.
Measures
Cortisol
Saliva samples were gathered from both spouses at five time points during the lab session. Cortisol levels in saliva reflect reactivity from about 15 to 20 min prior to the time of measurement (Stansbury & Gunnar, 1994). Saliva samples were collected with this lag time in mind, with the aim of capturing responses to the conflict discussion stressor. The first two samples were given prior to the conflict discussion (about 30 min and 1 hr 10min into the lab session), the next sample was provided 10 min after the conflict discussion ended and captured the acute cortisol level during the conflict discussion. The final two samples were provided at 30 min and 60 min after the conflict discussion. As medications can affect salivary cortisol (Granger, Hibel, Fortunato, & Kapelewski, 2009), participants listed all medications they had taken in the 24 hours prior to the lab visit.
Following guidelines provided by Salimetrics, LLC, participants were asked to “passively drool down a straw and into a small plastic vial” with their heads tilted forward until the required amount of saliva was collected. The vial was sealed and immediately placed in frozen storage (−85°C) until samples were shipped on dry ice to be assayed. Each saliva sample was divided into two vials and separately assayed for salivary cortisol with a highly sensitive enzyme immunoassay (Salimetrics, PA). Thus, each sample had two cortisol measures, resulting in a total of 10 values for the five samples. The test used 25µL of saliva per determination and had a lower limit of sensitivity of .003 µg/dl, a standard curve range from .012 µg/dl to 3.0 µg/dl, an average intra-assay coefficient of variation of 3.5%, and an average interassay coefficient of variation of 5.1%. Method accuracy determined by spike and recovery averaged 100.8%, and linearity determined by serial dilution averaged 91.7%.
Four saliva samples did not have the sufficient volume needed to be properly assayed (2 each visit). Cortisol values that were greater than or equal to 4 µg/dl (8 at T1, 5 at T2) were excluded from analyses, as these are beyond the plausible range (Aardal & Holm, 1995). Values below .003 (3 at T1, 0 at T2) were also excluded because they are considered too low to be detectable. With these exclusions, 20 cortisol values were treated as missing in the final dataset, comprising <1% of the total samples (with .8% missing at T1, and .4% missing at T2). The cortisol values were quite positively skewed and kurtotic, and required a base-10 logarithmic transformation in order to symmetrize the data to meet the assumption of normality required for statistical analysis (Tukey, 1977). Table 1 provides descriptive statistics for the cortisol samples.
Table 1.
Raw and Transformed Values for Cortisol Samples, Lab Visits 1 and 2
| Lab Visit 1 | Lab Visit 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| M | SD | Min | Max | M | SD | Min | Max | ||
| Wives’ Samples | |||||||||
| 1 | µg/dl | .106 | .119 | .017 | 1.205 | .100 | .102 | .016 | 1.301 |
| log10(µg/dl) | −1.078 | .269 | −1.769 | .081 | −1.075 | .235 | −1.796 | .114 | |
| 2 | µg/dl | .086 | .073 | .014 | .513 | .085 | .060 | .012 | .488 |
| log10(µg/dl) | −1.165 | .282 | −1.876 | −.291 | −1.139 | .236 | −1.921 | −.312 | |
| 3 | µg/dl | .070 | .067 | .005 | .552 | .072 | .068 | .008 | .581 |
| log10(µg/dl) | −1.262 | .297 | −2.274 | −.258 | −1.235 | .265 | −2.100 | −.236 | |
| 4 | µg/dl | .066 | .064 | .004 | .505 | .072 | .072 | .013 | .694 |
| log10(µg/dl) | −1.295 | .297 | −2.286 | −.297 | −1.232 | .254 | −1.886 | −.159 | |
| 5 | µg/dl | .059 | .052 | .004 | .498 | .077 | .168 | .008 | 1.732 |
| log10(µg/dl) | −1.327 | .290 | −2.393 | −.303 | −1.293 | .305 | −2.097 | .238 | |
| Hµsbands’ Samples | |||||||||
| 1 | µg/dl | .121 | .122 | .011 | 1.113 | .128 | .160 | .015 | 1.441 |
| log10(µg/dl) | −1.034 | .311 | −1.982 | .046 | −1.040 | .325 | −1.861 | .159 | |
| 2 | µg/dl | .096 | .120 | .003 | 1.222 | .107 | .174 | .016 | 1.956 |
| log10(µg/dl) | −1.151 | .318 | −1.942 | .086 | −1.137 | .332 | −1.784 | .291 | |
| 3 | µg/dl | .071 | .060 | .007 | .498 | .093 | .146 | .008 | 1.456 |
| log10(µg/dl) | −1.259 | .316 | −2.155 | −.303 | −1.217 | .351 | −2.126 | .163 | |
| 4 | µg/dl | .074 | .106 | .006 | .984 | .087 | .136 | .010 | .970 |
| log10(µg/dl) | −1.287 | .326 | −2.251 | −.007 | −1.235 | .335 | −2.009 | −.014 | |
| 5 | µg/dl | .067 | .110 | .004 | 1.027 | .078 | .120 | .008 | 1.107 |
| log10(µg/dl) | 1.365 | .356 | −2.397 | .011 | −1.289 | .347 | −2.130 | .044 | |
Note: Approximate timing of saliva samples was as follows, by sample: 1) 30 min after lab entry; 2) 1 hr, 10 min after lab entry; 3) 10 min after conflict discussion (thus reflecting acute cortisol level during conflict); 4) 30 min after conflict; 5) 60 min after conflict.
Cohabitation length
Spouses reported whether they had lived together prior to marriage and, if they had, the length of time they had cohabited. Spouses’ separate reports were averaged to create one measure of cohabitation length, measured in the metric of years, M =2.36, SD =2.36, Range 0 – 13.75 years. The variable was positively skewed and required a square root transformation to symmetrize the data, (M =1.31, SD =.80).
Relationship satisfaction
Relationship satisfaction at T1 was measured using the satisfaction subscale from the Dyadic Adjustment Scale (DAS; Spanier, 1976). The subscale has ten items about aspects of relationship satisfaction that are summed, with a possible range from 0–50. Reliability for this sample was adequate (wives, α= .75; husbands, α= .77). Spouses’ separate reports were averaged to create a couple-level measure of satisfaction, as our study was geared toward a couple-level process (convergence). Note that spouses’ reports were highly correlated (r = .614, p < .001), lending support to our choice to use a dyadic measure of this construct. The satisfaction variable was not sufficiently skewed to require transformation, M = 42.48, SD = 3.28.
Analytic Strategy
There were three steps in the analyses. In the first step, growth curve modeling was used to estimate a trajectory of change in cortisol over the five samples obtained during the first lab visit for each spouse. Based on the study design, the conflict discussion represented a quasi-experimental manipulation expected to change the course of participants’ cortisol trajectories. The trajectories of change were expected to be discontinuous and were therefore modeled as a piecewise growth model, discussed in depth below. In the second step, a difference score model was used to obtain estimates of the relative difference between spouses’ cortisol trajectories for each dyad. This model is based on a technique modeling latent differences between partners’ scores at each measurement occasion using multilevel modeling (Sayer & Klute, 2005). The third step used a growth model to estimate change in cortisol similarity (modeled as absolute discrepancy) from the first to the second lab visits. Characteristics of the couple from their first lab visit (cohabitation length and T1 relationship satisfaction) were used as predictors of convergence over time.
The Hierarchical Linear Modeling program (HLM7; Raudenbush, Bryk, & Congdon, 2011) was used for all analyses. HLM models the two sources of non-independence found in our data: the correlations among the repeated measures, and the correlations between members of the same dyad (Raudenbush, Brennan, & Barnett, 1995; Kenny, Kashy, & Cook, 2006). Maximum likelihood (ML) estimation was used within the multilevel modeling framework in HLM, which provided model-based estimates for missing values at Level 1. ML estimation is one of the recommended ways for appropriately handling missing data (Allison, 2009). HLM does not allow, however, for missing data at level 2. The few missing data points at level 2 (at most 3 missing values per predictor) were replaced with variable means.
Results
To compare the degree of dependency within couples, on average, intraclass correlations (ICCs) were estimated across all cortisol samples per lab visit. In the first lab visit, the average correlation within couples was 40.22% (ICC=.4022). In the second lab visit, the average was 43.34% (ICC=.4334). The increase in the ICC is in line with the convergence hypotheses in that the within-couple correlation, or dependency, increased from the first to the second lab visit. These descriptive statistics are limited, however, in that they do not directly test whether this increase was statistically significant, and also do not allow us to test which couple-level characteristics predicted increases in dependency in cortisol responses. Thus, we moved to a modeling strategy that allowed us to model, rather than simply account for, cortisol similarity within dyads.
We first modeled growth curves for the husbands’ and wives’ cortisol responses around to the conflict discussion. This statistical model is a version of the one used in Beck et al. (2013).2 The piecewise growth model was defined by three parameters: an intercept that represented the expected value of cortisol when time is equal to zero, a linear slope that represented the change in cortisol from the first sample given early in lab visit up to the conflict discussion, and a linear slope that represented the change in cortisol from the conflict discussion to the end of the lab visit. Piecewise models are often used when an intervening event or experimental manipulation shifts the expected rate of change (Raudenbush & Bryk, 2002). An important issue in piecewise modeling is the choice of the transition point, which is the time point at which the linear slope is permitted to vary. Because the conflict discussion was a planned manipulation with a hypothesized effect on cortisol across the laboratory session, the cortisol change trajectory was allowed to vary at that point, and the time variable was centered so that we could predict cortisol levels during the conflict discussion as the intercept of the trajectory. Time elapsed was scaled to a metric of hours; the specific coding used for each trajectory is provided in Table S2 in the online supplement. A multivariate outcomes model (Raudenbush et al., 1995) was estimated, with husbands’ and wives’ cortisol trajectories modeled simultaneously to appropriately account for the dependency in their cortisol scores. Equations for the piecewise trajectory models were:
| Level 1 |
| Level 2 |
The level-1 model is the within-couple model that uses three separate parameters to capture the trajectory for each spouse: an intercept, reflecting the cortisol level during the conflict discussion (“Intercept”), change in cortisol up until the conflict discussion (“Piece 1”), and change in cortisol after the conflict discussion (“Piece 2”).3 Model estimates for both the fixed effects (the averages), and the variance of the random effects (the variability) for both lab visits are presented in Table 2. The Level 2 unconditional equations are shown above, with residual errors (u’s) that allow trajectories to vary across participants. Also at Level 2 (not shown in equations), we included all controls that were significant predictors either at T1 or T2, in addition to a variable controlling for time of day.4 Significant controls for wives were hormonal birth control, analgesics, and antibiotics; for husbands, they were corticosteroids, anti-depressant or anxiety medications, attention-deficit/hyperactivity disorder medications, and proton pump inhibitors.
Table 2.
Fixed and Random Effects for Husbands’ and Wives’ Cortisol Trajectories, Lab Visits 1 and 2
| Lab Visit 1 | Lab Visit 2 | |||
|---|---|---|---|---|
| Husband | Wife | Husband | Wife | |
| Fixed Effects | ||||
| Intercept | −1.118*** (.031) |
−1.288*** (.029) |
−1.114*** (.028) |
−1.209*** (.023) |
| Time of Day | −.133*** (.022) |
−.066** (.019) |
−.136*** (.022) |
−.041* (.016) |
| H_Corticosteriod Use | −.174 (.124) |
-- | −.279* (.137) |
|
| H_Antacid Use | −.137* (.098) |
-- | −.137 (.116) |
|
| H_Stimulant Use | .476† (.272) |
-- | .714*** (.163) |
|
| H_Proton Inhibitor Use | −.071 (.113) |
-- | −.140 (.137) |
|
| W_Contraceptive Use | .174*** (.035) |
.060† (.034) |
||
| W_Analgesic Use | .311 (.165)† |
.196* (.086) |
||
| W_Antbiotic Use | −.018 (.096) |
−.619** (.208) |
||
| Piece 1 (Pre-Conflict Slope) |
−.179*** (.014) |
−.153*** (.012) |
−.152*** (.011) |
−.121*** (.011) |
| Piece 2 (Post-Conflict Slope) |
−.142*** (.020) |
−.082*** (.017) |
−.082*** (.015) |
−.082*** (.018) |
| Random Effectsa | ||||
| Intercept | .089*** | .070*** | .086*** | .052*** |
| Piece 1 | .029*** | .021*** | .017*** | .018*** |
| Piece 2 | .057*** | .042*** | .026*** | .046*** |
Note: p < .10
p < .05
p < .01
p < .001.
Level 1 variance excluded because heterogeneous variances were estimated for husbands’ and wives’ trajectories.
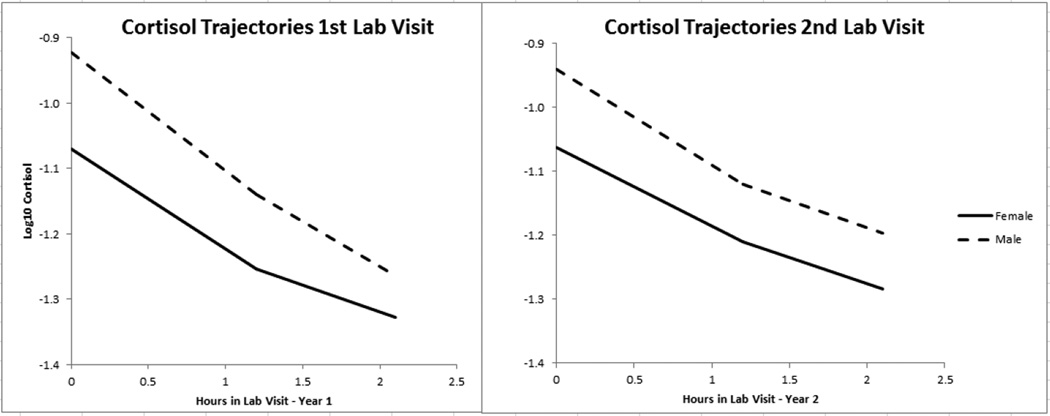
As displayed under the row 2 of the “Fixed Effects” in Table 2, the pre-conflict slope was negative at both lab visits, indicating that cortisol was decreasing from entry up to the conflict discussion. This trajectory course is consistent with the T1 cortisol trajectories modeled in the Beck et al. (2013) study, which showed that couples entered the laboratory with higher cortisol levels, in part due to anticipation of the conflict discussion. The general downward trend in cortisol slopes is consistent with other studies of cortisol trajectories, which model the natural circadian decline in cortisol (Chaplin et al., 2012; Robles, Shaffer, Malarkey, & Kiecolt-Glaser, 2006). As cortisol is declining, in general, at this time of day, we expected that the conflict discussion would disrupt, or flatten, this natural decline if effective as a stressor. As displayed in Table 2, the cortisol slope following the conflict discussion was also negative but flatter than the slope prior to the conflict discussion, indicating that the decline in cortisol indeed slowed after the conflict discussion. The average cortisol trajectories are graphed in Figure 2. Note that the average couple’s cortisol trajectories at T1 appear more discrepant than at T2, again providing descriptive evidence that couples’ cortisol response patterns converged over time. Estimates for each individual’s trajectory parameters (post-estimation scores, or Empirical Bayes (EB) coefficients) were used in the next step of the analyses.
Figure 2.
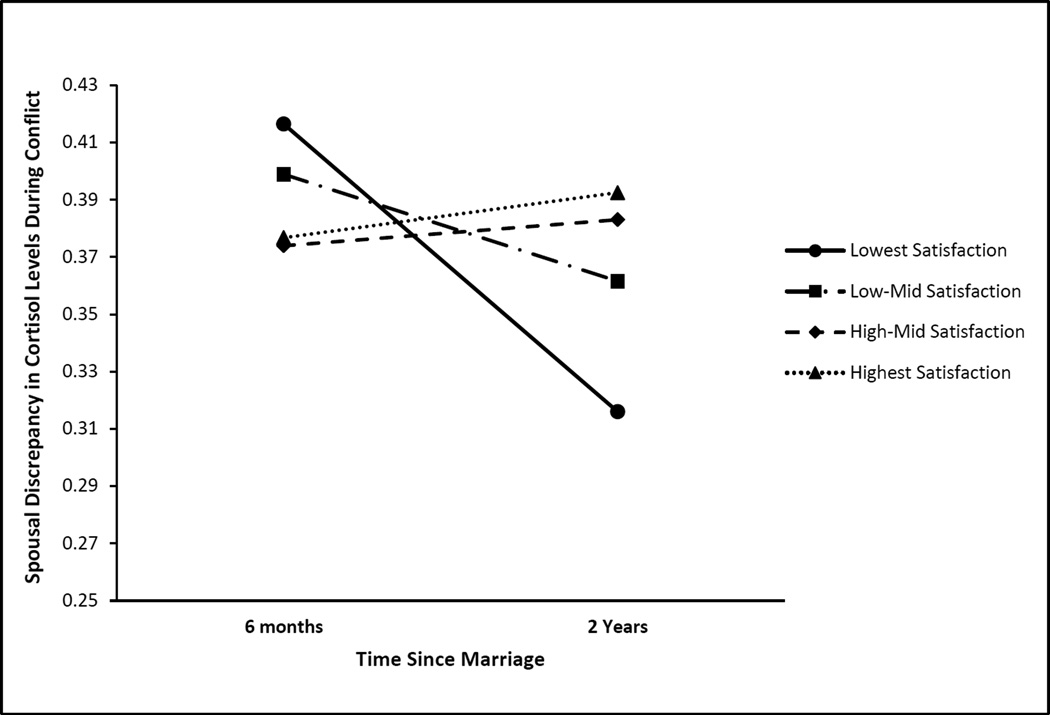
Satisfaction moderates spousal convergence in cortisol levels during conflict over 1.5 years. We found no general convergence in spouses’ cortisol levels during the conflict discussions from lab visit 1 (about 6 months into their marriage) to lab visit 2 (about 2 years into their marriage), but did find that relationship satisfaction at 6 months moderated spousal convergence in acute cortisol levels during conflict. Simple slopes analyses were conducted for each quartile of couples, from lowest to highest satisfied couples. As in many studies of newlywed couples, satisfaction reports were quite high on average (M = 42.5 on a scale of 0–50). Still, there was variability in satisfaction. The “Lowest” quartile of couples had satisfaction values ranging from 29.5 to 40, indicating at least some dissatisfaction in their relationship. Couples in the upper three quartiles had satisfaction ratings above 40. The ranges for these quartiles were as follows: “Low-Mid” satisfaction scores ranged from 40.5 to 42.5, “High-Mid” satisfaction scores from 43 to 44.5, and “Highest” satisfaction scores from 45 to 49. Results showed that only the lowest satisfaction quartile of couples showed significant convergence in acute conflict cortisol levels over time (solid line), γ = −.100, p =.027. Convergence slopes for all other satisfaction levels (i.e., above the 25th percentile) were not significantly different from zero.
In the second step of analyses, the differences between spouses’ cortisol trajectories were estimated simultaneously in a multivariate outcomes difference score model (e.g., Lyons & Sayer, 2005). This model estimated a dyadic average and difference for each aspect of spouses’ cortisol trajectories (acute level during the conflict discussion, and slopes before and after the conflict). This modeling procedure was applied separately to the data from the two lab visits. At level-1, the model estimated two coefficients per trajectory parameter, one capturing the couple average (the model intercept) and one capturing the signed difference between the two scores (the slope). Wives’ values were coded −.5 and husbands’ values coded .5. Equations were:
| Level 1 |
| Level 2 |
Estimates for the average couple and the variability across couples for both models are presented in Table S2 of the online supplement. Empirical Bayes’ (EB) coefficient estimates of average and differences in cortisol qualities were output as residuals for use in the final step of analyses. The final step of modeling provided results for our first hypothesis, that across the lab visits from T1 to T2 there would be increased cortisol similarity over time (convergence). Estimates of absolute discrepancy in each growth parameter were used as input for the final modeling step, in which discrepancy in cortisol parameters at T1 were compared with the discrepancies from T2. Thus, the EB coefficients for the signed difference for each couple were transformed into absolute discrepancies. The distribution of the discrepancies was positively skewed; a square root transformation was used to symmetrize the distribution to meet normality assumptions.
Lower cortisol discrepancy values indicated more similarity (less discrepancy), and higher scores indicated less similarity (more discrepancy). These scores were used as dependent variables in a model testing for change in absolute discrepancy over time (we hypothesized a decline in discrepancy, indicating convergence). A multivariate outcomes model was used to estimate change in cortisol discrepancy simultaneously for cortisol level during the conflict, change in cortisol prior to conflict (Piece 1), and change following the conflict (Piece 2). The independent variable in this model was Time, coded −.5 for lab visit, and +. 5 for lab visit 2. Equations for this model were:
| Level 1 |
| Level 2 |
In these equations, β1, β3, and β5 (“Average”) represent average level of spousal discrepancy across lab visits for each cortisol trajectory parameter, and β2, β4, and β6 (“Convergence”) represent change in dyadic cortisol discrepancy from the first to the second lab visit. As displayed in Table 3, change in discrepancies were negative and significantly different from zero, for both the cortisol slope prior to, and the slope following, the conflict stressor. Spouses’ cortisol levels during the discussion did not become more similar, on average. These findings indicated that the absolute discrepancies in how couples responded to the conflict discussion decreased over time, providing evidence for convergence in cortisol responses to the stressor.5 There was significant variability around this average cortisol convergence, however, supporting the addition of predictors to the model to find out if relationship-level characteristics systematically explained some of this variability. See Figure S1 and further description in online supplement, which illustrates the substantial variability around average convergence.
Table 3.
Longitudinal Convergence in Couples’ Cortisol Discrepancies: Reactivity Level During Conflict, Pre-Conflict Slope, and Post-Conflict Slope
| Fixed Effects | Coefficient | SE | t | p |
|---|---|---|---|---|
| Cortisol Level During Conflict | ||||
| Dyadic Average Discrepancy, γ10 | .474 | (.012) | 38.063 | <.001 |
| Discrepancy Change (Convergence), γ20 | −.025 | (.021) | −1.151 | .251 |
| Pre-Conflict Cortisol Slope | ||||
| Dyadic Average Discrepancy γ30 | .308 | (.008) | 40.380 | <.001 |
| Discrepancy Change (Convergence), γ40 | −.046 | (.015) | −3.007 | .003 |
| Post-Conflict Cortisol Slope | ||||
| Dyadic Average Discrepancy, γ50 | .350 | (.009) | 37.109 | <.001 |
| Discrepancy Change (Convergence), γ60 | −.062 | (.019) | 3.214 | .002 |
| Random Effects | Variance Component |
χ2 | p | |
| Cortisol Level Discrepancy Average, u1 | .028 | 5753.720 | <.001 | |
| Cortisol Level Discrepancy Change, u2 | .080 | 4221.191 | <.001 | |
| Pre-Conflict Slope Discrepancy Average, u3 | .009 | 1074.314 | <.001 | |
| Pre-Conflict Slope Discrepancy Change, u4 | .035 | 1053.882 | <.001 | |
| Post-Conflict Slope Discrepancy Average, u5 | .013 | 948.916 | <.001 | |
| Pre-Conflict Slope Discrepancy Change, u6 | .055 | 980.175 | <.001 | |
| Model Deviance (df) | −2195.903 (27) | |||
Hypothesis 2 Results: Moderators of Cortisol Convergence
Finally, predictors were added to the baseline model to test whether couple-level characteristics moderated convergence in spouses’ cortisol responses. Cohabitation length was associated with higher rates of cortisol convergence on the pre-conflict cortisol slope, γ = −.040, p = .061. This was the only significant association for cohabitation length (see Table S3 in the online supplement). In partial support of our hypothesis, lower marital satisfaction was associated with higher rates of convergence over time (γ = .013, p = .041) in cortisol levels during the conflict discussion, but not the shape of the trajectories around the discussion. To probe the nature of this interaction, we tested the significance of the cortisol level convergence at different levels of satisfaction. Results from these follow-up simple slopes analyses showed that only those couples with the lowest satisfaction levels (i.e., in the lowest quartile) showed significant convergence in cortisol levels during the conflict discussion (γ = −.100, p =.027). Results showed no significant conflict cortisol convergence for couples in the highest (γ = .016, p = .707), high middle (γ = −.037, p = .381), or low middle satisfaction quartiles (γ = .009, p = .820). Additional follow-up analyses showed that this effect was similar for both spouses’ reports (low satisfaction couples’ convergence by wives’ reports γ = −.090, p =.063, and husbands’ reports γ = −.107, p =.013). See Figure 2.
Exploration of conflict discussion affect and behavior
To aid interpretation of the low satisfaction findings, we conducted exploratory follow-up analyses to test whether self-reported anger6 or observer-rated hostility7 during the first conflict discussion were associated with convergence in conflict cortisol levels over time. Husbands’ reports of anger during the conflict were associated with convergence in conflict cortisol levels in a similar pattern of association as the low satisfaction results, with more anger associated with greater conflict cortisol level convergence (γ = −.055, p = .003). Wives’ reports of anger during the conflict, however, did not significantly predict conflict cortisol convergence (γ = −.002, p = .904). Both husbands’ and wives’ high proportions of hostile behaviors, as indicated by observer coding of the first conflict discussion, were associated with convergence in conflict cortisol levels over time (wives’ γ = - .101, p = .035; husbands’ γ = −.116, p = .040). Thus, multiple measures support the interpretation that dissatisfied, more conflictual couples showed convergence in their acute cortisol levels during the conflict discussion.
Discussion
This study found that spouses become more similar in the shape of their cortisol trajectories around a conflict stressor in the early years of marriage. While prior research found covariation in spouses’ cortisol over a few days at one time period in their relationship, our study is the first to test whether similarity increased over a longer period of time (i.e., over about a year and a half) and in response to a defined event (a conflict discussion). Our results indicated that newlywed spouses become more similar in their cortisol trajectories around a conflict stressor as their relationship matures. Significant convergence in conflict cortisol levels, however, did not occur in the entire sample, but only couples low in relationship satisfaction.
Significant variability was found in cortisol convergence in spouses’ cortisol trajectories and in conflict cortisol levels. For example, while couples, on average, became more similar in their cortisol trajectories, some couples became divergent, and other couples showed much stronger convergence than the average. This finding supports the notion that couple-level characteristics can inform an understanding of which couples are more versus less influential on partners’ responses to stressful interactions. Indeed, in the final step of analyses, we found relationship-level predictors of this variability.
We found evidence that the longer couples had lived together, the more similar their cortisol trajectories from the first to the second lab visit. This finding dovetails with research indicating that time spent in proximity to one’s spouse on a given day increased spousal covariation in cortisol (Papp et al., 2013; Saxbe & Repetti, 2010), and is aligned with the notion that partners who have spent more time living together have had more chances to influence each other (Repetti et al., 2011). Note that we applied a root transformation to cohabitation length, and therefore the effect of cohabitation length on cortisol convergence was not linear: effects on cortisol trajectory convergence were most pronounced in the early years of cohabitation, with the decreasing increments for each additional year that couples had lived together prior to marriage. This finding is in line with theory and research suggesting that the early years of romantic relationships are a time of greater mutual influence than later years. Note too that cohabitation length predicted change only in the pre-conflict slope of the cortisol trajectory, even though we had expected it to impact the post-conflict slope as well. One possible explanation for the unexpected restriction of this finding to the pre-conflict slope is that this slope may carry more information about spouses’ natural decline in cortisol at the end of the day, before it was disrupted by the conflict stressor. The fact that couples become more attuned in their cortisol slopes at the end of the day may not be maladaptive, but instead a marker of increased similarity due to shared environment and mutual influence found across multiple health domains (Meyler et al., 2007). By contrast, we found evidence that moderators of convergence in acute cortisol levels during the conflict corresponded with maladaptive relationship processes.
We found evidence that acute tuning of cortisol levels during conflict discussion was associated with lower satisfaction, which is an established risk factor for other adverse health outcomes (e.g., Holt-Lunstad et al., 2010). This finding is also consistent with prior findings from daily diary studies indicating that covariation in cortisol responses may signal unhealthy processes within relationships (Liu et al., 2013; Saxbe & Repetti, 2010). Our follow-up analyses of self-reported anger (reported directly after the conflict discussion) and observer coded hostility showed a similar pattern of results: higher anger and hostility during the conflict discussion were associated with cortisol level convergence, but not trajectory shape convergence, from the first to the second lab visits. We interpret this finding as a signal of acute physiological synchrony within the 15 min conflict stressor itself. Perhaps during conflicts, couples who engage in greater negative behaviors show matched stress responses that feed into negative communication patterns. Physiological measures that can provide multiple measures within the conflict discussion, such as heart rate or skin response measures, may provide further illumination of this process. For example, it may be that a particular hostile behavior in one spouse leads to increased stress responses in the other, which in turn potentiates a hostile behavior from that spouse. More fine-grained sequencing of physiological and behavior measures within the conflict discussion would be needed to test such speculations. The present findings, however, suggest that increased similarity in acute cortisol levels during the conflict discussion are a feature of dissatisfied couples who show more hostility and report more anger during conflict. Thus, we believe we have identified one context in which increased cortisol similarity is maladaptive: becoming more similar in acute cortisol levels during the conflict discussion was associated with negative relationship behaviors, and thus may carry subsequent negative consequences for health.
Note that only husbands’ (not wives’) self-reported anger significantly related to conflict cortisol level convergence. Given that the observer-coded hostility behaviors showed significant associations for both husbands’ and wives’ hostility during the conflict discussion, it does not appear that there is a gender difference at the level of actions during the conflict. It is possible that wives’ perceptions of anger are less closely linked with physiological measures of similarity than husbands’ perceptions. It is also plausible that the lack of association for wives’ self-reported anger was due to the poor measurement of post-conflict anger via only a single item, though future work examining gender differences in anger perception-cortisol associations will be needed to support this claim.
Considering these results together provides clues about the conditions under which cortisol convergence is beneficial or maladaptive for health. Cortisol trajectory convergence around the conflict discussion was not associated with low satisfaction, anger, or hostility during the conflict discussion. We interpret the overall finding of cortisol trajectory convergence and cohabitation findings as the result of general mutual influence or shared experience resulting in greater similarity and not necessarily carrying negative consequences for health. This pattern is in contrast to convergence in acute cortisol levels during the conflict discussion. Only a subset of couples, those reporting lower levels of satisfaction, and higher anger and hostility during the conflict, showed convergence in cute cortisol levels during the conflict stressor. This suggests there is utility in distinguishing between health implications of convergence in cortisol trajectories versus convergence in acute cortisol levels during conflict. One possibility is that becoming more similar in stressor response style (i.e., cortisol trajectories around a stressor) is simply a phenomenon of shared experience and mutual influence, but becoming more similar in cortisol levels during a stressor is associated with negative health outcomes. These findings suggest that convergence in shape of change around a conflict stressor simply reflects the increased similarity hypothesized by some researchers of health concordance (Meyler et al., 2007) and is not necessarily “bad” for health, whereas becoming attuned on cortisol levels during conflict may be associated with negative health outcomes.
The multilevel analytic strategy used in this study differs from that used in previous studies of cortisol covariation (Liu et al., 2013; Papp et al., 2013; Saxbe & Repetti, 2010). It is a novel extension of the traditional dyadic specification in that it emphasizes dyad-level constructs of discrepancy and convergence in an attempt to map mutual influence over time. Our goal was to model, rather than simply account for, interdependence within dyads. Such modeling allowed for direct tests of whether couples become more similar over time and the addition of predictors to test why some couples demonstrate stronger bidirectional influence than others.
This study has several limitations. As in any observational study, some of the longitudinal effects may have resulted from other factors, such as habituation to the laboratory visit, rather than to true changes in the relationship. We note that couples did engage in a new conflict discussion of their own choosing in the second lab visit. In addition, cortisol values were not significantly lower at the follow-up session, suggesting that the findings cannot be explained by practice effects alone. It is also possible that the convergence in cortisol responses occurs specifically in response to spousal conflict but does not extend to other types of stressors. While our findings are similar to findings from prior daily diary studies, the methodology used in our study focuses on a particular known stressor. Other sources of covariation found in daily life are not examined in the present work, and further investigation of longitudinal convergence patterns across multiple stressor contexts is needed to determine the scope of the increased similarity observed in the interpersonal conflict context addressed here. Still, the replication of cortisol linkage findings across stress-task versus daily diary methodologies shows that they are robust to study design differences. Still, we acknowledge the potential bias in the literature toward positive results; it is likely that there are unpublished studies that found no direct associations between spouses’ cortisol responses, and it is important for future research to consider in which contexts linkages do or do not occur.
Clearly, what is of greatest importance to health researchers is the identification of mutual influence patterns that are particularly maladaptive as targets for intervention, or adaptive mutual influence patterns that buffer spouses from adverse health outcomes. For example, it is possible that those with higher reactivity in general exert more influence on their partners than those with less reactive cortisol responses, or that certain pairings (e.g., one spouse with a flattened trajectory in response to conflict paired with a spouse who is highly reactive) are associated with poor health outcomes. Cortisol pattern convergence may itself be a mechanism by which behavior affects physiological similarity, which in turn is associated with greater likelihood of developing other health problems. Because our study focused on changes in spousal similarity in cortisol responses, it did not directly address which patterns of cortisol trajectory similarity present health risk. This issue is not limited to our study, however: it is a feature of the literature in general. That is, there is no single characteristic trajectory shape of maladaptive stressor response; rather it depends on contextual factors (see Pietromonaco & Powers, 2015). Both high cortisol reactivity to stressors, as well as blunted patterns of cortisol response to stressors, have been linked with adverse outcomes (Fisher, Kim, Bruce, & Pears, 2012). Our examination of significant, and increasing, similarity in spouses’ cortisol responses emphasizes the importance of considering the close relationships of individuals when looking at the real-world chronic factors that shape HPA function. We have demonstrated that convergence occurs in cortisol trajectories over the first two years of marriage, however, the precise patterns and pairings that are beneficial or harmful for health will need to be identified in further research examining the connection between spouses’ convergence in cortisol patterns and each partner’s health or disease outcomes over time.
Finally, while this study provides a framework for testing whether significant convergence took place and identified moderators of this process, it did not directly test mechanisms underlying convergence. Several theorists have discussed reasons for similarity, or covariation, in spouses’ physiological responses. They have suggested that similarities in physiological responses may result from behavior during time spent together, emotion contagion, experiencing similar stressors such as conflicts, engaging in similar joint activities (e.g., intense or relaxing), or through similar sleep patterns, eating habits, or exercise (e.g., Beck, Pietromonaco, DeBuse, Powers, & Sayer, 2013; Butner, Diamond, & Hicks, 2007; Sbarra & Hazan, 2008). Conflict, for example, may evoke particular behavior patterns (e.g., negative affect reciprocity, hostility, avoidance) that are shared by the couple members and that lead to similar physiological responses in both partners. Although the current work does not allow us to identify the precise mechanism underlying convergence of cortisol trajectories, we believe that the findings for cortisol levels during the conflict provide some direction for future research. Conflict, for example, may evoke particular behavior patterns (e.g., negative affect reciprocity, hostility, avoidance) that are shared by the couple members and that lead to similar physiological responses in both partners. It is a limitation of the present work that we do not identify corresponding longitudinal changes in behavioral or affective aspects of the relationship to further probe potential mechanisms of cortisol convergence. The next step for research in this area will be to pinpoint the mechanisms that produce physiological linkages between spouses, and to identify when these linkages are beneficial, and when they are harmful. Nevertheless, demonstrating that spouses’ cortisol trajectories around the conflict stressor became more similar, on average, is an important step in establishing that spouses show increasing linkages in their cortisol trajectories over time. It will rest on future work to identify potential behavioral or physiological mechanisms that may be responsible for cortisol convergence.
This study provided longitudinal evidence that spouses become more similar in their cortisol trajectories around a conflict stressor in the early years of marriage. We found evidence to support the claim that through repeated interactions and shared experience spouses become increasingly more similar to one another in how they respond to interpersonal stressors. These increasing linkages in stress responses in turn may have implications for other shared health risks within long-term marriages. Convergence in cortisol levels during the conflict was found only for couples who were less satisfied and showed more hostility during the conflict discussion. Findings contribute to a growing body of evidence that patterns of physiological functioning are affected by the marital context. By extension, findings support the notion that interventions aimed at improving relationship functioning may also improve individual health and well-being.
Supplementary Material
Figure 1.
Cortisol trajectories for the average couple at the 1st lab visit (about 6 months into couples’ marriages) & the 2nd lab visit (about 2 years into couples’ marriages). These were piecewise trajectories, allowed to have different slopes before and after the conflict discussion. Models estimating these trajectories controlled for significantly associated medications as well as time of day effects.
Acknowledgments
This work is based on data from a larger study of biopsychosocial factors in early marriage and was part of the first author’s dissertation. Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number R01 CA133908 (Pietromonaco, PI; Powers, Co-PI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Thanks also to the Center for Research on Families for a dissertation fellowship supporting work on this project. Preliminary results were presented at annual meetings of the International Association of Relationship Research (2012) and the Association for Psychological Science (2014).
Footnotes
Researchers have used different terms to describe the phenomenon of similarity, such as attunement (Ruttle, Serbin, Stack, Schwartzman, & Shirtcliff, 2011), synchrony (Diamond & Aspinwall, 2003), and coregulation (Saxbe & Repetti, 2010). See Butler (2011) for a more complete discussion of the terminology issues in this literature.
The primary difference between the two models is that Beck et al. (2013) included an additional saliva sample taken at participants’ homes to enable modeling of cortisol reactivity both to the laboratory situation as well as the conflict discussion. Because we were interested only in the conflict discussion’s effect on cortisol, we omitted the home sample from our models.
The piecewise model provided a significantly better fit to the data than a simple linear trajectory for both lab visit 1, Δχ2(Δdf= 13) =2187.922, p < .001; and lab visit 2, Δχ2(Δdf= 13) =2064.974, p < .001. These findings support the use of piecewise model for the cortisol trajectories, indicating that the conflict stressor significantly disrupted the natural evening decline in cortisol.
As a preliminary step, we estimated models with single medication control indicators (coded as present = 1 or absent =0) separately for barbiturates, corticosteroids, allergy medications, antidepressant or antianxiety medications, attention deficit/hyperactivity medications, analgesics, proton pump inhibitors, antibiotics, anti-inflammatories, and hormonal birth control (wives only). Significant controls from these preliminary models were retained and added simultaneously to the models presented in this study. In order to make valid comparisons across study years, we used identical controls for both years.
To address concerns about testing for change using only two time points, we performed two analyses to rule out the possibility that our convergence findings were the result of regression to the mean. First, we reversed the direction of time in the model. Methodologists recommend that researchers using a regressed change approach reverse the time points in the analysis to ensure that the significant findings hold (e.g., Campbell & Kenny, 1999). We demonstrated that the approach to the analysis of change used in our convergence analyses (a two point growth model) simply reversed the sign of the change in dyadic discrepancies over time; coefficient values were identical but with opposite signs. This is one advantage of modeling change via growth curve modeling as opposed to a regressed change approach. We also assessed for selection effects. We tested whether couples who did not return were less extreme in cortisol values as compared to those who did. If found, such differences would represent a selection problem, in which our subsample was a group with extreme values, who (by the property of regression to the mean) would be more likely to have middling values at the next measurement point. If the subsample of couples used in our study represented those who were more discrepant at year 1, our finding that in general couples became less discrepant could plausibly be due to selection effects – meaning that couples in the subsample were more extremely discrepant by chance at the first visit, and thus more likely to be less discrepant at the second lab visit by chance. To address this potential issue, we conducted comparisons of absolute raw discrepancies between couples’ cortisol samples. There were no significant differences in dyadic discrepancies between our subsample and the group who did not return for a 2nd lab visit (see final rows of Table S1 in the online supplement for t statistics). With these analyses, we find no evidence to indicate that regression to the mean issues were responsible for our findings.
After the conflict discussion, participants completed ratings about their feelings during the interaction, including how angry they felt (from 1=“not at all” to 7=“extremely”). Spouses’ T1 reports were retained as separate variables due to their low correlation r = .259, p < .001 (M = 1.57, SD = 1.03, for husbands, and M = 1.84, SD = 1.33, for wives).
Observer-rated T1 hostility from the Rapid Marital Interaction Coding System (RMICS; Heyman, 2004) was used in expoloratory analyses. The percentage of hostile “turns” out of total behaviors during the conflict was computed. As it was highly skewed with a large number of zeros, the variable could not be used as a continuous predictor. Spouses’ hostility behaviors were recoded as binary indicators either indicating “none” of the behavior (41.5% of husbands, 39.9% wives) a “low” proportion of the behavior (.01–9.9% of total behaviors; 44.3% for husbands, 39.9% for wives) or “high” proportion (> 10% of total behaviors; 14.2% for husbands, 20.2% for wives).
Contributor Information
Holly Laws, Department of Psychiatry, Yale University School of Medicine.
Aline G. Sayer, Department of Psychological and Brain Sciences, University of Massachusetts Amherst
Paula R. Pietromonaco, Department of Psychological and Brain Sciences, University of Massachusetts Amherst
Sally I. Powers, Department of Psychological and Brain Sciences, University of Massachusetts Amherst
REFERENCES
- Aardal E, Holm AC. Cortisol in saliva - Reference ranges and relation to cortisol in serum. European Journal of Clinical Chemistry and Clinical Biochemistry. 1995;33:927–932. doi: 10.1515/cclm.1995.33.12.927. [DOI] [PubMed] [Google Scholar]
- Abercrombie HC, Giese-Davis J, Sephton S, Epel ES, Turner-Cobb JM, Spiegel D. Flattened cortisol rhythms in metastatic breast cancer patients. Psychoneuroendocrinology. 2004;29(8):1082–1092. doi: 10.1016/j.psyneuen.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Allison Paul D. Missing Data. In: Millsap RE, Maydeu-Olivares A, editors. The SAGE Handbook of Quantitative Methods in Psychology. Thousand Oaks, CA: Sage Publications Inc; 2009. pp. 72–89. [Google Scholar]
- Anderson C, Keltner D, John OP. Emotional convergence between people over time. Journal of Personality and Social Psychology. 2003;84:1054–1068. doi: 10.1037/0022-3514.84.5.1054. [DOI] [PubMed] [Google Scholar]
- Barnett R, Steptoe A, Gareis KC. Marital-role quality and stress-related psychobiological indicators. Annals of Behavioral Medicine. 2005;30:36–43. doi: 10.1207/s15324796abm3001_5. [DOI] [PubMed] [Google Scholar]
- Beck LA, Pietromonaco PR, DeBuse CJ, Powers SI, Sayer AG. Spouses’ attachment pairings predict neuroendocrine, behavioral, and psychological responses to marital conflict. Journal of Personality and Social Psychology. 2013;105:388–424. doi: 10.1037/a0033056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benazon NR, Coyne JC. Living with a depressed spouse. Journal of Family Psychology. 2000;14:71–79. [PubMed] [Google Scholar]
- Berkman LF, Glass T, Brissette I, Seeman TE. From social integration to health: Durkheim in the new millennium. Social Science & Medicine. 2000;51(6):843–857. doi: 10.1016/s0277-9536(00)00065-4. [DOI] [PubMed] [Google Scholar]
- Butler EA. Temporal interpersonal emotion systems: The “TIES” that form relationships. Personality and Social Psychology Review. 2011;15:367–393. doi: 10.1177/1088868311411164. [DOI] [PubMed] [Google Scholar]
- Campbell DT, Kenny DA. A primer on regression artifacts. Vol. 3. Guilford Publications; 1999. [Google Scholar]
- Chaplin TM, Sinha R, Simmons JA, Healy SM, Mayes LC, Hommer RE, Crowley MJ. Parent-adolescent conflict interactions and adolescent alcohol use. Addictive Behaviors. 2012;37:605–612. doi: 10.1016/j.addbeh.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone RD, Low MJ, Elmquist JK, Cameron JL. In: Neuroendocrinology. Williams Textbook of Endocrinology. 10th ed. Larsen PR, Kronenberg HM, Melmed S, Polonsky KS, editors. Philadelphia, PA: Saunders; 2002. 2002. pp. 81–176. [Google Scholar]
- DeSantis AS, DiezRoux AV, Hajat A, Aiello AE, Golden SH, Jenny NS, Shea S. Associations of salivary cortisol levels with inflammatory markers: The Multi-Ethnic Study of Atherosclerosis. Psychoneuroendocrinology. 2012;37(7):1009–1018. doi: 10.1016/j.psyneuen.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond LM. Contributions of psychophysiology to research on adult attachment: Review and recommendations. Personality and Social Psychology Review. 2001;5:276–295. [Google Scholar]
- Diamond LM, Aspinwall LG. Emotion regulation across the life span: An integrative perspective emphasizing self-regulation, positive affect, and dyadic processes. Motivation and Emotion. 2003;27:125–156. [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dorn LD, Lucke JF, Loucks TL, Berga SL. Salivary cortisol reflects serum cortisol: analysis of circadian profiles. Annals of Clinical Biochemistry. 2007;44(3):281–284. doi: 10.1258/000456307780480954. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Kim HK, Bruce J, Pears KC. Cumulative effects of prenatal substance exposure and early adversity on foster children’s HPA-axis reactivity during a psychosocial stressor. International Journal of Behavioral Development. 2012;36(1):29–35. doi: 10.1177/0165025411406863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser J, editors. Handbook of human stress and immunity. San Diego, CA: Academic Press; 1994. [Google Scholar]
- Gonzaga GC, Campos B, Bradbury T. Synchrony, convergence, and relationship satisfaction in dating and married couples. Journal of Personality and Social Psychology. 2007;93:34–48. doi: 10.1037/0022-3514.93.1.34. [DOI] [PubMed] [Google Scholar]
- Gottman JM. Marital interaction: Experimental investigations. London: Academic Press; 1979. [Google Scholar]
- Gouin J-P, Kiecolt-Glaser JK, Malarkey WB, Glaser R. The influence of anger expression on wound healing. Brain, Behavior, and Immunity. 2008;22(5):699–708. doi: 10.1016/j.bbi.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DA, Hibel LC, Fortunato CK, Kapelewski CH. Medication effects on salivary cortisol: Tactics and strategy to minimize impact in behavioral and developmental science. Psychoneuroendocrinology. 2009;34:1437–1448. doi: 10.1016/j.psyneuen.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Grynderup MB, Kolstad HA, Mikkelsen S, Andersen JH, Bonde JP, Buttenschøn HN, Hansen ÅM. A two-year follow-up study of salivary cortisol concentration and the risk of depression. Psychoneuroendocrinology. 2013;38(10):2042–2050. doi: 10.1016/j.psyneuen.2013.03.013. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: A meta-analytic review. PLoS Medicine. 2010;7:1–20. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huston TL, Melz H. The case for (promoting) marriage: The devil is in the details. Journal of Marriage and Family. 2004;66:943–958. [Google Scholar]
- Huston TL, Niehuis S, Smith SE. The early marital roots of conjugal distress and divorce. Current Directions in Psychological Science. 2001;10:116–119. [Google Scholar]
- Jaremka LM, Glaser R, Malarkey WB, Kiecolt-Glaser JK. Marital distress prospectively predicts poorer cellular immune function. Psychoneuroendocrinology. 2013;38:2713–2719. doi: 10.1016/j.psyneuen.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner T, Katz J. Contagion of depressive symptoms and mood: Meta-analytic review and explanations from cognitive, behavioral, and interpersonal viewpoints. Clinical Psychology: Science and Practice. 1999;6:149–164. [Google Scholar]
- Kenny DA, Kashy DA, Cook WL. Dyadic data analysis. New York: Guilford; 2006. [Google Scholar]
- Kiecolt-Glaser JK, Bane C, Glaser R, Malarkey WB. Love, marriage, and divorce: Newlyweds’ stress hormones foreshadow relationship changes. Journal of Consulting and Clinical Psychology. 2003;71:176–188. doi: 10.1037//0022-006x.71.1.176. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Newton TL. Marriage and health: His and hers. Psychological Bulletin. 2001;127:472–503. doi: 10.1037/0033-2909.127.4.472. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’-a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1–2):76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Laurent HK, Powers SI, Granger DA. Refining the multisystem view of the stress response: Coordination among cortisol, alpha-amylase, and subjective stress in response to relationship conflict. Physiology & Behavior. 2013;119:52–60. doi: 10.1016/j.physbeh.2013.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Rovine MJ, Klein L, Almeida DM. Synchrony of diurnal cortisol pattern in couples. Journal of Family Psychology. 2013;27:579–588. doi: 10.1037/a0033735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons KS, Sayer AG. Using multilevel modeling in caregiving research. Aging & Mental Health. 2005;9:189–195. doi: 10.1080/13607860500089831. [DOI] [PubMed] [Google Scholar]
- Meyler D, Stimpson JP, Peek M. Health concordance within couples: A systematic review. Social Science & Medicine. 2007;64:2297–2310. doi: 10.1016/j.socscimed.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Papp LM, Pendry P, Simon CD, Adam EK. Spouses’ cortisol associations and moderators: Testing physiological synchrony and connectedness in everyday life. Family Process. 2013;52:284–298. doi: 10.1111/j.1545-5300.2012.01413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AC, Ginty AT, Hughes BM. The other side of the coin: Blunted cardiovascular and cortisol reactivity are associated with negative health outcomes. International Journal of Psychophysiology. 2013;90(1):1–7. doi: 10.1016/j.ijpsycho.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Pietromonaco PR, DeBuse CJ, Powers SI. Does attachment get under the skin? Adult romantic attachment and cortisol responses to stress. Current Directions in Psychological Science. 2013;22:63–68. doi: 10.1177/0963721412463229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietromonaco PR, Powers SI. Attachment and health-related physiological stress processes. Current Opinion in Psychology. 2015;1:34–39. doi: 10.1016/j.copsyc.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietromonaco PR, Uchino B, Dunkel Schetter C. Close relationship processes and health: Implications of attachment theory for health and disease. Health Psychology. 2013;32:499–513. doi: 10.1037/a0029349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers SI, Pietromonaco PR, Gunlicks M, Sayer A. Dating couples’ attachment styles and patterns of cortisol reactivity and recovery in response to a relationship conflict. Journal of Personality and Social Psychology. 2006;90:613–628. doi: 10.1037/0022-3514.90.4.613. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Brennan RT, Barnett RC. A multivariate hierarchical model for studying psychological change within married couples. Journal of Family Psychology. 1995;9:161–174. [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. 2nd. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- Raudenbush SW, Bryk AS, Congdon R. HLM 7 for Windows [Computer software] Lincolnwood, IL: Scientific Software International, Inc; 2011. [Google Scholar]
- Rehman US, Gollan J, Mortimer AR. The marital context of depression: Research, limitations, and new directions. Clinical Psychology Review. 2008;28:179–198. doi: 10.1016/j.cpr.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Repetti RL, Wang S, Saxbe DE. Adult health in the context of everyday family life. Annals of Behavioral Medicine. 2011;42:285–293. doi: 10.1007/s12160-011-9293-x. [DOI] [PubMed] [Google Scholar]
- Robles TF, Shaffer VA, Malarkey WB, Kiecolt-Glaser JK. Positive behaviors during marital conflict: Influences on stress hormones. Journal of Social and Personal Relationships. 2006;23:305–325. [Google Scholar]
- Rodriguez AJ, Margolin G. Wives’ and Husbands’ Cortisol Reactivity to Proximal and Distal Dimensions of Couple Conflict. Family Process. 2013;52(3):555–569. doi: 10.1111/famp.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy MP, Kirschbaum C, Steptoe A. Psychological, cardiovascular, and metabolic correlates of individual differences in cortisol stress recovery in young men. Psychoneuroendocrinology. 2001;26(4):375–391. doi: 10.1016/s0306-4530(00)00061-5. [DOI] [PubMed] [Google Scholar]
- Ruttle PL, Serbin LA, Stack DM, Schwartzman AE, Shirtcliff EA. Adrenocortical attunement in mother-child dyads: Importance of situational and behavioral characteristics. Biological Psychology. 2011;88:104–111. doi: 10.1016/j.biopsycho.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Saxbe DE, Repetti RL, Nishina A. Marital satisfaction, recovery from work, and diurnal cortisol among men and women. Health Psychology. 2008;27:15–25. doi: 10.1037/0278-6133.27.1.15. [DOI] [PubMed] [Google Scholar]
- Saxbe DE, Repetti RL. For better or worse? Coregulation of couples’ cortisol levels and mood states. Journal of Personality and Social Psychology. 2010;98:92–103. doi: 10.1037/a0016959. [DOI] [PubMed] [Google Scholar]
- Sayer AG, Klute M. Analyzing couples and families: Multilevel methods. In: Bengtson V, Acock A, Allen K, Dilworth-Anderson P, Klein DM, editors. Sourcebook of family theory & research. Thousand Oaks, CA; Sage Publications, Inc; 2005. pp. 289–313. [Google Scholar]
- Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, Colditz GA. Night-shift work and risk of colorectal cancer in the nurses’ health study. Journal of the National Cancer Institute. 2003;95:825–828. doi: 10.1093/jnci/95.11.825. http://jnci.oxfordjournals.org/ [DOI] [PubMed] [Google Scholar]
- Smith TW, Uchino BN, Berg CA, Florsheim P. Marital discord and coronary artery disease: A comparison of behaviorally defined discrete groups. Journal of Consulting and Clinical Psychology. 2012;80:87–92. doi: 10.1037/a0026561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanier GB. Measuring dyadic adjustment: New scales for assessing the quality of marriage and similar dyads. Journal of Marriage and the Family. 1976;38:15–28. [Google Scholar]
- Stadler G, Snyder KA, Horn AB, Shrout PE, Bolger NP. Close relationships and health in daily life: a review and empirical data on intimacy and somatic symptoms. Psychosomatic Medicine. 2012;74(4):398–409. doi: 10.1097/PSY.0b013e31825473b8. [DOI] [PubMed] [Google Scholar]
- Stansbury K, Gunnar MR. Adrenocortical activity and emotion regulation. Monographs of the Society for Research in Child Development. 1994;59:108–134. 250–283. [PubMed] [Google Scholar]
- Thompson A, Bolger N. Emotional transmission in couples under stress. Journal of Marriage and the Family. 1999:38–48. [Google Scholar]
- Tukey JW. Exploratory data analysis. Reading, MA: Addison-Wesley Publishing Company; 1977. [Google Scholar]
- Uchino BN, Bosch JA, Smith TW, Carlisle M, Birmingham W, Bowen KS…, O’Hartaigh B. Relationships and cardiovascular risk: Perceived spousal ambivalence in specific relationship contexts and its links to inflammation. Health Psychology. 2013;32:1067–1075. doi: 10.1037/a0033515. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.