Abstract
Increased calcium influx through L-type voltage-gated calcium channels (L-VGCC) has been implicated in the neuronal dysfunction underlying age-related memory declines. In the present study we sought to test the specific role of Cacna1c (which encodes Cav1.2) in modulating age-related memory dysfunction. Short-term, spatial, and contextual/emotional memory was evaluated in young and aged, wild-type as well as mice with one functional copy of Cacna1c (haploinsufficient), using the novel object recognition, Y-maze, and passive avoidance tasks respectively. Hippocampal expression of Cacna1c mRNA was measured by quantitative polymerase chain reaction (qPCR). Aging was associated with object recognition and contextual/emotional memory deficits and a significant increase in hippocampal Cacna1c mRNA expression. Cacna1c haploinsufficiency was associated with decreased Cacna1c mRNA expression in both young and old animals. However, haploinsufficient mice did not manifest an age-related increase in expression of this gene. Behaviorally, Cacna1c haploinsufficiency prevented object recognition deficits during aging in both male and female mice. A significant correlation between higher Cacna1c levels and decreased object recognition performance was observed in both sexes. We have also observed a sex-dependent protective role of decreased Cacna1c levels in contextual/emotional memory loss, specifically in male mice. These data provide evidence for an association between increased hippocampal Cacna1c expression and age-related cognitive decline. Additionally, they indicate an interaction between the Cacna1c gene and sex in the modulation of age-related contextual memory declines.
Keywords: aging, memory, cognition, mice, hippocampus
INTRODUCTION
Aging has been widely associated with cognitive impairments and greater vulnerability for the development of neurodegenerative disorders (Raz et al., 1998; Hedden & Gabrieli, 2004; Mattson & Magnus, 2006). There is a need to identify novel targets for the development of pharmacotherapies to maintain cognitive integrity in the elderly. The ‘calcium hypothesis of aging’ proposes a key role for an altered neuronal calcium homeostasis in mediating changes in neuronal development associated with aging (Khachaturian, 1987; Landfield, 1987). Subsequent studies have shown that chronic elevation of calcium influx has been implicated in aging-associated neuronal death (Disterhoft et al., 1994). L-VGCC current densities are significantly elevated in hippocampal neurons of old compared to young rats (Landfield, 1994; Campbell et al., 1996; Thibault & Landfield, 1996; Disterhoft et al., 2004; Wang & Mattson, 2014) and surface L-VGCCs expression levels have been found to be increased in aged hippocampus (Nunez-Santana et al., 2014). However, there is conflicting evidence for aging-induced altered L-VGCC expression levels; increased (Herman et al., 1998; Chen et al., 2000; Veng & Browning, 2002), no change (Blalock et al., 2003; Kadish et al., 2009; Nunez-Santana et al., 2014) or decreased expression levels (Rowe et al., 2007) have been previously observed.
The L-VGCC family consists of four distinct channels referred to as Cav1.1-Cav1.4. While Cav1.2 and Cav1.3, have both been identified in the brain, Cav1.2 accounts for about 80% in the rodent brain (Hell et al., 1993; Sinnegger-Brauns et al., 2009). The gene coding for the pore-forming alpha-1C subunit of Cav1.2 is Cacna1c (Soldatov, 1994). To date, the hypothesis that selectively decreasing Cav1.2 or Cacna1c improves age-related changes in cognitive performance has not been tested. Moreover, since sex differences in cognitive decline during aging have been identified (see Gur & Gur, 2002), we investigated the role of the Cacna1c gene in age-associated memory decline by comparing the effects of Cacna1c haploinsufficiency on cognitive behavioral function between male and female mice. We have previously shown that young Cacna1c haploinsufficient mice have an ~50% decreased Cav1.2 protein levels in the hippocampus as well as decreased L-VGCC current density in CA1 compared to their wild-type littermates (Dao et al., 2010). Our data reveal a correlation between increased age-related hippocampal Cacna1c mRNA expression and impaired short-term memory in the novel object recognition task, which were prevented in both male and female Cacna1c haploinsufficient mice. We also found that Cacna1c haploinsufficiency prevented age-related declines in emotional/contextual memory (passive avoidance) in male but not female mice. These findings suggest a relationship between hippocampal Cacna1c and age-related memory deficits and indicate a possible sex-dependent mechanism underlying this calcium channel's role in emotional-associated cognitive impairment.
METHODS AND MATERIALS
Mice
Male and female wild-type and Cacna1c haploinsufficient mice were obtained and generated as previously described by (Dao et al., 2010). Founder male and female heterozygous Cacna1c knockout mice originally developed by Deltagen Inc. (San Mateo, CA) were obtained from Jackson Laboratories (Bar Harbor, ME). They were then backcrossed to C57BL/6J for at least five generations prior to arrival, and greater than eight additional generations in our laboratory. All the mice used in the present study were bred in-house by breeding heterozygous males, generated in our own colony, and wild-type C57BL/6J females obtained from The Jackson Laboratories. At the time of initial behavioral testing the age of the animals was 4-5 months for the young groups and 17-18 months for the aged groups.
Ethical standards
All experimental procedures were approved by the University of Maryland Animal Care and Use Committee and were conducted in full accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Genotyping
DNA was extracted from tail clips by Proteinase K digestion followed by isopropanol precipitation. Genotype was determined through PCR amplification of a common 400bp product and knockout-specific 650bp product using three different primers:
-
(i)
a common sense primer, 5' TCTCTCCCACCTCGCACGCCGAATC 3'
-
(ii)
a wild-type specific anti-sense primer, 5' CACGACTGGCCTCTACTGCTCTTGAC 3'
-
(iii)
a knockout-specific anti-sense primer, 5' GACGAGTTCTTCTGAGGGGATCGATC 3'
Cacna1c expression
All the animals used in the behavioral studies were euthanized by decapitation five days following behavioral testing (see Fig. 1). Brains were collected and 1 mm coronal sections were cut at Bregma −2.30 using a mouse brain matrix. Dorsal hippocampi were then dissected and subsequently immediately frozen on dry ice, and stored at −80°C. The dorsal hippocampus was collected since it has been associated with cognitive functions, while the ventral hippocampus has been mainly related to stress, emotion and affective behaviors (see Fanselow & Dong, 2010). Total RNA was isolated from left hippocampus of mice by a phenol-chloroform-alcohol method using RNAzol (Molecular Research Center Inc, OH, USA) and the Directzol RNA kit (Zymo Research, CA, USA) following manufacturer protocols. Shipton et al., (2014) showed dissociation between the left and right hippocampus in the regulation of hippocampal-dependent memory and in particular, hippocampus-dependent associative spatial long-term memory has been shown to specifically require the left but not right hippocampus in mice. On-column DNase I digestion was included during the RNA extraction to eliminate potential DNA contamination. Four hundred ng of total RNA per sample were reverse transcribed into cDNA in a 20μL reaction volume using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA) according to the manufacturer's instructions. The cDNA product was diluted in nuclease-free water (1:5) and the RT-PCR reaction was conducted using the SensiFAST SYBR LowROX kit (Bioline, USA) in 10 μL total volume. The RT-PCR reaction was run on a ViiA7 platform (Lifetechnologies Inc, USA) with a three-step cycling program as follows: an initial denaturation of 20 sec at 95° C followed by 40 cycles with a denaturation step of 1 sec at 95° C, an annealing and an extension step of 20 sec at 62°C with the optics on at this last step. A melting curve was executed at the end of the amplification step. The primers for Cacna1c were designed using open source program Primer3 (Koressaar & Remm, 2007; Untergasser et al., 2012) and synthesized by IDT Inc, USA corresponding to the following sequences. The primers for Cacna1c were designed using open source program Primer3 (Koressaar & Remm, 2007; Untergasser et al., 2012) and synthesized by IDT Inc, USA corresponding to the following sequences: Forward TCA CCA TTG CCT CCG AAC ATT A; and Reverse GGG CTT TAT TGG CTG TGT CTT G. Three set of primers were used for reference genes and obtained from the PrimerTime™qPCRPrimers of IDT Tfrc: Mm.PT.56a.13036034, RPLP0: Mm.PT.56a.8566742.g and RPL13: Mm.PT.56a.4260941. All primer sets generated a single melting peak and were confirmed as a single amplification product by gel electrophoresis. All three reference genes were included with Cacna1c on each PCR run performed in triplicate. Relative expression was determined using the 2−ΔΔCT method (Livak & Schmittgen, 2001), using the geometric mean CT value of the three reference genes for normalization. Values are expressed as fold change with respect to wild-type young males used as reference group.
Figure 1. Behavioral and tissue collection sequence.

Novel object recognition (NOR); Passive avoidance (PA).
Behavioral studies
All mice were tested in all the behavioral tests. Experiments progressed from relatively least stressful to more stressful (i.e., Y-maze, novel object recognition and then passive avoidance; Fig. 1). Due to the large number of mice studied, the groups were randomly assessed in three experimental cohorts that were combined for data analysis.
Y-maze (spontaneous alternation task)
To assess the effects of aging and Cacna1c haploinsufficiency on working memory, we used the Y-maze paradigm (Coburn-Litvak et al., 2003; Dudchenko, 2004; Bannerman et al., 2008; Gotz & Ittner, 2008). The Y-maze apparatus (Stoelting Co., IL, US) consists of three identical arms (5 × 35 cm) joined in the middle thus forming a “Y” shape. The Y-maze protocol was based on previously published methods (Sarnyai et al., 2000). Briefly, mice were placed into the “start” arm of the maze and thereafter explored the apparatus for eight min. All the experimental trials were recorded with a digital video-camera. The videos were then scored by a trained observer blind to the experimental groups. Number of total arm entries and sequence of arm entries were measured. Percent alternations were calculated as: [the number of consecutive entries into three different compartments, divided by the total alternations (number of arm entries minus 2)] × 100.
Novel object recognition (NOR)
We assessed short-term recognition memory using the NOR task (Balderas et al., 2008; Moore et al., 2013). The NOR apparatus consists of a Plexiglas open-field chamber (40×9×23 cm) and was carried out under dim light conditions (10 LUX). The NOR behavioral testing consisted of three different sessions across two days: During the habituation phase (Day 1), the animals explored the NOR apparatus for 30 min in the absence of objects. Twenty-four hours later, during the familiarization session (Day 2), two identical objects were fixed on the floor of the apparatus symmetrically 8.5cm from the wall and the animals were allowed to explore the objects for 30 min. The objects were either two 50ml clear glass conical flasks (4.5 cm bottom diameter × 7 cm height) or two white-painted small glass vials (2.5 cm bottom diameter × 6 cm height). After familiarization with the “familiar” objects, mice were immediately returned to their home cages. Following a 30-min delay, mice were placed back into the NOR apparatus, in which one of the “familiar” objects used during the familiarization session was replaced by a “novel” object (retention phase). Mice were permitted to freely explore the objects for a period of six min. During both familiarization and retention sessions the objects were used in a counterbalanced between-groups manner. All the sessions were videotaped by a digital video-camera. The retention sessions were manually scored by a trained observer blind to the experimental groups, using the AnoStar scoring software (Cleversys Inc., VA, US). Mice were considered to be interacting with the objects when their head was facing the object in a pre-set distance of ≤ 1 cm. A discrimination ratio was calculated as the time a mouse was interacting with the novel object divided by the total time of interaction with both the objects during the retention phase (Bevins & Besheer, 2006).
Passive avoidance
Passive avoidance is a memory task based on emotional/contextual learning (Ogren, 1985). The passive avoidance task was performed as previously described (Yamada et al., 2003), with minor modifications. The apparatus consists of a two-chambered light-dark shuttle box (34 cm height × 37 cm width × 18 cm depth; Coulbourn Instruments, PA, USA) interconnected with a guillotine door. The experimental protocol consisted of two sessions; the training and the retention session, conducted 24 hours apart. During the training session, the animals were individually placed into the light compartment (800 LUX) of the apparatus and explored the light compartment for a 30-sec adaptation period with the door between the light and dark compartments closed. After the adaptation period, the door between the two compartments was automatically raised and mice were given a further five min to explore the compartment during which the latency of crossing to the dark compartment was measured. Upon entering the dark compartment, the guillotine door was closed and three sec later an inescapable foot-shock (0.32 mA, 2 sec duration) was delivered through the grid floor. Thirty sec after the foot-shock animals were immediately returned to their home cage. On the retention day (24 hours following training) mice were placed into the light compartment of the apparatus and after a 30-sec delay the door between the two compartments was raised. The latency to enter the dark compartment was automatically measured by the Coulbourn Instruments software. The trial was terminated after five min when the animal did not cross into the dark compartment.
Statistical Analysis
All values are expressed as the mean ± SEM. All data were analyzed by three-way analysis of variance (ANOVA) for factors ‘sex’, ‘age’ and genotype. For the behavioral studies, where three-way ANOVA did not reveal an effect of ‘sex’, data from both male and female mice were combined and a two-way ANOVA was then performed. ANOVAs were followed by LSD post-hoc comparison when statistical significance was reached (i.e., p<0.05). Values outside of the group mean ±2 SD were considered statistical outliers and excluded from all the analyses. All statistical analyses were performed using Statistica v10 (StatSoft Inc., USA).
RESULTS
Spatial memory was not altered in the Y-maze in aged mice
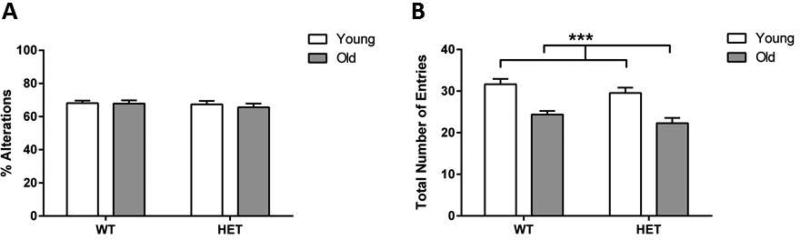
We assessed the effects of aging on both spontaneous alternation behaviors (percent alternation and total arm entries) in the Y-maze (WT male young: n=13; WT male old: n=13; HET male young: n=12; HET male old: n=11; WT female young: n=14; WT female old: n=12; HET female young: n=10; HET female old: n=7). A three-way ANOVA did not reveal any effect of ‘age’ (F[1,86]=0.10; p=0.75), ‘genotype’ (F[1,86]=0.22; p=0.64), ‘sex’ (F[1,86]=0.89; p=0.35), or an ‘age’ x ‘genotype’ x ‘sex’ interaction (F[1,86]=0.10; p=0.75) in spontaneous % alternation of mice. None of the other interactions were significant. Because there was no significant effect of sex we combined data from the males and females. Two-way ANOVA for percent alterations for both male and female mice combined (Fig. 2A) similarly did not show any significant effect of ‘age’ (F[1,89]=0.63; p=0.43), ‘genotype’ (F[1,89]=0.27; p=0.60), or a ‘age’ x ‘genotype’ interaction (F[1,89]=0.18; p=0.68). However, three-way ANOVA showed that aging was associated with a significant decrease in the total arm entries (age effect: F[1,86]=36.68; p<0.001), irrespective of ‘genotype’ (F[1,86]=3.13; p=0.08), ‘sex’ (F[1,86]=3.02; p=0.09), ‘age’ x ‘genotype’ x ‘sex’ interaction (F[1,86]=2.80; p=0.1), or any of the other interactions. Two-way ANOVA analysis of total arm entries on the combined data from both male and female mice showed a significant effect of ‘age’ (F[1,89]=36.97; p<0.001), but not ‘genotype’ (F[1,89]=3.12; p=0.08) or an ‘age’ x ‘genotype’ interaction (F[1,89]=0.0003; p=0.99) (Fig. 2B).
Figure 2. Y-maze performance.
(A) Percent alternations in the Y-maze were measured in both wild-type (WT) and Cacna1c haploinsufficient (HET) mice. Aging did not induce any significant changes in percent alternations in mice. (B) Total number of entries into the arms of the Y-maze apparatus showed a significant decrease in exploratory activity in aged WT and Cacna1c HET mice irrespective of their genotype. Data represent mean ± SEM (n = 7-14/group); *** p<0.001.
Aging-related decrease in novel object recognition was prevented in Cacna1c haploinsufficient mice
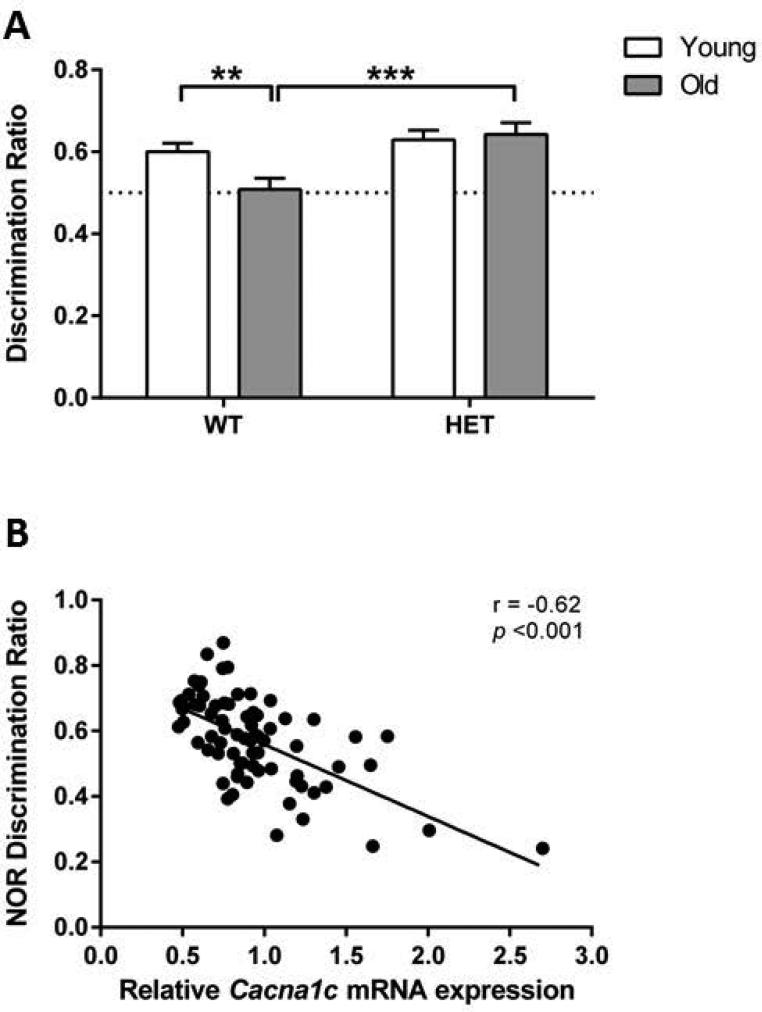
In the NOR task a three-way ANOVA revealed a significant effect of ‘genotype’ (F[1,82]=11.21; p<0.01) and an ‘age’ x ‘genotype’ interaction (F[1,82]=5.30; p<0.05), but no significant effect of ‘age’ (F[1,82]=1.66; p=0.20), ‘sex’ (F[1,82]=0.56; p=0.46), or any of the other interactions (WT male young: n=12; WT male old: n=13; HET male young: n=13; HET male old: n=10; WT female young: n=13; WT female old: n=12; HET female young: n=10; HET female old: n=7). In order to examine Cacna1c haploinsufficiency effects on aging and genotype, we performed a two-way ANOVA by combining data from male and female mice (Fig. 3A). A two-way ANOVA showed a significant effect of ‘genotype’ (F[1,86]=10.24; p<0.01) and a significant ‘age’ x ‘genotype’ interaction (F[1,86]=4.25; p<0.05). Post-hoc comparison indicated a significant decrease in the discrimination ratio of WT old mice compared with WT young mice (p<0.01). In contrast, in Cacna1c haploinsufficient mice there was no significant effect of age on the discrimination ratio (p=0.74).
Figure 3. Novel object recognition memory performance.
(A) Novel object recognition (NOR) discrimination ratio in young as well as old wild-type (WT) and Cacna1c haploinsufficient (HET) mice. Aging induced novel object recognition impairment in WT but not Cacna1c haploinsufficient (HET) mice, as indicated by a decreased discrimination ratio specifically in aged WT mice. (B) Correlation analyses between NOR discrimination ratio and relative Cacna1c levels in the hippocampus indicated a significant negative correlation. Data represent mean ± SEM (n = 7-14/group); ** p<0.01, *** p<0.001.
Cacna1c haploinsufficiency prevented aging-related reductions in contextual/emotional memory in passive avoidance learning in male but not female mice
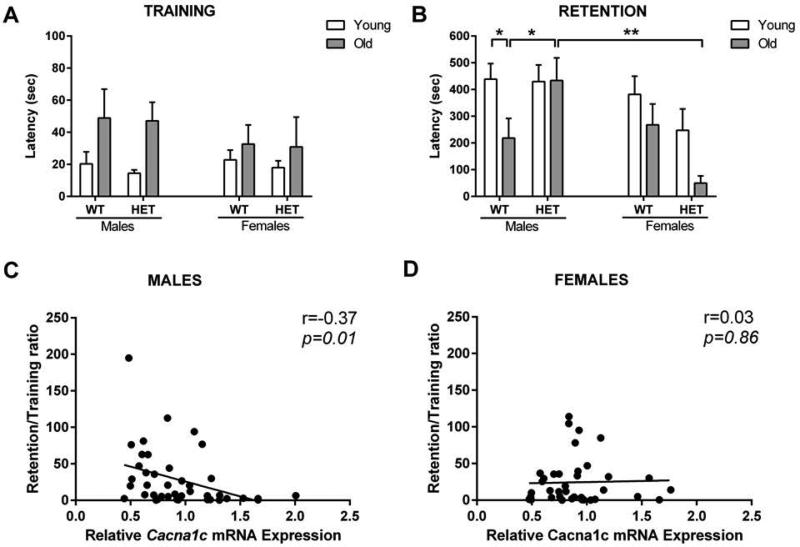
During the training session of the passive avoidance task (Fig. 4A), a three-way ANOVA revealed a significant effect of ‘age’ (F[1,84]=8.06; p<0.01) but not ‘genotype’ (F[1,84]=0.08; p=0.77), ‘sex’ (F[1,84]=0.47; p=0.50), or an ‘age’ x ‘genotype’ x ‘sex’ interaction (F[1,84]=0.01; p=0.90). None of the other interactions were significant. In contrast, during the retention phase (Fig. 4B), a three-way ANOVA revealed significant effects of ‘age’ (F[1,84]=6.28; p<0.05), ‘sex’ (F[1,84]=7.40; p<0.01), and a ‘sex’ x ‘genotype’ interaction (F[1,84]=7.03; p<0.01) in the latency for the animals to cross in the previously footshock-conditioned dark compartment of the passive avoidance apparatus during the retention phase (Fig. 4B) (WT male young: n=13; WT male old: n=13; HET male young: n=13; HET male old: n=10; WT female young: n=14; WT female old: n=13; HET female young: n=10; HET female old: n=6). There was no overall significant effect of genotype (F[1,84]=0.49; p=0.49) and the other interactions were not significant. Post-hoc analysis indicated a significant decrease in light-dark latency in WT old male mice compared to WT young male controls (p<0.05). Cacna1c haploinsufficiency prevented aging-induced contextual/emotional memory impairment as indicated by the prolonged light-dark latency of HET old male mice in the passive avoidance learning task compared with WT old male mice (p<0.05).
Figure 4. Passive avoidance task performance.
(A) Aging increased the baseline latency to cross from the light to the dark compartment in both wild-type (WT) and Cacna1c haploinsufficient (HET) mice during the training session of the passive avoidance task (age effect: p< 0.01). (B) Aged WT, but not Cacna1c HET mice manifested impairment in passive avoidance learning, as indicated by decreased retention latency. In contrast, Cacna1c haploinsufficiency was not protective in the passive avoidance learning in aged female mice. Correlation analyses between ratio retention/training latency in the passive avoidance task and relative hippocampal Cacna1c levels indicated a negative correlation in (C) male but not (D) female mice. Data represent mean ± SEM (n = 7-14/group); * p<0.05, ** p<0.01.
Effects of ageing on hippocampal Cacna1c expression
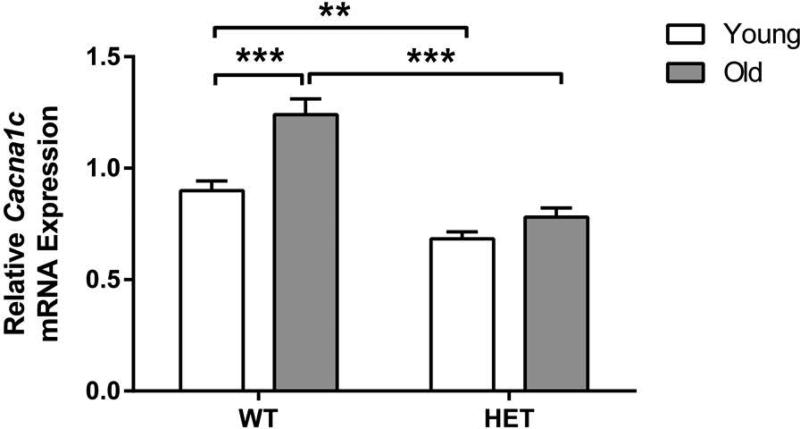
Relative Cacna1c expression was measured in hippocampal sections (Fig. 5). A three-way ANOVA showed a significant effect of ‘genotype’ (F[1,73]=38.70; p<0.001), ‘age’ (F[1,73]=17.40; p<0.001) and a ‘genotype’ x ‘age’ interaction (F[1,73]=4.46; p<0.05), but no significant effect of ‘sex’ (F[1,73]=0.05; p=0.82). None of the other interactions were significant (WT male young: n=10; WT male old: n=12; HET male young: n=12; HET male old: n=9; WT female young: n=12; WT female old: n=11; HET female young: n=9; HET female old: n=6). Two-way ANOVA analysis of relative Cacna1c expression on combined data from male and female mice combined revealed significant effect of ‘age’ (F[1,77]=17.60; p<0.001), ‘genotype’ (F[1,77]=41.73; p<0.001) and an ‘age’ x ‘genotype’ interaction (F[1,77]=5.41; p<0.05). Post-hoc analysis demonstrated a significant aging-induced increase in relative hippocampal Cacna1c expression specifically in WT mice (p<0.001). In Cacna1c haploinsufficient mice, Cacna1c levels were significantly lower compared to WT in young mice (p<0.01). Relative Cacna1c levels were significantly lower in Cacna1c haploinsufficient old mice compared to their WT old counterparts (p<0.001).
Figure 5. Hippocampal Cacna1c expression.
Aging induced a significant increase in relative Cacna1c expression in the hippocampus of wild-type (WT) but not Cacna1c haploinsufficient (HET) mice. Relative Cacna1c levels did not change during aging in HET mice. Data represent mean ± SEM (n = 6-13/group); * p<0.05, ** p<0.01, *** p<0.001.
Correlation analysis between Cacna1c expression and object recognition discrimination ratio revealed that object recognition memory and hippocampal Cacna1c levels are negatively correlated (Fig 3B). Importantly, This was similarly true in both male (r = −0.58, p<0.001) and female (r = −0.49, p<0.01) mice and within-group correlation analyses were also significant (data not shown).
Correlation analyses between Cacna1c expression and the ratio of retention/training latency time supported a negative correlation between emotional/contextual memory and hippocampal Cacna1c levels in male (r= −0.37, p=0.01; Fig. 4C), but not female (r= 0.03, p=0.86; Fig. 4D), mice. Similarly, correlation analysis between Cacna1c expression and passive avoidance retention latency time, uncorrected for training latency, also revealed that emotional/contextual memory and hippocampal Cacna1c levels are negatively correlated in male (r = −0.35, p<0.05; data not shown), but not female (r = 0.14, p=0.40; data not shown), mice.
DISCUSSION
Our data indicate age-related declines in object recognition memory in both male and female wild-type aged mice, which were prevented by genetic deletion of a single Cacna1c allele. Importantly, we also observed a robust negative correlation between Cacna1c expression in the hippocampus and short-term object recognition memory. We also showed that Cacna1c haploinsufficiency had a protective role in age-related emotional/contextual memory declines in male but not female mice. NOR (Burke et al., 2010), and passive avoidance (Zanotti et al., 1989; Knauber & Muller, 2000; Fiore et al., 2002) tasks have been previously used to assess memory declines during aging in rodents. While the hippocampus is primarily known for its involvement in spatial memory (Broadbent et al., 2004), it has been also shown to have a role in object recognition memory (O'Brien et al., 2006), as well as memory associated with aversive tasks (i.e., passive avoidance here) (e.g. Izquierdo et al., 1992). Our findings support the involvement of the Cacna1c gene in the hippocampus to at least partly underlie short-term object recognition memory and sex-dependently contextual/emotional memory. On the other hand, our data do not support an effect of aging on Y-maze spatial memory. This is not surprising as Y-maze has not been associated with age-related memory declines (Arendash et al., 2001).
Pre-existing evidence reveals robust alternations in neuronal calcium homeostasis during aging. In particular, influx of calcium through L-VGCC is significantly elevated in the hippocampus of aged rodents (Campbell et al., 1996; Thibault & Landfield, 1996; Wang & Mattson, 2014). An increase in calcium voltage-activated currents has been also found to occur in hippocampal CA1 neurons of aged rodents (Landfield & Pitler, 1984; Pitler & Landfield, 1990; Kerr & Abraham, 1993; Campbell et al., 1996) and rabbits (Moyer et al., 1992; Disterhoft et al., 1993). In addition, increased age-dependent cell death has also been associated with an increased density of L-VGCCs in long-term hippocampal cell cultures (Porter et al., 1997). Moreover, Davare and Hell, (2003) demonstrated increased cAMP-dependent protein kinase phosphorylation-induced activation of L-VGCC Cav1.2 (S1928) in the hippocampus of aged rats. While Nunex-Santana et al. have recently found a decrease of L-VGCCs in whole hippocampal tissue lysates, they also showed an age-related increase of S1928 phosphorylation in the dentate gyrus (Nunez-Santana et al., 2014). Our findings also support a role of L-VGCC during aging, since we have observed significant increases of Cacna1c mRNA levels in the hippocampus of aged mice. While previous reports have similarly found age-related increased hippocampal Cacna1c expression levels (Herman et al., 1998; Chen et al., 2000), there are other reports of either no change (Blalock et al., 2003; Kadish et al., 2009; Nunez-Santana et al., 2014) or decreased Cacna1c levels (Rowe et al., 2007). These discrepancies between the findings might be due to differences in analysis and/or methods used. Indeed, some of the aforementioned studies relied on gene array analysis and did not confirm the Cacna1c findings by other methods. Whether in our animals age-related Cacna1c expression alternations are region- and/or cell-specific remain to be determined. Furthermore, a limitation of our study is that we measured only Cacna1c mRNA concentrations, and not Cav1.2 protein levels or electrophysiological function of channels. We focused on mRNA because it is more readily quantifiable in the >90 samples between eight experimental groups utilized in our study. The significant correlations between Cacan1c levels and cognitive performance suggests that hippocampal mRNA Cacna1c levels predict function in our mouse line.
Increased age-related calcium influx through L-VGCC has been hypothesized to be detrimental to memory processes (e.g. Lynch, 2004). In the present study we provide further evidence that elevated hippocampal L-VGCC mRNA levels is associated with cognitive declines during aging. Findings from previous studies in Alzheimer's disease patients as well as animal models of cognitive dysfunction suggest that elevated hippocampal calcium influx is associated with synaptic dysfunction and neuronal degeneration, which might lead to cognitive impairments (Sandin et al., 1990; Mattson & Magnus, 2006). Enhanced L-VGCC function during aging has been shown to be at least partly responsible for the increase in after-hyperpolarization in the hippocampus, in particular the CA1 region, leading to robust alternations in synaptic plasticity (Norris et al., 1998b; a; Thibault et al., 2001). Several studies have demonstrated that L-type channel antagonists were able to reverse learning deficits in aged rodents (Levere & Walker, 1992; Ingram et al., 1994; Riekkinen et al., 1997; Batuecas et al., 1998; Veng et al., 2003), rabbits (Deyo et al., 1989a; Deyo et al., 1989b; Kowalska & Disterhoft, 1994; Solomon et al., 1995; Woodruff-Pak et al., 1997; Rose et al., 2007; Hopp et al., 2014), non-human primates (Sandin et al., 1990) and humans (Ban et al., 1990). These findings have been suggested to at least partly involve a compensatory action of L-VGCC antagonism over age-induced altered calcium metabolism (Gibson & Peterson, 1987). In contrast, in young intact animals, L-VGCC antagonism revealed contradictory effects. For instance, nimodipine administration enhanced memory performance in young chicks at low doses, while it induced an amnesic effect at high doses in a visual discrimination task (Deyo et al., 1990). Nifedipine also impaired retention in the same task (Deyo et al., 1992). Nimodipine administration was shown to improve spatial working memory in the 8-arm radial maze in your rats (Levy et al., 1991). Clements et al., (1995) showed no effects of nimodipine, nifedipine or amlodipine on passive avoidance or visual discrimination learning tasks in young chicks. Moreover, Quartermain et al., (1993) showed improvements of memory on an emotional-response learning task in amlodipine-treated young mice. Finally, in the inhibitory avoidance task, administration of nifedipine was found to be beneficial on memory retention in young rats (Quevedo et al., 1998). Importantly, selectively targeting L-VGCC blockage to the hippocampus is sufficient to enhance spatial memory (Quevedo et al., 1998; Kim et al., 2011). However, since existing L-type channel blockers inhibit all L-VGCC subtypes, none of these previous studies showed specificity to a particular LVGCC. Also, whether L-VGCC involvement in learning and memory processes is age-specific is not clear.
As such, the present study is the first, to our knowledge, to report beneficial effects of decreased hippocampal Cacna1c expression on age-associated memory impairment, as well as sex-dependent effects in emotional/contextual memory. In particular, we observed prevention of age-related memory decline in male, but not female, Cacna1c haploinsufficient mice in the passive avoidance memory task, and a significant correlation between increased Cacna1c expression and impaired passive avoidance learning specifically in male mice. Sex differences in learning and memory retention have been attributed to hormone-induced differences in hippocampal morphology and function (McEwen, 1997; Romeo et al., 2004; Hajszan et al., 2007). Notably, in the present study, we did not observe differences in Cacna1c expression in the two sexes, suggesting that a possible interaction between gonadal hormones and Cacna1c gene might have driven the observed sex-dependent behavioral effects in the passive avoidance task. This is consistent with the finding that β-estradiol directly potentiates calcium influx via Cav1.2 via the dihydropyridine binding site (Sarkar et al., 2008) and that Cav1.2 is a target of estradiol in vivo (Brewer et al., 2009).
In contrast with the novel object recognition and passive avoidance tasks, we demonstrated that aged mice did not manifest spatial working deficits in the Y-maze task, regardless of sex or genotype. These results are in line with previous findings by Arendash et al., (2001), who did not observe any impairment in Y-maze alternations between young (5-7 months) and old mice (15-17 months). However, we have observed aging-related decreases in total arm entries in both male and female mice, irrespective of genotype. Decreased total arm entries in the Y-maze paradigm indicates decreased spontaneous locomotor activity (e.g. Luszczki et al., 2005). Impaired prefrontal cortex activation has been implicated in hypo-locomotor and decreased gait speed effects of aging in humans (Andreescu et al., 2011).
Of translational relevance, an intronic single nucleotide polymorphism (SNP; rs1006737) in human CACNA1C has been associated with a number of brain functional changes including hippocampal performance (Bhat et al., 2012). These data include impaired working memory as well as decreased fractional anisotropy values within the hippocampus, which was directly associated with poorer learning performance in healthy individuals (Zhang et al., 2012; Dietsche et al., 2014; Heck et al., 2014). Healthy individuals carrying the CACNA1C rs1006737 risk allele showed reduced bilateral activation in the hippocampus during episodic memory recall and decreased functional coupling between the right and left hippocampus (Erk et al., 2010). Similarly, in a recent study, healthy carriers of CACNA1C risk allele showed lower activation of the right hippocampus during episodic memory encoding or retrieval (Krug et al., 2014). Notably, Erk et al., (2014) showed that during an episodic memory task there was a significant reduction of hippocampal activation in healthy first-degree relatives of CACNA1C risk allele carriers with major depression or bipolar disorder, indicating a possible familial risk. Moreover, Paulus et al., (2014) using functional magnetic resonance imaging to measure neural activation during a working memory task, reported a positive association of fronto-hippocampal connectivity in healthy risk allele carriers. This same SNP has also been associated with differences in expression of CACNA1C in both human postmortem brain and in induced neurons (Bigos et al., 2010; Gershon et al., 2014; Roussos et al., 2014; Yoshimizu et al., 2014), though the direction of effect and functional relevance has been reported to be both increased and decreased depending upon the study and brain region assessed. Together, these studies support the CACNA1C gene as a key modulator of hippocampal-related learning and memory processes. However, the effects of rs1006737 on age-associated learning in humans have yet to be studied.
Our data provide evidence that decreased Cacna1c expression has a protective role in the modulation of age-related cognitive declines and support an interaction between Cacna1c, sex, and memory impairment. Overall, our data provide additional support for the ‘calcium hypothesis’ of aging (Khachaturian, 1987; Landfield, 1987).
ACKNOWLEDGEMENTS
This study was supported by grant MH093967 to TDG. The sponsors had no involvement in the design of the study and in the collection, analysis and interpretation of the data, nor in the writing of the manuscript and the decision to submit this article for publication. We thank Nikolai Soldatov for comments on the manuscript.
Footnotes
FINANCIAL DISCLOSURES
P. Zanos, S. Bhat, R.J. Smith, C.E. Terrillion, L.H. Tonelli, and T.D. Gould declare no conflict of interest and derive no financial interest from this research.
REFERENCES
- Andreescu C, Gross JJ, Lenze E, Edelman KD, Snyder S, Tanase C, Aizenstein H. Altered cerebral blood flow patterns associated with pathologic worry in the elderly. Depress Anxiety. 2011;28:202–209. doi: 10.1002/da.20799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendash GW, King DL, Gordon MN, Morgan D, Hatcher JM, Hope CE, Diamond DM. Progressive, age-related behavioral impairments in transgenic mice carrying both mutant amyloid precursor protein and presenilin-1 transgenes. Brain Res. 2001;891:42–53. doi: 10.1016/s0006-8993(00)03186-3. [DOI] [PubMed] [Google Scholar]
- Balderas I, Rodriguez-Ortiz CJ, Salgado-Tonda P, Chavez-Hurtado J, McGaugh JL, Bermudez-Rattoni F. The consolidation of object and context recognition memory involve different regions of the temporal lobe. Learn Mem. 2008;15:618–624. doi: 10.1101/lm.1028008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban TA, Morey L, Aguglia E, Azzarelli O, Balsano F, Marigliano V, Caglieris N, Sterlicchio M, Capurso A, Tomasi NA, et al. Nimodipine in the treatment of old age dementias. Prog Neuropsychopharmacol Biol Psychiatry. 1990;14:525–551. doi: 10.1016/0278-5846(90)90005-2. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Niewoehner B, Lyon L, Romberg C, Schmitt WB, Taylor A, Sanderson DJ, Cottam J, Sprengel R, Seeburg PH, Kohr G, Rawlins JN. NMDA receptor subunit NR2A is required for rapidly acquired spatial working memory but not incremental spatial reference memory. J Neurosci. 2008;28:3623–3630. doi: 10.1523/JNEUROSCI.3639-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batuecas A, Pereira R, Centeno C, Pulido JA, Hernandez M, Bollati A, Bogonez E, Satrustegui J. Effects of chronic nimodipine on working memory of old rats in relation to defects in synaptosomal calcium homeostasis. Eur J Pharmacol. 1998;350:141–150. doi: 10.1016/s0014-2999(98)00250-7. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nat Protoc. 2006;1:1306–1311. doi: 10.1038/nprot.2006.205. [DOI] [PubMed] [Google Scholar]
- Bhat S, Dao DT, Terrillion CE, Arad M, Smith RJ, Soldatov NM, Gould TD. CACNA1C (Cav1.2) in the pathophysiology of psychiatric disease. Prog Neurobiol. 2012;99:1–14. doi: 10.1016/j.pneurobio.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigos KL, Mattay VS, Callicott JH, Straub RE, Vakkalanka R, Kolachana B, Hyde TM, Lipska BK, Kleinman JE, Weinberger DR. Genetic variation in CACNA1C affects brain circuitries related to mental illness. Arch Gen Psychiatry. 2010;67:939–945. doi: 10.1001/archgenpsychiatry.2010.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blalock EM, Chen KC, Sharrow K, Herman JP, Porter NM, Foster TC, Landfield PW. Gene microarrays in hippocampal aging: statistical profiling identifies novel processes correlated with cognitive impairment. J Neurosci. 2003;23:3807–3819. doi: 10.1523/JNEUROSCI.23-09-03807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer LD, Dowling AL, Curran-Rauhut MA, Landfield PW, Porter NM, Blalock EM. Estradiol reverses a calcium-related biomarker of brain aging in female rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:6058–6067. doi: 10.1523/JNEUROSCI.5253-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci U S A. 2004;101:14515–14520. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Wallace JL, Nematollahi S, Uprety AR, Barnes CA. Pattern separation deficits may contribute to age-associated recognition impairments. Behav Neurosci. 2010;124:559–573. doi: 10.1037/a0020893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell LW, Hao SY, Thibault O, Blalock EM, Landfield PW. Aging changes in voltage-gated calcium currents in hippocampal CA1 neurons. J Neurosci. 1996;16:6286–6295. doi: 10.1523/JNEUROSCI.16-19-06286.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KC, Blalock EM, Thibault O, Kaminker P, Landfield PW. Expression of alpha 1D subunit mRNA is correlated with L-type Ca2+ channel activity in single neurons of hippocampal “zipper” slices. Proc Natl Acad Sci U S A. 2000;97:4357–4362. doi: 10.1073/pnas.070056097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements MP, Rose SP, Tiunova A. omega-Conotoxin GVIA disrupts memory formation in the day-old chick. Neurobiol Learn Mem. 1995;64:276–284. doi: 10.1006/nlme.1995.0010. [DOI] [PubMed] [Google Scholar]
- Coburn-Litvak PS, Pothakos K, Tata DA, McCloskey DP, Anderson BJ. Chronic administration of corticosterone impairs spatial reference memory before spatial working memory in rats. Neurobiol Learn Mem. 2003;80:11–23. doi: 10.1016/s1074-7427(03)00019-4. [DOI] [PubMed] [Google Scholar]
- Dao DT, Mahon PB, Cai X, Kovacsics CE, Blackwell RA, Arad M, Shi J, Zandi PP, O'Donnell P, Knowles JA, Weissman MM, Coryell W, Scheftner WA, Lawson WB, Levinson DF, Thompson SM, Potash JB, Gould TD. Mood disorder susceptibility gene CACNA1C modifies mood-related behaviors in mice and interacts with sex to influence behavior in mice and diagnosis in humans. Biol Psychiatry. 2010;68:801–810. doi: 10.1016/j.biopsych.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davare MA, Hell JW. Increased phosphorylation of the neuronal L-type Ca(2+) channel Ca(v)1.2 during aging. Proc Natl Acad Sci U S A. 2003;100:16018–16023. doi: 10.1073/pnas.2236970100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyo R, Panksepp J, Conner RL. Nimodipine alters acquisition of a visual discrimination task in chicks. Behav Neural Biol. 1990;53:149–152. doi: 10.1016/0163-1047(90)90339-8. [DOI] [PubMed] [Google Scholar]
- Deyo RA, Nix DA, Parker TW. Nifedipine blocks retention of a visual discrimination task in chicks. Behav Neural Biol. 1992;57:260–262. doi: 10.1016/0163-1047(92)90262-3. [DOI] [PubMed] [Google Scholar]
- Deyo RA, Straube KT, Disterhoft JF. Nimodipine facilitates associative learning in aging rabbits. Science. 1989a;243:809–811. doi: 10.1126/science.2916127. [DOI] [PubMed] [Google Scholar]
- Deyo RA, Straube KT, Moyer JR, Jr., Disterhoft JF. Nimodipine ameliorates aging-related changes in open-field behaviors of the rabbit. Exp Aging Res. 1989b;15:169–175. doi: 10.1080/03610738908259771. [DOI] [PubMed] [Google Scholar]
- Dietsche B, Backes H, Laneri D, Weikert T, Witt SH, Rietschel M, Sommer J, Kircher T, Krug A. The impact of a CACNA1C gene polymorphism on learning and hippocampal formation in healthy individuals: a diffusion tensor imaging study. Neuroimage. 2014;89:256–261. doi: 10.1016/j.neuroimage.2013.11.030. [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Moyer JR, Jr., Thompson LT. The calcium rationale in aging and Alzheimer's disease. Evidence from an animal model of normal aging. Ann N Y Acad Sci. 1994;747:382–406. doi: 10.1111/j.1749-6632.1994.tb44424.x. [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Moyer JR, Jr., Thompson LT, Kowalska M. Functional aspects of calcium-channel modulation. Clin Neuropharmacol. 1993;16(Suppl 1):S12–24. doi: 10.1097/00002826-199316001-00003. [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Wu WW, Ohno M. Biophysical alterations of hippocampal pyramidal neurons in learning, ageing and Alzheimer's disease. Ageing Res Rev. 2004;3:383–406. doi: 10.1016/j.arr.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Dudchenko PA. An overview of the tasks used to test working memory in rodents. Neurosci Biobehav Rev. 2004;28:699–709. doi: 10.1016/j.neubiorev.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Erk S, Meyer-Lindenberg A, Schmierer P, Mohnke S, Grimm O, Garbusow M, Haddad L, Poehland L, Muhleisen TW, Witt SH, Tost H, Kirsch P, Romanczuk-Seiferth N, Schott BH, Cichon S, Nothen MM, Rietschel M, Heinz A, Walter H. Hippocampal and frontolimbic function as intermediate phenotype for psychosis: evidence from healthy relatives and a common risk variant in CACNA1C. Biol Psychiatry. 2014;76:466–475. doi: 10.1016/j.biopsych.2013.11.025. [DOI] [PubMed] [Google Scholar]
- Erk S, Meyer-Lindenberg A, Schnell K, Opitz von Boberfeld C, Esslinger C, Kirsch P, Grimm O, Arnold C, Haddad L, Witt SH, Cichon S, Nothen MM, Rietschel M, Walter H. Brain function in carriers of a genome-wide supported bipolar disorder variant. Arch Gen Psychiatry. 2010;67:803–811. doi: 10.1001/archgenpsychiatry.2010.94. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore M, Triaca V, Amendola T, Tirassa P, Aloe L. Brain NGF and EGF administration improves passive avoidance response and stimulates brain precursor cells in aged male mice. Physiol Behav. 2002;77:437–443. doi: 10.1016/s0031-9384(02)00875-2. [DOI] [PubMed] [Google Scholar]
- Gershon ES, Grennan K, Busnello J, Badner JA, Ovsiew F, Memon S, Alliey-Rodriguez N, Cooper J, Romanos B, Liu C. A rare mutation of CACNA1C in a patient with bipolar disorder, and decreased gene expression associated with a bipolar-associated common SNP of CACNA1C in brain. Mol Psychiatry. 2014;19:890–894. doi: 10.1038/mp.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson GE, Peterson C. Calcium and the aging nervous system. Neurobiol Aging. 1987;8:329–343. doi: 10.1016/0197-4580(87)90072-8. [DOI] [PubMed] [Google Scholar]
- Gotz J, Ittner LM. Animal models of Alzheimer's disease and frontotemporal dementia. Nat Rev Neurosci. 2008;9:532–544. doi: 10.1038/nrn2420. [DOI] [PubMed] [Google Scholar]
- Gur RE, Gur RC. Gender differences in aging: cognition, emotions, and neuroimaging studies. Dialogues Clin Neurosci. 2002;4:197–210. doi: 10.31887/DCNS.2002.4.2/rgur. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajszan T, Milner TA, Leranth C. Sex steroids and the dentate gyrus. Prog Brain Res. 2007;163:399–415. doi: 10.1016/S0079-6123(07)63023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck A, Fastenrath M, Ackermann S, Auschra B, Bickel H, Coynel D, Gschwind L, Jessen F, Kaduszkiewicz H, Maier W, Milnik A, Pentzek M, Riedel-Heller SG, Ripke S, Spalek K, Sullivan P, Vogler C, Wagner M, Weyerer S, Wolfsgruber S, de Quervain DJ, Papassotiropoulos A. Converging genetic and functional brain imaging evidence links neuronal excitability to working memory, psychiatric disease, and brain activity. Neuron. 2014;81:1203–1213. doi: 10.1016/j.neuron.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Hell JW, Westenbroek RE, Warner C, Ahlijanian MK, Prystay W, Gilbert MM, Snutch TP, Catterall WA. Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel alpha 1 subunits. J Cell Biol. 1993;123:949–962. doi: 10.1083/jcb.123.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Chen KC, Booze R, Landfield PW. Up-regulation of alpha1D Ca2+ channel subunit mRNA expression in the hippocampus of aged F344 rats. Neurobiol Aging. 1998;19:581–587. doi: 10.1016/s0197-4580(98)00099-2. [DOI] [PubMed] [Google Scholar]
- Hopp SC, D'Angelo HM, Royer SE, Kaercher RM, Adzovic L, Wenk GL. Differential rescue of spatial memory deficits in aged rats by L-type voltage-dependent calcium channel and ryanodine receptor antagonism. Neuroscience. 2014;280:10–18. doi: 10.1016/j.neuroscience.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram DK, Joseph JA, Spangler EL, Roberts D, Hengemihle J, Fanelli RJ. Chronic nimodipine treatment in aged rats: analysis of motor and cognitive effects and muscarinic-induced striatal dopamine release. Neurobiol Aging. 1994;15:55–61. doi: 10.1016/0197-4580(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, da Cunha C, Rosat R, Jerusalinsky D, Ferreira MB, Medina JH. Neurotransmitter receptors involved in post-training memory processing by the amygdala, medial septum, and hippocampus of the rat. Behav Neural Biol. 1992;58:16–26. doi: 10.1016/0163-1047(92)90847-w. [DOI] [PubMed] [Google Scholar]
- Kadish I, Thibault O, Blalock EM, Chen KC, Gant JC, Porter NM, Landfield PW. Hippocampal and cognitive aging across the lifespan: a bioenergetic shift precedes and increased cholesterol trafficking parallels memory impairment. J Neurosci. 2009;29:1805–1816. doi: 10.1523/JNEUROSCI.4599-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr DS, Abraham WC. Comparison of associative and non-associative conditioning procedures in the induction of LTD in CA1 of the hippocampus. Synapse. 1993;14:305–313. doi: 10.1002/syn.890140408. [DOI] [PubMed] [Google Scholar]
- Khachaturian ZS. Hypothesis on the regulation of cytosol calcium concentration and the aging brain. Neurobiol Aging. 1987;8:345–346. doi: 10.1016/0197-4580(87)90073-x. [DOI] [PubMed] [Google Scholar]
- Kim R, Moki R, Kida S. Molecular mechanisms for the destabilization and restabilization of reactivated spatial memory in the Morris water maze. Mol Brain. 2011;4:9. doi: 10.1186/1756-6606-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauber J, Muller WE. Subchronic treatment with prazosin improves passive avoidance learning in aged mice: possible relationships to alpha1-receptor up-regulation. J Neural Transm. 2000;107:1413–1426. doi: 10.1007/s007020070005. [DOI] [PubMed] [Google Scholar]
- Koressaar T, Remm M. Enhancements and modifications of primer design program Primer3. Bioinformatics. 2007;23:1289–1291. doi: 10.1093/bioinformatics/btm091. [DOI] [PubMed] [Google Scholar]
- Kowalska M, Disterhoft JF. Relation of nimodipine dose and serum concentration to learning enhancement in aging rabbits. Exp Neurol. 1994;127:159–166. doi: 10.1006/exnr.1994.1090. [DOI] [PubMed] [Google Scholar]
- Krug A, Witt SH, Backes H, Dietsche B, Nieratschker V, Shah NJ, Nothen MM, Rietschel M, Kircher T. A genome-wide supported variant in CACNA1C influences hippocampal activation during episodic memory encoding and retrieval. Eur Arch Psychiatry Clin Neurosci. 2014;264:103–110. doi: 10.1007/s00406-013-0428-x. [DOI] [PubMed] [Google Scholar]
- Landfield PW. 'Increased calcium-current' hypothesis of brain aging. Neurobiol Aging. 1987;8:346–347. doi: 10.1016/0197-4580(87)90074-1. [DOI] [PubMed] [Google Scholar]
- Landfield PW. Increased hippocampal Ca2+ channel activity in brain aging and dementia. Hormonal and pharmacologic modulation. Ann N Y Acad Sci. 1994;747:351–364. doi: 10.1111/j.1749-6632.1994.tb44422.x. [DOI] [PubMed] [Google Scholar]
- Landfield PW, Pitler TA. Prolonged Ca2+-dependent afterhyperpolarizations in hippocampal neurons of aged rats. Science. 1984;226:1089–1092. doi: 10.1126/science.6494926. [DOI] [PubMed] [Google Scholar]
- Levere TE, Walker A. Old age and cognition: enhancement of recent memory in aged rats by the calcium channel blocker nimodipine. Neurobiol Aging. 1992;13:63–66. doi: 10.1016/0197-4580(92)90010-u. [DOI] [PubMed] [Google Scholar]
- Levy A, Kong RM, Stillman MJ, Shukitt-Hale B, Kadar T, Rauch TM, Lieberman HR. Nimodipine improves spatial working memory and elevates hippocampal acetylcholine in young rats. Pharmacol Biochem Behav. 1991;39:781–786. doi: 10.1016/0091-3057(91)90164-w. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luszczki JJ, Wojcik-Cwikla J, Andres MM, Czuczwar SJ. Pharmacological and behavioral characteristics of interactions between vigabatrin and conventional antiepileptic drugs in pentylenetetrazole-induced seizures in mice: an isobolographic analysis. Neuropsychopharmacology. 2005;30:958–973. doi: 10.1038/sj.npp.1300602. [DOI] [PubMed] [Google Scholar]
- Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nat Rev Neurosci. 2006;7:278–294. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Hormones as regulators of brain development: life-long effects related to health and disease. Acta Paediatr Suppl. 1997;422:41–44. doi: 10.1111/j.1651-2227.1997.tb18343.x. [DOI] [PubMed] [Google Scholar]
- Moore SJ, Deshpande K, Stinnett GS, Seasholtz AF, Murphy GG. Conversion of short-term to long-term memory in the novel object recognition paradigm. Neurobiol Learn Mem. 2013;105:174–185. doi: 10.1016/j.nlm.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer JR, Jr., Thompson LT, Black JP, Disterhoft JF. Nimodipine increases excitability of rabbit CA1 pyramidal neurons in an age-and concentration-dependent manner. J Neurophysiol. 1992;68:2100–2109. doi: 10.1152/jn.1992.68.6.2100. [DOI] [PubMed] [Google Scholar]
- Norris CM, Halpain S, Foster TC. Alterations in the balance of protein kinase/phosphatase activities parallel reduced synaptic strength during aging. J Neurophysiol. 1998a;80:1567–1570. doi: 10.1152/jn.1998.80.3.1567. [DOI] [PubMed] [Google Scholar]
- Norris CM, Halpain S, Foster TC. Reversal of age-related alterations in synaptic plasticity by blockade of L-type Ca2+ channels. J Neurosci. 1998b;18:3171–3179. doi: 10.1523/JNEUROSCI.18-09-03171.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez-Santana FL, Oh MM, Antion MD, Lee A, Hell JW, Disterhoft JF. Surface L-type Ca2+ channel expression levels are increased in aged hippocampus. Aging Cell. 2014;13:111–120. doi: 10.1111/acel.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien N, Lehmann H, Lecluse V, Mumby DG. Enhanced context-dependency of object recognition in rats with hippocampal lesions. Behav Brain Res. 2006;170:156–162. doi: 10.1016/j.bbr.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Ogren SO. Evidence for a role of brain serotonergic neurotransmission in avoidance learning. Acta Physiol Scand Suppl. 1985;544:1–71. [PubMed] [Google Scholar]
- Paulus FM, Bedenbender J, Krach S, Pyka M, Krug A, Sommer J, Mette M, Nothen MM, Witt SH, Rietschel M, Kircher T, Jansen A. Association of rs1006737 in CACNA1C with alterations in prefrontal activation and fronto-hippocampal connectivity. Hum Brain Mapp. 2014;35:1190–1200. doi: 10.1002/hbm.22244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitler TA, Landfield PW. Aging-related prolongation of calcium spike duration in rat hippocampal slice neurons. Brain Res. 1990;508:1–6. doi: 10.1016/0006-8993(90)91109-t. [DOI] [PubMed] [Google Scholar]
- Porter NM, Thibault O, Thibault V, Chen KC, Landfield PW. Calcium channel density and hippocampal cell death with age in long-term culture. J Neurosci. 1997;17:5629–5639. doi: 10.1523/JNEUROSCI.17-14-05629.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartermain D, Hawxhurst A, Ermita B, Puente J. Effect of the calcium channel blocker amlodipine on memory in mice. Behav Neural Biol. 1993;60:211–219. doi: 10.1016/0163-1047(93)90390-4. [DOI] [PubMed] [Google Scholar]
- Quevedo J, Vianna M, Daroit D, Born AG, Kuyven CR, Roesler R, Quillfeldt JA. L-type voltage-dependent calcium channel blocker nifedipine enhances memory retention when infused into the hippocampus. Neurobiol Learn Mem. 1998;69:320–325. doi: 10.1006/nlme.1998.3822. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon FM, Head D, Dupuis JH, Acker JD. Neuroanatomical correlates of cognitive aging: evidence from structural magnetic resonance imaging. Neuropsychology. 1998;12:95–114. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- Riekkinen M, Schmidt B, Kuitunen J, Riekkinen P., Jr. Effects of combined chronic nimodipine and acute metrifonate treatment on spatial and avoidance behavior. Eur J Pharmacol. 1997;322:1–9. doi: 10.1016/s0014-2999(96)00976-4. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Waters EM, McEwen BS. Steroid-induced hippocampal synaptic plasticity: sex differences and similarities. Neuron Glia Biol. 2004;1:219–229. doi: 10.1017/S1740925X05000086. [DOI] [PubMed] [Google Scholar]
- Rose GM, Ong VS, Woodruff-Pak DS. Efficacy of MEM 1003, a novel calcium channel blocker, in delay and trace eyeblink conditioning in older rabbits. Neurobiol Aging. 2007;28:766–773. doi: 10.1016/j.neurobiolaging.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Roussos P, Mitchell AC, Voloudakis G, Fullard JF, Pothula VM, Tsang J, Stahl EA, Georgakopoulos A, Ruderfer DM, Charney A, Okada Y, Siminovitch KA, Worthington J, Padyukov L, Klareskog L, Gregersen PK, Plenge RM, Raychaudhuri S, Fromer M, Purcell SM, Brennand KJ, Robakis NK, Schadt EE, Akbarian S, Sklar P. A role for noncoding variation in schizophrenia. Cell Rep. 2014;9:1417–1429. doi: 10.1016/j.celrep.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe WB, Blalock EM, Chen KC, Kadish I, Wang D, Barrett JE, Thibault O, Porter NM, Rose GM, Landfield PW. Hippocampal expression analyses reveal selective association of immediate-early, neuroenergetic, and myelinogenic pathways with cognitive impairment in aged rats. J Neurosci. 2007;27:3098–3110. doi: 10.1523/JNEUROSCI.4163-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandin M, Jasmin S, Levere TE. Aging and cognition: facilitation of recent memory in aged nonhuman primates by nimodipine. Neurobiol Aging. 1990;11:573–575. doi: 10.1016/0197-4580(90)90120-o. [DOI] [PubMed] [Google Scholar]
- Sarkar SN, Huang RQ, Logan SM, Yi KD, Dillon GH, Simpkins JW. Estrogens directly potentiate neuronal L-type Ca2+ channels. Proc Natl Acad Sci U S A. 2008;105:15148–15153. doi: 10.1073/pnas.0802379105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnyai Z, Sibille EL, Pavlides C, Fenster RJ, McEwen BS, Toth M. Impaired hippocampal-dependent learning and functional abnormalities in the hippocampus in mice lacking serotonin(1A) receptors. Proc Natl Acad Sci U S A. 2000;97:14731–14736. doi: 10.1073/pnas.97.26.14731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipton OA, El-Gaby M, Apergis-Schoute J, Deisseroth K, Bannerman DM, Paulsen O, Kohl MM. Left-right dissociation of hippocampal memory processes in mice. Proc Natl Acad Sci U S A. 2014;111:15238–15243. doi: 10.1073/pnas.1405648111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnegger-Brauns MJ, Huber IG, Koschak A, Wild C, Obermair GJ, Einzinger U, Hoda JC, Sartori SB, Striessnig J. Expression and 1,4-dihydropyridine-binding properties of brain L-type calcium channel isoforms. Mol Pharmacol. 2009;75:407–414. doi: 10.1124/mol.108.049981. [DOI] [PubMed] [Google Scholar]
- Soldatov NM. Genomic structure of human L-type Ca2+ channel. Genomics. 1994;22:77–87. doi: 10.1006/geno.1994.1347. [DOI] [PubMed] [Google Scholar]
- Solomon PR, Wood MS, Groccia-Ellison ME, Yang BY, Fanelli RJ, Mervis RF. Nimodipine facilitates retention of the classically conditioned nictitating membrane response in aged rabbits over long retention intervals. Neurobiol Aging. 1995;16:791–796. doi: 10.1016/0197-4580(95)00093-t. [DOI] [PubMed] [Google Scholar]
- Thibault O, Hadley R, Landfield PW. Elevated postsynaptic [Ca2+]i and L-type calcium channel activity in aged hippocampal neurons: relationship to impaired synaptic plasticity. J Neurosci. 2001;21:9744–9756. doi: 10.1523/JNEUROSCI.21-24-09744.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault O, Landfield PW. Increase in single L-type calcium channels in hippocampal neurons during aging. Science. 1996;272:1017–1020. doi: 10.1126/science.272.5264.1017. [DOI] [PubMed] [Google Scholar]
- Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG. Primer3--new capabilities and interfaces. Nucleic Acids Res. 2012;40:e115. doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veng LM, Browning MD. Regionally selective alterations in expression of the alpha(1D) subunit (Ca(v)1.3) of L-type calcium channels in the hippocampus of aged rats. Brain Res Mol Brain Res. 2002;107:120–127. doi: 10.1016/s0169-328x(02)00453-9. [DOI] [PubMed] [Google Scholar]
- Veng LM, Mesches MH, Browning MD. Age-related working memory impairment is correlated with increases in the L-type calcium channel protein alpha1D (Cav1.3) in area CA1 of the hippocampus and both are ameliorated by chronic nimodipine treatment. Brain Res Mol Brain Res. 2003;110:193–202. doi: 10.1016/s0169-328x(02)00643-5. [DOI] [PubMed] [Google Scholar]
- Wang Y, Mattson MP. L-type Ca2+ currents at CA1 synapses, but not CA3 or dentate granule neuron synapses, are increased in 3xTgAD mice in an age-dependent manner. Neurobiol Aging. 2014;35:88–95. doi: 10.1016/j.neurobiolaging.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Chi J, Li YT, Pak MH, Fanelli RJ. Nimodipine ameliorates impaired eyeblink classical conditioning in older rabbits in the long-delay paradigm. Neurobiol Aging. 1997;18:641–649. doi: 10.1016/s0197-4580(97)00159-0. [DOI] [PubMed] [Google Scholar]
- Yamada K, Santo-Yamada Y, Wada K. Stress-induced impairment of inhibitory avoidance learning in female neuromedin B receptor-deficient mice. Physiol Behav. 2003;78:303–309. doi: 10.1016/s0031-9384(02)00979-4. [DOI] [PubMed] [Google Scholar]
- Yoshimizu T, Pan JQ, Mungenast AE, Madison JM, Su S, Ketterman J, Ongur D, McPhie D, Cohen B, Perlis R, Tsai LH. Functional implications of a psychiatric risk variant within CACNA1C in induced human neurons. Mol Psychiatry. 2014 doi: 10.1038/mp.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanotti A, Valzelli L, Toffano G. Chronic phosphatidylserine treatment improves spatial memory and passive avoidance in aged rats. Psychopharmacology (Berl) 1989;99:316–321. doi: 10.1007/BF00445550. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Shen Q, Xu Z, Chen M, Cheng L, Zhai J, Gu H, Bao X, Chen X, Wang K, Deng X, Ji F, Liu C, Li J, Dong Q, Chen C. The effects of CACNA1C gene polymorphism on spatial working memory in both healthy controls and patients with schizophrenia or bipolar disorder. Neuropsychopharmacology. 2012;37:677–684. doi: 10.1038/npp.2011.242. [DOI] [PMC free article] [PubMed] [Google Scholar]