Abstract
Introduction
As many as 30% of SCS patients fail to obtain long term pain coverage, even with the strictest parameters of a successful trial, unremarkable psychological assessment, and ideal placement of the permanent device. Why these patients either never receive adequate benefit or lose benefit remains elusive.
Methods
We perform a retrospective review of our prospective database of SCS patients undergoing surgery for routine indications. Six month post-operative follow-up data was available for 57 patients. Two providers who routinely saw the patients were asked to independently grade the patient’s outcome in a blinded fashion on a Global Outcome Ratings scale of 1 to 10, with 5 being 50% improvement at six months post-operation. A score of less than 5 was deemed a failure. The impact of Body Mass Index (BMI), random drug screen results, workers’ compensation status, depression, and smoking were assessed.
Results
We report a phi correlation of 0.350 between smoking and failure (p=0.017). Smoking status is correlated with both lead migration revisions (phi =0.269) (p=0.044) and with revision due to new pain symptoms (phi=0.241) (p=0.072). Further, there is a trend of correlation (phi=0.289) between drug use and patients (n=3) who underwent device removal (p=0.045). In this cohort, worker’s compensation status, BMI, and depression did not impact outcome.
Conclusions
Tobacco use correlates with less success with SCS at 6-month follow-up. Whether that is because of issues with healing and our transmission of signals to the periphery warrants further exploration. This data provide further evidence that tobacco cessation is important to surgical results.
Keywords: spinal cord stimulation, tobacco, illegal drug use
Introduction
Chronic pain is a debilitating biological and psychological condition that affects nearly one third of the American population with an annual cost of $560 to $635 billion from associated health care costs and lost productivity (1). The crisis of prescription opioid drug overmedication has driven many pain physicians to seek alternative treatment modalities, such as spinal cord stimulation. Spinal cord stimulation (SCS) is an invasive technique used to provide relief of certain types of chronic pain when medical therapies fail. SCS results in meaningful pain relief for fifty to seventy-percent of well-selected patients and has minor rates of complications (2). Additionally SCS improves quality of life (3). However, while SCS can provide significant benefit for many patients, failures occur. These can include lead migration, onset of new pain symptoms, and other complaints causing patients to request lead explantation.
Previous studies have identified non-modifiable risk factors. Researchers have found a strong relationship between psychiatric co-morbidities and a poor response to SCS treatment (3). In one systematic review, 92.0% of studies exhibited a positive relationship between one or more psychological factors and poor treatment outcome (3). Evidence also suggests that longer pain duration prior to intervention was predictive of poorer outcomes (5). Additionally, SCS has been more successful in patients whose pain did not follow a surgical procedure than in patients who had multiple surgical procedures prior to their first implant (6). In patients who did have previous surgical procedures, a greater success rate was found when the duration of time to implantation was shorter (7). While non-modifiable risk factors are helpful in selecting SCS candidates and minimizing complications, data regarding modifiable risk factors may further add to this growing body of literature. In this study, we primarily consider modifiable factors that may lead to poor SCS outcomes presenting within 6 months of operation..
Methods
All patients who were undergoing SCS surgery were offered participation in this IRB-approved study. We prospectively collect data from all SCS patients. This study includes data from all consented patients who reached six-month follow up. Two providers who routinely saw the patients were asked to independently grade the patient’s outcome in a blinded fashion on a Global Outcome Ratings scale of 1 to 10, with 5 being 50% improvement at six months post-operation. This rating is determined based on patient’s post-operative care visit at 6 months and takes into consideration patient’s level of pain, physical mobility and functional capacity, and overall satisfaction. This scale offers an overall rating of the patient’s surgical outcome. A score less than 5 was deemed a failure, based on the targeted 50% improvement with this therapy. Patients who required explantation for reasons other than infection were also considered failures. We report two infections following surgery. Demographic information, explantation, and revision statuses were also recorded.
The impact of body mass index (BMI), smoking status, illegal drug use, psychiatric factors, workman’s compensation status, past surgical history, and age was assessed were retrospectively assessed through examination of the history, physical, and pre-operative psychological evaluation. A urine drug screen was obtained during pre-operative evaluation only. Analysis of collected data was performed using correlation analyses.
Results
Demographics of patients are included in Table 1. At six month follow of 57 patients, 5 failures were documented with 3 devices explanted. This excludes 2 patients who underwent removal due to infection. Six devices necessitated revision- 4 secondary to the development of new pain (N=2 at internal pulse generator site, and N=2 in the region being treated due to the device) and 2 due to lost coverage after migration. Analysis reveals a significant correlation between smoking status and recreational drug use on patient outcomes.
Table 1.
Patient demographics including surgery indication and lead location where failure is based on Global Outcome Ratings Scale of less than 5 and success is greater than 5.
| FAILURE | SUCCESS | ||
|---|---|---|---|
| AGE | Mean +Range | 47.8+ 25.0 | 51.2 + 53 |
| GENDER | Male: Female | 3:7 | 20:27 |
| LEAD LOCATION | Cervical % | 50% | 13.5% |
| Thoracic% | 50% | 78.4% | |
| Hybrid% | 0% | 8.1% | |
| INDICATION | FBSS % | 60% | 37.8% |
| CRPS % | 20% | 37.8% | |
| Neuritis % | 20% | 24.3% |
Our cohort consisted of 20 smokers and 3 drug users. In smokers, 2 patients required explantation, 2 required revision due to migration, and 3 required revision due to onset of new pain.
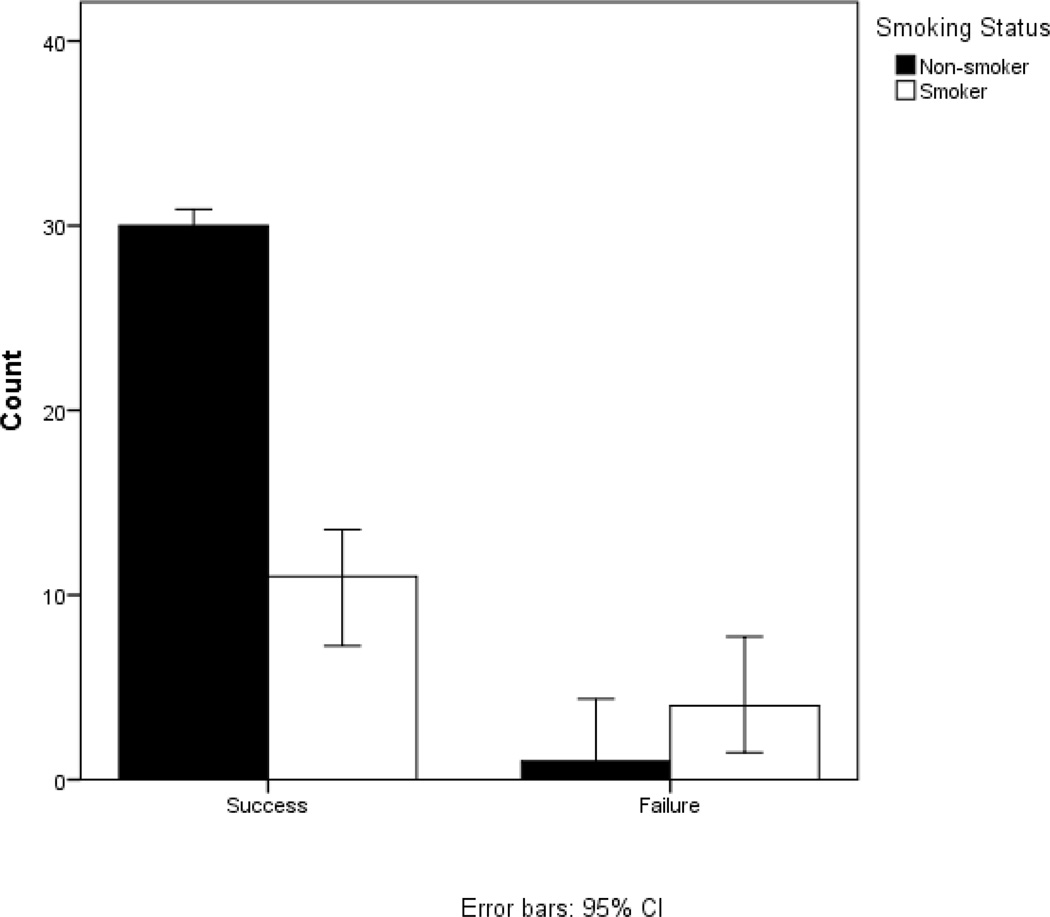
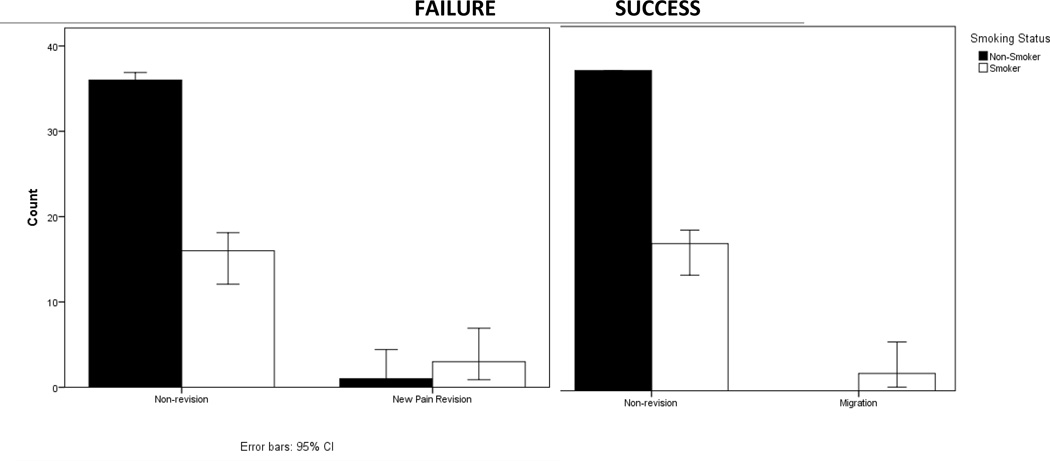
Smoking status has an impact on both scores of failure on the global outcomes rating scale and revision status. Smoking status is correlated with failure (global outcome rating scale <5) in patients where phi=0.353 (p=0.017) (Figure 1). Smoking status is correlated with both lead migration revisions (phi =0.269) (p=0.044) and with revision due to new pain symptoms (phi=0.241) (p=0.072) (Figure 2). We note a trend of smoking correlating with depression in our patient population (R=0.240) (p=0.075). We did not find this trend of depression in our drug users.
Figure 1.
Smokers compose 80% of the failure group and 33% of success group. Smoking status is correlated with failure (global outcome rating scale <5) in patients where phi=0.353 (p=0.017).
Figure 2.
Smokers compose only 31% of non-revision patients. They compose 100% of patients requiring revision due to migration and 75% of patients undergoing revision due to new pain symptoms. Smoking status is correlated with both lead migration revisions (phi =0.269) (p=0.044) and with revision due to new pain symptoms (phi=0.241) (p=0.072)
Analysis did not reveal any significant impact of workers’ compensation status, BMI, age, history of back surgery, or depression on SCS patient outcomes at six-month follow-up.
Discussion
In this study, we aim to determine the causes of early SCS failure, defined as poor response to treatment within 6 months of implant. We found that smoking and illegal drug use significantly correlated with early SCS failure. We note a trend of depression in smokers within our patient pool. BMI, psychiatric factors, WC status, past surgical history, and age did not. Patients’ illegal drug use in our study included marijuana or cocaine. Though we routinely obtain urine drug screens on all our patients, we have typically not precluded surgery for marijuana use. In the patient that used cocaine, UDS at the time of surgery was negative.
Physiological factors
It is unclear whether smoking and illegal drug use led to early failure for the same reasons. Tobacco use is known to impede wound healing and to have many negative physiological effects. This has been supported in many retrospective studies. Researchers found that wound contraction is enhanced in smokers and this may occur due to alterations in myofibroblast function (8). Also, many studies concluded that smoking attenuates epidermal regeneration and neovascularization (8). Smoking also has a temporary detrimental vasoactive effect on peripheral tissue blood flow, oxygenation, and aerobe metabolism (8). Healing is stunted, too, due to oxidative stress, which has been seen to affect the physiological, inflammatory, and proliferative healing response to smoking (8). Interestingly, smoking cessation will rapidly restore oxygen levels in the tissue but many other effects from smoking are irreversible. In conclusion, because smoking temporarily reduces tissue perfusion and oxygenation, impairs inflammatory cell functions and oxidative bactericidal mechanisms, and attenuates reparative cell functions including synthesis and deposition of collagen, smokers are at a greater risk for ineffective healing at the site of the lead and the pulse generator. It may also predispose to painful incisions (8).
Nicotine may specifically increase how painful smokers perceive stimuli. Though there is evidence of improved pain in non-smokers with spinal cord injury (SCI) pain, the impact on smokers is inconclusive. Musculoskeletal and neuropathic pain increase with nicotine exposure. Further, smoking has also been associated with increased levels of musculoskeletal pain in the limbs and backs of non-SCI patients (9).
The role of marijuana and cocaine on early failure is less clear. Marijuana has been linked with impaired neural connectivity in specific brain regions including the precuneus and fimbria, an area of the hippocampus (10). These brain areas are important because they are involved in functions that require a high degree of integration and are involved in learning and memory respectively and may alter how external stimuli are perceived. Cocaine directly affects the hypothalamus and affects the cerebral arteries, which can lead to vasoconstriction both in the brain and in the periphery (11) Studies suggest that cocaine users are at risk of experiencing decreased pain tolerance (12). Further investigation is needed into how drug use affects physiological factors associated with early SCS failure.
Psychological factors
Both drug use and smoking correlate with depression and anxiety, which are independent risk factors for poorer SCS outcomes (3). We noted a trend that our smokers had more depression. However, other factors are also likely to predispose patients dependent on substances to have difficulties with surgery. Marijuana, for instance impairs cognitive and motor functions and can lead to a lack of motivation and the inability to pay attention (10). Further, providing drug users with appropriate care is difficult because of sometimes aggressive and/or manipulative behaviors. Personality disorders are often comorbid (13).
When patients are in chronic pain, it is difficult to ask them to reduce euphoria producing substances that are often used to self-medicate pain and psychological issues. Further work needs to be done to identify the impact of additional counseling and psychiatric services in this patient population to reduce reliance on substances and improve SCS outcomes.
Anecdotal evidence from hospital-based tobacco cessation consult service reveals many patients are receptive to forming quit plans. Often, these patients have many prior failed quit attempts due to limited knowledge and training. A short, educational dialogue which includes strategies for behavioral modification and nicotine replacement therapy options frequently inspires these patients to renew their efforts to quit smoking and increases their chances for success. A three-minute physician dialogue yields a 10.2% statistically significant increase in cessation rates (14). However, while 89 % of surgical residents inquire about smoking status, only 38% feel prepared to counsel their patients (15). This suggests a need for continued tobacco cessation training for residents.
We acknowledge that our study is limited by sample size making our results suggestive rather than conclusive. Our hope is that this data may be used to further physician-patient dialogue and be helpful for future systemic reviews. Additionally, we rely on patients reporting smoking status to practitioner that may result in underreporting. Finally, drug screens are only performed before surgery and may not capture illegal drug use occurring during the recovery process.
Conclusion
Tobacco and recreational drug use correlates with early failure of SCS by 6 months. We suspect these substances lead to failure both due to psychological and physiological factors, which differ, based on substance. Further research is needed on the effects of marijuana and cocaine on wound healing, and if poor wound healing leads to less SCS success. Our findings allow physicians to better counsel patients regarding tobacco and drug cessation prior to surgery.
Footnotes
Conflicts of interest: Dr. Pilitsis is a consultant for Medtronic, Boston Scientific and St. Jude and receives grant support from Medtronic, Boston Scientific, St. Jude and NIH 1R01CA166379. All other authors have no conflict of interest or financial disclosures related directly to this manuscript.
Authorship Statement: Dr. Julie Pilitsis, Priscilla De La Cruz, Steven Roth, Steven Lange, Jessica Haller, Chris Fama, and Meghan Wilock designed and conducted the study including patient recruitment, data collection, and data analysis. Priscilla De La Cruz, Chris Fama, and Dr. Julie Pilitsis prepared the manuscript draft. All authors approved the final manuscript.
References
- 1.National Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. 2011 Retrieved from http://www.iom.edu/Reports/2011/Relieving-Pain-in-America-A-Blueprint-for-transforming-Prevention-Care-Education-Research.aspx. [PubMed]
- 2.Bernstein CA. Spinal cord stimulation for chronic pain. 2006 Jul 07; Retrieved from http://www.spine-health.com/treatment/back-surgery/spinal-cord-stimulation-chronic-pain.
- 3.Celestin J, Edwards RR, Jamison RN. Pretreatment psychosocial variables as predictors of outcomes following lumbar surgery and spinal cord stimulation: A systematic review and literature synthesis. Pain Medicine. 2009;10(4):639–653. doi: 10.1111/j.1526-4637.2009.00632.x. [DOI] [PubMed] [Google Scholar]
- 4.Schu S, Slotty PJ, Bara G, von Knop M, Edgar D, Vesper J. A Prospective, Randomised, Double-blind, Placebo-controlled Study to Examine the Effectiveness of Burst Spinal Cord Stimulation Patterns for the Treatment of Failed Back Surgery Syndrome. Neuromodulation: Technology at the Neural Interface. 2014 doi: 10.1111/ner.12197. [DOI] [PubMed] [Google Scholar]
- 5.Taylor RS, Desai MJ, Rigoard P. Predictors of pain relief following spinal cord stimulation in chronic back and leg pain and failed back surgery syndrome: A systematic review and meta-regression analysis. Wiley Periodicals Inc. 2013;2(1):1–17. doi: 10.1111/papr.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar K, Toth C, Rahul KN. Epidural spinal cord stimulation for treatment of chronic pain— some predictors of success. a 15-year experience. Elsevier Inc. 1998;50:110–121. doi: 10.1016/s0090-3019(98)00012-3. [DOI] [PubMed] [Google Scholar]
- 7.Taylor RS, Van Buyten JP, Buchser E. spinal cord stimulation for chronic back and leg pain and failed back surgery syndrome: A systematic review and analysis of prognostic factors. SPINE. 2004;30(1):152–160. doi: 10.1097/01.brs.0000149199.68381.fe. [DOI] [PubMed] [Google Scholar]
- 8.Soresnsen LT. Wound healing and infection in surgery: the pathophysiological impact of smoking, smoking cessation, and nicotine replacement therapy. Annals of Surgery. 2012;255(6):1069–1075. doi: 10.1097/SLA.0b013e31824f632d. [DOI] [PubMed] [Google Scholar]
- 9.Richardson EJ, Ness TJ, Redden DT, Stewart CC, Richards JS. Effects of Nicotine on Spinal Cord Injury Pain Vary Among Subtypes of Pain and Smoking Status: Results From a Randomized, Controlled Experiment. The Journal of Pain. 2012;13(12):1206–1214. doi: 10.1016/j.jpain.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Volkow ND, Baler RD, Compton WM. Adverse health effects of marijuana use. NEJM Knowledge. 2014 doi: 10.1056/NEJMra1402309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enevoldson TP. Recreational Drugs And Their Neurological Consequences. Journal of Neurology, Neurosurgery & Psychiatry. 2004;75(suppl_3):iii9–iii15. doi: 10.1136/jnnp.2004.045732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Compton MA. Cold-pressor pain tolerance in opiate and cocaine abusers: Correlates of drug type and use status. Journal of Pain and Symptom Management. 1994;9(7):462–473. doi: 10.1016/0885-3924(94)90203-8. [DOI] [PubMed] [Google Scholar]
- 13.Laroche F, Rostaing S, Aubrun F, Perrot S. Pain management in heroin and cocaine users. Joint Bone Spine. 2012;79(5):446–450. doi: 10.1016/j.jbspin.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Fiore MC, Jorenby DE, Schensky AE, et al. Smoking status as the new vital sign: Effect on assessment and intervention in patients who smoke. Mayo Clin Proc. 1995;70:209–213. doi: 10.4065/70.3.209. [DOI] [PubMed] [Google Scholar]
- 15.Krupski WC, Nguyen HT, Jones DN, Wallace H, Whitehill TA, Nehler MR. Smoking cessation counseling: a missed opportunity for general surgery trainees. J Vasc Surg. 2002;36(2):257–262. doi: 10.1067/mva.2002.125030. discussion 262. [DOI] [PubMed] [Google Scholar]