Abstract
AIM: Nitrative and oxidative DNA damage such as 8-nitroguanine and 8-oxo-7,8-dihydro-2’-deoxyguanosine (8-oxodG) formation has been implicated in initiation and/ or promotion of inflammation-mediated carcinogenesis. The aim of this study is to clarify whether these DNA lesions participate in the progression of intrahepatic cholangiocarcinoma.
METHODS: We investigated the relation of the formation of 8-nitroguanine and 8-oxodG and the expression of hypoxia-inducible factor-1α (HIF-1α) with tumor invasion in 37 patients with intra-hepatic cholangiocarcinoma.
RESULTS: Immunohistochemical analyses revealed that 8-nitroguanine and 8-oxodG formation occurred to a much greater extent in cancerous tissues than in non-cancerous tissues. HIF-1α could be detected in cancerous tissues in all patients, suggesting low oxygen tension in the tumors. HIF-1α expression was correlated with inducible nitric oxide synthase (iNOS) expression (r = 0.369 and P = 0.025) and 8-oxodG formation (r = 0.398 and P = 0.015). Double immunofluorescence study revealed that iNOS and HIF-1α co-localized in cancerous tissues. Notably, the formation of 8-oxodG was correlated significantly with lymphatic invasion (r = 0.386 and P = 0.018). Moreover, 8-nitroguanine and 8-oxodG in non-cancerous tissues were associated significantly with neural invasion (P = 0.042 and P = 0.026, respectively). These results suggest that reciprocal activation between HIF-1α and iNOS mediates persistent DNA damage, which induces tumor invasiveness via mutations, resulting in poor prognosis.
CONCLUSION: The formation of 8-nitroguanine and 8-oxodG plays an important role in multiple steps of genetic changes leading to tumor progression, including invasiveness.
Keywords: 8-Nitroguanine; 8-Oxo-7,8-dihydro-2’-deoxyg-uanosine; Hypoxia-inducible factor-1α; Cholangiocarcinoma; Tumor invasion
INTRODUCTION
Intra-hepatic cholangiocarcinoma (ICC), an adenocarcinoma originating from intra-hepatic bile duct epithelium, presents in most cases with an extremely poor prognosis[1]. The highest proportional incidence of ICC is observed in the north-east region of Thailand, in which Opisthorchis viverrini (OV) infection is endemic[2,3]. Thus, molecular markers serving as predictive factors are needed to provide effective therapy as well as to understand the underlying mechanisms of ICC carcinogenesis.
Infections are well accounted for association with several types of cancer including ICC through chronic inflammation[4,5]. Production of a large amount of reactive oxygen species (ROS) and reactive nitrogen species (RNS), such as nitric oxide (NO), is associated with an increased risk of human cancer[6-8]. 8-Oxo-7,8-dihydro-2’-deoxyguanosine (8-oxodG), a marker of DNA damage by ROS[9], is a mutagenic lesion leading to G→T transversions[10,11], which are frequently found in tumor relevant genes in a variety of cancers. NO generation by inducible nitric oxide synthase (iNOS) is triggered during infection and inflammation[12]. Overproduction of NO leads to the generation of various RNS, causing nitrative DNA damage such as 8-nitroguanosine formation[13]. 8-nitroguanine undergoes spontaneous depurination, which leads to apurinic sites in DNA[13]. The resulting apurinic sites in DNA can also lead to G→T transversions[14]. Recently, we have reported 8-nitroguanine formation in the liver of hamsters re-infected with OV[15-17]. We have also demonstrated the accumulation of 8-nitroguanine in human gastric epithelium induced by Helicobacter pylori infection, which is associated with gastric cancer[18]. Thus, we have proposed that 8-nitroguanine and 8-oxodG can be biomarkers of initiation and/or promotion in cases of carcinogenesis mediated by inflammation. However, the role of 8-nitroguanine and 8-oxodG in tumor progression in ICC has not been elucidated.
Tumor growth induces hypoxia, which is associated with poor prognosis[19]. Tumor cells adapt to hypoxia by increasing the synthesis of hypoxia-inducible factor-1α (HIF-1α), which mediates transcription of various genes, including vascular endothelial growth factor, glucose transporter 1, lactate dehydrogenase and iNOS[19], in solid tumors. It remains to be clarified whether HIF-1α is associated with DNA base lesions and tumor invasion, resulting in poor prognosis with ICC patients.
To evaluate prognostic factors in ICC carcinogenesis, we investigated the formation of 8-nitroguanine and 8-oxodG, and the expression of iNOS and HIF-1α in the liver of ICC patients by immunohistochemistry. We raised a highly specific antibody against 8-nitroguanine without cross reaction by immunizing with an 8-nitroguanine-aldehyde-rabbit serum albumin conjugate. We investigated the association of DNA damage in the liver tissue with neural and lymphatic invasion in ICC patients.
MATERIALS AND METHODS
Subjects
This study was approved by the Ethics Group of the Human Research Committee, Khon Kaen University, Thailand. Patients undergoing surgical resection of hepatogastrointestinal cancer in 1998 and 1999 at the Department of Surgery, Faculty of Medicine, Khon Kaen University, Thailand, were asked to volunteer for this study. Informed consent was obtained from each subject. Gross appearance of 37 surgical specimens was classified as periductal-infiltrating (11 patients) or mass-forming (26 patients) types. In addition, nine healthy individuals of accidental cause of death were used as controls.
Specimen collection and storage
ICC cases were clarified by physicians using clinical finding and laboratory investigation such as tumor markers, including α-fetoprotein, carcinoembryonic antigen and carbohydrate antigen 19-9, X-ray and histological examination. Liver tissues in both cancerous and adjacent non-cancerous region were obtained from the same patients in all cases. Sections were immediately frozen in liquid nitrogen and stored at -80°C until analysis. The International Union against Cancer TNM classification and staging system were used for tumor assessment. Liver function test and complete blood count were performed by the hospital laboratory using standard protocols.
Reagents
Highly sensitive and specific anti-8-nitroguanine rabbit polyclonal antibody was raised as described previously[17]. Mouse monoclonal anti-8-oxodG antibody was purchased from Japan Institute for the Control of Aging (Fukuroi, Japan). Rabbit polyclonal anti-iNOS antibody and mouse monoclonal anti-HIF-1α antibody were purchased from Calbiochem-Novabiochem Corporation (Darmstadt, Germany). Alexa 594-labeled antibody against rabbit IgG and Alexa 488-labeled antibody against mouse IgG were obtained from Molecular Probes Inc. (Eugene, OR, USA).
Immunohistological staining
Immunohistochemical staining was performed by using immunoperoxidase methods. Sections (thickness, 6 μm) were deparaffinized in xylene and rehydrated in descending gradations of ethanol. To enhance the immunostaining, sections were placed in citrate buffer (pH 6) and microwaved intermittently for up to 10 min for antigen unmasking. Endog-enous peroxidase was quenched by immersion in 30 mL/L hydrogen peroxide (H2O2) for 30 min, and then blocked with 10 mg/L skimmed milk for 30 min. These sections were treated with the primary antibodies (2 μg/mL for 8-nitroguanine and 5 μg/mL for 8-oxodG) overnight at room temperature. The slides were then washed, and incubated for 3 h with goat anti-rabbit IgG antibody, followed by the treatment with peroxidase–anti-peroxidase complex (1:200) for 1 h for detection of 8-nitroguanine. To detect 8-oxodG, the slides were treated with HRP-conjugated goat anti-mouse IgG antibody (1:200). The immunostaining were developed by using 3,3-diaminobenzidine tetrahydrochloride as a chromogen for 15 min.
The expression of iNOS and HIF-1α was assessed by using double immunofluorescence technique as described previously[18]. Briefly, the sections were treated with anti-iNOS antibody (1:300) and anti-HIF-1α antibody (1:500) and then treated with Alexa 594-labeled antibody against rabbit IgG and Alexa 488-labeled antibody against mouse IgG (1:400 each). The results were analyzed by using a laser scanning microscope.
The following scores were assigned to each specimen according to the degree of staining: 0, negative; +, less than 25% (minimal); ++, 25-50% (moderate); and +++, more than 50% (strong) in the cells of tissue sections.
Histopathological study
Histopathological study was performed by hematoxylin and eosin staining in paraffin sections as described previously[20]. Neural invasion and lymphatic invasion were assessed by standard method[21].
Examination of specific IgG antibody against OV antigen and OV eggs
OV-specific IgG antibody was determined by ELISA technique[22]. Crude somatic antigen of OV was prepared from hamsters infected with 100 metacercariae after 4 mo as described previously[20]. The OV egg count was determined from 1 g of feces using the formalin-ethyl acetate concentration technique[22].
Statistical analysis
Significant differences were analyzed by the χ2-test. Spearman’s rank correlation coefficient served to analyze correlations for qualitative data, while Pearson’s correlation coefficient was used for quantitative data. P values less than 0.05 were considered to be statistically significant.
RESULTS
Clinical data of ICC patients
ICC samples, comprising 37 matched cancerous and adjacent noncancerous tissues and 9 healthy subjects who died from accidental cases, were collected at the time of surgery. ICC patients included 26 men and 11 women with the mean age 53 ± 11 years. These subjects were verified by histopathological study and comprised of well (15 patients, including 2 patients of papillary), moderately (11 patients) and poorly (11 patients) differentiated adenocarcinoma. The gross appearance was periductal infiltrating (11 patients) and mass forming (26 patients). Tumor size was 3-6 cm (22 patients), 7-10 cm (4 patients) and > 10 cm (4 patients). The average of hemoglobin and hematocrit of all patients was 11.7 ± 2.3% and 34.7 ± 6.5%, respectively. Most of the patients (22 out of 37, 59.5%) had total bilirubin less than 1 mg% without jaundice, and other patients had total bilirubin of 1-10 mg% (5 patients) and > 14 mg% (10 patients). Sixty percent was positive for OV antibody and 30% was positive for OV egg, without adult worm in the liver. All patients had no history of infection with hepatitis virus and exposure to aflatoxin.
Formation of 8-oxodG and 8-nitroguanine
Immunohistochemical staining demonstrated that 8-oxodG and 8-nitroguanine formation was observed in inflammatory cells within the liver to a greater extent in cancer patients than in healthy subjects. 8-oxodG and 8-nitroguanine were formed to a much greater extent in cancerous tissues than that in non-cancerous tissues. 8-oxodG formation was observed in tumor cells and inflammatory cells, whereas 8-nitroguanine was formed mainly in inflammatory cells and weakly in tumor cells in cancerous tissues (Figure 1). We have confirmed that the anti-8-nitroguanine antibody is highly sensitive and specific by a dot immunobinding assay and absorption test[17].
Figure 1.
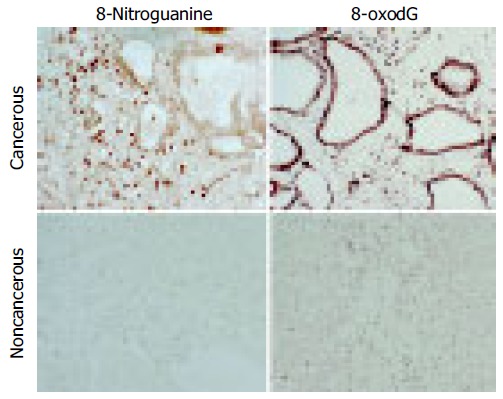
Localization of 8-oxodG and 8-nitroguanine in cancerous and noncancerous liver tissues in an ICC patient with a well-differentiated adenocarcinoma. Formation of 8-oxodG and 8-nitroguanine was assessed by immunohistochemistry using an immunoperoxidase method. Paraffin sections (6 μm thickness) were incubated with a rabbit polyclonal anti-8-nitroguanine antibody and a mouse monoclonal anti-8-oxodG antibody. The original magnification is 200×.
Expression of HIF-1α and iNOS
Double immunofluorescence staining revealed that HIF-1α and iNOS expression could be detected in all cancerous tissues and was found to a greater extent than in non-cancerous tissues. Expression of HIF-1α and iNOS was found in the tumor tissues especially bile duct epithelial cells (Figure 2). Slight immunoreactivity of iNOS was observed in inflammatory cells, especially Kupffer cells, in healthy subjects.
Figure 2.
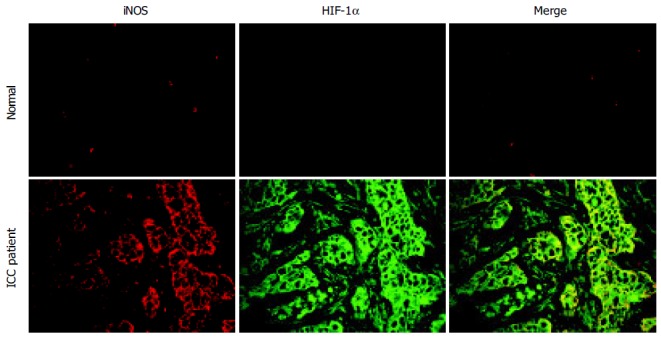
Localization of iNOS and HIF-1α in cancerous liver tissues in an ICC patient with poorly-differentiated adenocarcinoma. The expression of iNOS and HIF-1α was assessed by using double immunofluorescence technique. Paraffin sections were treated with anti-iNOS and anti-HIF-1α antibodies. The original magnification is 400×.
The correlation of 8-oxodG and 8-nitroguanine formation and iNOS expression with tumor invasion
Table 1 exhibits the relationship of DNA damage with lymphatic and neural invasion in ICC patients. Immuno-reactivity for 8-oxodG and 8-nitroguanine was stronger in cancerous tissues than in the adjacent non-cancerous tissues (P < 0.05). HIF-1α could be detected in all cancerous tissues. HIF-1α expression was correlated with iNOS expression (r = 0.369 and P = 0.025) and 8-oxodG formation (r = 0.398 and P = 0.015) in cancerous tissues. Furthermore, iNOS expression was significantly correlated with the formation of 8-oxodG (r = 0.584 and P = 0.00015) and 8-nitroguanine (r = 0.328 and P = 0.047). 8-oxodG formation in cancerous tissues was also significantly correlated with increased lymphatic invasion (r = 0.386, P =0 .018). The formation of 8-nitroguanine and 8-oxodG in non-cancerous tissues was significantly associated with neural invasion (P = 0.042 and P = 0.026, respectively). Neural invasion was associated with poor survival by generalized Wilcoxon test (P = 0.021) using the Kaplan-Meier method (Figure 3). In addition, in cancerous tissues, formation of 8-oxodG and 8-nitroguanine was positively correlated with serum ALT, AST and alkaline phosphatase (ALP) (r = 0.76, 0.58, and 0.96 for 8-oxodG and r = 0.63, 0.58, and 0.86 for 8-nitroguanine, respectively). Moreover, iNOS expression was also associated with ALT, AST, ALP (r = 0.71, 0.97 and 0.82, respectively).
Table 1.
Relationship of DNA damage with lymphatic and neural invasion in ICC patients
| Immuno- histological grading |
8-oxodG
Number of
patients (%) |
8-Nitroguanine
Number of
patients (%) |
HIF-1α
Number of
patients (%) |
Number (%)
of patients
with
lymphatic
invasion (8-oxodG) |
Number (%)
of patients
with
lymphatic
invasion (8-nitroguanine) |
Number (%) of
patients with
neural
invasion
(8-oxodG) |
Number (%)
of patients
with
neural
invasion (8-nitroguanine) |
||
| Cancerous | Non-cancer | Cancerous | Non-cancer | Cancerous | Cancerous | Cancerous | Non-cancer | Non-cancer | |
| ICC (37) | |||||||||
| 0 | - | 5 (13.51) | 11 (29.73) | 17 (46) | - | - | 9/11 (81.82) | 1/5 (20) | 8/17 (47.1) |
| + | 11 (29.73) | 17 (45.94) | 13 (35.14) | 19 (51.4) | 10 (27.03) | 6/11 (54.55) | 10/13 (76.92) | 14/17 (82.4) | 16/19 (84.2) |
| ++ | 11 (29.73) | 10 (27.03) | 8 (21.62) | 1 (2.70) | 18 (48.65) | 10/11 (90.91) | 6/8 (75) | 5/10 (50) | 0/1 (0) |
| +++ | 15 (40.54) | 5 (13.51) | 5 (13.51) | - | 9 (24.32) | 14/15 (93.33) | 5/5 (100) | 4/5 (80) | |
| P-value | P = 0.004 | P = 0.007 | P = 0.018 | P = 0.679 | P = 0.042 | P = 0.026 | |||
| Healthy (9) | |||||||||
| 0 | 2 (22.22) | 7 (77.77) | |||||||
| + | 7 (77.77) | 2 (22.2) | |||||||
Non-cancer = noncancerous tissues. 0 = no positive cell, + = positive in few areas or cells, ++ = moderately positive and +++ = predominately positive. ICC samples, comprising 37 matched cancerous and adjacent noncancerous tissues and 9 healthy subjects who died from accidental cases were collected at the time of surgery. ICC patients included 26 men and 11 women with the mean age 53±11 years. These subjects were verified by histopathological study and comprised well- (15 patients, including 2 patients of papillary), moderately- (11 patients) and poorly (11 patients) differentiated adenocarcinoma. The International Union Against Cancer TNM classification and staging system were used for tumor assessment.
Figure 3.
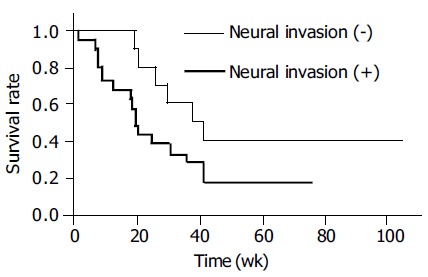
Overall survival curve of ICC patients. Thin line, neural invasion evaluated as -; thick line, neural invasion evaluated as + (Kaplan-Meier method).
DISCUSSION
Our results showed that both 8-nitroguanine and 8-oxodG were formed in cancerous tissues to a much greater extent than the adjacent non-cancerous tissues. Moreover, the formation of these DNA lesions was correlated with neural and lymphatic invasion. 8-oxodG was formed in tumor cells and inflammatory cells, whereas 8-nitroguanine was observed mainly in inflammatory cells and weakly in tumor cells. These results are consistent with a model in which tumor cells encourage inflammatory cell infiltration in cancerous areas; inflammatory cells then induce 8-nitroguanine and 8-oxodG formation via NO and superoxide anion radical production[23].
The present study revealed that 8-nitroguanine and 8-oxodG formation was related with serum ALT, AST, and ALP activity. This result is supported by the report showing that the number of 8-oxodG-positive hepatocytes was correlated with ALT and AST in liver diseases[24]. These findings can be explained by assuming that increase in NO and ROS not only causes DNA damage, but also induces epithelial bile duct and hepatocyte injury, resulting in an increase in hepatobiliary enzyme activities.
Our results showed that HIF-1α was associated with iNOS expression and 8-oxodG formation in cancerous tissues. This result is confirmed by immunohistochemistry showing that iNOS and HIF-1α co-localized in cancerous tissues. HIF-1α could be detected in all cancerous tissues, suggesting low oxygen consumption in tumor tissues. Tumor cells adapt to hypoxia by increasing the synthesis of HIF-1α, which mediates transcription of various genes, including iNOS[19]. iNOS expression was correlated with both 8-nitroguanine and 8-oxodG formation. Therefore, we hypot-hesize that tumor hypoxia induces HIF-1α expression, and then HIF-1α mediates iNOS expression, resulting in nitrative and oxidative DNA damage. On the other hand, an increase in NO production through iNOS expression not only causes DNA damage[25] but also induces the accumulation and activation of HIF-1α[26,27]. Therefore, these findings lead to an idea that reciprocal activation between HIF-1α and iNOS mediates persistent DNA damage. Hypoxia in tumor cells increases the generation of mitochondrial H2O2 at complex III[28], which may lead to 8-oxodG formation. Interestingly, we found that 8-oxodG formation in cancerous tissues was significantly correlated with lymphatic invasion. In addition, the formation of 8-nitroguanine and 8-oxodG in non-cancerous tissues was significantly associated with neural invasion. These results suggest that DNA damage participates in tumor invasion and/or poor survival. The accumulation of DNA base lesions may contribute to genetic instability and mutations, leading to tumor progression[14,29]. Recent data have expanded the concept that inflammation is a critical component of tumor progression[4]. It has been reported that iNOS expression is associated with poor survival in cancer patients[30,31]. Tumor-derived NO promoted tumor growth and metastasis by enhancing invasive, angiogenic and migratory capacities of tumor cells[32-34]. Our findings raise the idea that NO-mediated DNA damage plays the key role in tumor progression and poor survival. Together, HIF-1α may contribute to tumor progression[35] and subsequent poor prognosis[36] via a nitrative and oxidative DNA damage-dependent mechanism. Therefore, our results and these reports lead to an idea that tumor hypoxia mediates HIF-1α expression, followed by iNOS expression, and nitrative and oxidative DNA damage, which induces tumor invasiveness via mutations. Tumor growth facilitates tumor hypoxia and subsequent expression of HIF-1α and iNOS to cause DNA damage in addition to DNA damage mediated by inflammatory cells, resulting in tumor development. Tumor tissues may undergo development through repetition of this process, resulting in invasion and metastasis, leading to poor prognosis.
The combination of previous data with the results of this study, proposes a model in which 8-nitroguanine and 8-oxodG induced by inflammation and hypoxia may participate in tumor invasion, functioning in combination with DNA damage-independent mechanisms, including NO-mediated promotion of migratory, invasive and angiogenic properties[37]. We previously reported that OV infection induced the formation of 8-oxodG and 8-nitroguanine through chronic inflammation at the initiation and/or promotion step of carcinogenesis[16]. In addition, we hypothesize that 8-nitroguanine and 8-oxodG may also contribute to progression of carcinogenesis mediated by chronic OV infection. In this study, we demonstrated earlier that 8-nitroguanine was formed by cancerous tissues in humans, and this observation strongly supports our hypothesis. In conclusion, the formation of 8-nitroguanine and 8-oxodG is mediated by reactive species generated endogenously in the process of tumor hypoxia. These DNA lesions could contribute to multiple steps of carcinogenesis, leading to tumor progression, including invasiveness.
ACKNOWLEDGMENTS
The author also thanks all members of the Liver Fluke and Cholangiocarcinoma Research Centers’ staff at the Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand, for their kind help in the collection of samples.
Footnotes
Science Editor Guo SY Language Editor Elsevier HK
Supported by the Khon Kaen University Research Fund in Thailand and Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan
References
- 1.Gores GJ. Cholangiocarcinoma: current concepts and insights. Hepatology. 2003;37:961–969. doi: 10.1053/jhep.2003.50200. [DOI] [PubMed] [Google Scholar]
- 2.IARC Working Group. Infection with liver flukes (Opisthorchis viverrini, Opisthorchis felineus and Clonorchis sinensis) IARC Monogr Eval Carcinog Risks Hum. 1994;61:121–175. [PMC free article] [PubMed] [Google Scholar]
- 3.Sripa B. Pathobiology of opisthorchiasis: an update. Acta Trop. 2003;88:209–220. doi: 10.1016/j.actatropica.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer. 2003;3:276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 6.Wiseman H, Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem J. 1996;313(Pt 1):17–29. doi: 10.1042/bj3130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hofseth LJ, Hussain SP, Wogan GN, Harris CC. Nitric oxide in cancer and chemoprevention. Free Radic Biol Med. 2003;34:955–968. doi: 10.1016/s0891-5849(02)01363-1. [DOI] [PubMed] [Google Scholar]
- 8.Dedon PC, Tannenbaum SR. Reactive nitrogen species in the chemical biology of inflammation. Arch Biochem Biophys. 2004;423:12–22. doi: 10.1016/j.abb.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 9.Kasai H, Crain PF, Kuchino Y, Nishimura S, Ootsuyama A, Tanooka H. Formation of 8-hydroxyguanine moiety in cellular DNA by agents producing oxygen radicals and evidence for its repair. Carcinogenesis. 1986;7:1849–1851. doi: 10.1093/carcin/7.11.1849. [DOI] [PubMed] [Google Scholar]
- 10.Shibutani S, Takeshita M, Grollman AP. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature. 1991;349:431–434. doi: 10.1038/349431a0. [DOI] [PubMed] [Google Scholar]
- 11.Bruner SD, Norman DP, Verdine GL. Structural basis for recognition and repair of the endogenous mutagen 8-oxoguanine in DNA. Nature. 2000;403:859–866. doi: 10.1038/35002510. [DOI] [PubMed] [Google Scholar]
- 12.Ohshima H, Tatemichi M, Sawa T. Chemical basis of inflammation-induced carcinogenesis. Arch Biochem Biophys. 2003;417:3–11. doi: 10.1016/s0003-9861(03)00283-2. [DOI] [PubMed] [Google Scholar]
- 13.Yermilov V, Rubio J, Ohshima H. Formation of 8-nitroguanine in DNA treated with peroxynitrite in vitro and its rapid removal from DNA by depurination. FEBS Lett. 1995;376:207–210. doi: 10.1016/0014-5793(95)01281-6. [DOI] [PubMed] [Google Scholar]
- 14.Loeb LA, Loeb KR, Anderson JP. Multiple mutations and cancer. Proc Natl Acad Sci USA. 2003;100:776–781. doi: 10.1073/pnas.0334858100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinlaor S, Yongvanit P, Hiraku Y, Ma N, Semba R, Oikawa S, Murata M, Sripa B, Sithithaworn P, Kawanishi S. 8-nitroguanine formation in the liver of hamsters infected with Opisthorchis viverrini. Biochem Biophys Res Commun. 2003;309:567–571. doi: 10.1016/j.bbrc.2003.08.039. [DOI] [PubMed] [Google Scholar]
- 16.Pinlaor S, Ma N, Hiraku Y, Yongvanit P, Semba R, Oikawa S, Murata M, Sripa B, Sithithaworn P, Kawanishi S. Repeated infection with Opisthorchis viverrini induces accumulation of 8-nitroguanine and 8-oxo-7,8-dihydro-2'-deoxyguanine in the bile duct of hamsters via inducible nitric oxide synthase. Carcinogenesis. 2004;25:1535–1542. doi: 10.1093/carcin/bgh157. [DOI] [PubMed] [Google Scholar]
- 17.Pinlaor S, Hiraku Y, Ma N, Yongvanit P, Semba R, Oikawa S, Murata M, Sripa B, Sithithaworn P, Kawanishi S. Mechanism of NO-mediated oxidative and nitrative DNA damage in hamsters infected with Opisthorchis viverrini: a model of inflammation-mediated carcinogenesis. Nitric Oxide. 2004;11:175–183. doi: 10.1016/j.niox.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Ma N, Adachi Y, Hiraku Y, Horiki N, Horiike S, Imoto I, Pinlaor S, Murata M, Semba R, Kawanishi S. Accumulation of 8-nitroguanine in human gastric epithelium induced by Helicobacter pylori infection. Biochem Biophys Res Commun. 2004;319:506–510. doi: 10.1016/j.bbrc.2004.04.193. [DOI] [PubMed] [Google Scholar]
- 19.Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 20.Sripa B, Kaewkes S. Localisation of parasite antigens and inflammatory responses in experimental opisthorchiasis. Int J Parasitol. 2000;30:735–740. doi: 10.1016/s0020-7519(00)00054-0. [DOI] [PubMed] [Google Scholar]
- 21.Nakanuma Y, Harada K, Ishikawa A, Zen Y, Sasaki M. Anatomic and molecular pathology of intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2003;10:265–281. doi: 10.1007/s00534-002-0729-3. [DOI] [PubMed] [Google Scholar]
- 22.Elkins DB, Sithithaworn P, Haswell-Elkins M, Kaewkes S, Awacharagan P, Wongratanacheewin S. Opisthorchis viverrini: relationships between egg counts, worms recovered and antibody levels within an endemic community in northeast Thailand. Parasitology. 1991;102 Pt 2:283–288. doi: 10.1017/s0031182000062600. [DOI] [PubMed] [Google Scholar]
- 23.Chazotte-Aubert L, Oikawa S, Gilibert I, Bianchini F, Kawanishi S, Ohshima H. Cytotoxicity and site-specific DNA damage induced by nitroxyl anion (NO(-)) in the presence of hydrogen peroxide. Implications for various pathophysiological conditions. J Biol Chem. 1999;274:20909–20915. doi: 10.1074/jbc.274.30.20909. [DOI] [PubMed] [Google Scholar]
- 24.Ichiba M, Maeta Y, Mukoyama T, Saeki T, Yasui S, Kanbe T, Okano J, Tanabe Y, Hirooka Y, Yamada S, et al. Expression of 8-hydroxy-2'-deoxyguanosine in chronic liver disease and hepatocellular carcinoma. Liver Int. 2003;23:338–345. doi: 10.1034/j.1478-3231.2003.00868.x. [DOI] [PubMed] [Google Scholar]
- 25.Jadeski LC, Chakraborty C, Lala PK. Role of nitric oxide in tumour progression with special reference to a murine breast cancer model. Can J Physiol Pharmacol. 2002;80:125–135. doi: 10.1139/y02-007. [DOI] [PubMed] [Google Scholar]
- 26.Mateo J, García-Lecea M, Cadenas S, Hernández C, Moncada S. Regulation of hypoxia-inducible factor-1alpha by nitric oxide through mitochondria-dependent and -independent pathways. Biochem J. 2003;376:537–544. doi: 10.1042/BJ20031155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas DD, Espey MG, Ridnour LA, Hofseth LJ, Mancardi D, Harris CC, Wink DA. Hypoxic inducible factor 1alpha, extracellular signal-regulated kinase, and p53 are regulated by distinct threshold concentrations of nitric oxide. Proc Natl Acad Sci USA. 2004;101:8894–8899. doi: 10.1073/pnas.0400453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000;275:25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 29.Olinski R, Gackowski D, Rozalski R, Foksinski M, Bialkowski K. Oxidative DNA damage in cancer patients: a cause or a consequence of the disease development? Mutat Res. 2003;531:177–190. doi: 10.1016/j.mrfmmm.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Ekmekcioglu S, Ellerhorst J, Smid CM, Prieto VG, Munsell M, Buzaid AC, Grimm EA. Inducible nitric oxide synthase and nitrotyrosine in human metastatic melanoma tumors correlate with poor survival. Clin Cancer Res. 2000;6:4768–4775. [PubMed] [Google Scholar]
- 31.Aaltoma SH, Lipponen PK, Kosma VM. Inducible nitric oxide synthase (iNOS) expression and its prognostic value in prostate cancer. Anticancer Res. 2001;21:3101–3106. [PubMed] [Google Scholar]
- 32.Jadeski LC, Chakraborty C, Lala PK. Nitric oxide-mediated promotion of mammary tumour cell migration requires sequential activation of nitric oxide synthase, guanylate cyclase and mitogen-activated protein kinase. Int J Cancer. 2003;106:496–504. doi: 10.1002/ijc.11268. [DOI] [PubMed] [Google Scholar]
- 33.Lala PK, Orucevic A. Role of nitric oxide in tumor progression: lessons from experimental tumors. Cancer Metastasis Rev. 1998;17:91–106. doi: 10.1023/a:1005960822365. [DOI] [PubMed] [Google Scholar]
- 34.Siegert A, Rosenberg C, Schmitt WD, Denkert C, Hauptmann S. Nitric oxide of human colorectal adenocarcinoma cell lines promotes tumour cell invasion. Br J Cancer. 2002;86:1310–1315. doi: 10.1038/sj.bjc.6600224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Semenza GL. Hypoxia, clonal selection, and the role of HIF-1 in tumor progression. Crit Rev Biochem Mol Biol. 2000;35:71–103. doi: 10.1080/10409230091169186. [DOI] [PubMed] [Google Scholar]
- 36.Koukourakis MI, Giatromanolaki A, Brekken RA, Sivridis E, Gatter KC, Harris AL, Sage EH. Enhanced expression of SPARC/osteonectin in the tumor-associated stroma of non-small cell lung cancer is correlated with markers of hypoxia/acidity and with poor prognosis of patients. Cancer Res. 2003;63:5376–5380. [PubMed] [Google Scholar]
- 37.Lala PK, Chakraborty C. Role of nitric oxide in carcinogenesis and tumour progression. Lancet Oncol. 2001;2:149–156. doi: 10.1016/S1470-2045(00)00256-4. [DOI] [PubMed] [Google Scholar]


