Abstract
AIM: To study the effect of proton pump inhibitor (PPI) treatment on patients with reflux esophagitis and its in vivo effect on apoptosis, p53- and epidermal growth factor receptor (EGFR) expression.
METHODS: After informed consent was obtained, gastric biopsies of the antrum were taken from patients with reflux oesophagitis prior to and after 6 mo of 20 mg omeprazole (n = 14) or 40 mg esomeprazole (n = 12) therapy. Patients did not take any other medications known to affect the gastric mucosa. All patients were Helicobacter pylori negative as confirmed by rapid urease test and histology, respectively. Cell proliferation, apoptosis, EGFR, and p53 expression were measured by immunohistochemical techniques. At least 600 glandular epithelial cells were encountered and results were expressed as percentage of total cells counted. Was considered statistically significant.
RESULTS: Although there was a trend towards increase of cell proliferation and EGFR expression both in omeprazole and esomeprazole treated group, the difference was not statistically significant. Neither apoptosis nor p53 expression was affected.
CONCLUSION: Long-term PPI treatment does not significantly increase gastric epithelial cell proliferation and EGFR expression and has no effect on apoptosis and p53 expression.
Keywords: Proton pump inhibitor, Omeprazole, Esomeprazole, Cell proliferation, Apoptosis, p53 expression, Epidermal growth factor receptor
INTRODUCTION
Long-term PPI therapy is suggested to be the best treatment for gastro-esophageal reflux disease. Administration of PPI causes profound and continuous hypochlorhydria by selective inhibition of the proton pump (H+/K+-ATPase) in gastric parietal cells[1]. It has been shown in animal studies that long-term omeprazole treatment reversibly increases epidermal cell proliferation and suppresses its differentiation in rats[2,3].
Apoptosis normally plays a role complementing prolifer-ation and is also considered to be essential for the maintenance of gastro-intestinal homeostasis and health[4]. Disturbance in the balance between these two processes may predispose to either cell loss with mucosal damage or cell accumulation and cancer development[5].
However, several studies have investigated the effects of omeprazole on gastric mucosa, but there is no information available about the effect of the first single-isomer, esome-prazole, on gastric epithelial cell proliferation, apoptosis, p53-and EGFR expression.
The proliferating cell nuclear antigen (PCNA) technique is an accepted method for measurement of cell proliferation. PCNA is the co-factor of DNA-polymerase and can be detected mostly in the late G1 and S phases, but it is also present in every phase of the cell cycle.
The terminal deoxynucleotidyl (TdT)-mediated deoxyuri-dinetriphosphate (dUTP) nick end labelling (TUNEL) method has been accepted for the detection of apoptotic cells[6].
Abnormalities in p53 expression represent the most common molecular change not only in cancer, but also in precancerous gastric lesions, including gastric dysplasia[7,8]. An increased wild-type p53 expression may also represent a cellular response to DNA damage.
Epithelial growth factor (EGF) is a potent mitogenic peptide, which plays a crucial role in promoting gastric epithelial cell migration, proliferation and differentiation. The increased local production of EGF leads to over expression of EGFR[9-11].
The aim of the present study was to measure the cell turnover (cell proliferation and apoptosis), p53- and EGFR expression by immunohistochemistry in gastric biopsy samples during long-term omeprazole and esomeprazole treatment.
MATERIALS AND METHODS
Patients
To analyze the effect of PPI therapy on cell kinetics pattern of the gastric mucosa, we studied patients with gastro-esophageal reflux disease. A total of 26 patients (14 males and 12 females, mean age 46.2 ± 16.5 years) took part in the study. All patients gave written informed consent.
Biopsies were taken in each subject during upper endoscopy from the antrum (lesser curvature, 3 cm from the pylorus). Additional biopsies were taken during endoscopy for the histological evaluation of their Helicobacter pylori (H pylori) status[12].
Patients were treated in an open label study continuously with omeprazole (20 mg/d) or esomeprazole (40 mg/d) for 6 mo. Fourteen patients were on omeprazole and 12 patients on esomeprazole therapy.
Patients did not receive any other medication known to affect the gastric mucosa, but stable medication for hypertension or other diseases such as hypercholesterinemia, non-insulin dependent diabetes mellitus, etc. was allowed.
Patients were classified by the Los Angeles classification (15 patients had grade A, and 11 had grade B). None of the patients had Los Angeles grades C, D, or Barrett esophagus. Exclusion criteria were active H pylori infection and presence of intestinal metaplasia, since it has been established in previous studies that gastric epithelial cell proliferation is enhanced, if intestinal metaplasia or H pylori infection is present[13-15]. Since histology may miss initial focal microscopical lesions of intestinal metaplasia, small intestine mucus antigen (SIMA) and large intestine mucus antigen (LIMA), each indicates intestinal metaplasia in the gastric biopsy samples, were measured by immunohistochemical technique in all samples. To exclude H pylori infection, in addition to histology, both rapid urease test during endoscopy and urease breath test were performed.
Biopsies taken at 0 and 6 mo in both the omeprazole-and esomeprazole-treated groups were assessed.
Neither patients treated with omeprazole nor patients on esomeprazole therapy had endoscopic changes in the stomach or duodenum or H pylori infection when biopsy was taken. No intestinal metaplasia was found in the samples.
For immunohistochemistry, all biopsy specimens were fixed in buffered formalin and embedded in paraffin. Four micron thick sections were cut and mounted on glass slides.
Proliferation-PCNA immunohistochemistry
The four micron thick tissue sections were dewaxed and rehydrated. Antigen unmasking was carried out in citrate buffer pH 6.0 by microwave heat treatment (3 min 750 W and 3 min 370 W), and samples were cooled down in PBS for 20 min. Endogenous peroxidase activity was blocked by incubation for 30 min at room temperature in 3% hydrogen peroxide. After being washed thrice in PBS for 3 min, the slides were incubated with optimally diluted PCNA antibody (Clone: PC10, DAKO) for 15 min at room temperature in a humidified chamber. After being washed thrice in PBS, signal conversion was carried out with the LSAB2 system (DAKO: K0672) as described in the manual. Hematoxylin co-staining was done.
Apoptosis-TUNEL immunohistochemistry
After deparaffinization in xylene and rehydration through graded ethanol, antigen unmasking was carried out in citrate buffer pH 6.0 by microwave heat treatment (5 min 750 W), and samples were cooled down in PBS for 20 min. Samples were digested with nuclease free proteinase K for 20 min at room temperature. After being washed twice in PBS, samples were covered with 30 μL TUNEL dilution label and 50 μL TUNEL reaction mixture (5 μL Tdt enzyme solution and 45 μL dUTP label solution). Samples were incubated for 120 min at 37°C in a dark humidified chamber. After being washed thrice in PBS, endogenous peroxidase activity was blocked by incubation for 30 min in 3% hydrogen peroxide at room temperature in a dark humidified chamber. After being washed twice in PBS, non-specific blocking was carried out with 1% BSA-PBS solution for 10 min at room temperature in a dark humidified chamber. After redundant BSA was removed with pipette, samples were covered with 50 μL converter-POD antibody and incubated for 60 min at 37°C in a dark humidified chamber. After being washed thrice with PBS, 50 μL DAB solution (5 μL DAB substrate and 45 μL peroxide buffer) was added to each sample and signal conversion was checked by light microscopy. Finally, haematoxylin co-staining was done.
P53 immunohistochemistry
The four micron thick tissue sections were deparaffinized in xylene, rehydrated through graded ethanol. Antigen unmasking was carried out by microwave heat treatment (samples in plastic jars containing citrate buffer pH 6.0 were put into a preheated (95-99°C) plastic water bath and heated with 500 W for 15 min), and samples were cooled down in PBS for 20 min. Endogenous peroxidase activity was blocked by incubation for 30 min at room temperature in 3% hydrogen peroxide. After being washed thrice in PBS for 3 min, the slides were incubated with optimally diluted p53 antibody (Clone: DO-7, DAKO) at 37°C for 30 min in a humidified chamber. After being washed thrice in PBS, signal conversion was carried out with the LSAB2 system (DAKO) as described in the manual. Hematoxylin co-staining was done.
EGFR immunohistochemistry
After deparaffinization, antigen unmasking was carried out by nuclease free proteinase K digestion for 20 min at room temperature. After being washed twice in PBS, endogenous peroxidase activity was blocked by incubation for 30 min at room temperature in 3% hydrogen peroxide. After being washed thrice in PBS for 3 min, non-specific blocking was done with 1% BSA-PBS solution for 10 min at room temperature. Then, the slides were incubated with diluted EGFR antibody (1 μL EGFR antibody and 40 μL PBS) (Clone: H-11, DAKO) at 37°C for 60 min in a humidified chamber. After being washed thrice in PBS, signal conversion was carried out with the LSAB2 system (DAKO) as described in the manual. Hematoxylin co-staining was done.
Counting
Known immunohistochemically-positive tissue sections were used as positive controls, and negative control sections were processed immunohistochemically after the primary antibody was replaced by PBS. None of these control sections exhibited immunoreactivity.
Axially, at least 800 (mainly 1 000) crypt epithelial cells within well-oriented crypts were counted in each sample under light microscope (40X objective). The labelling index (LI) was defined as a percentage of the positive nuclei over the total nuclei counted. The evaluation of staining intensity (i.e. number of positive cells) for PCNA, TUNEL, p53 and EGFR was performed by two investigators independently, without knowledge of the histology and the results of the other investigator. There was less than 5 % variance between the results of two counts.
SIMA, LIMA immunohistochemistry
The four micron thick tissue sections were dewaxed and rehydrated, reacted with anti-SIMA and -LIMA mAbs, stained by indirect immunoperoxidase methods, and counterstained with hematoxylin, including appropriate controls. The deparaffinized sections were blocked with 5% BSA (diluted in PBS) for 5 min, drained and incubated with the diluted mouse antibodies for 20 min. After two 5-min washings with PBS, the sections were covered with horseradish-peroxidase-labelled rabbit anti-mouse immunoglobulin (Serotec, UK), then washed twice for 5 min with PBS. Sections from all blocks were also stained with hematoxylin-eosin (H&E) for 2-10 min.
The immunoperoxidase-stained slides were then viewed under a light microscope, and assessed under code, by two observers. Scores of 0-3 were assigned to intensity of reactivity (weak, 1; moderate, 2; strong, 3) and distribution (restricted, < 25% positive, 1; patchy, 25-75%, 2; and diffuse, > 75%, 3) for each of the antibodies, in serial sections of specimens.
Statistical analysis
Statistical analysis with one-way ANOVA, LSD test and correlation analysis were performed by the Statistica for Windows 4.3 program package. P<0.05 was considered statistically significant.
RESULTS
Proliferation index, apoptosis, p53- and EGFR expression prior to and after 6 mo of omeprazole or esomeprazole therapy are shown in Tables 1 and 2, respectively.
Table 1.
Effect of long-term omeprazole therapy on gastric epithelial cell kinetics (mean±SD)
| Omeprazole (n = 14) | ||
| Endoscopy (mo) | 0 | 6 |
| Proliferation index (%) | 40.9 ± 13.8 | 54.1 ± 16.6 |
| Apoptosis (%) | 45.3 ± 8.7 | 49.5 ± 10.3 |
| P53 (%) | 28.7 ± 4.3 | 28.9 ± 12.7 |
| EGFR (%) | 17.6 ± 11.9 | 21.9 ± 7.9 |
Table 2.
Effect of long-term esomeprazole therapy on gastric epithelial cell kinetics (mean±SD)
| Esomeprazole (n = 12) | ||
| Endoscopy (mo) | 0 | 6 |
| Proliferation index (%) | 39.6 ± 8.7 | 52.8 ± 10.4 |
| Apoptosis (%) | 43.5 ± 9.8 | 48.9 ± 9.8 |
| P53 (%) | 29.2 ± 10.7 | 29.6 ± 11.5 |
| EGFR (%) | 16.8 ± 8.1 | 22.3 ± 8.1 |
There was no difference between the effects of omeprazole and esomeprazole therapy on gastric epithelial cell kinetics (Figure 1). There was no statistically significant difference in any of the investigated parameters between the samples taken at the beginning and those taken after 6 mo of PPI treatment. Cell parameters were not significantly affected by age and sex (data not shown).
Figure 1.
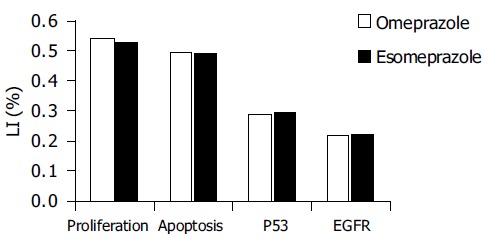
No difference between the effects of omeprazole and esomeprazole on cell turnover of gastric mucosa.
Although there was a trend towards increase of cell proliferation and EGFR expression in both omeprazole-and esomeprazole-treated groups, the difference was not statistically significant.
We found alterations only in the localization of immunohistochemical staining density prior to and after PPI therapy.
In a non-affected normal gastric mucosa, the greatest density of PCNA positive cells was found in the neck cell compartment (Figure 2). The greatest increment in cell proliferation in response to PPI therapy, occurred in the gland compartment of the gastric mucosa. The increase was limited to the deepest portions of the crypts (Figure 3). In both, prior to and after PPI administration, parietal cells did not express PCNA.
Figure 2.
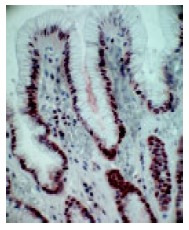
Greatest density of PCNA positive cells (brown colored cells) found in neck cell compartment of non-affected gastric mucosa (400x magnification).
Figure 3.
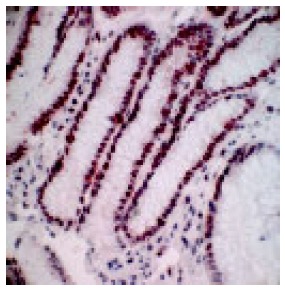
Greatest increase in PCNA positive cells (brown colored cells) observed in gland compartment of gastric mucosa after PPI therapy (400x magnification).
In a non-affected normal gastric mucosa, positive EGFR immunoreactivity was observed in parietal cells and mucous neck cells of the gastric fundic glands. EGFR was localized to the basolateral cell membrane, but not to the apical luminal membrane (Figure 4). After PPI administration, a strong positive EGFR immunoreactivity was observed at the basolateral membrane, in cytoplasm and supranuclear area of mucosal cells (Figure 5A). Positive EGFR expression was also found in some parietal cells (in cytoplasm and plasma membrane), but it was generally weaker compared to the neck cells (Figure 5B).
Figure 4.
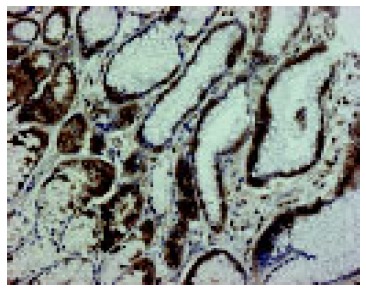
Positive EGFR immunoreactivity (brown color) in parietal cells and mucus neck cells of fundic glands (200x magnification).
Figure 5.
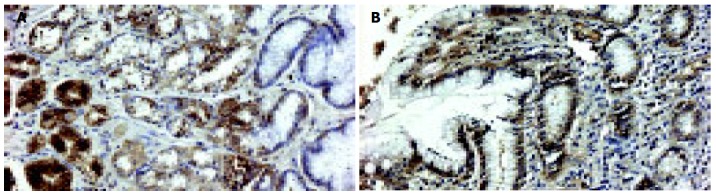
EGFR immunostaining (A) and immunoreactivity (B) after PPI therapy (200×magnification).
No dysplasia was observed after 6 mo of follow-up in any of the patients receiving either omeprazole or esomeprazole therapy.
DISCUSSION
Maintaining cell turnover is a key feature in organs with high metabolism such as the gastric mucosa. Higher cell turnover may lead to tumor formation while the suppressed state results in ulcer development[4].
Omeprazole-induced potent acid suppression may lead to sustained and profound hypochlorhydria, which is often associated with hypergastrinemia[16]. In majority of animal studies, it has been shown that long-term omeprazole treatment reversibly increases epithelial cell proliferation and suppresses its differentiation[2,3]. In other studies, however, neither cell proliferation is higher[17,18], nor increased gastrinoma or other tumor formation is observed during long-term PPI treatment[19]. Additional data suggest that gastrin enhances growth of normal and malignant colonic cells in vitro and may be linked to the development of colorectal cancer[20-24]. On the other hand, animal and clinical studies do not support the role of omeprazole-induced hyperga-strinemia in gastro-intestinal neoplasia development[25,26] and no increased incidence of colon cancer has been found in patients with either pernicious anemia or gastrinomas[27-29].
The risk of hypergastrinemia induced by long-term PPI therapy is still ambiguous and of concern to many clinicians.
This study analyzed the gastric epithelial cell kinetics in patients with gastro-esophageal reflux disease during long-term PPI treatment using immunohistochemical techniques. We investigated the effect of two different proton pump inhibitors: omeprazole and esomeprazole.
Although esomeprazole has a higher bio-availability than omeprazole and provides more pronounced inhibition of acid secretion compared to all other clinically available proton pump inhibitors, we found no difference between these drugs in terms of their effects on the gastric epithelium.
Our results confirmed the previous observations[17,18] that cell proliferation is not significantly altered during long-term PPI therapy. Although there was a trend towards increase of both cell proliferation and EGFR expression, the difference was not statistically significant. Previous studies have indicated that PCNA positive cells are nearly always positive for EGFR[10]. The accompanied increase of EGFR expression to a higher PCNA activity in this study has therefore been expected.
The fact that the trend towards increase in gastric cell proliferation and EGFR expression is not accompanied with a parallel increase in apoptosis and p53 expression also supports the conclusion that there is no significant change in cell turnover during chronic administration of any PPI. No dysplasia or neoplasia was observed in any of the samples obtained during this study.
In the present study, in a non-affected normal gastric mucosa, positive EGFR immunoreactivity was identified in the mucous neck cells and parietal cells of the fundic glands, and the staining was localized only on the basolateral cell membrane, which is in agreement with previous studies[3,11].
Gray et al[3] suggested that the greatest increment in cell proliferation in response to the increased gastrin drive occurs in the gland compartment of gastric mucosa. Our findings have confirmed their observations. After long-term PPI administration, we observed the greatest increase in PCNA positive cells mainly in the gland compartment. Although there is a proportionally greater increase in proliferation in the gland compartment compared to that in the mucous neck cell compartment, the neck cell compartment remains the main source of new cell formation.
Several studies have shown an intense EGFR expression during ulcer healing[10,11]. In our study, after administration of PPI a strong positive EGFR immunoreactivity was observed predominantly in some mucous neck cells of the proliferative zone compared to the weaker staining density in parietal cells. The EGFR immunoreactivity was localized not only on the basolateral membrane of these cells, but also appeared in cytoplasm and supranuclear area.
EGFR is a good immunohistochemical marker for the detection of altered gastric epithelial cell function. The presence of EGFR on cells of the proliferative zone clearly indicates that they are the targets for the proliferation-stimulating action of EGF.
Parietal cells express EGFR but not PCNA. Presence of EGFR in the parietal cells is not associated with cell proliferation, but is consistent with a potent inhibitory action of EGF on gastric acid secretion.
We demonstrated that long-term PPI treatment did not significantly increase gastric epithelial cell proliferation and EGFR expression and had no effect on apoptosis and p53 expression. We found alterations only in the localization of immunohistochemical staining density during chronic PPI administration.
Our results have confirmed the previous observations that cell proliferation is not significantly altered during long-term PPI therapy. In addition no alterations in cellular response and no disturbance in the balance between cell proliferation and apoptosis are found, the maintenance of gastro-intestinal hemostasis is ensured and there is no risk for progression of hyperplasia to dysplasia in patients during chronic PPI administration.
These data suggest that 6-mo treatment with proton pump inhibitors is not associated with cell proliferation abnormalities of the gastric antral mucosa, which is a further argument for the safety of PPIs.
Footnotes
Science Editor Wang XL Language Editor Elsevier HK
Supported by the National Science Foundation (OTKA Grant No: T 034345)
References
- 1.Iijima K, Sekine H, Koike T, Imatani A, Ohara S, Shimosegawa T. Long-term effect of Helicobacter pylori eradication on the reversibility of acid secretion in profound hypochlorhydria. Aliment Pharmacol Ther. 2004;19:1181–1188. doi: 10.1111/j.1365-2036.2004.01948.x. [DOI] [PubMed] [Google Scholar]
- 2.Kakei N, Ichinose M, Tatematsu M, Shimizu M, Oka M, Yahagi N, Matsushima M, Kurokawa K, Yonezawa S, Furihata C. Effects of long-term omeprazole treatment on adult rat gastric mucosa--enhancement of the epithelial cell proliferation and suppression of its differentiation. Biochem Biophys Res Commun. 1995;214:861–868. doi: 10.1006/bbrc.1995.2366. [DOI] [PubMed] [Google Scholar]
- 3.Gray MR, Darnton SJ, Hunt JA, Irlam RW, Nemeth J, Wallace HM. Accelerated gastric epithelial proliferation. Gut. 1995;36:522–527. doi: 10.1136/gut.36.4.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Que FG, Gores GJ. Cell death by apoptosis: basic concepts and disease relevance for the gastroenterologist. Gastroenterology. 1996;110:1238–1243. doi: 10.1053/gast.1996.v110.pm8613014. [DOI] [PubMed] [Google Scholar]
- 5.Yoshimura T, Shimoyama T, Tanaka M, Sasaki Y, Fukuda S, Munakata A. Gastric mucosal inflammation and epithelial cell turnover are associated with gastric cancer in patients with Helicobacter pylori infection. J Clin Pathol. 2000;53:532–536. doi: 10.1136/jcp.53.7.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Labat-Moleur F, Guillermet C, Lorimier P, Robert C, Lantuejoul S, Brambilla E, Negoescu A. TUNEL apoptotic cell detection in tissue sections: critical evaluation and improvement. J Histochem Cytochem. 1998;46:327–334. doi: 10.1177/002215549804600306. [DOI] [PubMed] [Google Scholar]
- 7.Sasano H, Date F, Imatani A, Asaki S, Nagura H. Double immunostaining for c-erbB-2 and p53 in human stomach cancer cells. Hum Pathol. 1993;24:584–589. doi: 10.1016/0046-8177(93)90236-a. [DOI] [PubMed] [Google Scholar]
- 8.Shiao YH, Rugge M, Correa P, Lehmann HP, Scheer WD. p53 alteration in gastric precancerous lesions. Am J Pathol. 1994;144:511–517. [PMC free article] [PubMed] [Google Scholar]
- 9.Playford RJ, Boulton R, Ghatei MA, Bloom SR, Wright NA, Goodlad RA. Comparison of the effects of transforming growth factor alpha and epidermal growth factor on gastrointestinal proliferation and hormone release. Digestion. 1996;57:362–367. doi: 10.1159/000201358. [DOI] [PubMed] [Google Scholar]
- 10.Abe S, Sasano H, Katoh K, Ohara S, Arikawa T, Noguchi T, Asaki S, Yasui W, Tahara E, Nagura H, et al. Immunohistochemical studies on EGF family growth factors in normal and ulcerated human gastric mucosa. Dig Dis Sci. 1997;42:1199–1209. doi: 10.1023/a:1018897922644. [DOI] [PubMed] [Google Scholar]
- 11.Tarnawski A, Stachura J, Durbin T, Sarfeh IJ, Gergely H. Increased expression of epidermal growth factor receptor during gastric ulcer healing in rats. Gastroenterology. 1992;102:695–698. doi: 10.1016/0016-5085(92)90123-g. [DOI] [PubMed] [Google Scholar]
- 12.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Unger Z, Molnár B, Szaleczky E, Törgyekes E, Müller F, Zágoni T, Tulassay Z, Prónai L. Effect of Helicobacter pylori infection and eradication on gastric epithelial cell proliferation and apoptosis. J Physiol Paris. 2001;95:355–360. doi: 10.1016/s0928-4257(01)00048-1. [DOI] [PubMed] [Google Scholar]
- 14.Ruzsovics A, Unger Z, Molnar B, Pronai L, Tulassay Z. Effect of Helicobacter pylori infection on epidermal growth factor receptor (EGFR) expression and cell proliferation of gastric epithelial mucosa: correlation to macroscopic and microscopic diagnosis. Int J Exp Pathol. 2002;83:257–263. doi: 10.1046/j.1365-2613.2002.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan XG, Kelleher D, Fan XJ, Xia HX, Keeling PW. Helicobacter pylori increases proliferation of gastric epithelial cells. Gut. 1996;38:19–22. doi: 10.1136/gut.38.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ligumsky M, Lysy J, Siguencia G, Friedlander Y. Effect of long-term, continuous versus alternate-day omeprazole therapy on serum gastrin in patients treated for reflux esophagitis. J Clin Gastroenterol. 2001;33:32–35. doi: 10.1097/00004836-200107000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Biasco G, Mordenti P, Brandi G, Paganelli GM, Santucci R, Miglioli M. Cell kinetics of the gastric mucosa of patients treated with omeprazole. Am J Gastroenterol. 1996;91:621–622. [PubMed] [Google Scholar]
- 18.Li H, Helander HF. Parietal cell kinetics after administration of omeprazole and ranitidine in the rat. Scand J Gastroenterol. 1995;30:205–209. doi: 10.3109/00365529509093264. [DOI] [PubMed] [Google Scholar]
- 19.Klinkenberg-Knol EC, Nelis F, Dent J, Snel P, Mitchell B, Prichard P, Lloyd D, Havu N, Frame MH, Romàn J, et al. Long-term omeprazole treatment in resistant gastroesophageal reflux disease: efficacy, safety, and influence on gastric mucosa. Gastroenterology. 2000;118:661–669. doi: 10.1016/s0016-5085(00)70135-1. [DOI] [PubMed] [Google Scholar]
- 20.Sirinek KR, Levine BA, Moyer MP. Pentagastrin stimulates in vitro growth of normal and malignant human colon epithelial cells. Am J Surg. 1985;149:35–39. doi: 10.1016/s0002-9610(85)80006-4. [DOI] [PubMed] [Google Scholar]
- 21.Smith JP, Wood JG, Solomon TE. Elevated gastrin levels in patients with colon cancer or adenomatous polyps. Dig Dis Sci. 1989;34:171–174. doi: 10.1007/BF01536047. [DOI] [PubMed] [Google Scholar]
- 22.Seitz JF, Giovannini M, Gouvernet J, Gauthier AP. Elevated serum gastrin levels in patients with colorectal neoplasia. J Clin Gastroenterol. 1991;13:541–545. doi: 10.1097/00004836-199110000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Chu M, Nielsen FC, Franzén L, Rehfeld JF, Holst JJ, Borch K. Effect of endogenous hypergastrinemia on gastrin receptor expressing human colon carcinoma transplanted to athymic rats. Gastroenterology. 1995;109:1415–1420. doi: 10.1016/0016-5085(95)90625-8. [DOI] [PubMed] [Google Scholar]
- 24.Renga M, Brandi G, Paganelli GM, Calabrese C, Papa S, Tosti A, Tomassetti P, Miglioli M, Biasco G. Rectal cell proliferation and colon cancer risk in patients with hypergastrinaemia. Gut. 1997;41:330–332. doi: 10.1136/gut.41.3.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graffner H, Singh G, Chaudry I, Milsom JW. Omeprazole-induced hypergastrinemia does not influence growth of colon carcinoma. Dig Dis Sci. 1992;37:485–489. doi: 10.1007/BF01307567. [DOI] [PubMed] [Google Scholar]
- 26.Pinson DM, Havu N, Sztern MI, Mattsson H, Looney GA, Kimler BF, Hurwitz A. Drug-induced hypergastrinemia: absence of trophic effects on colonic carcinoma in rats. Gastroenterology. 1995;108:1068–1074. doi: 10.1016/0016-5085(95)90204-x. [DOI] [PubMed] [Google Scholar]
- 27.Brinton LA, Gridley G, Hrubec Z, Hoover R, Fraumeni JF. Cancer risk following pernicious anaemia. Br J Cancer. 1989;59:810–813. doi: 10.1038/bjc.1989.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Talley NJ, Chute CG, Larson DE, Epstein R, Lydick EG, Melton LJ. Risk for colorectal adenocarcinoma in pernicious anemia. A population-based cohort study. Ann Intern Med. 1989;111:738–742. doi: 10.7326/0003-4819-111-9-738. [DOI] [PubMed] [Google Scholar]
- 29.Orbuch M, Venzon DJ, Lubensky IA, Weber HC, Gibril F, Jensen RT. Prolonged hypergastrinemia does not increase the frequency of colonic neoplasia in patients with Zollinger-Ellison syndrome. Dig Dis Sci. 1996;41:604–613. doi: 10.1007/BF02282349. [DOI] [PubMed] [Google Scholar]


