Abstract
AIM: To detect the expression of a proliferation-related ligand on human hepatocellular carcinoma (HCC) cell lines (SK-Hep1, HLE and HepG2) and in culture medium.
METHODS: APRIL expression was analyzed by Western blotting in HCC cell lines. Effects of APRIL to cell count and angiogenesis were analyzed, too.
RESULTS: Recombinant human APRIL (rhAPRIL) increased cell viability of HepG2 cells and, in HUVEC, rhAPRIL provided slight tolerance to cell death from serum starvation. Soluble APRIL (sAPRIL) from HLE cells increased after serum starvation, but did not change in SK-Hep1 or HepG2 cells. These cells showed down-regulation of VEGF after incubation with anti-APRIL antibody. Furthermore, culture medium from the HCC cells treated with anti-APRIL antibody treatment inhibited tube formation of HUVECs.
CONCLUSION: Functional expression of APRIL might contribute to neovascularization via an upregulation of VEGF in HCC.
Keywords: A proliferation-inducing ligand, HCC, VEGF, Cell proliferation, Neovascularization
INTRODUCTION
Cytokines regulate cellular proliferation and differentiation by binding to their specific receptors on target cells[1]. Tumor necrosis factor (TNF) is the prototypic member of a family of cytokines playing an important role in immune regulation and cancer[2,3].
A proliferation-inducing ligand (APRIL), a new member of the TNF family, is reported to stimulate tumor cell growth[4-6], modulate tumor cell apoptosis[7-9], or activate nuclear factor-kappa B (NF-κ B)[10,11]. APRIL also is associated with regulation of humoral immunity[6,12]. APRIL is a type II membrane protein, which is typical of the TNF ligand family member[4]. The sequence of the extracellular domain of APRIL shows homology with FasL, TNFα, LTβ, TRAIL, TWEAK, and TRANCE[4]. In particular, APRIL shares significant homology with B-lymphocyte stimulator (BLys)[13]. Though the TNF ligand family is synthesized into membrane-bound proteins, of which several are cleaved into a soluble form, APRIL is processed intracellularly and secreted from the cell surface[14].
The expression of APRIL mRNA or protein has been detected in many tumor cells and tissues[4,5,7], but is almost undetectable in normal tissues[5]. Hence, APRIL may provide a significant growth advantage to malignant cells. Because soluble APRIL can stimulate tumor cell growth[1], APRIL may provide its signal in an autocrine and/or paracrine mode[5].
APRIL binds to two TNF receptor families, transmembrane activator and CAML interactor (TACI) and B cell maturation antigen (BMCA) with relatively high affinity[6,10,15]. It is clear that TACI and BCMA intracellular signaling is similar to that of other TNF receptor homologs without a death domain[16,17]. However, the signaling transduction pathway triggered by APRIL that prolongs lymphocyte survival is still not characterized[18]. A soluble form of BCMA inhibits APRIL activity and decreases tumor cell proliferation[19]. NZB/WF1 mice, which develop chronic autoimmune disease, can inhibit development of proteinuria and prolongation of survival after being treated with soluble TACI-Ig fusion protein treatment[20]. These observations imply the importance of APRIL via these receptors with tumor cell or lymphocyte survival. However, in Jurkat cells, APRIL induces cell death and binds to other TNF family receptors[7]. An additional APRIL-specific receptor expressed on cell surfaces other than TACI or BMCA, is implied adenocarcinoma or fibroblast tumor cells lacking any detectable BCMA or TACI expression even though these cells respond to growth stimulatory effects of APRIL[5,19]. However, the function of APRIL in tumor cells is not well elucidated.
Therefore, we investigated the function of APRIL in HCC cell lines and human umbilical vein endothelial cells (HUVEC). We further investigated the expression of APRIL in HCC cells and evaluated the effects of APRIL on neovascularization.
MATERIALS AND METHODS
Cell lines and reagents
Human umbilical vein endothelial cells (HUVECs), and human HCC cell lines, HepG2 and SK-Hep1 cells were purchased from American Type Culture Collection (Rockville, MD, USA). Human HCC cell line, HLE was purchased from the Health Science Research Resource Bank (Osaka, Japan). HCC cell lines were cultured in Dulbecco’s modified Eagle’s medium (Dainippon Pharmaceutical Co., Ltd., Osaka, Japan) at 37°C. All media were supplemented with 1% penicillin/streptomycin (GIBCO BRL, Grand Island, NY, USA) and 10% heat-inactivated fetal calf serum (GIBCO BRL). HUVECs were cultured in HuMedia-EG2 (KURABO, Osaka, Japan). Recombinant human APRIL (rhAPRIL) was purchased from ALEXIS Biochemicals (Switzerland). Anti-APRIL polyclonal antibody was purchased from ψ ProSci Incorporated (Poway, CA, USA). Anti- vascular endothelial growth factor (VEGF) mAb and anti-α-tubulin mAb were purchased from Oncogene Research Products (Boston, MA, USA). Normal rabbit IgG was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Recombinant human VEGF (rhVEGF) was purchased from Peprotech EC Ltd. (London, UK).
Assessment of count of HCC cells and HUVECs
To assess the viability of HCC cells and HUVECs, the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay was performed. The cells were plated at a density of 5×103 cells/well in 96-well microtiter plates (Corning Glass Works, Corning, NY, USA) and each plate was incubated for 24 h at 37°C in 50 mL/L CO2. Reagents were added and the plates were incubated for the indicated time. The live-cell count was determined using a Cell Titer 96 assay kit (Promega, Madison, WI, USA) according to the manufacturer’s instructions. The absorbance of each well was measured at 570 nm with a microtiter plate reader (Bio-Rad Laboratories, Hercules, CA, USA).
Western blotting
Expression of APRIL was analyzed by Western blotting. HCC cells were harvested and lysed in lysis buffer (50 mmol/L Tris-HCl, pH 8, 150 mmol/L NaCl, 5 mmol/L EDTA, 1% NP-40, 1 mmol/L phenylmethyl-sulfonyl fluoride) on ice. Protein content was measured using a Bio-Rad protein assay kit (Bio-Rad Laboratories). Equal amounts of protein from each extract were separated by 14% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose membranes (Toyo Roshi, Tokyo, Japan). Blots were blocked by incubation in 5% non-fat dried milk in Tris buffered saline (TBS) overnight at 4°C and probed for 2 h at room temperature with primary antibody. The immunoblots were then probed with horseradish peroxidase-conjugated immunoglobulin (Ig) G (1:2 000 diluted with 5% non-fat dried milk in Tris-HCl; pH 7.5 and 0.05% Tween 20). Signal was detected with an ECL kit (Amersham Pharmacia Biotech, Buckinghamshire, UK).
Angiogenesis assay
Matrigel (BD Biosciences, MA) was placed in an 8-well Lab-tek II chamber slide (NUNCTM Brand Products, Denmark) (100 μL/well) and allowed to set at 37°C for 30 min. HUVECs were added to each well (4 × 104) and incubated in the culture medium of HCC cells with rabbit IgG or anti-APRIL polyclonal antibody at 37 × for 6 h in a 50 mL/L CO2 atmosphere. Tube formation was observed by microscopy (OLYMPUS, Tokyo, Japan).
RESULTS
APRIL expressions in human HCC cell lines
APRIL expression in 3 human HCC cell lines (SK-Hep1, HLE, HepG2) was investigated. Bands corresponding to the expression in 42 ku unprocessed form of APRIL were observed in all human HCC cell lines (Figure 1). SK-Hep1 or HepG2 cells, were similar, but weaker in HLE cells. In addition to the unprocessed form, a lower molecular weight form (17 ku) was observed in HCC cell lysates and supernatants (Figure 1). This was thought to be a soluble form of APRIL. The soluble form of APRIL was observed in the three HCC cell lysates and supernatants. The soluble form of APRIL was most strongly expressed in supernatant from SK-Hep1 cells.
Figure 1.

APRIL expression in human HCC cell lines and their supernatants by Western blotting. Closed arrowhead indicates the expression of unprocessed form of APRIL (42 ku) and open arrowhead indicates the soluble form of APRIL (17 ku).
Effect of recombinant human APRIL (rhAPRIL) in human HCC cell lines and HUVECs
We analyzed the effect of rhAPRIL on proliferation of human HCC cells. After 72-h incubation, the HCC cell counts slightly increased at the concentrations of 1, 10, or 100 ng/mL (Figure 2A), the greatest effects were observed at concentration of 1 ng/mL in HepG2 cells.
Figure 2.
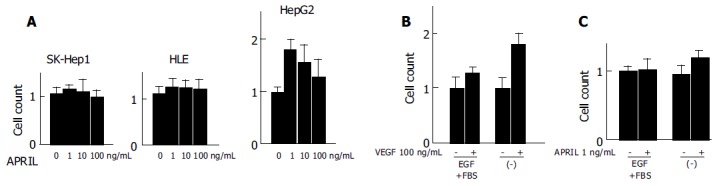
Effects of rhAPRIL stimulation on viability of human HCC cells (A) and HUVECs (B and C) incubated at different time periods.
Since HCC is generally a hypervascular tumor[21] and gains its hypervascularity during dedifferentiation and progression[22], we investigated the effect of rhAPRIL stimulation on the proliferation of HUVECs.
An increase in proliferation at the concentration of 100 ng/mL rhVEGF was observed in the presence or absence of FBS and EGF (Figure 2B). In particular, rhVEGF effectively inhibited cell death induced by cell starvation. The cell viability of HUVECs slightly increased with the stimulation of 1 ng/mL rhAPRIL and the same concentration of rhAPRIL inhibited cell death with starvation (Figure 2C). From these results, we confirmed that APRIL could induce cell proliferation and cell death inhibition in HCC cells and HUVECs, but we also observed that the effect of APRIL on HCC cell lines was not so potent.
APRIL could mediate regulation of cell growth, induce XIAP expression and decrease caspase activity[23]. We examined the expression of apoptosis-related proteins, including FLICE/caspase-8 inhibitory protein (FLIP), X-chromosome-linked inhibitor of apoptosis (XIAP), BclxL, Apaf-1, and smac in HCC cell lines and HUVECs after APRIL stimulation. However, these apoptosis-related proteins did not show changes in expression levels after APRIL stimulation (data not shown).
Regulation of APRIL in human HCC cell lines
We examined the expression of APRIL after serum starvation in HCC cells and HUVECs (Figure 3). Serum starvation increased the expression of both the unprocessed and soluble forms of APRIL in HLE. In SK-Hep1 and HepG2 cells, serum starvation did not induce upregulation of APRIL, however its expression was maintained. sAPRIL eventually disappeared after 24’h of serum starvation in SK-Hep1 cells, whereas HepG2 cells maintained sAPRIL expression.
Figure 3.
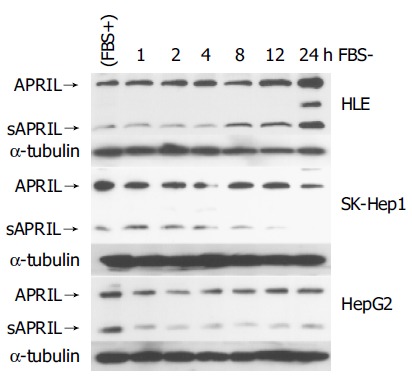
Regulation of APRIL expression in HCC cells. Closed arrowhead indicates the expression of unprocessed form of APRIL. Open arrowhead indicates soluble form of APRIL.
APRIL was associated with VEGF expression in HCC cell lines
We observed APRIL expression and regulation by serum starvation in human HCC cell lines. In particular, the serum starvation induced APRIL expression in HLE cells. VEGF might be associated with angiogenesis or tumor progression[24-31] and VEGF expression could be induced by stress, for example hypoxia or ischemia, in HCC cells[24,25,28,29]. All HCC cell lines showed expression of VEGF with serum starvation, although the modulation of VEGF expression differed among three cell lines (Figure 4A). In SK-Hep1 and HepG2 cells, VEGF expression was stable for 12 h. In contrast, VEGF expression in HLE cells was not observed until 48 h and was less than that in SK-Hep1 or HepG2 cells.
Figure 4.
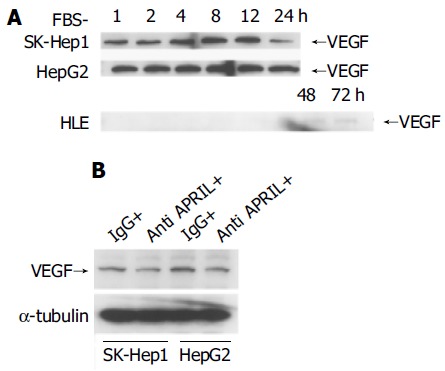
Regulation of VEGF expression with starvation in HCC cells incubated with FBS-negative culture (A) and 10 μg/mL anti-APRIL polyclonal antibody or normal rabbit IgG (B).
Because APRIL was expressed constitutively in SK-Hep1 and HepG2 and upregulated in HLE cells after starvation, we considered that APRIL might be associated with VEGF expression in HCC cells. So, we used anti-APRIL antibody to bind to sAPRIL in culture medium and to inhibit APRIL effects mediated by an autocrine or paracrine system. As shown in Figure 4B, blockade of APRIL by anti-APRIL antibody induced downregulation of VEGF expression in SK-Hep1 and HepG2 cells, compared to controls with normal IgG.
Finally, we observed that culture medium from the HCC cells incubated with anti-APRIL polyclonal antibody inhibited endothelial cell tube formation in matrigel (Figure 5).
Figure 5.
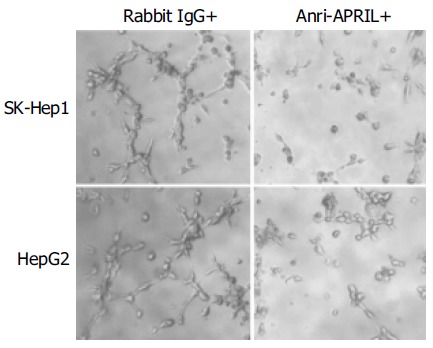
Effect of APRIL on in vitro endothelial cell tube formation.
DISCUSSION
Though HCC is the most common primary cancer of the liver and preceded by chronic hepatitis and cirrhosis, the mechanism of hepatocarcinogenesis or tumor progression is complex and not well understood. Cell killing and stimulation of mitosis leading to cell transformation or chromosomal instability induced by recombinogenic proteins during chronic hepatitis may cause carcinogenesis of HCC[32].
HCC is resistant to conservative chemotherapy and difficult to induce apoptosis with TNF family members[33]. On the other hand, TNF families, including TNF, TRAIL or Fas, can induce activation of NF-κB[33-41] and the tumor itself expresses these ligands[42,43]. These phenomena suggest that TNF families contribute to tumor progression by autocrine or paracrine systems. In this study, we demonstrated that APRIL, a TNF family member, expressed in HCC cell lines, including SK-Hep1, HLE and HepG2, and sAPRIL was detected in culture medium of these HCC cell lines. However, addition of rhAPRIL did not induce any significant effect on viability of HCC cell lines, except for HepG2 cells stimulated with 1 ng/mL rhAPRIL. Because APRIL can induce both cell proliferation[4,5,19] and death[7] of malignant tumors, the result of present study may reflect that APRIL does not induce cell death so much as proliferation in HCC cells. In contrast to other TNF members, which are processed from the cell surface, APRIL is processed intracellularly and sAPRIL is purified[14]. sAPRIL is used to stimulate tumor cells and induce cell proliferation in previous studies[4,14]. The unprocessed form of APRIL may have less ability to induce tumor cell proliferation than sAPRIL.
APRIL enhances the growth of several tumor lines, however, the effect is more prominent at the point of tumor implantation than established tumor[23]. Because expanding solid tumors, including HCC, are advanced with insufficient nutrition or oxygen, they have to get new vascular support to grow. In this study, HUVECs did not show significant cell proliferation or apoptosis inhibition after APRIL stimulation. But HLE cells showed upregulation of APRIL after 24 h starvation, and further prolonged starvation induced upregulation of VEGF. In SK-Hep1 and HepG2 cells, starvation did not significantly induce the expression of APRIL, however, starvation promptly induced upregulation of VEGF. These results suggest that APRIL may be associated with expression of VEGF and induction of neovascularization in HCC. Further more, blockade of APRIL activity by anti-APRIL antibody induced downregulation of VEGF and inhibited tube formation of HUVECs. These results support the notion that neovascularization and progression of HCC may be induced by APRIL via VEGF expression.
Until now, some factors including VEGF have been identified as mediators of angiogenesis associated with tumors. With regard to HCC, recent studies indicate that angiopoietin[44,45] is a target of angiogenesis. APRIL may not be critical for HCC progression as a direct effector, but plays an important role in neovascularization of HCC.
Footnotes
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
References
- 1.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 2.Beutler B. TNF, immunity and inflammatory disease: lessons of the past decade. J Investig Med. 1995;43:227–235. [PubMed] [Google Scholar]
- 3.Bazzoni F, Beutler B. The tumor necrosis factor ligand and receptor families. N Engl J Med. 1996;334:1717–1725. doi: 10.1056/NEJM199606273342607. [DOI] [PubMed] [Google Scholar]
- 4.Hahne M, Kataoka T, Schröter M, Hofmann K, Irmler M, Bodmer JL, Schneider P, Bornand T, Holler N, French LE, et al. APRIL, a new ligand of the tumor necrosis factor family, stimulates tumor cell growth. J Exp Med. 1998;188:1185–1190. doi: 10.1084/jem.188.6.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ware CF. APRIL and BAFF connect autoimmunity and cancer. J Exp Med. 2000;192:F35–F38. doi: 10.1084/jem.192.11.f35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu G, Boone T, Delaney J, Hawkins N, Kelley M, Ramakrishnan M, McCabe S, Qiu WR, Kornuc M, Xia XZ, et al. APRIL and TALL-I and receptors BCMA and TACI: system for regulating humoral immunity. Nat Immunol. 2000;1:252–256. doi: 10.1038/79802. [DOI] [PubMed] [Google Scholar]
- 7.Kelly K, Manos E, Jensen G, Nadauld L, Jones DA. APRIL/TRDL-1, a tumor necrosis factor-like ligand, stimulates cell death. Cancer Res. 2000;60:1021–1027. [PubMed] [Google Scholar]
- 8.Roth W, Wagenknecht B, Klumpp A, Naumann U, Hahne M, Tschopp J, Weller M. APRIL, a new member of the tumor necrosis factor family, modulates death ligand-induced apoptosis. Cell Death Differ. 2001;8:403–410. doi: 10.1038/sj.cdd.4400827. [DOI] [PubMed] [Google Scholar]
- 9.Novak AJ, Bram RJ, Kay NE, Jelinek DF. Aberrant expression of B-lymphocyte stimulator by B chronic lymphocytic leukemia cells: a mechanism for survival. Blood. 2002;100:2973–2979. doi: 10.1182/blood-2002-02-0558. [DOI] [PubMed] [Google Scholar]
- 10.Marsters SA, Yan M, Pitti RM, Haas PE, Dixit VM, Ashkenazi A. Interaction of the TNF homologues BLyS and APRIL with the TNF receptor homologues BCMA and TACI. Curr Biol. 2000;10:785–788. doi: 10.1016/s0960-9822(00)00566-2. [DOI] [PubMed] [Google Scholar]
- 11.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, Cerutti A. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3:822–829. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stein JV, López-Fraga M, Elustondo FA, Carvalho-Pinto CE, Rodríguez D, Gómez-Caro R, De Jong J, Martínez-A C, Medema JP, Hahne M. APRIL modulates B and T cell immunity. J Clin Invest. 2002;109:1587–1598. doi: 10.1172/JCI15034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore PA, Belvedere O, Orr A, Pieri K, LaFleur DW, Feng P, Soppet D, Charters M, Gentz R, Parmelee D, et al. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999;285:260–263. doi: 10.1126/science.285.5425.260. [DOI] [PubMed] [Google Scholar]
- 14.López-Fraga M, Fernández R, Albar JP, Hahne M. Biologically active APRIL is secreted following intracellular processing in the Golgi apparatus by furin convertase. EMBO Rep. 2001;2:945–951. doi: 10.1093/embo-reports/kve198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Y, Bressette D, Carrell JA, Kaufman T, Feng P, Taylor K, Gan Y, Cho YH, Garcia AD, Gollatz E, et al. Tumor necrosis factor (TNF) receptor superfamily member TACI is a high affinity receptor for TNF family members APRIL and BLyS. J Biol Chem. 2000;275:35478–35485. doi: 10.1074/jbc.M005224200. [DOI] [PubMed] [Google Scholar]
- 16.Xia XZ, Treanor J, Senaldi G, Khare SD, Boone T, Kelley M, Theill LE, Colombero A, Solovyev I, Lee F, et al. TACI is a TRAF-interacting receptor for TALL-1, a tumor necrosis factor family member involved in B cell regulation. J Exp Med. 2000;192:137–143. doi: 10.1084/jem.192.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hatzoglou A, Roussel J, Bourgeade MF, Rogier E, Madry C, Inoue J, Devergne O, Tsapis A. TNF receptor family member BCMA (B cell maturation) associates with TNF receptor-associated factor (TRAF) 1, TRAF2, and TRAF3 and activates NF-kappa B, elk-1, c-Jun N-terminal kinase, and p38 mitogen-activated protein kinase. J Immunol. 2000;165:1322–1330. doi: 10.4049/jimmunol.165.3.1322. [DOI] [PubMed] [Google Scholar]
- 18.MacLennan I, Vinuesa C. Dendritic cells, BAFF, and APRIL: innate players in adaptive antibody responses. Immunity. 2002;17:235–238. doi: 10.1016/s1074-7613(02)00398-9. [DOI] [PubMed] [Google Scholar]
- 19.Rennert P, Schneider P, Cachero TG, Thompson J, Trabach L, Hertig S, Holler N, Qian F, Mullen C, Strauch K, et al. A soluble form of B cell maturation antigen, a receptor for the tumor necrosis factor family member APRIL, inhibits tumor cell growth. J Exp Med. 2000;192:1677–1684. doi: 10.1084/jem.192.11.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gross JA, Johnston J, Mudri S, Enselman R, Dillon SR, Madden K, Xu W, Parrish-Novak J, Foster D, Lofton-Day C, et al. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature. 2000;404:995–999. doi: 10.1038/35010115. [DOI] [PubMed] [Google Scholar]
- 21.Francis IR, Agha FP, Thompson NW, Keren DF. Fibrolamellar hepatocarcinoma: clinical, radiologic, and pathologic features. Gastrointest Radiol. 1986;11:67–72. doi: 10.1007/BF02035035. [DOI] [PubMed] [Google Scholar]
- 22.Sugimachi K, Tanaka S, Terashi T, Taguchi K, Rikimaru T, Sugimachi K. The mechanisms of angiogenesis in hepatocellular carcinoma: angiogenic switch during tumor progression. Surgery. 2002;131:S135–S141. doi: 10.1067/msy.2002.119365. [DOI] [PubMed] [Google Scholar]
- 23.Mackay F, Schneider P, Rennert P, Browning J. BAFF AND APRIL: a tutorial on B cell survival. Annu Rev Immunol. 2003;21:231–264. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- 24.Mise M, Arii S, Higashituji H, Furutani M, Niwano M, Harada T, Ishigami S, Toda Y, Nakayama H, Fukumoto M, et al. Clinical significance of vascular endothelial growth factor and basic fibroblast growth factor gene expression in liver tumor. Hepatology. 1996;23:455–464. doi: 10.1053/jhep.1996.v23.pm0008617424. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki H, Seto K, Shinoda Y, Mori M, Ishimura Y, Suematsu M, Ishii H. Paracrine upregulation of VEGF receptor mRNA in endothelial cells by hypoxia-exposed hep G2 cells. Am J Physiol. 1999;276:G92–G97. doi: 10.1152/ajpgi.1999.276.1.G92. [DOI] [PubMed] [Google Scholar]
- 26.Yoshiji H, Kuriyama S, Hicklin DJ, Huber J, Yoshii J, Miyamoto Y, Kawata M, Ikenaka Y, Nakatani T, Tsujinoue H, et al. KDR/Flk-1 is a major regulator of vascular endothelial growth factor-induced tumor development and angiogenesis in murine hepatocellular carcinoma cells. Hepatology. 1999;30:1179–1186. doi: 10.1002/hep.510300509. [DOI] [PubMed] [Google Scholar]
- 27.Kang MA, Kim KY, Seol JY, Kim KC, Nam MJ. The growth inhibition of hepatoma by gene transfer of antisense vascular endothelial growth factor. J Gene Med. 2000;2:289–296. doi: 10.1002/1521-2254(200007/08)2:4<289::AID-JGM116>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 28.Baek JH, Jang JE, Kang CM, Chung HY, Kim ND, Kim KW. Hypoxia-induced VEGF enhances tumor survivability via suppression of serum deprivation-induced apoptosis. Oncogene. 2000;19:4621–4631. doi: 10.1038/sj.onc.1203814. [DOI] [PubMed] [Google Scholar]
- 29.von Marschall Z, Cramer T, Höcker M, Finkenzeller G, Wiedenmann B, Rosewicz S. Dual mechanism of vascular endothelial growth factor upregulation by hypoxia in human hepatocellular carcinoma. Gut. 2001;48:87–96. doi: 10.1136/gut.48.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng IO, Poon RT, Lee JM, Fan ST, Ng M, Tso WK. Microvessel density, vascular endothelial growth factor and its receptors Flt-1 and Flk-1/KDR in hepatocellular carcinoma. Am J Clin Pathol. 2001;116:838–845. doi: 10.1309/FXNL-QTN1-94FH-AB3A. [DOI] [PubMed] [Google Scholar]
- 31.Shimamura T, Saito S, Morita K, Kitamura T, Morimoto M, Kiba T, Numata K, Tanaka K, Sekihara H. Detection of vascular endothelial growth factor and its receptor expression in human hepatocellular carcinoma biopsy specimens. J Gastroenterol Hepatol. 2000;15:640–646. doi: 10.1046/j.1440-1746.2000.02201.x. [DOI] [PubMed] [Google Scholar]
- 32.Hino O, Kajino K, Umeda T, Arakawa Y. Understanding the hypercarcinogenic state in chronic hepatitis: a clue to the prevention of human hepatocellular carcinoma. J Gastroenterol. 2002;37:883–887. doi: 10.1007/s005350200149. [DOI] [PubMed] [Google Scholar]
- 33.Yamanaka T, Shiraki K, Sugimoto K, Ito T, Fujikawa K, Ito M, Takase K, Moriyama M, Nakano T, Suzuki A. Chemotherapeutic agents augment TRAIL-induced apoptosis in human hepatocellular carcinoma cell lines. Hepatology. 2000;32:482–490. doi: 10.1053/jhep.2000.16266. [DOI] [PubMed] [Google Scholar]
- 34.Hsu H, Xiong J, Goeddel DV. The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell. 1995;81:495–504. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 35.Hsu H, Shu HB, Pan MG, Goeddel DV. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 36.Wajant H, Haas E, Schwenzer R, Muhlenbeck F, Kreuz S, Schubert G, Grell M, Smith C, Scheurich P. Inhibition of death receptor-mediated gene induction by a cycloheximide-sensitive factor occurs at the level of or upstream of Fas-associated death domain protein (FADD) J Biol Chem. 2000;275:24357–24366. doi: 10.1074/jbc.M000811200. [DOI] [PubMed] [Google Scholar]
- 37.Bodmer JL, Burns K, Schneider P, Hofmann K, Steiner V, Thome M, Bornand T, Hahne M, Schröter M, Becker K, et al. TRAMP, a novel apoptosis-mediating receptor with sequence homology to tumor necrosis factor receptor 1 and Fas(Apo-1/CD95) Immunity. 1997;6:79–88. doi: 10.1016/s1074-7613(00)80244-7. [DOI] [PubMed] [Google Scholar]
- 38.Chinnaiyan AM, O'Rourke K, Yu GL, Lyons RH, Garg M, Duan DR, Xing L, Gentz R, Ni J, Dixit VM. Signal transduction by DR3, a death domain-containing receptor related to TNFR-1 and CD95. Science. 1996;274:990–992. doi: 10.1126/science.274.5289.990. [DOI] [PubMed] [Google Scholar]
- 39.Ponton A, Clément MV, Stamenkovic I. The CD95 (APO-1/Fas) receptor activates NF-kappaB independently of its cytotoxic function. J Biol Chem. 1996;271:8991–8995. doi: 10.1074/jbc.271.15.8991. [DOI] [PubMed] [Google Scholar]
- 40.Okano H, Shiraki K, Inoue H, Kawakita T, Saitou Y, Enokimura N, Yamamoto N, Sugimoto K, Murata K, Nakano T. Fas stimulation activates NF-kappaB in SK-Hep1 hepatocellular carcinoma cells. Oncol Rep. 2003;10:1145–1148. [PubMed] [Google Scholar]
- 41.Shiraki K, Takase K, Nakano T. The emerging role of caspase inhibitors in gastrointestinal cancers. J Gastroenterol. 2002;37:323–331. doi: 10.1007/s005350200045. [DOI] [PubMed] [Google Scholar]
- 42.Shiraki K, Tsuji N, Shioda T, Isselbacher KJ, Takahashi H. Expression of Fas ligand in liver metastases of human colonic adenocarcinomas. Proc Natl Acad Sci USA. 1997;94:6420–6425. doi: 10.1073/pnas.94.12.6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Inoue H, Shiraki K, Yamanaka T, Ohmori S, Sakai T, Deguchi M, Okano H, Murata K, Sugimoto K, Nakano T. Functional expression of tumor necrosis factor-related apoptosis-inducing ligand in human colonic adenocarcinoma cells. Lab Invest. 2002;82:1111–1119. doi: 10.1097/01.lab.0000027838.69455.39. [DOI] [PubMed] [Google Scholar]
- 44.Mitsuhashi N, Shimizu H, Ohtsuka M, Wakabayashi Y, Ito H, Kimura F, Yoshidome H, Kato A, Nukui Y, Miyazaki M. Angiopoietins and Tie-2 expression in angiogenesis and proliferation of human hepatocellular carcinoma. Hepatology. 2003;37:1105–1113. doi: 10.1053/jhep.2003.50204. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka S, Sugimachi K, Yamashita Y, Shirabe K, Shimada M, Wands JR, Sugimachi K. Angiogenic switch as a molecular target of malignant tumors. J Gastroenterol. 2003;38 Suppl 15:93–97. [PubMed] [Google Scholar]


