Abstract
AIM: To investigate the clinical values of serum free insulin-like growth factor II (IGF-II) levels and IGF-II mRNA in hepatocellular carcinoma (HCC) tissues and peripheral blood for diagnosis of HCC and monitoring of extrahepatic metastasis.
METHODS: Total RNAs were extracted from HCC tissues or peripheral blood mononuclear cells from patients with HCC, liver diseases devoid of cancer, non-hepatic tumors, and healthy controls, respectively. IGF-II cDNAs were synthesized through random primers and reverse-transcriptase, amplified by polymerase chain reaction (PCR), and confirmed by DNA sequencing analysis. Serum free IGF-II levels in patients with different liver diseases were analyzed by an enzyme-linked immunosorbent assay.
RESULTS: The amplified fragments of IGF-II mRNA by RT-PCR were identical to originally designed ones with a size of 170 bp and confirmed by sequencing analysis. The dilution experiments revealed that the lowest sensitivity of our system was 2 ng/L of total RNA. The positive frequencies of IGF-II mRNA were 100% in HCC tissues, 53.3% in para-cancerous tissues, and 0% in non-cancerous tissues, respectively. The serum free IGF-II levels were significantly higher in HCC than those in chronic hepatitis or liver cirrhosis. The positive frequency of circulating IGF-II mRNA was 34.2% in HCC, no amplified fragment was found in other liver diseases, extrahepatic tumors, and normal controls, respectively. The circulating IGF-II mRNA correlated with the stage of HCC, and its positive rate was 100% in HCC with extrahepatic metastasis and 35.5% in HCC with AFP-negative. No significant correlation was found between tumor sizes and circulating IGF-II mRNA fragment.
CONCLUSION: The abnormal expressions of free IGF-II and IGF-II mRNA are useful tumor markers for HCC diagnosis, differentiation of extrahepatic metastasis and monitoring postoperative recurrence.
Keywords: IGF-II, Hepatocellular carcinoma
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common and rapidly fatal malignancies worldwide, and has been ranked as the second cancer killer in China since the 1990s, particularly in the eastern and southern areas, including the inshore area of the Yangtze River[1,2]. Major risk factors for HCC in these areas are exposure to aflatoxin B1 (AFB1) and infection by hepatitis viruses[3]. HCC prognosis is poor and early detection is of utmost importance[4]. Treatment options are severely limited by the frequent presence of metastases[2]. Although serum α-fetoprotein (AFP) is a useful tumor marker for the detection and monitoring of HCC development, the false-negative rate with AFP level alone may be as high as 40% for patients with small size HCC[5]. However, if hepatocyte-specific mRNAs are detected in the circulation, it is possible to infer the presence of circulating, presumably malignant liver cells and to predict the likelihood of hematogenous metastasis[6,7].
Insulin-like growth factor II (IGF-II) is a mitogenic polypeptide closely related to insulin. Its gene has complex regulation of transcription, resulting in multiple mRNAs initiated by different promoters[8,9]. IGF-II is speculated to serve as an autocrine growth factor in various cancers, because they often coexpress IGF-II and IGF-I receptors. IGF-II is a kind of fetal growth factor and highly expressed during hepatocarcinogenesis[10,11] and reexpression of IGF-II gene has recently been described in HCC[12,13]. HCC is generally considered to be a hypervascular tumor. Although hepatic arterial embolization is widely used as an effective treatment of HCC on the basis of hypervascul-arization of HCC, IGF-II may play an important role in the development of neovascularization of HCC, because IGF-II substantially increases vascular endothelial growth factor (VEGF) mRNA and protein levels in a time-dependent manner in human hepatoma cells[14]. The induction of VEGF by IGF-II was further increased by hypoxia, and IGF-II may be a hypoxia-inducible angiogenic factor in HCC and stimulates the growth of HCC cells in vitro[15,16]. Park et al[17] reported that most of the cirrhotic and HCC tissues express IGF-II. However, little is known of the circulating IGF-II in HCC.
In order to investigate the expression of IGF-II-mRNA in patients with liver diseases, we analyzed IGF-II-mRNA in tissues and peripheral blood of patients with HCC by reverse-transcriptase polymerase chain reaction (RT-PCR), and estimated the clinical values of circulating IGF-II mRNA as a peripheral blood tumor marker in diagnosis, differential diagnosis, and hematogenous metastasis of HCC.
MATERIALS AND METHODS
Patients
We studied 111 patients (100 males and 11 females) with HCC treated at Affiliated Hospital of Nantong University, China. The patients’ ages ranged from 25 to 80 years (median, 48.3 years). Ninety patients (81%) had a history of cirrhosis, and 32 (29%) had a history of chronic hepatitis. Moreover, 85.6% (95/111) had hepatitis B surface antigen (HBsAg) carriers, 10.8% (12/111) had antibody to hepatitis C virus (anti-HCV, second generation antibody, Shanghai, China) and 14.4% (16/111) antibody to hepatitis G virus by enzyme-linked immunosorbent assay (ELISA, Beijing, China), respectively. Other cases studied included 30 patients with chronic viral hepatitis (23 males and 7 females), 30 patients with acute hepatitis (18 males and 12 females), 25 patients with cirrhosis (16 males and 9 females), 25 patients with non-liver tumors (6 with lung cancer, 6 with gastric cancer, 3 with esophageal cancer, 3 with breast cancer, 3 with colon cancer, 2 with cervical cancer, 2 with pancreatic cancer), and 25 healthy individuals with hepatitis B markers (HBsAg, HBcAb, HBV-DNA, and anti-HCV)-negative and normal serum alanine aminotransferase (ALT) levels from the Nantong Central Blood Bank as a control group.
All patients were diagnosed by blood biochemical tests, viral histology and B-ultrasonic examination. All peripheral blood samples were collected in the morning, with anti-clot heparin, and peripheral blood mononuclear cells were separated immediately, according to the method as described previously[18]. Serum AFP concentrations ranged from 30 to 2 600 μg/L (median, 418 μg/L) and exceeding 50 μg/L was taken as a positive result. AFP-mRNA in peripheral blood was also detected in this study as described elsewhere[7]. The diagnosis of HCC and viral hepatitis was based on the criteria proposed by Chinese National Collaborative Cancer Research Group[19] and the 2000 Prevention and Cure Scheme of Viral Hepatitis[20], respectively.
Tissue specimens
Fresh tissue specimens including cancerous, paracancerous, and non-cancerous tissues were collected from 30 patients who underwent operations for liver cancers at the Affiliated Hospital of Nantong University, China. The tissue specimens were immediately frozen in liquid nitrogen and kept at -85°C until used. The patients included 25 men and 5 women, ranging from 22 to 70 years.
Isolation of total RNA and synthesis of cDNA
Total RNAs were isolated from peripheral blood mononuclear cells and from liver tissues by the guanidine thiocyanate method with RNAzole reagent (Promega) and purified as described elsewhere[21]. RNAs were dissolved in tromethamine-HCl buffer (10 mmol/L, pH 8.0) containing EDTA 10 mmol/L. The concentration of total RNAs was measured by optical density at 260 nm in an ultraviolet spectrophotometer (Shimadzu UV-2201 type, Kyoto, Japan), and calculated μg/mg wet tissue, and it was stored at -85°C. For synthesis of cDNA, 2 μg of total RNAs was denatured in the presence of random hexamers (200 pmol/L, Promega, Madison, WI, USA) at 95°C for 5 min and incubated with moloney murine leukemia virus reverse-transcriptase (GIBCO, BRL) at 23°C for 10 min, 42°C for 60 min and 95°C for 10 min, then on ice for 5 min, and stored at -20°C for PCR analysis.
Amplification of nested polymerase chain reaction
The resulting cDNA was amplified by a nested PCR with two pairs of primers. The oligonucleotides were designed according to IGF-II sequence[22] and synthesized with synthesizer (Model 381 A, Applied Biosystems, Foster City, CA, USA). The sequences of the 2 external primer pairs used for the initial PCR amplification were IGF-II-1(sense), 5’-ATGGGAATGCCAATGGGGAAG-3’ (nt 251-271) and IGF-II-2(antisense), 5’-CTTGCCCACGGGGTATC-TGGG-3’ (nt 566-586), the size of amplified gene fragment was 336 bp. The sequences of the two internal primer pairs used for the second PCR amplification were IGF-II-3 (sense), 5’-TGCTGCATTGCTGCTTACCG-3’ (nt 311-330) and IGF-II-4 (anti-sense), 5’-AGGTCACAGCTGCGG AAACA-3’ (nt 461-480). PCR amplification consisted of initial denaturation at 94°C for 5 min, followed by 94°C for 25 s, 55°C for 30 s, and 72°C for 90 s for 30 cycles. The final product of nested PCR was 170 bp. Human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) genome[23] was used as a control. Primer sequence for GAPDH was GAPDH-1 (sense), 5’-ACCACAGT-CCATGCCATCAC-3’ (nt 601-620) and GAPDH-2 (antisense), 5’-TCCACCACCCTGTTGCTGT A-3’ (nt 1 033-1 052), the product of PCR was 452 bp (GAPDH gene transcript, 40 pmol/L). The PCR products were electrophoresed on 2% agarose gels with ethidium bromide staining. The fragment sizes were evaluated using PCR markers (Promega) as molecular weight standards.
Sequencing of PCR products
The 170 bp amplified product of human IGF-II genome was purified with the Montage PCR centrifugal filter devices (Millipore, MA, USA) according to the instruction of protocol. One microgram DNA was used for preparation of sequencing reaction and directly sequenced using the MegaBACE DNA analysis system in MegaBACE DNA sequencer with the DYEnamic ET Dye Terminator Cycle Sequencing Kit (Amersham Biosciences, NJ, USA), following their protocol. The sequences were edited using the MegaBACE Sequence Analyzer Version 3.0 program (Amersham Biosciences) and aligned with the amplified sequences of IGF-II genome, HCC tissue and circulating IGF-II.
Detection of serum free IGF-II protein level
The levels of serum free IGF-II protein in patients with chronic diseases were detected by an enzymatically amplified two-step sandwich-type immunoassay (ACTIVETM Free IGF-II ELISA, TX). In this assay, standards, controls and serum samples were incubated in microtitration wells, which had been coated with anti-IGF-II antibody. After incubation and washing, the wells were treated with another anti-free IGF-II detection antibody labeled with the enzyme horseradish peroxidase (HRP). After a second incubation and washing step, the wells were incubated with the substrate tetramethylbenzidine (TMB). An acidic stopping solution was then added and the degree of enzymatic turnover of the substrate was determined by dual wavelength absorbance measurement at 450 nm and 620 nm. The absorbance measured was directly proportional to the concentration of free IGF-II present. A set of free IGF-II standards was used to draw a standard curve of absorbance vs free IGF-II concentration from which the free IGF-II concentrations in the serum samples can be calculated according to the ELISA routine method.
Statistical analysis
All patients were divided into six groups: HCC, acute hepatitis, chronic hepatitis, cirrhosis, extrahepatic tumor, and normal subjects. Hepatoma tissues were divided into three groups: cancerous, para-cancerous and non-cancerous tissues. Results are expressed as mean±SD. Differences between different groups were assessed by the Student’s t test or the χ2 test. P < 0.05 was considered to be significant.
RESULTS
Amplification of IGF-II mRNA and sensitivity of detection
The fragments of IGF-II genome were amplified by a nested PCR assay from human hepatoma tissues and circulating blood of patients with HCC (Figure 1). The sizes of amplified fragments were identical to the original designed ones, which were 336 bp in single-step PCR, and 170 bp in nested-PCR. Differences between single-step PCR and nested PCR for amplified IGF-II mRNA were compared in 111 peripheral blood samples. The detecting frequency of IGF-II-mRNA was 6.3% (7/111) by single-step PCR and 34.2% (38/111) by nested-PCR. The incidence of nested PCR for IGF-II mRNA amplification was significantly higher than that in single-step PCR (P < 0.05). Total RNAs (2 mg/L) extracted from hepatoma tissues were diluted 10-2-10-8 times and amplified by nested-PCR, and the lowest sensitivity of the assay was 2 ng/L of total RNA (Figure 1A). The positive fragments of IGF-II genome were found distinctly from HCC tissues or peripheral blood of patients with HCC (Figure 1C) and could not be detected from non-cancerous tissues of HCC patients or from peripheral blood of patients with acute hepatitis, chronic hepatitis, liver cirrhosis, and extrahepatic tumors. By sequence analysis, the nucleotide homologies of amplified IGF-II gene fragments from HCC tissue and peripheral blood were identical to the cited sequence of human IGF-II genome (Figure 2)[22].
Figure 1.
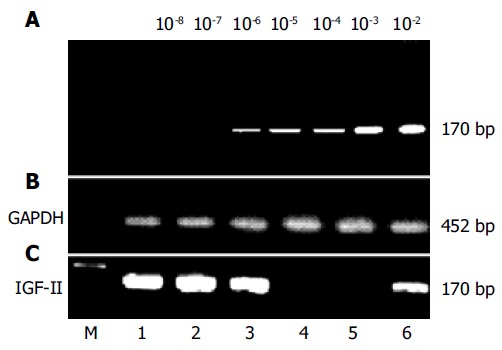
Amplification of IGF-II genomes from human hepatoma tissues or circulating blood samples of patients with hepatocellular carcinoma. IGF-II mRNAs were synthesized according to IGF-II cDNA with random hexamers and moloney murine leukemia virus reverse-transcriptase, and detected with different primer pairs by nested PCR (170 bp). The positive fragments of IGF-II genome were found distinctly in hepatoma tissues or in peripheral blood of patients with hepatocellular carcinoma. A: the sensitive limitation of our detection system (2 ng/L), using total RNA with 10-2-10-8 fold dilution and then amplified by nested PCR; B: the amplified fragments (452 bp) of glyceraldehyde-3-phosphate dehydrogenase genome from liver tissues or peripheral blood as controls; C: the amplification of IGF-II genomes in liver tissues (No. 1-4) or circulating blood (No. 5-6). No. 1-2, the positively amplified fragments of IGF-II mRNA from cancerous tissues of patients with hepatocellular carcinoma; No. 3, the positively amplified fragments of IGF-II mRNA from para-cancerous tissue of patients with hepatocellular carcinoma; No. 4, no positively amplified fragment from non-cancerous tissue of patients with hepatocellular carcinoma; No. 5, no positively amplified fragment from circulating blood of patients with liver cirrhosis, and No. 6, the positively amplified fragment from peripheral blood of patients with hepatocellular carcinoma. GAPDH: glyceraldehyde-3-phosphate dehydrogenase. M: DNA molecular weight marker.
Figure 2.

Alignment of nucleotide sequences of the amplified fragments of IGF-II genome from cancerous tissue or circulating blood in patients with HCC by sequence analysis. Origin: the cited sequence (170 bp, nt 311-480) of human
Expression of total RNA and IGF-II mRNA in HCC
Different expression of total RNA was found in different parts of HCC tissues. The total RNA concentrations were significantly lower in HCC tissues than in self-control surrounding tissues (P < 0.05) or non-cancerous tissues (P < 0.01), respectively (Table 1). However, the expression of IGF-II mRNA amplified by nested-PCR was 100% in cancerous tissues, 53.3% in its surrounding tissues, and 0% in its non-cancerous tissues, respectively. The positive frequencies of IGF-II mRNA in HCC tissues or its para-cancerous tissues were significantly higher than those in its non-cancerous tissues (P < 0.01), respectively.
Table 1.
Alterations of total RNA (mean±SD) and amplification of IGF-II mRNA in cancerous, para-cancerous, and non-cancerous liver tissues
| Groups | n | Total RNA level (μg/mg wet tissue) |
IGF-II mRNA |
|
| Positive | % | |||
| Cancerous tissues | 30 | 13.4 ± 8.4 | 30 | 100 |
| Para-cancerous tissues | 30 | 20.7 ± 14.6a | 16 | 53.3b |
| Non-cancerous tissues | 30 | 25.0 ± 20.2b | 0 | 0b |
P<0.05,
P<0.01 vs the cancerous tissue group.
Circulating free IGF-II level in patients with chronic liver diseases
The levels of serum free IGF-II protein in patients with chronic liver diseases were investigated in this study, and the results are shown in Table 2. Of the 166 cases with chronic liver disease, the level of circulating free IGF-II protein was significantly higher (P < 0.01) in HCC patients (75.7%, 84/111) than in patients with liver cirrhosis (28%, 7/25) or with chronic hepatitis (26.7%, 8/30). Also, the levels of serum AFP in patients with HCC (64.9%, 72/111) were significantly higher (P < 0.01) than in patients with liver cirrhosis (36%, 9/25) or with chronic hepatitis (23.3%, 7/30).
Table 2.
Levels of serum free IGF-II and AFP in patients with chronic liver diseases
| Groups | n |
Free IGF-II (μg/L) |
AFP (μg/L) |
||
| mean±SD | > 6.0 (%) | mean±SD | > 50 (%) | ||
| CH | 30 | 4.1 ± 2.4 | 8 (26.7) | 32.0 ± 23.7 | 7 (23.3) |
| LC | 25 | 5.5 ± 1.7 | 7 (28.0) | 34.7 ± 39.8 | 9 (36) |
| HCC | 111 | 6.7 ± 1.8 | 84 (75.7) b | 417.5 ± 274.1 | 72 (64.9) b |
P<0.01 vs the chronic hepatitis group or the liver cirrhosis group. HCC: hepatocellular carcinoma; LC: liver cirrhosis; CH: chronic hepatitis.
Detection of circulating IGF-II mRNA in HCC
Amplification of IGF-II mRNA in peripheral blood of patients with liver diseases or with extrahepatic tumors in comparison with AFP mRNA is shown in Table 3. Although the serum free IGF-II level increased in patients with chronic hepatitis or with liver cirrhosis, the circulating IGF-II mRNAs only were detected in HCC patients. The frequency of peripheral blood IGF-II mRNA was 34.2% in patients with HCC, no amplified fragments of circulating IGF-II mRNA could be detected in patients with benign liver diseases, extrahepatic tumors, and normal controls, respectively. The incidence of circulating IGF-II mRNA was lower than that of AFP mRNA in patients with HCC, but it was a more specific circulating marker for HCC diagnosis. Of the 111 cases with HCC, both of AFP mRNA and IGF-II mRNA were detected in 28 patients (25.2%, 28/111), and only 27.0% of HCC cases were positive for AFP mRNA (30/111) or only IGF-II mRNA was detected in 10 patients (9%, 10/111). The combined analysis of circulating AFP mRNA and IGF-II mRNA was useful for diagnosis and differential diagnosis of HCC.
Table 3.
Analysis of IGF-II-mRNA and AFP-mRNA in peripheral blood of patients with different liver diseases or non-liver tumors
| Groups | n |
IGF-II mRNA |
AFP mRNA |
Both |
|||
| Positive | % | Positive | % | Positive | % | ||
| HCC | 111 | 38 | 34.2 | 58 | 52.3 | 68 | 61.3 |
| LC | 25 | 0 | 0b | 5 | 20.0b | 5 | 20.0b |
| CH | 30 | 0 | 0b | 2 | 6.7b | 2 | 6.7b |
| AH | 30 | 0 | 0b | 0 | 0b | 0 | 0b |
| ET | 25 | 0 | 0b | 0 | 0b | 0 | 0b |
| NC | 25 | 0 | 0b | 0 | 0b | 0 | 0b |
P<0.01 vs the hepatocellular carcinoma group. HCC: hepatocellular carcinoma; LC: liver cirrhosis; CH: chronic hepatitis; AH: acute hepatitis; ET: extrahepatic tumor; NC: normal control.
Circulating IGF-II mRNA in HCC metastasis
Of the 111 patients with HCC, the relationship between circulating IGF-II mRNA and HCC stages and metastasis is analyzed in Table 4. The fragments of circulating IGF-II mRNA could be detected in any stage of HCC development. No significant differences of IGF-II mRNA were found between HCC stage I and II. The incidence of IGF-II mRNA in HCC stage III was 45.5% (30/66), significantly higher than that in early-stage (stage I or II) HCC. The fragments of IGF-II mRNA could be detected in all HCC patients with extrahepatic metastasis (100%). However, no significant difference of peripheral blood IGF-II mRNA was found between HCC with intra-hepatic metastasis and without intra-hepatic metastasis (P > 0.05).
Table 4.
Relationship between peripheral blood IGF-II mRNA or AFP mRNA and HCC stage or metastasis
| Groups | n |
IGF-II mRNA |
AFP mRNA |
||
| Positive | % | Positive | % | ||
| HCC Stage I | 14 | 2 | 14.3a | 5 | 35.7a |
| Stage II | 31 | 6 | 19.4a | 8 | 25.8a |
| Stage III | 66 | 30 | 45.5 | 47 | 71.2 |
| Intra-hepatic metastasis | |||||
| With | 67 | 27 | 40.3 | 44 | 65.7c |
| Without | 44 | 11 | 25 | 18 | 40.9 |
| Extrahepatic metastasis | |||||
| With | 13 | 13 | 100b | 13 | 100b |
| Without | 98 | 25 | 25.5 | 53 | 54.1 |
P<0.05 vs the HCC stage III group.
P<0.01,
P<0.05 vs the non-metastasis group. HCC: hepatocellular carcinoma.
Circulating IGF-II mRNA with AFP level and tumor size
We divided the 111 HCC patients into two groups according to serum AFP level: the positive frequency of circulating IGF-II mRNA fragment was 35.3% (6/17) in cases with AFP less than 50 μg/L, and 34.0% (32/94) in cases with AFP more than or equal to 50 μg/L, without significant differences between the two groups (P > 0.05). In addition, we found that the incidence of peripheral blood IGF-II mRNA was 31.8% (7/22) in cases with diameters of tumor less than 5 cm, and 34.8% (31/89) in cases with diameters of tumor more than or equal to 5 cm, respectively.
DISCUSSION
Hepatocellular carcinoma is one of the most common forms of malignant cancer with the 4th highest mortality rate worldwide[24]. Major risk factors for the development of HCC include chronic infections with hepatitis B or C virus, alcohol consumption, exposure to dietary aflatoxin B1, hereditary liver disease or liver cirrhosis of any etiology[25]. Recent studies have discovered changes in the IGF axis that affect the molecular pathogenesis of HCC, including the autocrine production of IGFs, IGF binding proteins (IGFBPs), IGFBP proteases, and IGF receptor expression. Characteristic alterations detected in HCC and hepatoma cell lines comprise the overexpression of IGF-II and IGF-I receptor emerging as critical events in malignant transformation and growth of tumors[26,17]. IGF-II is a polypeptide hormone secreted by many organs of the fetus. Very little information is available on the expression of IGF-II mRNA in HCC. In the present study, the total RNA levels and IGF-II mRNA in different parts of HCC tissues, the expression of peripheral blood IGF-II mRNA, and the level of serum free IGF-II protein were investigated in patients with various liver diseases.
The sinusoidal cells in para-cancerous cirrhotic nodule tissues and the malignant hepatocytes in HCC tissues expressed IGF-II. As we know, liver cirrhotic nodules are the precancerous lesion of HCC, so it is suggested that, in the precancerous condition, IGF-II mediated hepatocyte proliferation mainly via IGF1R by a paracrine mechanism. IGF-II mRNA was distributed in the cytoplasm of hepatocytes and overexpressed in HCC tissues[27,28]. IGF-II could be secreted by hepatoma cells themselves and stimulate their proliferation via an autocrine mechanism[29,30]. In order to analyze IGF-II expression, the fragments of IGF-II genome in HCC tissues were amplified by the sensitive nested PCR and confirmed by analysis of IGF-II sequences. Although different expression levels of total RNA were found in different parts of HCC tissues, the expression of IGF-II mRNA was detected in all of HCC tissues, half in its para-cancerous cirrhotic tissues, and none in its non-cancerous tissues (Table 1), respectively. Some differences in RNA level between tumor and cirrhotic tissues were quite informative.
The observations that HCC cells expressed less IGF-I than control liver cells, whereas IGF-II expression was higher in a high proportion of HCC cells[31,32], are consistent with previous reports[33,34] which showed that IGF-I mRNA levels were lower in HCC as compared with adjacent non-tumorous hepatic tissue, whereas IGF-II mRNA was higher in tumor tissues compared with normal liver tissues. The molecular mechanisms responsible for reduction in IGF-I and reactivation of IGF-II in HCC remain to be determined. These results support growth factor-dependent HCC development and provide novel prognosis markers after HCC surgery.
IGFs are potent autocrine and paracrine mitogens for liver cancer cell proliferation, and their bioactivity is reduced by IGFBP-3. Human embryonic liver cell lines expressed IGF-II also, suggesting that hepatoma cells may regain some embryonic development characteristics like AFP secretion[35,36]. A smaller proportion of IGF-II is associated with other IGFBPs, while less than 5% of IGF-II exists as unbound or free IGF-II that is believed to be the biologically active fraction of IGF-II[37], capable of binding the type 2 IGF receptor[38]. IGF-II present in the ternary complex is not easily dissociated, however IGF-II contained in low molecular weight binding complexes has a rapid turnover and may be the source of much of the detected free IGF-II. The levels of serum free IGF-II protein were significantly higher in HCC group than those in liver cirrhosis or in chronic hepatitis group (Table 2). The data indicated that serum free IGF-II secreted from HCC cells may act as an angiogenic factor for the hypervascularization of HCC.
Serum AFP is a diagnostic marker of HCC, but its significance in the early diagnosis of HCC is unclear and the positive rate is not high[5,7,39]. The fragments of peripheral blood IGF-II mRNA were amplified by PCR and its clinical significances in patients with liver diseases were analyzed in the present study (Table 3). Although the serum free IGF-II level increased in patients with chronic hepatitis or liver cirrhosis, the circulating IGF-II mRNA only was detected in HCC patients. The frequency of IGF-II mRNA was not so high in patients with HCC, yet it was more specific (100%) for HCC diagnosis than that of peripheral blood AFP mRNA. No amplified fragments of circulating IGF-II mRNA could be detected in patients with benign liver diseases, extrahepatic tumors, and normal controls. The fragments of circulating IGF-II mRNA could be detected in all HCC patients with extrahepatic metastasis (100%), and like circulating AFP mRNA, could provide markers of severity and prognosis after HCC resection. The analyses of peripheral blood AFP-mRNA and IGF-II mRNA were more specific and more sensitive tumor markers for detecting and monitoring a few of circulating HCC hepatocytes.
In conclusion, the present data indicate that the expression levels of IGF-II mRNA were different in different parts of HCC liver tissues, and IGF-II mRNA could only be detected in peripheral blood of HCC patients. The frequency of circulating IGF-II mRNA and its diagnostic value increased with clinical stage of HCC and with distant metastases of HCC. The circulating IGF-II mRNA could be a useful molecular marker for HCC diagnosis, especially in monitoring extrahepatic metastases of tumor cells. Further studies will allow us to quantitate IGF-II mRNA in liver tissues and peripheral blood, and to explore the molecular mechanisms responsible for reactivation of IGF-II in development of HCC.
Footnotes
Science Editor Zhu LH and Guo SY Language Editor Elsevier HK
Supported by a grants-in-aid from the Key Project of Medical Science from Jiangsu Province, China, No. RC2003100
References
- 1.Peto J. Cancer epidemiology in the last century and the next decade. Nature. 2001;411:390–395. doi: 10.1038/35077256. [DOI] [PubMed] [Google Scholar]
- 2.Qin LX, Tang ZY. The prognostic molecular markers in hepatocellular carcinoma. World J Gastroenterol. 2002;8:385–392. doi: 10.3748/wjg.v8.i3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yao DF, Horie C, Horie T, Shimizu I, Meng XY, Ito S. Virological features of hepatitis C virus infection in patients with liver diseases in the inshore area of the Yangtze River. Tokushima J Exp Med. 1994;41:49–56. [PubMed] [Google Scholar]
- 4.Shimizu I, Yao DF, Horie C, Yasuda M, Shiba M, Horie T, Nishikado T, Meng XY, Ito S. Mutations in a hydrophilic part of the core gene of hepatitis C virus in patients with hepatocellular carcinoma in China. J Gastroenterol. 1997;32:47–55. doi: 10.1007/BF01213296. [DOI] [PubMed] [Google Scholar]
- 5.Yao D, Jiang D, Huang Z, Lu J, Tao Q, Yu Z, Meng X. Abnormal expression of hepatoma specific gamma-glutamyl transferase and alteration of gamma-glutamyl transferase gene methylation status in patients with hepatocellular carcinoma. Cancer. 2000;88:761–769. [PubMed] [Google Scholar]
- 6.Kar S, Carr BI. Detection of liver cells in peripheral blood of patients with advanced-stage hepatocellular carcinoma. Hepatology. 1995;21:403–407. [PubMed] [Google Scholar]
- 7.Yao DF, Dong ZZ, Yang DM, Zhu YS, Jiang DR, Lu JX. Pe-ripheral blood AFP mRNA amplification in the diagnosis and differential diagnosis of hepatocellular carcinoma. Zhonghua Putong Waike Zazhi. 2000;15:474–477. [Google Scholar]
- 8.Kim KW, Bae SK, Lee OH, Bae MH, Lee MJ, Park BC. Insulin-like growth factor II induced by hypoxia may contribute to angiogenesis of human hepatocellular carcinoma. Cancer Res. 1998;58:348–351. [PubMed] [Google Scholar]
- 9.Lee YI, Lee S, Das GC, Park US, Park SM, Lee YI. Activation of the insulin-like growth factor II transcription by aflatoxin B1 induced p53 mutant 249 is caused by activation of transcription complexes; implications for a gain-of-function during the formation of hepatocellular carcinoma. Oncogene. 2000;19:3717–3726. doi: 10.1038/sj.onc.1203694. [DOI] [PubMed] [Google Scholar]
- 10.Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339–346. doi: 10.1038/ng0802-339. [DOI] [PubMed] [Google Scholar]
- 11.Aihara T, Noguchi S, Miyoshi Y, Nakano H, Sasaki Y, Nakamura Y, Monden M, Imaoka S. Allelic imbalance of insulin-like growth factor II gene expression in cancerous and precancerous lesions of the liver. Hepatology. 1998;28:86–89. doi: 10.1002/hep.510280113. [DOI] [PubMed] [Google Scholar]
- 12.Fiorentino M, Grigioni WF, Baccarini P, D'Errico A, De Mitri MS, Pisi E, Mancini AM. Different in situ expression of insulin-like growth factor type II in hepatocellular carcinoma. An in situ hybridization and immunohistochemical study. Diagn Mol Pathol. 1994;3:59–65. doi: 10.1097/00019606-199403010-00010. [DOI] [PubMed] [Google Scholar]
- 13.Scharf JG, Dombrowski F, Ramadori G. The IGF axis and hepatocarcinogenesis. Mol Pathol. 2001;54:138–144. doi: 10.1136/mp.54.3.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bae MH, Lee MJ, Bae SK, Lee OH, Lee YM, Park BC, Kim KW. Insulin-like growth factor II (IGF-II) secreted from HepG2 human hepatocellular carcinoma cells shows angiogenic activity. Cancer Lett. 1998;128:41–46. doi: 10.1016/s0304-3835(98)00044-5. [DOI] [PubMed] [Google Scholar]
- 15.Kang-Park S, Lee YI, Lee YI. PTEN modulates insulin-like growth factor II (IGF-II)-mediated signaling; the protein phosphatase activity of PTEN downregulates IGF-II expression in hepatoma cells. FEBS Lett. 2003;545:203–208. doi: 10.1016/s0014-5793(03)00535-0. [DOI] [PubMed] [Google Scholar]
- 16.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 17.Park BC, Huh MH, Seo JH. Differential expression of transforming growth factor alpha and insulin-like growth factor II in chronic active hepatitis B, cirrhosis and hepatocellular carcinoma. J Hepatol. 1995;22:286–294. doi: 10.1016/0168-8278(95)80281-9. [DOI] [PubMed] [Google Scholar]
- 18.Ijichi M, Takayama T, Matsumura M, Shiratori Y, Omata M, Makuuchi M. alpha-Fetoprotein mRNA in the circulation as a predictor of postsurgical recurrence of hepatocellular carcinoma: a prospective study. Hepatology. 2002;35:853–860. doi: 10.1053/jhep.2002.32100. [DOI] [PubMed] [Google Scholar]
- 19.The Liver Cancer Committee of Chinese Anticancer Association. Diagnostic criteria of primary hepatocellular carcinoma. Zhonghua Ganzangbing Zazhi. 2000;8:135. [Google Scholar]
- 20.The Group of Viral Hepatitis Research (2000, Xian) The Pre-vention and Cure Scheme of Viral Hepatitis. Zhonghua Ganzangbing Zazhi. 2000;8:324–329. [Google Scholar]
- 21.Kanashiro CA, Schally AV, Groot K, Armatis P, Bernardino AL, Varga JL. Inhibition of mutant p53 expression and growth of DMS-153 small cell lung carcinoma by antagonists of growth hormone-releasing hormone and bombesin. Proc Natl Acad Sci USA. 2003;100:15836–15841. doi: 10.1073/pnas.2536558100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rall LB, Scott J, Bell GI. Human insulin-like growth factor I and II messenger RNA: isolation of complementary DNA and analysis of expression. Methods Enzymol. 1987;146:239–248. doi: 10.1016/s0076-6879(87)46026-6. [DOI] [PubMed] [Google Scholar]
- 23.Benham FJ, Povey S. Members of the human glyceraldehyde-3-phosphate dehydrogenase-related gene family map to dispersed chromosomal locations. Genomics. 1989;5:209–214. doi: 10.1016/0888-7543(89)90048-7. [DOI] [PubMed] [Google Scholar]
- 24.Ito S, Yao DF, Nii C, Horie T, Kamamura M, Nishikado T, Honda H, Shibata H, Shimizu I, Meng XY. Incidence of hepatitis C virus (HCV) antibodies and HCV-RNA in blood donors and patients with liver diseases in the inshore area of the Yangtze River. J Gastroenterol Hepatol. 1994;9:245–249. doi: 10.1111/j.1440-1746.1994.tb01718.x. [DOI] [PubMed] [Google Scholar]
- 25.Orito E, Mizokami M. Hepatitis B virus genotypes and hepatocellular carcinoma in Japan. Intervirology. 2003;46:408–412. doi: 10.1159/000075000. [DOI] [PubMed] [Google Scholar]
- 26.Scharf JG, Braulke T. The role of the IGF axis in hepatocarcinogenesis. Horm Metab Res. 2003;35:685–693. doi: 10.1055/s-2004-814151. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, Chan EK. Autoantibodies to IGF-II mRNA binding protein p62 and overexpression of p62 in human hepatocellular carcinoma. Autoimmun Rev. 2002;1:146–153. doi: 10.1016/s1568-9972(02)00030-7. [DOI] [PubMed] [Google Scholar]
- 28.Huynh H, Chow PK, Ooi LL, Soo KC. A possible role for insulin-like growth factor-binding protein-3 autocrine/paracrine loops in controlling hepatocellular carcinoma cell proliferation. Cell Growth Differ. 2002;13:115–122. [PubMed] [Google Scholar]
- 29.Lee S, Park U, Lee YI. Hepatitis C virus core protein transactivates insulin-like growth factor II gene transcription through acting concurrently on Egr1 and Sp1 sites. Virology. 2001;283:167–177. doi: 10.1006/viro.2001.0892. [DOI] [PubMed] [Google Scholar]
- 30.Ng IO, Lee JM, Srivastava G, Ng M. Expression of insulin-like growth factor II mRNA in hepatocellular carcinoma. J Gastroenterol Hepatol. 1998;13:152–157. doi: 10.1111/j.1440-1746.1998.tb00630.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhang N, Siegel K, Odenthal M, Becker R, Oesch F, Dienes HP, Schirmacher P, Steinberg P. The role of insulin-like growth factor II in the malignant transformation of rat liver oval cells. Hepatology. 1997;25:900–905. doi: 10.1002/hep.510250419. [DOI] [PubMed] [Google Scholar]
- 32.Su Q, Liu YF, Zhang JF, Zhang SX, Li DF, Yang JJ. Expression of insulin-like growth factor II in hepatitis B, cirrhosis and hepatocellular carcinoma: its relationship with hepatitis B virus antigen expression. Hepatology. 1994;20:788–799. doi: 10.1002/hep.1840200404. [DOI] [PubMed] [Google Scholar]
- 33.Scharf JG, Ramadori G, Dombrowski F. Analysis of the IGF axis in preneoplastic hepatic foci and hepatocellular neoplasms developing after low-number pancreatic islet transplantation into the livers of streptozotocin diabetic rats. Lab Invest. 2000;80:1399–1411. doi: 10.1038/labinvest.3780147. [DOI] [PubMed] [Google Scholar]
- 34.Su JJ, Qin GZ, Yan RQ, Huang DR, Yang C, Lotlikar PD. The expression of insulin-like growth factor II, hepatitis B virus X antigen and p21 in experimental hepatocarcinogenesis in tree shrews. Ann Acad Med Singapore. 1999;28:62–66. [PubMed] [Google Scholar]
- 35.Sohda T, Iwata K, Soejima H, Kamimura S, Shijo H, Yun K. In situ detection of insulin-like growth factor II (IGF2) and H19 gene expression in hepatocellular carcinoma. J Hum Genet. 1998;43:49–53. doi: 10.1007/s100380050036. [DOI] [PubMed] [Google Scholar]
- 36.Uchida K, Kondo M, Takeda S, Osada H, Takahashi T, Nakao A, Takahashi T. Altered transcriptional regulation of the insulin-like growth factor 2 gene in human hepatocellular carcinoma. Mol Carcinog. 1997;18:193–198. [PubMed] [Google Scholar]
- 37.Ooasa T, Karasaki H, Kanda H, Nomura K, Kitagawa T, Ogawa K. Loss of imprinting of the insulin-like growth factor II gene in mouse hepatocellular carcinoma cell lines. Mol Carcinog. 1998;23:248–253. [PubMed] [Google Scholar]
- 38.Seo JH, Kim KW, Murakami S, Park BC. Lack of colocalization of HBxAg and insulin like growth factor II in the livers of patients with chronic hepatitis B, cirrhosis and hepatocellular carcinoma. J Korean Med Sci. 1997;12:523–531. doi: 10.3346/jkms.1997.12.6.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Funaki NO, Tanaka J, Seto SI, Kasamatsu T, Kaido T, Imamura M. Hematogenous spreading of hepatocellular carcinoma cells: possible participation in recurrence in the liver. Hepatology. 1997;25:564–568. doi: 10.1002/hep.510250312. [DOI] [PubMed] [Google Scholar]


