Abstract
Purpose
Urinary tract obstruction and reduced nephron number often occur together as a result of maldevelopment of kidneys and urinary tract. We wished to determine the role of nephron number on the adaptation of remaining nephrons of mice subjected to neonatal partial unilateral ureteral obstruction (UUO) and followed through adulthood.
Materials and Methods
Wild-type (WT) and Os/+ mice (with 50% fewer nephrons) were subjected to sham operation or partial UUO in the first 2 days of life. Additional mice underwent release of UUO at 7 days. All kidneys were harvested at 3 weeks (weaning) or 6 weeks (adulthood). Glomerular number and area, glomerulotubular junction integrity, proximal tubular volume fraction, and interstitial fibrosis were measured by histomorphometry.
Results
In the obstructed kidney, UUO caused additional nephron loss in Os/+ but not WT mice. Glomerular growth from 3 to 6 weeks was impaired by ipsilateral UUO and was not preserved by release in WT or Os/+. Proximal tubular growth was impaired and interstitial collagen was increased by ipsilateral UUO in all mice. These were attenuated by release of UUO in WT mice, but were not restored in Os/+ mice. UUO increased interstitial collagen in the contralateral kidney; release of UUO enhanced tubular growth and reduced interstitial collagen.
Conclusions
We conclude that UUO in early postnatal development impairs adaptation to reduced nephron number and induces additional nephron loss despite release of obstruction. Premature and low birth weight infants with congenital obstructive nephropathy are likely at increased risk for progression of chronic kidney disease.
Keywords: ureteral obstruction, growth and development, disease progression, nephrons
Introduction
Most pediatric chronic kidney disease (CKD) results from congenital anomalies of the kidneys and urinary tract.1 It is now recognized that of all patients developing renal failure as a result of congenital obstructive nephropathy, the majority are over 18 years of age.2 Brenner and his associates first demonstrated that reduction in nephron number accelerates the progression of renal disease by provoking a maladaptive response in the remaining nephrons, characterized by glomerular hypertrophy, hyperfiltration, and eventual sclerosis.3 Since then, the cellular and molecular basis for progression of CKD has undergone intense investigation, with emphasis on renal interstitial cellular infiltration and collagen matrix deposition.4 The animal model most widely used to elucidate the underlying mechanisms, complete unilateral ureteral obstruction (UUO), is characterized by widespread death of proximal tubular cells and formation of atubular glomeruli within 1-2 weeks.5,6 We have developed a technique to create variable partial, reversible ureteral obstruction in the mouse within the first 2 days of life, at which time nephrogenesis is incomplete.7 In this model, renal injury evolves from birth through to adulthood, and release of the obstruction allows recovery and remodeling of the renal parenchyma.7
Frequently associated with congenital renal anomalies, reduced nephron number at birth is an independent risk factor for adult CKD.8 We wished to determine the role of nephron number on the adaptation of remaining nephrons of mice subjected to neonatal partial UUO and followed through adulthood. In additional mice, the obstruction was released to examine the process of recovery and remodeling. Histomorphometry and immunohistochemical techniques were utilized to measure the effects of these variables on glomerular, tubular, and interstitial development and response to injury. The results reveal that congenital reduction in nephron number accelerates obstructive renal injury and impairs recovery when assessed in adults, despite early release of obstruction.
Materials and Methods
Experimental animals and surgical procedures
A total of 132 oligosyndactyly heterozygous (Os/+) mutant mice with reduced nephron number and their wild-type (WT) littermate controls were derived from B6.ROP/Le +/- and C57BL/6 breeding pairs (provided by Ashraf El-Meanawy, Milwaukee, WI). Sample sizes were based on prior studies utilizing this model.7 Pups were weaned at 21 days of age. The protocol was approved by the Institutional Animal Care and Use Committee of the University of Virginia. All surgical procedures were carried out using sterile technique under anesthesia with isoflurane and oxygen. Neonates were subjected to 0.2 mm diameter partial UUO or sham operation within the first 36 hours of life, and underwent either sham-operation or release of UUO 7 days later as previously described (Fig. 1).7,9
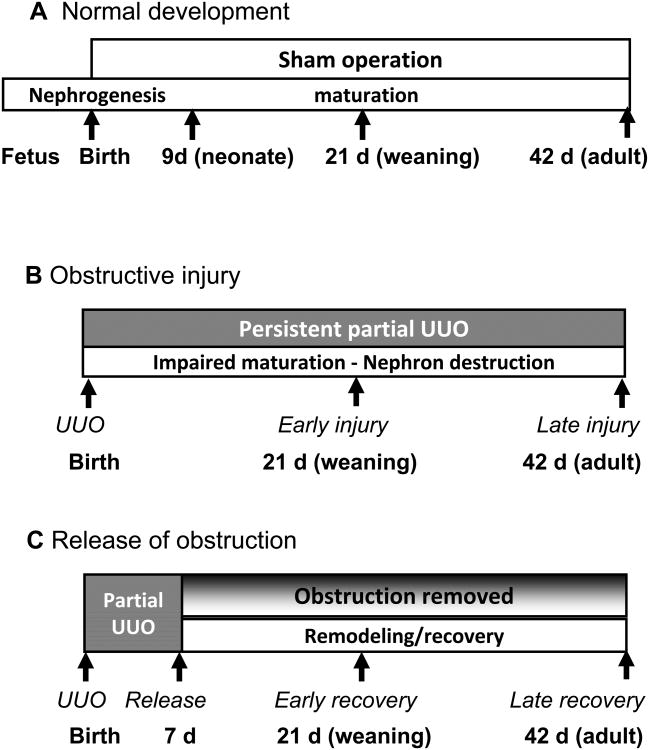
Figure 1. Experimental design.
Three groups of mice were studied; in each group, C57BL/6 wild-type mice were compared to Os/+ mice with a 50% congenital reduction in nephron number. Group A, normal renal development; Group B, obstructive injury; Group C, recovery from obstructive injury. Early recovery was determined at 21 days, and late recovery at 42 days of age.
As reported previously, plasma creatinine does not differ between Os/+ and +/+ mice or between uninephrectomized and normal mice of either strain.10 Because the right kidney remained intact and the left kidney was only partially obstructed in the present study, plasma creatinine was not measured. In 42 day-old mice (n = 17) with partial UUO or sham-operation, urine was collected prior to sacrifice for determination of protein11 and creatinine concentration (DetectX, Arbor Assays, An Arbor, MI). Both male and female mice were studied at 21 days of age (n = 15 WT, 20 Os/+), whereas only males were included at 42 days of age (n = 40 WT, 33 Os/+) because the fraction of glomeruli with tall parietal epithelial cells (Lotus lectin-positive glomeruli) is greater in males than females after 21 days of age.12 In all mice undergoing partial UUO, patency of the ureter was documented by passage into the bladder of India ink injected into the renal pelvis at the time of kidney harvest.7 Additional groups of non-operated mice were studied birth (n = 4 WT, 4 Os/+) and at 9 days of age (n = 12 WT, 4 Os/+).
Tissue fixation and embedment
Animals were anesthetized with pentobarbital sodium-phenytoin sodium solution (Euthasol: Virbac, Ft. Worth, TX). Kidneys were perfusion-fixed or immersed in phosphate-buffered formalin solution, embedded in paraffin and sectioned on a Leica RM2155 microtome.
For plastic embedment, kidneys were perfused with Hank's Balanced Salt Solution (HBSS) followed by 2.5% glutaraldehyde in HBSS, cut into 50-μm sections with a vibrating microtome, postfixed in osmium tetroxide, infiltrated with Poly/Bed 812 resin (Polysciences, Warrington, PA) and embedded on microscope slides.13 Semithin sections (0.1-0.2 μm) were cut on a Sorvall MT-2B ultramicrotome. All sections were examined with Leica DMLS light microscopes equipped with Olympus QColor 3 digital camera.
Staining
Morphological features were examined in paraffin sections stained with periodic-acid Schiff (PAS)/hematoxylin. Collagen was stained with picrosirius red. Lotus tetragonolobus agglutinin staining was utilized to identify proximal tubules and tall epithelial cells in Bowman's capsule characteristic of mature murine glomerulotubular junctions.5,6 Plastic sections were stained with alkaline toluidine blue solution.
Morphometry
Nephron number was determined by counting glomeruli in PAS-stained median sagittal sections, a technique validated by the disector technique.7,14,15 ImagePro Plus 5.1 or 7.0 image-analysis software (Media Cybernetics, Silver Spring, MD) was used to determine glomerular area by sampling the entire cortical thickness,16 as well as volume fraction of collagen and proximal tubules (VV(PT)).6 Proximal tubular maturation in the mouse is characterized by the acquisition of avid binding by Lotus tetragonolobus lectin in the first 3 weeks of life.17 Conversely, UUO leads to the progressive loss of Lotus lectin binding, a reflection of epithelial injury and cell death that begins at the glomerulotubular junction and extends down the proximal tubule with continued obstruction.5,17 The fraction of total glomeruli in a sagittal section that contains any Lotus lectin staining along Bowman's capsule, or “glomerulotubular junction index,” therefore represents the fraction of mature nephrons with functional integrity (a combined measure of maturation and injury). The proximal tubular volume fraction is calculated as the ratio of Lotus lectin-staining tubules to the total cortical parenchyma, and represents a measure of proximal tubular mass.6,17
Tissue collagen was measured in 20 microscopic fields of picrosirius red-stained sections, alternating between cortical and medullary zones. Parenchymal area was determined for digital images of median sagittal sections of kidneys scanned with a Nikon Super Coolscan 4000 slide scanner (Nikon, Tokyo, Japan). All morphometric measurements were made using tissue labeled with unique identifier numbers.
Statistical analysis
Comparisons between wild-type and Os/+ strains for individual parameters were made using Students t-test for normally distributed data, and Mann-Whitney rank-sum test for data not normally distributed. Comparisons between 3 treatment groups (sham, UUO and UUO-release) for each strain (wild-type or Os/+), age (21 or 42 days), and kidney (obstructed or contralateral) were made using one-way analysis of variance (ANOVA). For data passing equal variance test, ANOVA was followed by Holm-Sidak pairwise multiple comparison. For data not passing equal variance test, Kruskal-Wallis ANOVA was performed, followed by Dunn's pairwise multiple comparison. Comparisons of the overall effects of age, strain and UUO on each parameter were made using two-way analysis of variance. All statistical analyses were performed using SigmaStat software (Systat, San Jose, CA). Statistical significance was defined as p<0.05.
Results
Os/+ mouse kidneys have fewer, larger glomeruli than wild-type
As shown in Figures 2A – 2F, compared to the C57BL/6 wild-type kidney, glomeruli appear larger and more widely dispersed among larger tubules in the Os/+ kidney at 42 days of age. Detectable by 9 days of age, mean glomerular area is significantly greater in Os/+ than wild-type kidneys (Fig. 2G).
Figure 2. Growth and development in Os/+ and wild-type mice.
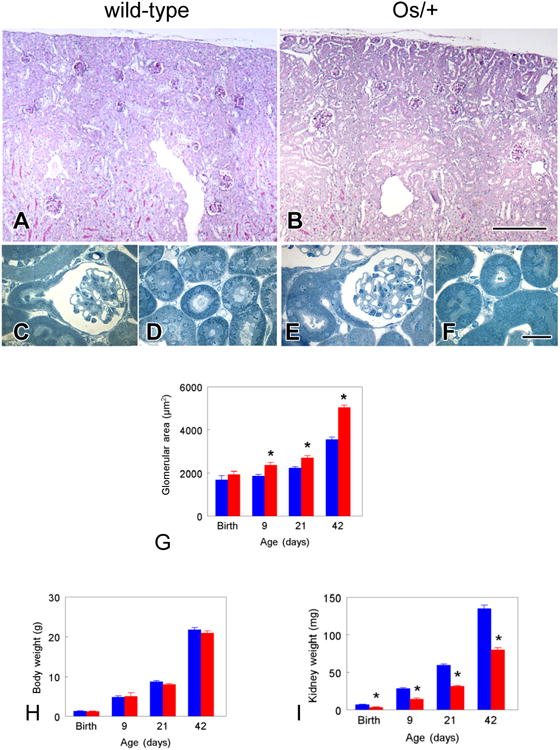
A and B. Survey micrographs of PAS-stained sections of 42-day old sham-operated mice, comparing glomerular size and distribution between the wild-type (A) and Os/+ (B) strains. Area measurements of the 14 glomeruli in the wild-type (A) average 3437 μm2, as opposed to the 4978 μm2 average for the eight glomeruli in the Os/+ kidney (B). C – F. Semithin plastic sections of glutaraldehyde-perfused kidneys from 42-day-old, sham-operated mice. C and D, wild-type mouse. C, Glomerulus with proximal tubule, showing typical extension of tall epithelial cells onto Bowman's capsule. D, transversely-sectioned proximal tubules in subcapsular cortex. E and F, Os/+ mouse. E, glomerulus and proximal tubule, with similar features to wild-type mouse, though overall dimensions of Os/+ glomeruli are larger. F, proximal tubules in cross-section, which are noticeably larger in diameter than those of the wild-type. G. Glomerular area. Glomeruli undergo compensatory growth detectable at 9 days and continuing through adulthood. H. Body weight. There is no difference in somatic growth between Os/+ and wild-type mice. I. Kidney weight in Os/+ mice from birth through adulthood remains approximately 50% lower than that of wild-type mice from birth through adulthood. Bars represent mean ± standard error. Blue bars, C57BL/6 wild-type; red bars, Os/+ mice. *p<0.05 by t-test vs. wild-type. Scale bar in B = 250 μm and applies to panels A and B. Scale bar in F = 25 μm and applies to panels C-F.
Normal body growth and urine protein excretion are maintained by Os/+ mice from birth to adulthood, and are not affected by UUO
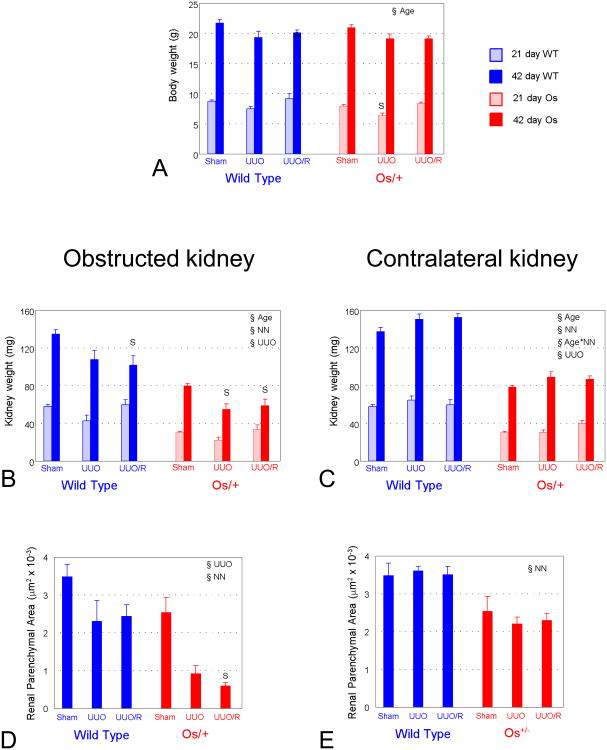
As shown in Figure 2G, somatic growth from birth through adulthood was not affected in Os/+ mice compared to wild-type animals. At 21 days, body weight of Os/+ mice with persistent UUO lagged behind that of sham-operated mice, but by 42 days, body weight was not affected by UUO, regardless of strain (Fig. 3A). Urine protein/creatinine ratio did not differ between groups: sham wild-type, 39.8±2.0; sham Os/+, 34.4±1.8; UUO wild-type, 39.5±26.1; UUO Os/+, 49.2±11.8 (not significant by 2-way ANOVA).
Figure 3. Somatic and renal growth in Os/+ and wild-type mice.
A. Body weight. B and C Kidney weight. D and E. Renal parenchymal area. Because variance in weight between left and right kidneys of sham-operated mice is <6%, only one kidney was examined for each sham-operated animal: these data are displayed in both obstructed and contralateral kidney columns. Bars represent mean ± standard error; light blue, 21 day wild-type; dark blue, 42 day wild-type; light red, 21 day Os/+, dark red, 42 day Os/+. §Age, p<0.05 by 2-way ANOVA within strain; §NN, nephron number p<0.05 by 2-way ANOVA within strain; Age*NN, interaction of age and nephron number, p<0.05 by 2-way ANOVA within strain; §UUO, p<0.05 by 2-way ANOVA within strain. S, p<0.05 by 1-way ANOVA vs. Sham, same strain.
Kidney growth is irreversibly impaired by UUO
Kidney weight remained significantly lower in Os/+ compared to wild-type mice from birth through 42 days of age (Fig. 2I). Kidney weight was not affected by 21 days of ipsilateral UUO, but continued growth to 42 days was impaired by persistent UUO in Os/+ mice, or following release of UUO, regardless of strain (Fig. 3B). Compared to sham-operated mice, weight of the contralateral kidney was increased 42 days after UUO, regardless of release of obstruction (p=0.001, 2-way ANOVA) (Fig. 3C). Renal parenchymal area following release of ipsilateral UUO was reduced by 29% compared to sham-operated kidneys of wild-type mice, and by 76% compared to sham-operated kidneys of Os/+ mice (Figs. 3D and 4A). Parenchymal area of the contralateral kidney was not affected by UUO or its release (Fig. 3E).
Figure 4. Recovery following release of partial UUO in wild-type and Os/+ mice.
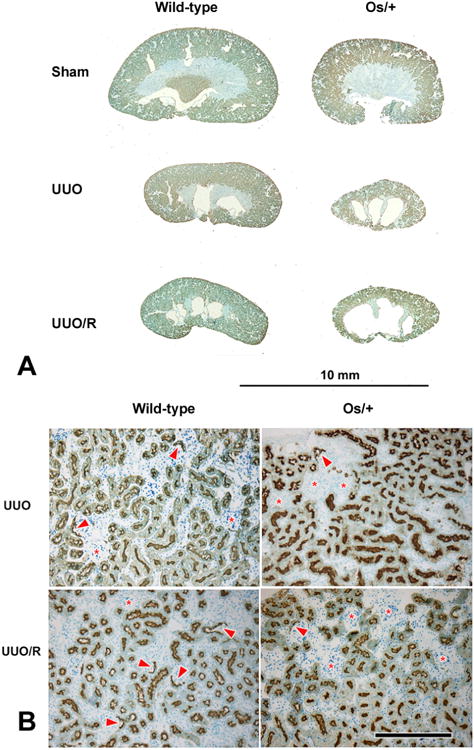
A. Comparison of wild-type and Os/+ kidneys. Median sagittal sections (Lotus tetragonolobus staining) of kidneys from 42-day-old animals. B. Details of cortical structure from the same UUO and UUO/R kidneys. In the wild-type mice, staining of Bowman's capsule with Lotus lectin (indicated by arrowheads), an indicator of intact nephrons, is superior to the Os/+ equivalents. In addition, release of ureteral obstruction results in a greater incidence of capsule lectin positivity in the wild-type, but not in the Os/+ (also see Fig. 5E). Persistently obstructed kidneys contain groups of crowded glomeruli with no Lotus lectin staining in their capsules (*). Scale bar in B = 250 μm and applies to all panels.
Glomerular growth is irreversibly impaired by UUO, and nephron number is further reduced by UUO in Os/+ mice
Glomerular area increased at a greater rate in Os/+ than in wild-type mice, with significant differences between strains detectable even at 9 days (Fig. 2D). This reveals that compensatory glomerular growth in Os/+ mice begins prior to the completion of nephron maturation.18 The maturational increase in glomerular area from 21 to 42 days was suppressed in the postobstructed kidney of wild-type mice, and compensatory glomerular growth of Os/+ mice was also impaired despite the release of UUO (Fig. 5A). There was no effect of UUO on glomerular area of the contralateral kidney in either strain (Fig. 5B).
Figure 5. Glomerular response to partial UUO and its release in wild-type and Os/+ mice.
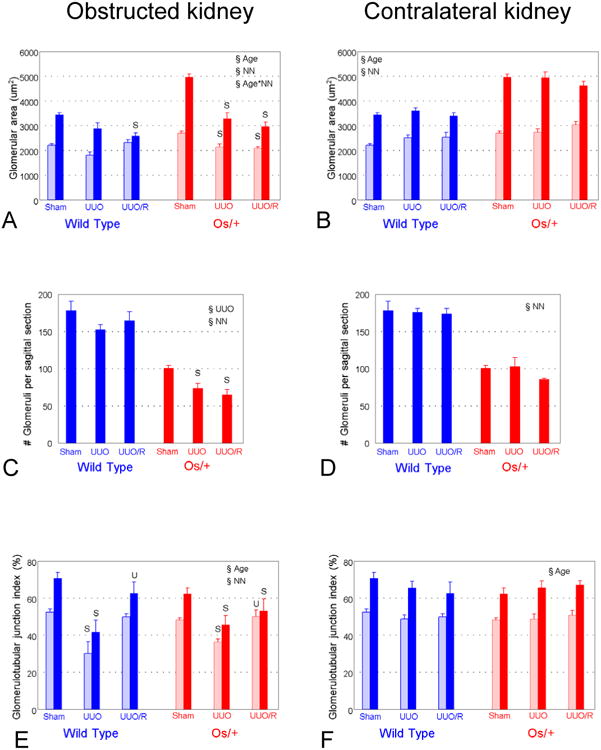
A and B. Glomerular area of the obstructed and contralateral kidney. B. Glomerular area. C and D. Number of glomeruli. E and F. Glomerulotubular junction index, or fraction of Lotus-staining glomeruli represents the fraction of mature intact glomerulotubular junctions. Symbols as for Figure 3. U, p<0.05 by 1-way ANOVA vs. UUO, same strain.
There was no effect of UUO on the number of glomeruli in the obstructed kidney of wild-type mice, whereas the number of glomeruli decreased further in the obstructed kidney of Os/+ mice, with no benefit from release of obstruction (Fig. 5C). There was no effect of UUO on the number of glomeruli in the contralateral kidney of mice of either strain (Fig. 5D).
Although UUO impairs maturation of the glomerulotubular junction in all mice, release of obstruction preserves maturation in wild-type mice only
Persistent partial UUO resulted in numerous glomeruli lacking capsular Lotus lectin staining, consistent with immature glomerulotubular junctions (Fig. 4B). As shown in Figure 5E, there was a significant increase in maturation of the glomerulotubular junction from 21 to 42 days in kidneys of sham-operated animals, which did not differ in contralateral kidneys of either strain (Fig. 5F). The fraction of mature glomerulotubular junctions was reduced in the obstructed kidney of wild-type mice at 21 and 42 days, but normalized with release of obstruction (Figs. 4B and 5E). A decreased fraction of mature glomerulotubular junctions observed in mice with persistent UUO is the result of a process of morphological alteration and selective death of cells in this nephron segment, culminating in the formation of atubular glomeruli.7 Although integrity of the glomerulotubular junction was similarly impaired by ipsilateral UUO in Os/+ mice, release of obstruction resulted in only partial recovery (Figs. 4B and 5E). There was no effect of UUO or its release on glomerular number, area, or glomerulotubular junction index of the contralateral kidney (Figs. 5B, 5D, 5F).
Proximal tubular volume fraction and interstitial collagen accumulation are inversely affected by UUO and its release in both obstructed and contralateral kidneys
Proximal tubular volume fraction increased with age in sham-operated mice, was suppressed by UUO, and resumed following release of obstruction in wild-type, but not Os/+ mice (Fig. 6A). Of note, interstitial collagen accumulated after 42 days of ipsilateral UUO, and decreased following release of obstruction in wild-type, but not Os/+ mice (Fig. 6C). Unexpectedly, the proximal tubular volume fraction was significantly greater in the contralateral kidney of both wild-type and Os/+ mice undergoing release of obstruction as compared to those with persistent UUO (Fig. 6B). This was accompanied by significant increases in interstitial collagen content in the contralateral kidney of both strains of mice with persistent UUO, with normalization of interstitial collagen in mice undergoing release of obstruction (Fig. 6D). There is a significant negative correlation between the volume fraction of interstitial collagen and proximal tubules in obstructed kidneys, and a similar trend in contralateral kidneys (Figs. 6E and 6F).
Figure 6. Proximal tubular and interstitial response to partial UUO and its release in wild-type and Os/+ mice.
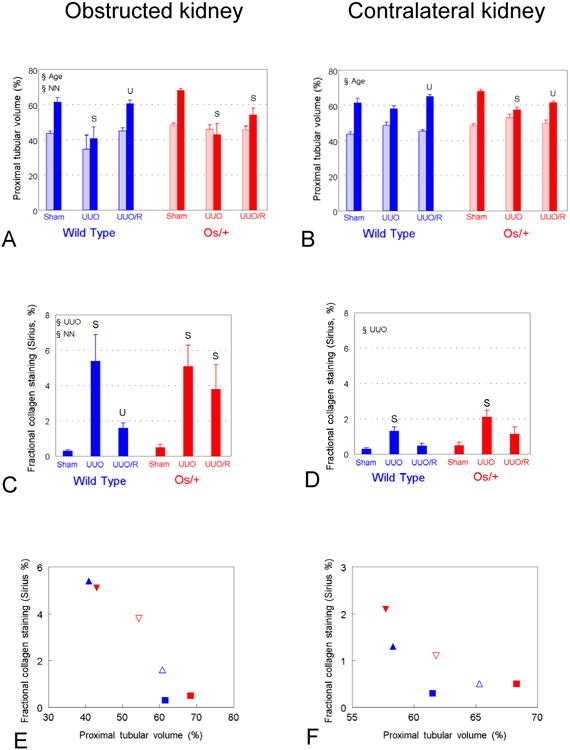
A and B. Proximal tubular volume fraction of renal parenchyma. C and D. Fractional interstitial collagen. E and F. Fractional interstitial collagen decreases as a function of proximal tubular volume fraction in both obstructed (E) (r = 0.95, p<0.001) and contralateral kidneys (F) (r = 0.75, p=0.09). Color symbols (A – E) as for Figures 3 and 5; E – F, squares = sham, closed triangles = UUO, open triangles = release. Symbols as for Figure 3. U, p<0.05 by 1-way ANOVA vs. UUO, same strain.
Discussion
Development of a model of congenital obstructive nephropathy
In infants with congenital obstructive nephropathy, there is an increased incidence of preterm birth and renal hypoplasia, both of which are associated with reduced nephron number.19,20 Since nephrogenesis in the mouse continues through the third day of life (followed by continuation of nephron maturation over the next three weeks), surgical partial UUO within 12-36 hours of birth is analogous to human ureteropelvic junction obstruction, the most common cause of obstructive nephropathy.18,21,22 This model allows control of the severity, timing, and duration of obstruction—all of which are significant determinants of clinical obstructive injury.7
The present study is the first to investigate, in an animal model from birth to adulthood, the consequences of reduced nephron number on the effects of persistent partial UUO as well as on recovery following release of obstruction. Study intervals of 21 and 42 days of age were selected because the former is the age of weaning, and the latter the age of sexual maturity in the mouse. This is relevant to human disease, since progression of chronic renal insufficiency in children is accelerated at the end of infancy and in early adolescence.23
Within 7 to 14 days of complete UUO in the adult mouse, proximal tubules undergo massive cell death, resulting in the formation of tubular fragments and atubular glomeruli.5 In contrast to the adult, proximal tubular cell death following complete UUO in the neonatal mouse is delayed past 14 days, with atubular glomeruli appearing only after 21 to 28 days.17 These observations led to the development of histomorphometric techniques that quantitate the integrity of the glomerulotubular junction and the proximal tubular volume fraction, as applied in the present study.6
Maturational and adaptive nephron growth are additive
The present study demonstrates that adaptive accelerated growth by glomeruli of Os/+ mice begins before the completion of nephron maturation. Despite the lower kidney weight in Os/+ compared to wild-type mice, the rate of kidney growth is similar in both strains, and glomerular and proximal tubular growth in sham-operated Os/+ mice continues to exceed that of wild-type mice throughout maturation (Figs. 5B and 6B). These observations imply a short-term preservation of adaptation to reduced nephron number in the adult.
Whereas nephron growth is normally accelerated in Os/+ mice, chronic UUO impairs adaptive growth of both glomeruli and tubules in the ipsilateral kidney (Figs. 5A and 6A). Although nephron number is not reduced by ipsilateral partial UUO in wild-type mice, the obstructed kidney of Os/+ mice suffers a further reduction in nephron number (Fig. 5C). This may result from impaired completion of nephrogenesis or subsequent loss of nephrons. Because nephron immaturity appears to be protective against proximal tubular damage due to UUO,17 accelerated maturation/growth of nephrons in both kidneys of Os/+ mice may increase their susceptibility to obstructive injury.
Reduced nephron number impairs recovery from neonatal UUO
Following release of obstruction, glomerular growth does not normalize regardless of nephron number (Fig. 5A). These results are consistent with a limited regenerative capacity for glomeruli. Although parietal epithelial cells of Bowman's capsule can replace damaged podocytes,24 the present study indicates that accelerated glomerular growth in Os/+ mice (in response to reduced nephron number) cannot be maintained following release of partial UUO.
There is complete proximal tubular recovery in wild-type, but impaired recovery in Os/+ mice (Fig. 6A). The close negative correlation between interstitial collagen and proximal tubular volume fraction suggests that extracellular matrix deposition is dependent on the severity of tubular injury (Fig. 6E).25 Thus, in early adulthood, renal interstitial fibrosis is reversible if tubular mass is preserved. However, reduction in nephron number blunts this response, underscored by persistence of significant interstitial collagen accumulation in Os/+ mice.
Increased weight of the contralateral kidney following 42 days of UUO (Fig. 3C) is consistent with compensatory growth regardless of strain or release of obstruction. This is likely due to increased interstitial collagen during UUO, and persistence of tubular hypertrophy after release of obstruction (Figs. 6B and 6D). Regardless of nephron number, compared to mice with persistent UUO, there is an increase in proximal tubular volume fraction in the contralateral kidney following release of UUO, paralleled by a concomitant decrease in interstitial collagen (Fig. 6B, 6D and 6F). These responses are consistent with reversal of oxidative proximal tubular stress in the kidney contralateral to a chronically obstructed neonatal kidney.17
Clinical implications of the mouse model
There is a great need for new predictors of progression of obstructive nephropathy. Correlation of outcome with renal pelvic dilatation or with renal scintigraphy is poor, and reliable biomarkers are not readily available.26 The most widely-used current index of progression of CKD, urinary protein excretion, has poor predictive value in early stages of the disease.27 The lack of sensitivity of urine protein/creatinine ratio in the present study contrasts with the reduction in renal mass in Os/+ mice with persistent or postobstructed UUO. However, these data suggest predictive value in the measurement of renal parenchymal area in patients by ultrasonography or magnetic resonance imaging. This has been borne out in male infants under 6 months of age with lower urinary tract obstruction: lower renal parenchymal area is associated with increased risk of renal failure later in life.28 To complement the measurement of renal parenchymal area, a promising new technique is being developed to measure the number and size of glomeruli using magnetic resonance imaging in whole human kidneys labeled with cationic ferritin.29
Conclusions
The present study reveals that congenital reduction in nephron number impairs recovery from obstructive injury, and contributes to additional nephron loss. These findings suggest that infants born with nephron number below the median (approximately 900,000 nephrons per kidney) are at increased risk for progressive renal injury due to obstructive nephropathy, the leading cause of chronic kidney disease in children. Key questions that require investigation include: 1) what are the signals responsible for adaptive glomerular and tubular growth before and after the completion of nephrogenesis; and 2) what are the determinants of the fate of individual cells responding to the injurious stimuli, leading either to remodeling/regeneration or cell death?
Acknowledgments
This project was supported by the Pediatric Center of Excellence in Nephrology, P50 DK096373 from the National Institutes of Health. M. Sergio was a Fulbright Visiting Scholar for Advanced Research 2011-1012. The technical assistance of Hiwat Abate, Jennifer R. Charlton, Thy Nguyen, Akif Shameem, and Lauren Simpkins is gratefully acknowledged. Portions of this study were presented at the annual meeting of the Pediatric Academic Societies, Boston, MA, April 2012, and the annual meeting of the American Society of Nephrology, Philadelphia, PA, November, 2014.
Abbreviations
- CKD
chronic kidney disease
- Os/+
oligosyndactyly heterozygote
- UUO
unilateral ureteral obstruction
- WT
wild-type
Footnotes
Publisher's Disclaimer: Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our subscribers we are providing this early version of the article. The paper will be copy edited and typeset, and proof will be reviewed before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to The Journal pertain.
References
- 1.Warady BA, Chadha V. Chronic kidney disease in children: the global perspective. Pediatr Nephrol. 2007;22:1999. doi: 10.1007/s00467-006-0410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wuhl E, van Stralen KJ, Verrina E, et al. Timing and outcome of renal replacement therapy in patients with congenital malformations of the kidney and urinary tract. Clin J Am Soc Nephrol. 2013;8:67. doi: 10.2215/CJN.03310412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hostetter TH, Olson JL, Rennke HG, et al. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. Am J Physiol. 1981;241:F85. doi: 10.1152/ajprenal.1981.241.1.F85. [DOI] [PubMed] [Google Scholar]
- 4.Chevalier RL, Forbes MS, Thornhill BA. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int. 2009;75:1145. doi: 10.1038/ki.2009.86. [DOI] [PubMed] [Google Scholar]
- 5.Forbes MS, Thornhill BA, Chevalier RL. Proximal tubular injury and rapid formation of atubular glomeruli in mice with unilateral ureteral obstruction: A new look at an old model. Am J Physiol Renal Physiol. 2011;301:F110. doi: 10.1152/ajprenal.00022.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forbes MS, Thornhill BA, Minor JJ, et al. Fight-or-flight: murine unilateral ureteral obstruction causes extensive proximal tubular degeneration, collecting duct dilatation, and minimal fibrosis. Am J Physiol Renal Physiol. 2012;303:F120. doi: 10.1152/ajprenal.00110.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thornhill BA, Forbes MS, Marcinko ES, et al. Glomerulotubular disconnection in neonatal mice after relief of partial ureteral obstruction. Kidney Int. 2007;72:1103. doi: 10.1038/sj.ki.5002512. [DOI] [PubMed] [Google Scholar]
- 8.Luyckx VA, Bertram JF, Brenner BM, et al. Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet. 2013;382:273. doi: 10.1016/S0140-6736(13)60311-6. [DOI] [PubMed] [Google Scholar]
- 9.Thornhill BA, Chevalier RL. Variable partial unilateral ureteral obstruction and its release in the neonatal and adult mouse. Methods Mol Biol. 2012;886:381. doi: 10.1007/978-1-61779-851-1_33. [DOI] [PubMed] [Google Scholar]
- 10.Zalups RK. The Os/+ mouse: A genetic animal model of reduced renal mass. Am J Physiol. 1993;264:F53. doi: 10.1152/ajprenal.1993.264.1.F53. [DOI] [PubMed] [Google Scholar]
- 11.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein-dye binding. Anal Biochem. 1976;72:248. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 12.Crabtree C. The structure of Bowman's capsule as an index of age and sex variations in normal mice. Anat Rec. 1941;79:395. [Google Scholar]
- 13.Forbes MS, Ghribi O, Herman MM, et al. Aluminum-induced dendritic pathology revisited: Cytochemical and electron microscopic studies of rabbit cortical pyramidal neurons. Ann Clin Lab Sci. 2002;32:75. [PubMed] [Google Scholar]
- 14.Dziarmaga A, Eccles M, Goodyer P. Suppression of ureteric bud apoptosis rescues nephron endowment and adult renal function in Pax2 mutant mice. J Am Soc Nephrol. 2006;17:1568. doi: 10.1681/ASN.2005101074. [DOI] [PubMed] [Google Scholar]
- 15.Chevalier RL, Thornhill BA, Chang AY, et al. Recovery from release of ureteral obstruction in the rat: Relationship to nephrogenesis. Kidney Int. 2002;61:2033. doi: 10.1046/j.1523-1755.2002.00359.x. [DOI] [PubMed] [Google Scholar]
- 16.Yoo KH, Thornhill BA, Forbes MS, et al. Compensatory renal growth due to neonatal ureteral obstruction: Implications for clinical studies. Pediatr Nephrol. 2006;21:368. doi: 10.1007/s00467-005-2119-y. [DOI] [PubMed] [Google Scholar]
- 17.Forbes MS, Thornhill BA, Galarreta CI, et al. Chronic unilateral ureteral obstruction in the neonatal mouse delays maturation of both kidneys and leads to late formation of atubular glomeruli. Am J Physiol Renal Physiol. 2013;305:F1736. doi: 10.1152/ajprenal.00152.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rumballe BA, Georgas KM, Combes AN, et al. Nephron formation adopts a novel spatial topology at cessation of nephrogenesis. Devel Biol. 2011;360:110. doi: 10.1016/j.ydbio.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez MM, Gomez AH, Abitbol CL, et al. Histomorphometric analysis of postnatal glomerulogenesis in extremely preterm infants. Pediatr Devel Pathol. 2004;7:17. doi: 10.1007/s10024-003-3029-2. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Z, Quinlan J, Hoy W, et al. A common RET variant is associated with reduced newborn kidney size and function. J Am Soc Nephrol. 2008;19:2027. doi: 10.1681/ASN.2007101098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartman HA, Lai HL, Patterson P. Cessation of renal morphogenesis in mice. Devel Biol. 2007;310:379. doi: 10.1016/j.ydbio.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosen S, Peters CA, Chevalier RL, et al. The kidney in congenital ureteropelvic junction obstruction: A spectrum from normal to nephrectomy. J Urol. 2008;179:1257. doi: 10.1016/j.juro.2007.11.048. [DOI] [PubMed] [Google Scholar]
- 23.Celedon CG, Bitsori M, Tullus K. Progression of chronic renal failure in childern with dysplastic kidneys. Pediatr Nephrol. 2007;22:1014. doi: 10.1007/s00467-007-0459-5. [DOI] [PubMed] [Google Scholar]
- 24.Ronconi E, Sagrinati C, Angelotti ML, et al. Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol. 2009;20:322. doi: 10.1681/ASN.2008070709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eddy AA. Can renal fibrosis be reversed? Pediatr Nephrol. 2005;20:1369. doi: 10.1007/s00467-005-1995-5. [DOI] [PubMed] [Google Scholar]
- 26.Chevalier RL. Biomarkers of congenital obstructive nephropathy: Past, present and future. J Urol. 2004;172:852. doi: 10.1097/01.ju.0000129542.22043.ef. [DOI] [PubMed] [Google Scholar]
- 27.Schanstra JP, Zurbig P, Alkhalaf A, et al. Diagnosis and prediction of CKD progression by assessment of urinary peptides. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2014050423.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pulido JE, Furth SL, Zderic SA, et al. Renal parenchymal area and risk of ESRD in boys with posterior urethral valves. Clin J Am Soc Nephrol. 2014;9:499. doi: 10.2215/CJN.08700813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beeman SC, Cullen-McEwen LA, Puelles VG, et al. MRI-based glomerular morphology and pathology in whole human kidneys. Am J Physiol Renal Physiol. 2014;306:F381. doi: 10.1152/ajprenal.00092.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]