Abstract
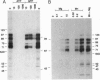
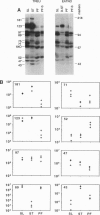
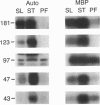
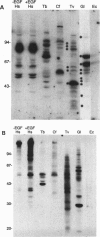
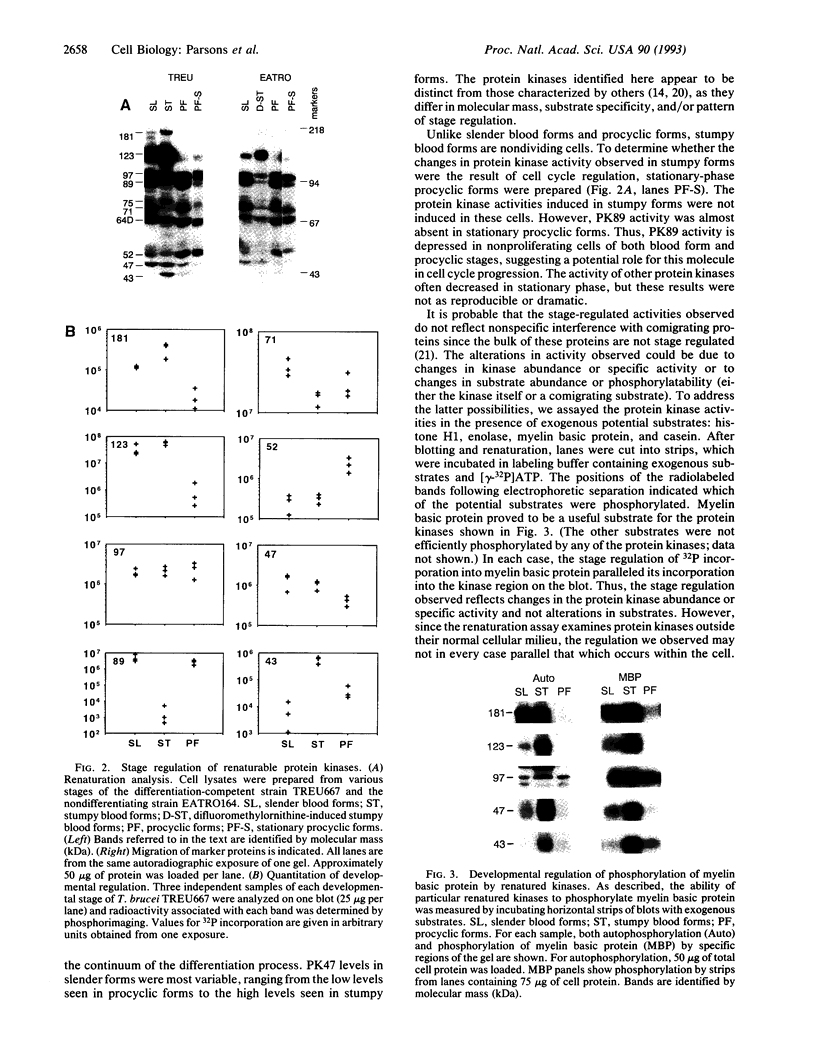
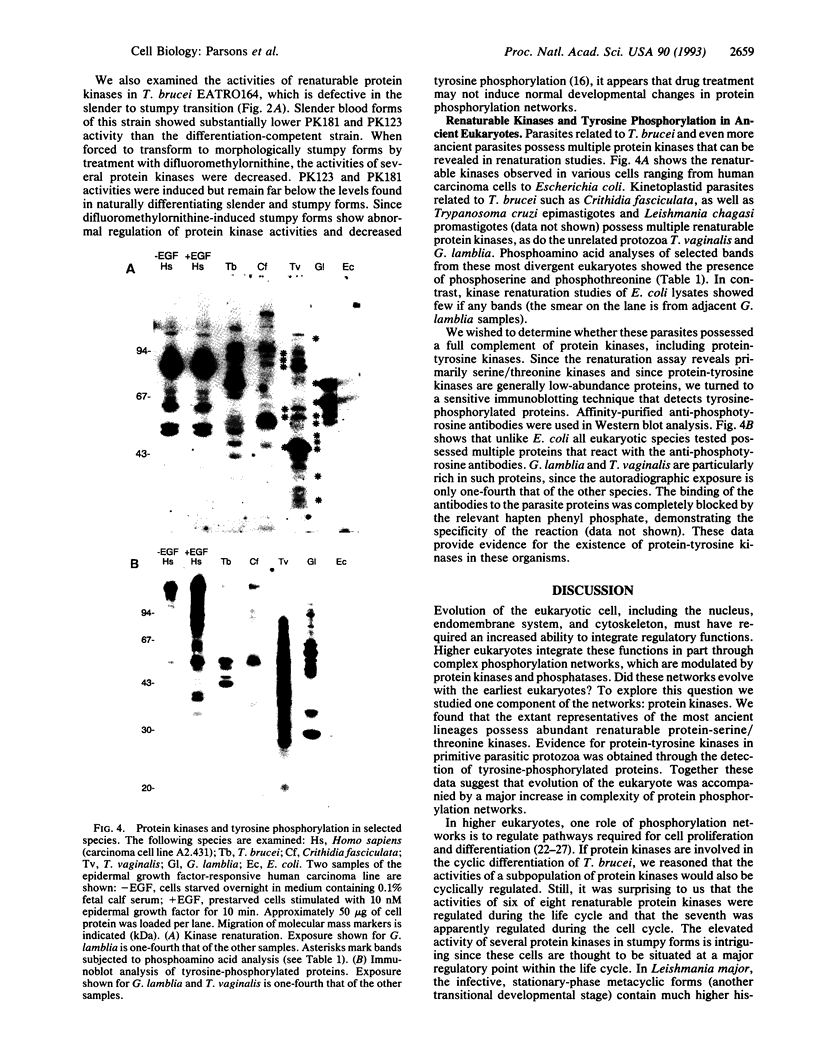
The role of protein kinases in organisms that diverged early in the eukaryotic lineage is relatively unexplored. In this study, we determined that primitive parasitic protozoa possess multiple protein-serine kinases and inferred the presence of protein-tyrosine kinases through sensitive immunoblotting techniques. To further explore the role of protein kinases in parasite development, we examined the activity of eight renaturable protein kinases during the life cycle of the protozoan parasite Trypanosoma brucei. The activities of six protein-serine/threonine kinases were regulated during development, with several distinct patterns of regulation. In addition, an 89-kDa protein kinase was detected in dividing cells but not in nondividing cells. Our data indicate that even the most primitive eukaryotes possess a large complement of protein kinases, including protein-tyrosine kinases as well as protein-serine/threonine kinases. The data further suggest that protein kinases may play a pivotal role in regulation of proliferation and differentiation in protozoa.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboagye-Kwarteng T., ole-MoiYoi O. K., Lonsdale-Eccles J. D. Phosphorylation differences among proteins of bloodstream developmental stages of Trypanosoma brucei brucei. Biochem J. 1991 Apr 1;275(Pt 1):7–14. doi: 10.1042/bj2750007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülow R., Nonnengässer C., Overath P. Release of the variant surface glycoprotein during differentiation of bloodstream to procyclic forms of Trypanosoma brucei. Mol Biochem Parasitol. 1989 Jan 1;32(1):85–92. doi: 10.1016/0166-6851(89)90132-1. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- DeVoti J., Seydoux G., Beach D., McLeod M. Interaction between ran1+ protein kinase and cAMP dependent protein kinase as negative regulators of fission yeast meiosis. EMBO J. 1991 Dec;10(12):3759–3768. doi: 10.1002/j.1460-2075.1991.tb04945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell J. E., Jr, Martin G. S. Thrombin stimulates the activities of multiple previously unidentified protein kinases in platelets. J Biol Chem. 1989 Dec 5;264(34):20723–20729. [PubMed] [Google Scholar]
- Gould K. L., Nurse P. Tyrosine phosphorylation of the fission yeast cdc2+ protein kinase regulates entry into mitosis. Nature. 1989 Nov 2;342(6245):39–45. doi: 10.1038/342039a0. [DOI] [PubMed] [Google Scholar]
- Harwood A. J., Hopper N. A., Simon M. N., Bouzid S., Veron M., Williams J. G. Multiple roles for cAMP-dependent protein kinase during Dictyostelium development. Dev Biol. 1992 Jan;149(1):90–99. doi: 10.1016/0012-1606(92)90266-j. [DOI] [PubMed] [Google Scholar]
- Hermoso T., Fishelson Z., Becker S. I., Hirschberg K., Jaffe C. L. Leishmanial protein kinases phosphorylate components of the complement system. EMBO J. 1991 Dec;10(13):4061–4067. doi: 10.1002/j.1460-2075.1991.tb04982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Johnston R. F., Pickett S. C., Barker D. L. Autoradiography using storage phosphor technology. Electrophoresis. 1990 May;11(5):355–360. doi: 10.1002/elps.1150110503. [DOI] [PubMed] [Google Scholar]
- Keith K., Hide G., Tait A. Characterisation of protein kinase C like activities in Trypanosoma brucei. Mol Biochem Parasitol. 1990 Nov;43(1):107–116. doi: 10.1016/0166-6851(90)90135-9. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay N. K., Saha A. K., Lovelace J. K., Da Silva R., Sacks D. L., Glew R. H. Comparison of the protein kinase and acid phosphatase activities of five species of Leishmania. J Protozool. 1988 Nov;35(4):601–607. doi: 10.1111/j.1550-7408.1988.tb04158.x. [DOI] [PubMed] [Google Scholar]
- Murphy W. J., Watkins K. P., Agabian N. Identification of a novel Y branch structure as an intermediate in trypanosome mRNA processing: evidence for trans splicing. Cell. 1986 Nov 21;47(4):517–525. doi: 10.1016/0092-8674(86)90616-1. [DOI] [PubMed] [Google Scholar]
- Müller M. Energy metabolism of protozoa without mitochondria. Annu Rev Microbiol. 1988;42:465–488. doi: 10.1146/annurev.mi.42.100188.002341. [DOI] [PubMed] [Google Scholar]
- Opperdoes F. R. Compartmentation of carbohydrate metabolism in trypanosomes. Annu Rev Microbiol. 1987;41:127–151. doi: 10.1146/annurev.mi.41.100187.001015. [DOI] [PubMed] [Google Scholar]
- Parsons M., Valentine M., Deans J., Schieven G. L., Ledbetter J. A. Distinct patterns of tyrosine phosphorylation during the life cycle of Trypanosoma brucei. Mol Biochem Parasitol. 1991 Apr;45(2):241–248. doi: 10.1016/0166-6851(91)90091-j. [DOI] [PubMed] [Google Scholar]
- Pawson T., Bernstein A. Receptor tyrosine kinases: genetic evidence for their role in Drosophila and mouse development. Trends Genet. 1990 Nov;6(11):350–356. doi: 10.1016/0168-9525(90)90276-c. [DOI] [PubMed] [Google Scholar]
- Richardson J. P., Beecroft R. P., Tolson D. L., Liu M. K., Pearson T. W. Procyclin: an unusual immunodominant glycoprotein surface antigen from the procyclic stage of African trypanosomes. Mol Biochem Parasitol. 1988 Dec;31(3):203–216. doi: 10.1016/0166-6851(88)90150-8. [DOI] [PubMed] [Google Scholar]
- Shapiro S. Z., Kimmel B. E. Differential protein synthesis during the life cycle of the protozoan parasite Trypanosoma brucei. J Protozool. 1987 Feb;34(1):58–62. doi: 10.1111/j.1550-7408.1987.tb03132.x. [DOI] [PubMed] [Google Scholar]
- Stuart K., Feagin J. E. Mitochondrial DNA of kinetoplastids. Int Rev Cytol. 1992;141:65–88. doi: 10.1016/s0074-7696(08)62063-x. [DOI] [PubMed] [Google Scholar]
- Sutton R. E., Boothroyd J. C. Trypanosome trans-splicing utilizes 2'-5' branches and a corresponding debranching activity. EMBO J. 1988 May;7(5):1431–1437. doi: 10.1002/j.1460-2075.1988.tb02960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner C. M., Barry J. D., Vickerman K. Loss of variable antigen during transformation of Trypanosoma brucei rhodesiense from bloodstream to procyclic forms in the tsetse fly. Parasitol Res. 1988;74(6):507–511. doi: 10.1007/BF00531626. [DOI] [PubMed] [Google Scholar]
- Vickerman K. Developmental cycles and biology of pathogenic trypanosomes. Br Med Bull. 1985 Apr;41(2):105–114. doi: 10.1093/oxfordjournals.bmb.a072036. [DOI] [PubMed] [Google Scholar]
- Vickerman K. On the surface coat and flagellar adhesion in trypanosomes. J Cell Sci. 1969 Jul;5(1):163–193. doi: 10.1242/jcs.5.1.163. [DOI] [PubMed] [Google Scholar]
- Vickerman K. Polymorphism and mitochondrial activity in sleeping sickness trypanosomes. Nature. 1965 Nov 20;208(5012):762–766. doi: 10.1038/208762a0. [DOI] [PubMed] [Google Scholar]
- Walter R. D. Multiple protein kinases from Trypanosoma gambiense. Hoppe Seylers Z Physiol Chem. 1978 May;359(5):601–606. doi: 10.1515/bchm.1978.359.1.601. [DOI] [PubMed] [Google Scholar]
- Webster P., Fish W. R. Endocytosis by African trypanosomes. II. Occurrence in different life-cycle stages and intracellular sorting. Eur J Cell Biol. 1989 Aug;49(2):303–310. [PubMed] [Google Scholar]