Abstract
AIM: To evaluate spiral computed tomography (CT) including virtual gastroscopy for diagnosis of gastric carcinoma in comparison with upper gastrointestinal series (UGI), fiberoptic gastroscopy (FG) and histopathology.
METHODS: Sixty patients with histologically proven gastric carcinoma (54 advanced and 6 early) were included in this study. The results of spiral CT were compared with those of UGI and FG. Two observers blindly evaluated images of spiral CT and UGI and video recording of FG with consensus in terms of diagnostic confidence with a five-point scale. Sensitivities of lesion detection, Borrmann's classification of spiral CT, UGI and FG, as well as the accuracy of TNM staging of spiral CT were determined by comparing them to surgical and histological findings.
RESULTS: The lesion detection rate was 98% (59/60), 95% (57/60) and 98% (59/60) for spiral CT, UGI and FG, respectively. There were no statistical differences in the detection sensitivity among the three techniques (P > 0.05). For the sensitivity in Borrmann's classification, spiral CT was higher than that of UGI (P = 0.025) and similar to that of FG (P > 0.05). The accuracy of spiral CT in staging the gastric carcinoma was 76.7%. Six cases of early gastric carcinoma were all detected by spiral CT as well as FG.
CONCLUSION: Spiral CT is equivalent to UGI and FG in the detection of gastric carcinoma, and superior to UGI but similar to FG in the Borrmann's classification of advanced gastric carcinoma. Spiral CT is more valuable than FG in the staging of gastric carcinoma.
INTRODUCTION
Fiberoptic gastroscopy (FG) is generally regarded as a standard test for detection of gastric cancer. Upper gastrointestinal series (UGI), however, still represents a routine or survey examination for imaging gastric abnormalities although it has certain limitations in clinical use. Even though the application of conventional computed tomography (CT) in staging gastric carcinoma has been introduced, the results are unsatisfactory[1-3]. Recently, spiral CT, including various three-dimensional (3D) reconstructions such as virtual endoscopy and axial source images (2D) has been used in imaging the alimentary tract[2-16]. While most reports in literature laid emphasis on colon polyps[11-12,14-15], there have been a few studies investigating gastric carcinoma with spiral CT[4-10]. In clinical practice, advanced gastric carcinoma is defined using Borrmann's classification, which is the pathological basis for UGI diagnosis, and the resectability of the tumor and prognosis of the patients are evaluated presurgically using TNM staging[17], which is one of the suggested applications of spiral CT.
In this study, 2D and 3D display techniques after spiral CT scan were cross-referenced. The role of this combined spiral CT technique was compared with that of UGI and FG in the detection and Borrmann's classification of gastric carcinoma, which, to our knowledge, has not been reported in literature. The staging of gastric carcinoma with spiral CT was also correlated with histopathology.
MATERIALS AND METHODS
Patients
During a 12-month period, 60 consecutive patients (36 males, 24 females) ranging from 27 to 79 years old (mean = 62 a.), who were diagnosed of gastric carcinoma with FG and subsequent biopsy were recruited. Among the 60 gastric carcinoma patients, 43 were undertaken gastrectomy or subtotal gastrectomy, 17 were undertaken surgical exploration only because of severe adhesion between tumor and surrounding tissues. This study was approved by the administrative authority of the university hospital and fully informed consent was obtained from each patient. Within one week after FG procedure, UGI was performed prior to spiral CT scanning on each patient.
Imaging acquisition
Spiral CT was performed with a Hispeed CT/i scanner (General Electric Medical Systems, Milwaukee, WI). All patients were fasted for 12 h. Before the CT scanning, 20 mg raceanisodamine hydrochloride was intramuscularly administered and two packs (6 g) of effervescent granules were taken orally. Usually patients were immediately placed on the scanning table in an oblique supine position. A scout projection was made to confirm the stomach to be distended by gas. If insufficient distention of stomach was found, half to one pack of effervescent granules was added and a scout projection was scanned again. Then, spiral CT was performed with a 3-mm collimation and a pitch of 1.2-1.5 mm during a single breath-hold of 22-33 s, which produced a 3D-volume acquisition that included the entire stomach. Tube current was 200-280 mA, voltage was 120 kVp and scan time was 1 second per rotation.
Barium double contrast technique with standard projection was used for UGI studies by an experienced radiologist. Standard FG examination was performed by an experienced gastrologist and the biopsy samples were processed as a clinical routine of pathology department.
Image reconstruction
3D-postprocessing modes including CT virtual gastroscopy (CTVG), surface shaded display (SSD) and "Raysum" Display (virtual double contrast barium study) were performed by a built-in workstation (Advantage Window 2.0, General Electric Medical Systems, Milwaukee, WI) after raw data reconstruction with 1 mm interval. For intraluminal views of the stomach, a default level of -525 (Hounsfield unit, HU) provided by the Navigator (GE Company) was chosen. SSD and "Raysum" display were obtained with a threshold of -311HU.
Image analysis
Sixty sets of hard copy of grayscale spiral CT images (including axial source images, CTVG, SSD, and "Raysum" display) and UGI radiograms from 60 patients were obtained by one radiologist and were randomly reviewed and scored with consensus by two other experienced radiologists who were blinded to clinical data, pathology, and the information of other imaging techniques. Video recording of FG examinations was reviewed and scored with consensus by two experienced gastrologists.
The diagnostic confidence, appearance, location, and size of suspected lesions on images of spiral CT, UGI and FG were recorded. For spiral CT, the observers made the final judgments after referring to all 4 techniques including CTVG, SSD, "Raysum" and axial source images. The lesions were then classified as early and advanced gastric carcinoma according to Borrmann's classification[2]. Based on spiral CT, TNM staging[16] of gastric carcinoma of each case was also noted. Diagnostic confidence for detecting a lesion was rated as 1, definitely no lesion; 2, probably not a lesion; 3, possible lesion; 4, probable lesion; 5, definite lesion[18]. Any image artifacts that degraded diagnostic confidence were also noted.
All findings of lesions with spiral CT, UGI and FG were further verified with the results of surgical exploration, dissected specimen and histology, which were used as the gold standard for detection, Borrmann's classification and spiral CT staging of gastric carcinoma.
Statistical analysis
Data entry procedures and statistical analysis were performed with a statistical software package (SAS for windows, version 6.12, SAS Institute, Cary, NC). By using only those lesions allocated with confidence rate of 3 or higher, the sensitivity of detection and classification of gastric carcinomas of each technique was assessed[18] (chi;2 test). Significant differences were considered when the P value was less than or equal to 0.05.
RESULTS
The classification, size and location of the gastric cancer in this study are summarized in Table 1. A complete set of image data of a patient is exemplified in Figure 1.
Table 1.
Classification, size and location of gastric carcinoma (n = 60)
| Gastric cancer classification Size of the tumor | Size ofthe Tumor | |||||||
|
Early |
Advanced* |
< 1 cm |
1-3 cm |
> 3 cm |
||||
|
Type 2c |
Type 3 |
Type 1 |
Type 2 |
Type 3 |
Type 4 |
|||
| 4 (6.7%) | 2 (3.3%) | 10(16.7%) | 25 (41.7%) | 14 (23.3%) | 5 (8.3%) | 6 | 24 | 30 |
| Location of the tumor | ||||||||
| Cardia area | Antrum | Great curv | Lesser curv | Antrum and body | ||||
| 29 | 18 | 5 | 5 | 3 | ||||
Notes: Numbers were patients. Curv. = curvature. *Borrmann's type of classification.
Figure 1.
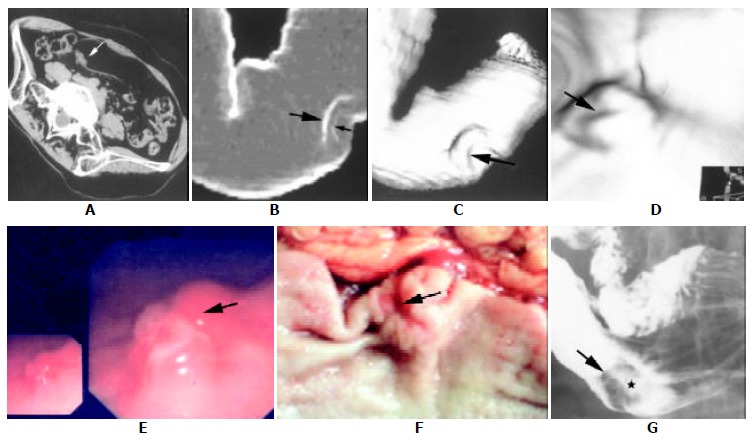
A complete set of image data of a patient. Spiral CT images (A-D), fiberoptic gastroscopy (E), surgical specimen (F) and corresponding barium study (G) from a patient (female, 48 a.) of Borrmann's type 2 and TNM stage 1 of advanced gastric carcinoma. (A), axial image (supine position with right side elevated) showed a focal irregular protruding (arrow) from the posterior wall of antrum. (B), "Raysum"display (virtual double contrast barium study) stressed double-margin changes at the greater curvature of antrum: tumor (large arrow) and ulcer (small arrow) margins. (C), SSD image showed depression at the antrum with a central ulcer (arrow). (D), CTVG image depicted an intraluminal irregular mass with a flat ulcer (arrow). The view angle was illustrated by the 2D image at lower right corner. (E), fiberoptic gastroscopic view showed a lobulated mass.(F), surgical specimen demon stratad a mass with a small central ulcer (arrow). (G), barium study revealed an intraluminal filling defect (arrows) with a flat ulcer (asterisk) at the greater curvature of antrum.
Lesion detection
In advanced gastric carcinoma, the lesion detection (with diagnostic confidence of 3 or higher) sensitivity of spiral CT was similar to that of UGI and FG (P > 0.05). In early gastric carcinoma, it was higher than that of UGI (P = 0.031) and similar to that of FG (Table 2). One false positive lesion (due to peristalsic wave at the lesser curvature) and one false negative lesion (with diagnostic confidence rate of 3 or lower) were noted in spiral CT. 3 and one false negative lesion was noted in UGI and FG, respectively (Table 3).
Table 2.
Comparison of sensitivities on gastric carcinoma between different techniques
|
Detection |
Classification |
||
| Early | Advanced | Advanced | |
| SCT | 100% | 98% | 77% |
| UGI | 33% | 95% | 47% |
| FG | 100% | 98% | 80% |
| Chi-Square test | |||
| SCT vs UGI | P = 0.031 | P > 0.05 | P = 0.025 |
| SCT vs FG | P > 0.05 | P > 0.05 | P > 0.05 |
Notes: SCT = spiral CT, UGI = upper gastrointestinal series, FG = fiberoptic gastroscopy.
Table 3.
Comparison of different techniques in erroneously interpreted lesions
| n | False positive | False negative |
Bormann's classification |
||
| Can not be classified | Erroneously classified | ||||
| SCT | 60 | 1 | 1 | 2 | 12 |
| UGI | 60 | 0 | 3 | 9 | 23 |
| FG | 60 | 0 | 1 | 0 | 12 |
Notes: Data were the number of lesions. SCT = spiral CT, UGI = upper gastrointestinal series, FG = fiberoptic gastroscopy.
Borrmann's classification
The ability of spiral CT to correctly classify the lesions according to Borrmann's classification was statistically higher than that of UGI (P = 0.025) and similar to that of FG (P > 0.05) (Table 2).
For spiral CT, 12/60 lesions were erroneously classified and 2/60 could not be classified which were attributed to image artifacts of respiration movement and fluid retention in stomach. For UGI, 23/60 lesions were erroneously classified and 9/60 could not be classified. For FG, 12/60 lesions were erroneously classified (Table 3). So, a total of 47 lesions were erroneously classified from spiral CT, UGI and FG. The rates of erroneously classified lesions in Borrmann's types 1, 2, 3, and early gastric carcinoma were 6.4% (3/47), 61.7% (29/47), 25.5% (12/47), and 6.4% (3/47), respectively. The majority of erroneously classified lesions occurred in Borrmann's type 2 (61.7%) and type 3 (25.5%). No erroneously classified lesion was found in Borrmann's type 4.
TNM staging of spiral CT
43 of 60 patients were included in the TNM staging. Of the 43 cases, stage I was found in 12, 6 of which were early gastric carcinoma, stage II in 7, stage III in 19 and stage IV in 5. The accuracy of spiral CT in TNM staging was 76.7% (33/43). 5 cases of overestimation and underestimation were noted respectively in spiral CT staging (Table 4).
Table 4.
Comparison of TNM staging of gastric carcinoma between spiral CT and pathology
| Pathological staging |
Spiral CT staging |
Total | |||
| 1 | 2 | 3 | 4 | ||
| 1 | 10 | 2 | 0 | 0 | 12 |
| 2 | 0 | 4 | 3 | 0 | 7 |
| 3 | 0 | 7 | 12 | 0 | 19 |
| 4 | 0 | 0 | 2 | 3 | 5 |
| Total | 10 | 13 | 17 | 3 | 43 |
DISCUSSION
The evaluation of gastric carcinoma by conventional barium studies is hampered by the following limitations: (1) the impossibility to produce endoscopic views, (2) the projective nature of the technique, (3) the quality of barium coating and the examiner's technique and skills. The disadvantage of FG examination is that it cannot evaluate the tumor involvement of gastric wall and surrounding tissues and therefore it has inability of TNM staging. Spiral CT has brought about additional possibilities for the evaluation of gastric tumors. Since the assessment of endoluminal morphology of gastric tumors is limited when only source images are used, 3D visualization techniques have been advocated[4-10]. In a recently published paper, the SSD and virtual double contrast imaging were compared with conventional barium studies and CTVG was compared with gastroscopy in various types of gastric pathologies respectively[6]. The present report represents a comprehensive clinical study that covers all three different 3D techniques of spiral CT (CTVG, SSD and virtual double contrast), as well as axial source images in comparison with UGI and FG focusing on the detection, classification and staging of gastric carcinoma.
We used two reconstruction algorithms for the 3D visualization of the stomach. One was SSD display, which provided outside opaque images of inner surface of the gastric wall generated with a thresholding technique and could be interpreted similarly as in single-contrast barium study[12]. The other was volume rendering technique including CTVG and virtual double contrast imaging as described elsewhere[12,13]. CTVG with perspective volume rendering technique produced intraluminal view similar to those obtained from fiberoptic gastroscopy. Virtual double contrast imaging displayed somewhat translucent images similar to those of double contrast barium study.
As different 3D techniques, the advantage of SSD and virtual double contrast display was to demonstrate the overall aspect and the contour changes of the stomach, but with poor mucosal information[6] (Figure 1 B, C), and CTVG offered the overview of the intraluminal tumors but with indiscernible surface coloration and extraluminal tumor invading (Figure 1D). Therefore, it is important to realize that information from 3D CT displays should be combined with that of source images in interpreting the gastric carcinoma clinically (Figure 1 A-D).
In our study, tumor detection sensitivities among spiral CT, UGI and FG were similar (P > 0.05). However, the sensitivity was higher with spiral CT than that with UGI (P = 0.031) and similar between SCG and FG (P > 0.05) in early gastric carcinoma. Only a few studies on the detection of early gastric carcinoma with spiral CT were reported, the results so far have been controversial[10]. The detection rate of early gastric carcinoma was 93.5% reported by Lee[10] with contrast enhanced SSD technique, and it was only 26% by spiral CT according to Fukuya[19]. Moreover, it was even lower with 3D CT than that of UGI according to Ogata[6]. In our study, spiral CT correctly detected all 6 cases of early gastric carcinomas, the detection rate (100%) was much higher than that of UGI (33.3%). However, the very limited number of cases with early gastric carcinoma in this series did not allow any conclusion in this regard.
The distribution and prevalence rate of gastric tumor according to Borrmann's classification in this study was slightly different from that reported, in which the most prevalent type was Borrmann's 3 and the most common site of tumor was the posterior wall of the antrum[8]. In our investigation, the most prevalent type was Borrmann's 2 and the most common site of tumor was the cardia and fundus. It has been reported that accurate classification of gastric tumor based on Borrmann's classification is possible with 3D CT[7,8]. Our study indicated that Borrmann's classification with spiral CT was better than that with UGI (P = 0.025), and similar to that of FG (P > 0.05). In this study, the majority of misclassification occurred in Borrmann's types 2 and 3, in which UGI accounted for a highest proportion of the misclassification rate. The main reasons were most likely due to the following: (1) in the present study, cardia or fundus carcinoma was found in the highest percentage of the patients (48.3%, 29/60), therefore Borrmann's type 2 or 3 tumors at cardia tended to be misclassified as type 1, because of the poor visualization of some small and flat ulcers within the tumor. (2) The limited capability of UGI in visualizing the ulcer and the infiltrating nature of tumors at the cardia was also a reason for the difficult differentiation between Borrmann's types 2 and 3. (3) On the contrary, Borrmann's type 2 or 3 at the antrum tended to be erroneously classified as type 4, because these tumors were poorly visualized by spiral CT [8].
The rational work out of treatment scheme and accurate evaluation of gastric carcinoma are mainly dependent on TNM staging before surgery. Spiral CT is a practical approach in TNM staging because it can visualize the gastric wall, surrounding tissue and organs. Many studies on gastric carcinoma staging with conventional CT have been reported so far. The results in the literature were various due to the different CT scanner and materials and methods used[20]. Using a spiral CT technique of thin slice thickness (3 mm) and plain scanning, staging accuracy of gastric carcinoma in this study reached 76.7%. This result was higher than that (72.0%) obtained by conventional CT scanning with contrast enhancement[21], and was lower than that (81%) obtained by triphase contrast enhancement of spiral CT[22]. Certainly, multiphase contrast enhanced spiral CT scan may further improve the staging of gastric carcinoma, the method we used, however, was simple, practical and more acceptable by the patients.
Some limitations of this study should be mentioned: (1) Since the size of more than half of the lesions in this group was large (> 3 cm), the conclusions drawn from the results might not be applicable to small or flat gastric lesions. (2) Because all the CT examinations were performed in patients with known gastric carcinoma, the specificity of different imaging techniques was not investigated in our study.
In summary, the present clinical study demonstrates that spiral CT is equal to UGI and FG in the detection of gastric carcinoma. For Borrmann's classification of gastric carcinoma, spiral CT scores better than UGI and similar to FG. Spiral CT is also of value in TNM staging of gastric carcinoma. It is suggested that spiral CT is an alternative to UGI and supplement to FG in gastric carcinoma diagnosis. However, further investigations on a larger scale are necessary to confirm the role of spiral CT in the evaluation of gastric carcinoma.
Footnotes
Supported by ECR 2000-EAR-ECR Research & Education Fund Fellowship Grant
Edited by Xu XQ and Wang XL
References
- 1.Sussman SK, Halvorsen RA, Illescas FF, Cohan RH, Saeed M, Silverman PM, Thompson WM, Meyers WC. Gastric adenocarcinoma: CT versus surgical staging. Radiology. 1988;167:335–340. doi: 10.1148/radiology.167.2.3357941. [DOI] [PubMed] [Google Scholar]
- 2.Fishman EK, Urban BA, Hruban RH. CT of the stomach: spectrum of disease. Radiographics. 1996;16:1035–1054. doi: 10.1148/radiographics.16.5.8888389. [DOI] [PubMed] [Google Scholar]
- 3.Balthazar EJ, Siegel SE, Megibow AJ, Scholes J, Gordon R. CT in patients with scirrhous carcinoma of the GI tract: imaging findings and value for tumor detection and staging. AJR Am J Roentgenol. 1995;165:839–845. doi: 10.2214/ajr.165.4.7676978. [DOI] [PubMed] [Google Scholar]
- 4.Lee DH, Ko YT. Gastric lesions: evaluation with three-dimensional images using helical CT. AJR Am J Roentgenol. 1997;169:787–789. doi: 10.2214/ajr.169.3.9275897. [DOI] [PubMed] [Google Scholar]
- 5.Wood BJ, O'Malley ME, Hahn PF, Mueller PR. Virtual endoscopy of the gastrointestinal system outside the colon. AJR Am J Roentgenol. 1998;171:1367–1372. doi: 10.2214/ajr.171.5.9798880. [DOI] [PubMed] [Google Scholar]
- 6.Ogata I, Komohara Y, Yamashita Y, Mitsuzaki K, Takahashi M, Ogawa M. CT evaluation of gastric lesions with three-dimensional display and interactive virtual endoscopy: comparison with conventional barium study and endoscopy. AJR Am J Roentgenol. 1999;172:1263–1270. doi: 10.2214/ajr.172.5.10227500. [DOI] [PubMed] [Google Scholar]
- 7.Lee DH, Ko YT. Advanced gastric carcinoma: the role of three-dimensional and axial imaging by spiral CT. Abdom Imaging. 1999;24:111–116. doi: 10.1007/s002619900456. [DOI] [PubMed] [Google Scholar]
- 8.Lee DH. Two-dimensional and three-dimensional imaging of gastric tumors using spiral CT. Abdom Imaging. 2000;25:1–6. doi: 10.1007/s002619910001. [DOI] [PubMed] [Google Scholar]
- 9.Lee DH. Three-dimensional imaging of the stomach by spiral CT. J Comput Assist Tomogr. 1998;22:52–58. doi: 10.1097/00004728-199801000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Zheng KE, Feng C, Ju SH, Sun J, Liu WH. CT virtual endoscopy: a preliminary clinical study in gastrointestinal tract. World Chinese J Digestol. 1999;7:629–631. [Google Scholar]
- 11.Springer P, Stöhr B, Giacomuzzi SM, Bodner G, Klingler A, Jaschke W, zur Nedden D. Virtual computed tomography colonoscopy: artifacts, image quality and radiation dose load in a cadaver study. Eur Radiol. 2000;10:183–187. doi: 10.1007/s003300050030. [DOI] [PubMed] [Google Scholar]
- 12.Rogalla P, Bender A, Bick U, Huitema A, Terwisscha van Scheltinga J, Hamm B. Tissue transition projection (TTP) of the intestines. Eur Radiol. 2000;10:806–810. doi: 10.1007/s003300051008. [DOI] [PubMed] [Google Scholar]
- 13.Rogalla P, Werner-Rustner M, Huitema A, van Est A, Meiri N, Hamm B. Virtual endoscopy of the small bowel: phantom study and preliminary clinical results. Eur Radiol. 1998;8:563–567. doi: 10.1007/s003300050434. [DOI] [PubMed] [Google Scholar]
- 14.Fletcher JG, Luboldt W. CT colonography and MR colonography: current status, research directions and comparison. Eur Radiol. 2000;10:786–801. doi: 10.1007/s003300051006. [DOI] [PubMed] [Google Scholar]
- 15.Hara AK, Johnson CD, Reed JE, Ehman RL, Ilstrup DM. Colorectal polyp detection with CT colography: two- versus three-dimensional techniques. Work in progress. Radiology. 1996;200:49–54. doi: 10.1148/radiology.200.1.8657944. [DOI] [PubMed] [Google Scholar]
- 16.Wu LF, Wang BZ, Feng JL, Cheng WR, Liu GR, Xu XH, Zheng ZC. Preoperative TN staging of esophageal cancer: comparison of miniprobe ultrasonography, spiral CT and MRI. World J Gastroenterol. 2003;9:219–224. doi: 10.3748/wjg.v9.i2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moss AA, Schnyder P, Marks W, Margulis AR. Gastric adenocarcinoma: a comparison of the accuracy and economics of staging by computed tomography and surgery. Gastroenterology. 1981;80:45–50. [PubMed] [Google Scholar]
- 18.Ward J, Chen F, Guthrie JA, Wilson D, Lodge JP, Wyatt JI, Robinson PJ. Hepatic lesion detection after superparamagnetic iron oxide enhancement: comparison of five T2-weighted sequences at 1.0 T by using alternative-free response receiver operating characteristic analysis. Radiology. 2000;214:159–166. doi: 10.1148/radiology.214.1.r00ja21159. [DOI] [PubMed] [Google Scholar]
- 19.Fukuya T, Honda H, Kaneko K, Kuroiwa T, Yoshimitsu K, Irie H, Maehara Y, Masuda K. Efficacy of helical CT in T-staging of gastric cancer. J Comput Assist Tomogr. 1997;21:73–81. doi: 10.1097/00004728-199701000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Miller FH, Kochman ML, Talamonti MS, Ghahremani GG, Gore RM. Gastric cancer. Radiologic staging. Radiol Clin North Am. 1997;35:331–349. [PubMed] [Google Scholar]
- 21.Minami M, Kawauchi N, Itai Y, Niki T, Sasaki Y. Gastric tumors: radiologic-pathologic correlation and accuracy of T staging with dynamic CT. Radiology. 1992;185:173–178. doi: 10.1148/radiology.185.1.1523303. [DOI] [PubMed] [Google Scholar]
- 22.Takao M, Fukuda T, Iwanaga S, Hayashi K, Kusano H, Okudaira S. Gastric cancer: evaluation of triphasic spiral CT and radiologic-pathologic correlation. J Comput Assist Tomogr. 1998;22:288–294. doi: 10.1097/00004728-199803000-00024. [DOI] [PubMed] [Google Scholar]


