Abstract
AIM: To study the role of Fas ligand (FasL) and Caspase-3 expression in carcinogenesis and progression of gastric cancer and molecular mechanisms of relevant immune escape.
METHODS: FasL and Caspase-3 expression was studied in adjacent epithelial cells, cancer cells and lymphocytes of primary foci, and cancer cells of metastatic foci from 113 cases of gastric cancer by streptavidin-biotin-peroxidase (S-P) immunohistochemistry. Expression of both proteins in cancer cells of primary foci was compared with clinicopathological features of gastric cancer. The relationship between FasL expression in cancer cells and Caspase-3 expression in cancer cells or infiltrating lymphocytes of primary foci was investigated.
RESULTS: Cancer cells of primary foci expressed FasL in 53.98% (61/113) of gastric cancers, more than their adjacent epithelial cells (34.51%, 39/113) (P = 0.003, χ2 = 8.681), while the expression of Caspase-3 in cancer cells of primary foci was detected in 32.74% (37/113) of gastric cancers, less than in the adjacent epithelial cells (50.44%, 57/113) (P = 0.007, χ2 = 7.286). Infiltrating lymphocytes of the primary foci showed positive immunoreactivity to Caspase-3 in 70.80% (80/113) of gastric cancers, more than their corresponding adjacent epithelial cells (P = 0.001, χ2 = 10.635) or cancer cells of primary foci (P = 0.000, χ2 = 32.767). FasL was less expressed in cancer cells of metastases (51.16%, 22/43) than in those of the corresponding primary foci (81.58%, 31/38) (P = 0.003, χ2 = 9.907). Conversely, Caspase-3 was more expressed in cancer cells of metastases (58.14%, 25/43) than in those of the corresponding primary foci (34.21%, 13/38) (P = 0.031, χ2 = 4.638). FasL expression was significantly correlated with tumor size (P = 0.035, rs = 0.276), invasive depth (P = 0.039, rs = 0.195), metastasis (P = 0.039, rs = 0.195), differentiation (P = 0.015, rs = 0.228) and Lauren’s classification (P = 0.038, rs = 0.196), but not with age or gender of patients, growth pattern or TNM staging of gastric cancer (P > 0.05). In contrast, Caspase-3 expression showed no correlation with any clinicopathological parameters described above in cancer cells of the primary foci (P > 0.05). Interestingly, FasL expression in primary gastric cancer cells paralleled to Caspase-3 expression in infiltrating lymphocytes of the primary foci (P = 0.016, χ2 = 5.825).
CONCLUSION: Up-regulated expression of FasL and down-regulated expression of Caspase-3 in cancer cells of primary foci play an important role in gastric carcinogenesis. As an effective marker to reveal the biological behaviors, FasL is implicated in differentiation, growth, invasion and metastasis of gastric cancer by inducing apoptosis of infiltrating lymphocytes. Chemical substances derived from the primary foci and metastatic microenvironment can inhibit the growth of metastatic cells by enhancing Caspase-3 expression and diminishing FasL expression.
INTRODUCTION
Gastric cancer is one of the commonest malignancies in China, and even in the world[1-7]. Although early diagnosis and treatment have somewhat improved the patients outcome, gastric cancer still remains the major killer among Chinese[8,9]. The stepwise transitions during gastric carcinogenesis and progression show that growth-limited gastric epithelial cells become immortalized and in turn exhibit malignant phenotypes[10,11]. It is obvious that the intrinsic regulatory systems for normal cell survival and death are perturbed in these sequential changes of gastric carcinogenesis and progression, so a further understanding of aberrant apoptosis of gastric epithelial cells and cancer cell will be of great significance in the prevention, diagnosis and treatment of gastric cancer.
Fas ligand (FasL) is a family member of tumor necrosis factor (TNF) and nerve growth factor (NGF), which maps to human chromosome 1[12]. When membrane FasL (mFasL) crosslinks with membrane Fas (mFas), cellular apoptosis is induced. However, soluble Fas is released into tumor microenvironment to neutralize FasL on tumor infiltrating lymphocytes and consequently blocks their apoptotic induction, leading to tumor immune escape[13]. Some matrix metalloproteinases can hydrolyze the mFasL into impotent soluble FasL, which can resist apoptosis-induced effect by lymphocytes[14]. In Fas/FasL pathway, association of Fas with FasL can activate the Fas-associated death domain (FADD) and make mitochondrion release cytochrome C[15], which eventually initiates the key Caspase-3 in catalyzing the specific cleavage of many important cellular proteins during apoptosis[16,17].
Capase-3/CPP32 is a member of the interleukin-1β-converting enzyme (ICE) family, which specifically cleaves substrates at the C-terminal of aspartic acid residues. Capase-3 includes two types of CPP32α and CPP32β with cysteine protease activity, and shows a high homology to the pro-apoptotic ced-3 of C elegans[18,19]. Several members of the Caspase family have been implicated in apoptosis, among which Caspase-3 is thought to act as a central mediator of programmed cell death (PCD) in mammalian cells[20]. Caspase-3 is synthesized as an inactive 32 kd proenzyme and processed during apoptosis into its active form that is composed of two subunits, p17-20 and p10-12. Activated Capase-3 is responsible for the cleavage of poly (ADP-ribose) polymerase (PARP), actin and sterol regulatory element binding protein (SREBP), which relate to apoptosis[19,21-23]. Inhibition of the CPP32-induced proteolytic breakdown of PARP has been demonstrated to result in the attenuation of apoptosis[15].
Previous study suggested that high expression of Fas and FasL was involved in gastrocarcinogenesis[24]. In the current study, we aimed to evaluate the expression of FasL and Caspase-3 in adjacent epithelial cells, cancer cells and lymphocytes of the primary foci, and cancer cells of the metastatic foci of gastric cancer and to find out if there is any relationship between their expressions in cancer cells of the primary foci and clinicopathological features of gastric cancer, as well as between FasL expression in cancer cells of the primary foci and Caspase-3 expression in cancer cells or its infiltrating lymphocytes of the primary foci in order to clarify the role of FasL and Caspase-3 expression in carcinogenesis and progression of gastric cancer and the molecular mechanism of relevant immune escape.
MATERIALS AND METHODS
Subjects
Surgical specimens of 113 gastric cancers were studied from the Second Affiliated Hospital of China Medical University from Sep. 1997 to Feb. 2001. None of the patients underwent radiotherapy or chemotherapy before operation. Adjacent mucosa and primary tumors of all the cases, as well as 43 metastases from 38 cases were fixed in 4% formaldehyde solution, embedded in paraffin and cut into 4 μm sections.
Evaluation of clinicopathological features
Hematoxylin-and-eosin-stained sections were examined by two pathologists to confirm the histological diagnosis and other microscopic characteristics. These cancers were histologically classified into differentiated and undifferentiated cancers. Their growth pattern was classified into mass, nest, or diffuse types, as reported previously[6]. Penetration of gastric wall, lymph node and distal metastases were routinely described in each patient. Tumor staging was assessed according to TNM classification system.
Immunohistochemistry
The representative and consecutive sections from each adjacent mucosa, primary and secondary tumor were immunostained with streptavidin-peroxidase technique (S-P kit, Zhongshan Biotech.). Anti-FasL antibody (Boster Biotech.) and anti-Caspase-3 antibody (DAKO Biotech.) were diluted in phosphate-buffered saline (PBS, 0.01 mol/L, pH7.4) at the dilution ratio of 1:50 and 1:100 respectively. All procedures were implemented according to the product illustration. For negative controls, sections were processed as the above but with PBS instead of the primary antibodies.
Evaluation of FasL and Caspase-3 staining
The immunoreactivity to FasL or Caspase-3 was localized in the cytoplasm. From 5 randomly selected representative fields of each section, one hundred cells were counted by two independent observers, who did not know the clinicopathological features of these gastric cancers. According to proportion of positive cells, the degree of staining achieved with their antibodies was graded as follows: negative (-), ≤ 5%; weakly positive (+), 5%-25%; moderately positive (++), 25%-50%; and strongly positive (+++), ≥ 50%.
Statistical analysis
Statistical evaluation was performed using chi-square to differentiate the rates of different groups and using Spearman’s test to analyze ranking correlation. P < 0.05 was considered statistically significant. SPSS 10.0 software for Windows was employed to analyze all data.
RESULTS
FasL and Capase-3 expression in adjacent epithelial cells, cancer cells and lymphocytes of the primary foci, and cancer cells of the metastatic foci of gastric cancer
Cancer cells of the primary foci expressed FasL in 53.98% (61/113) of gastric cancers, more than the adjacent epithelial cells (34.51%, 39/113) (P = 0.003, χ2 = 8.681), while Caspase-3 in cancer cells of primary foci was expressed in 32.74% (37/113) of gastric cancers, less than in the adjacent epithelial cells (50.44%, 57/113) (P = 0.007, χ2 = 7.286). Infiltrating lymphocytes in the primary foci showed strong immunoreactivity to Caspase-3 in 70.80% (80/113) of gastric cancers, more than that in the corresponding adjacent epithelium (P = 0.001, χ2 = 10.635) or cancer cells of the primary foci (P = 0.000, χ2 = 32.767). FasL was less expressed in cancer cells of metastases (51.16%, 22/43) than in those of the corresponding primary foci (81.58%, 31/38) (P = 0.003, χ2 = 9.907). Conversely, Caspase-3 was more positively expressed in cancer cells of metastases (58.14%, 25/43) than in those of the corresponding primary foci (34.21%, 13/38) (P = 0.031, χ2 = 4.638) (Table 1, Figure 1 Figure 2, Figure 3, Figure 4).
Table 1.
Expression of FasL and Caspase-3 in adjacent epithelial cells, cancer cells of primary and metastatic foci of gastric cancer
| n |
FasL expression |
Caspase-3 expression |
|||||
| - | +~+++ | PR(%) | - | +~+++ | PR(%) | ||
| Adjacent epithelial cells | 113 | 74 | 39 | 34.51 | 56 | 57 | 50.44 |
| Cancer cells of primary foci | 113 | 52 | 61 | 53.98a | 76 | 37 | 32.74b |
| Cancer cells of metastatic foci | 43 | 21 | 22 | 51.16c | 18 | 25 | 58.14d |
P = 0.003 (χ2 = 8.681, Pearson’ R = 0.176), vs adjacent epithelial cells;
P = 0.007 (χ2 = 7.286, Pearson’ R = 0.180) , vs adjacent epithelial cells;
P = 0.003 (χ2 = 9.907, Pearson’R = 0.245), vs cancer cells of the corresponding primary foci;
P = 0.031 (χ2 = 4.638, Pearson’R = 0.239), vs cancer cells of the corresponding primary foci; PR, positive rate.
Figure 1.
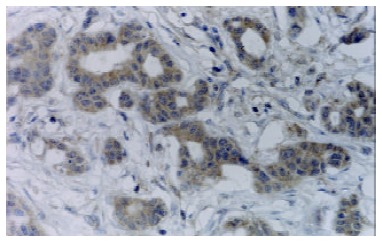
FasL was strongly expressed in moderately differentiated adenocarcinoma of stomach (+++). S-P × 400.
Figure 2.
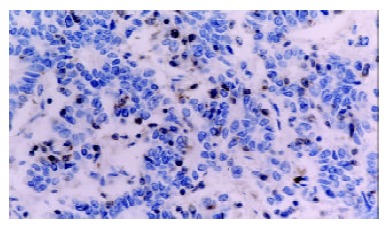
Caspase-3 was negatively expressed in poorly differentiated adenocarcinoma of stomach (-), while strongly positive in tumor infiltrating lymphocytes (+++). S-P × 400.
Figure 3.
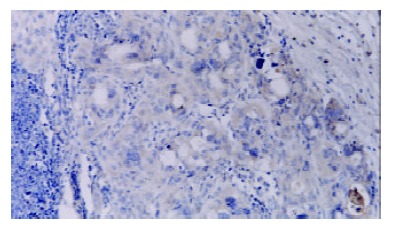
FasL was strongly expressed in gastric cancer cells of lymph node metastasis (+++). S-P × 400.
Figure 4.
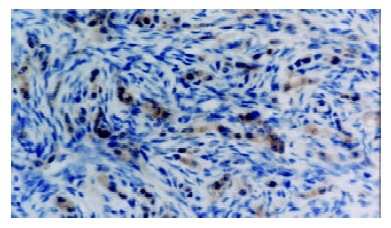
Caspase-3 was strongly expressed in gastric cancer cells of ovary metastasis (+++). S-P × 400.
Relationship between FasL and Caspase-3 expression in cancer cells of the primary foci and the clinicopathological features of gastric cancer
FasL expression was significantly correlated with tumor size (P = 0.035, rs = 0.276), invasive depth (P = 0.039, rs = 0.195), metastasis (P = 0.039, rs = 0.195), histological classification (P = 0.015, rs = 0.228) and Lauren's classification (P = 0.038, rs = 0.196) of gastric cancer in cancer cells of the primary foci, whereas not with growth pattern or TNM staging of gastric cancer, gender or age of patients (P > 0.05). Comparatively, Caspase-3 expression showed no significant correlation with tumor size, invasive depth, metastasis, histological classification, TNM staging, age and gender of patients in cancer cells of the primary foci (P > 0.05) (Table 2).
Table 2.
Relationship between the expression of FasL , Caspase-3 in cancer cells of primary foci and clinicopathological features of gastric cancer
| Clinicopathological features | n |
FasL expression |
Caspase-3 expression |
||||||||||
| - | + | ++ | +++ | PR(%) | P value | - | + | ++ | +++ | PR(%) | P value | ||
| Age | 0.318 | 0.414 | |||||||||||
| < 50 years | 33 | 13 | 7 | 6 | 7 | 60.61 | 21 | 3 | 3 | 6 | 36.36 | ||
| ≥ 50 years | 80 | 39 | 17 | 14 | 10 | 51.25 | 55 | 13 | 4 | 8 | 31.25 | ||
| Gender | 0.548 | 0.514 | |||||||||||
| Male | 83 | 37 | 16 | 16 | 14 | 55.42 | 57 | 12 | 5 | 9 | 31.32 | ||
| Female | 30 | 15 | 5 | 7 | 3 | 50.00 | 19 | 4 | 2 | 5 | 36.37 | ||
| Tumor size | 0.035 | 0.802 | |||||||||||
| < 4 cm | 47 | 27 | 8 | 7 | 5 | 42.55 | 33 | 3 | 5 | 6 | 29.79 | ||
| ≥ 4 cm | 66 | 25 | 13 | 16 | 12 | 62.12 | 43 | 13 | 2 | 8 | 34.84 | ||
| Invasive depth | 0.039 | 0.987 | |||||||||||
| Above submucosa | 26 | 16 | 4 | 4 | 2 | 38.46 | 18 | 3 | 3 | 2 | 30.76 | ||
| Muscularis propria | 34 | 16 | 6 | 8 | 4 | 52.94 | 22 | 6 | 0 | 6 | 32.35 | ||
| Below subserosa | 53 | 20 | 11 | 11 | 11 | 62.26 | 36 | 7 | 4 | 6 | 33.96 | ||
| Metastasis | 0.039 | 0.913 | |||||||||||
| - | 75 | 40 | 12 | 15 | 8 | 46.67 | 51 | 9 | 3 | 12 | 32.00 | ||
| + | 38 | 12 | 9 | 8 | 9 | 68.42 | 25 | 7 | 4 | 2 | 34.21 | ||
| TNM staging | 0.312 | 0.506 | |||||||||||
| O | 18 | 10 | 3 | 3 | 2 | 44.44 | 12 | 3 | 1 | 2 | 33.33 | ||
| I | 28 | 13 | 4 | 8 | 3 | 53.57 | 18 | 3 | 2 | 5 | 35.71 | ||
| II | 40 | 19 | 8 | 8 | 5 | 52.50 | 26 | 7 | 2 | 5 | 35.00 | ||
| III | 17 | 7 | 2 | 2 | 6 | 58.82 | 13 | 1 | 1 | 2 | 23.53 | ||
| IV | 10 | 3 | 4 | 2 | 1 | 70.00 | 7 | 2 | 1 | 0 | 30.00 | ||
| Growth pattern | 0.338 | 0.735 | |||||||||||
| Mass | 23 | 8 | 5 | 10 | 0 | 65.22 | 17 | 3 | 1 | 2 | 26.09 | ||
| Nest | 30 | 12 | 4 | 5 | 9 | 60.00 | 18 | 5 | 1 | 6 | 40.00 | ||
| Diffuse | 34 | 18 | 7 | 3 | 6 | 47.06 | 23 | 5 | 2 | 4 | 32.35 | ||
| Lauren'sclassification | 0.038 | 0.333 | |||||||||||
| Intestinal type | 36 | 12 | 7 | 13 | 4 | 66.67 | 27 | 3 | 1 | 5 | 25.00 | ||
| Diffuse type | 57 | 32 | 10 | 8 | 7 | 43.86 | 36 | 9 | 5 | 7 | 36.84 | ||
| Mixed type | 20 | 8 | 4 | 2 | 6 | 60.00 | 13 | 4 | 1 | 2 | 35.00 | ||
| Histological classification | 0.015 | 0.754 | |||||||||||
| Differentiated | 53 | 19 | 9 | 14 | 11 | 64.15 | 37 | 6 | 2 | 8 | 30.18 | ||
| Undifferentiated | 60 | 33 | 12 | 9 | 6 | 45.00 | 39 | 10 | 5 | 6 | 35.00 | ||
PR, positive rate.
Relationship between FasL and Caspase-3 expression in gastric cancer
Interestingly, our results showed FasL expression in cancer cells of the primary foci was positively associated with Caspase-3 expression in their infiltrating lymphocytes (P = 0.016, χ2 = 5.825), not with Caspase-3 expression in cancer cells of the primary foci (P > 0.05) (Table 3).
Table 3.
Relationship between FasL and Caspase-3 expression in gastric cancer
| FasL expression In CC of PF | n |
Caspase-3 expression in CC of PF |
Caspase-3 expression in ILC of PF |
||||
| - | +~+++ | PR(%) | - | +~+++ | PR(%) | ||
| - | 52 | 39 | 13 | 26.92 | 21 | 31 | 65.38 |
| +~+++ | 61 | 37 | 24 | 37.70 | 12 | 49 | 75.41a |
| PR(%) | 113 | 76 | 37 | 32.74 | 33 | 80 | 70.80 |
P = 0.016 (χ2 = 5.825, Pearson'P = 0.227), vs FasL-negative cases; CC, cancer cells; PF, primary foci; ILC, infiltrating lymphocytes; PR, positive rate.
DISCUSSION
As a transmembrane type II protein, FasL initiates PCD through activating FADD when coupled with mFas, so FasL greatly contributes to apoptotic induction in most cells[25]. During the course, Caspase-3 will be activated by a series of cascade reactions, and eventually DNase (CAD, CPAN, or DEF40) is activated, which belongs to the Mg2+-dependent endonuclease and acts as a killer in apoptosis. Some previous studies demonstrated that esophageal and colon cancers highly expressed FasL at the levels of mRNA and protein. Furthermore, increased FasL can bind to mFas in infiltrating lymphocytes, which is eliminated by inducing their apoptosis[26,27]. Therefore, cancer cells can form an immune escape.
In this study, we found that FasL and Caspase-3 were expressed in 34.51% (39/113) and 50.44% (57/113) of adjacent epithelial of gastric cancer respectively, most of which exhibited inflammation, regeneration, or intestinal metaplasia. Li et al[24] detected FasL expression in normal gastric mucosa and atrophic gastritis. It was previously documented that most cells from human organs showed positive immunoreactivity to Caspase-3, including cryptal cells of gastric pit. These data indicate that FasL and Caspase-3 play an important role in regulating the balance of cellular proliferation and apoptosis in gastric mucosa by inducing apoptosis.
Moreover, FasL is more frequently expressed in cancer cells of primary foci than in their adjacent epithelial cells (P < 0.05). Belluco et al[28] found that FasL was overexpressed in adenoma-adenocarcinoma of colorectum and FasL expression paralleled to malignant degrees. These results suggest that up-regulated expression of FasL contributes to gastric carcinogenesis. On the other hand, Capase-3 expression was less frequently detected in cancer cells of the primary foci, than in the adjacent epithelial cells (P < 0.05), as described previously[29]. Hoshi et al[30] also found the positive rate of Caspase-3 expression was lower in gastric cancers than in their adjacent mucosa and gastric adenoma, and was correlated negatively with proliferative index of Ki-67, as well as positively with apoptotic index labeled by TUNEL. These studies reveal that down-regulated expression of Capase-3 is implicated in gastric carcinogenesis.
Additionally, our results also indicated that Caspase-3 expression in infiltrating lymphocytes was found in 70.80% of gastric cancers, significantly more than in cancer cells and adjacent epithelial cells of their primary foci (P < 0.05). So most of primary gastric cancers showed strong expression of Capase-3 in infiltrating lymphocytes, whereas weak or no expression of Caspase-3 in primary cancer cells. Interestingly, we found that FasL expression in primary cancer cells was closely correlated with Caspase-3 expression in filtrating lymphocytes of the primary cancer (P < 0.05). Although FasL-positive gastric cancer expressed more Caspase-3 than FasL-negative one, the positive rates of Caspase-3 expression in FasL-positive and FasL-negative carcinomas were of no significant difference (P > 0.05). The more FasL was expressed in cancer cells, the more Caspase-3 was expressed in lymphocytes in the primary foci. Some investigators found that FasL-positive cancer showed increased apoptosis in its infiltrating lymphocytes and decreased apoptosis in cancer cells[31]. Our study showed that more expression of Caspase-3 in infiltrating lymphocytes of gastric cancer demonstrated its critical contribution to Fas-mediated immune escape. However, FasL-positive gastric cancer also showed high expression of Caspase-3, suggesting there were other mechanisms that played an important role in Fas-Caspase apoptotic pathway of gastric cancer cells.
In our study, FasL expression in cancer cells of primary foci was not correlated with gender or age of patients with gastric cancer (P > 0.05), suggesting that FasL expression in the primary cancer cells genetically did not depend on the gender or age of patients, and they therefore had no effect on relationship between FasL expression and patho-biological behaviors of gastric cancer. Our results showed that FasL expression in cancer cells of primary foci was closely associated with tumor size, invasive depth, metastasis, histological differentiation and Lauren's classification (P < 0.05), but not with growth pattern or TNM staging of gastric cancer (P > 0.05). Tsutsumi et al[32] found serum content of soluble FasL (sFasL) was correlated with invasive depth, lymph node and distal metastasis. These indicate that FasL is implicated in progression of gastric cancer by forming immune counterattack. Although FasL expression in primary cancer cells was not significantly associated with growth pattern of gastric cancer, higher expression of FasL in mass-type than in nest-type or diffuse-type gastric cancer revealed that increased FasL expression in cancer cells responsed to elevated infiltrating lymphocytes in gastric cancer with mass growth. Notably, FasL was expressed more in intestinal-type gastric cancers than in diffuse-type ones (P < 0.05), indicating that both types of gastric cancer have different histogenetic pathways. Furthermore, this study showed no relationship between down-regulated expression of Caspase-3 and clinicopathological features of gastric cancer described above (P > 0.05), suggesting that Caspase-3 expression is genetically independent of gender or age and is not implicated in progression.
FasL was significantly expressed in cancer cells of the primary foci than in corresponding metastatic foci (P < 0.05), while Caspase-3 was less expressed in cancer cells of primary foci than in corresponding metastatic foci of gastric cancer (P < 0.05), demonstrating that decreased ability of metastatic cancer cells to induce apoptosis of lymphocytes and increased apoptosis in metastatic cancer cells, might be influenced by tumor microenvironment. It was documented that metastases grew rapidly after the primary foci of tumor were removed, and a kind of substance from the primary foci could inhibit growth of metastases[33]. If so, another reason for this phenomenon might be that primary foci regulate the expression of Caspase-3 and FasL of metastatic cancer cells so as to suppress their ability to proliferate.
In conclusion, up-regulation of FasL expression and down-regulation of Caspase-3 expression in cancer cells of primary carcinoma are involved in gastric carcinogenesis. As an effective marker to reveal the patho-biological behaviors, FasL contributes to differentiation, growth, invasion and metastasis of gastric cancer by inducing apoptosis of infiltrating lymphocytes. Chemical substances from primary foci and metastatic microenvironment can induce apoptosis of metastatic cancer cells or weaken ability of metastatic cancer cells to counterattack adjacent lymphocytes by decreasing FasL expression and increasing Caspase-3 expression of metastastic cancer cells, eventually resulting in the inhibition of their growth.
ACKNOWLEDGMENTS
We thank Mrs Zhao Yuhong and Mrs Huang Yaming for their contribution to the preparation of this manuscript.
Footnotes
Edited by Zhang JZ
References
- 1.Zhou YN, Xu CP, Han B, Li M, Qiao L, Fang DC, Yang JM. Expression of E-cadherin and beta-catenin in gastric carcinoma and its correlation with the clinicopathological features and patient survival. World J Gastroenterol. 2002;8:987–993. doi: 10.3748/wjg.v8.i6.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fang DC, Luo YH, Yang SM, Li XA, Ling XL, Fang L. Mutation analysis of APC gene in gastric cancer with microsatellite instability. World J Gastroenterol. 2002;8:787–791. doi: 10.3748/wjg.v8.i5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song ZJ, Gong P, Wu YE. Relationship between the expression of iNOS, VEGF, tumor angiogenesis and gastric cancer. World J Gastroenterol. 2002;8:591–595. doi: 10.3748/wjg.v8.i4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao XX, Yin L, Sun ZC. The expression of hTERT mRNA and cellular immunity in gastric cancer and precancerosis. World J Gastroenterol. 2002;8:586–590. doi: 10.3748/wjg.v8.i4.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niu WX, Qin XY, Liu H, Wang CP. Clinicopathological analysis of patients with gastric cancer in 1200 cases. World J Gastroenterol. 2001;7:281–284. doi: 10.3748/wjg.v7.i2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xin Y, Li XL, Wang YP, Zhang SM, Zheng HC, Wu DY, Zhang YC. Relationship between phenotypes of cell-function differentiation and pathobiological behavior of gastric carcinomas. World J Gastroenterol. 2001;7:53–59. doi: 10.3748/wjg.v7.i1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang BJ, Sun RX, Lin H, Gao YF. Study on the risk factors of lymphatic metastasis and the indications of less in vasive operations in early gastric cancer. World J Gastroenterol. 2000;6:553–556. doi: 10.3748/wjg.v6.i4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jemal A, Thomas A, Murray T, Thun M. Cancer statistics, 2002. CA Cancer J Clin. 2002;52:23–47. doi: 10.3322/canjclin.52.1.23. [DOI] [PubMed] [Google Scholar]
- 9.Theuer CP. Asian gastric cancer patients at a southern California comprehensive cancer center are diagnosed with less advanced disease and have superior stage-stratified survival. Am Surg. 2000;66:821–826. [PubMed] [Google Scholar]
- 10.Schmidt PH, Lee JR, Joshi V, Playford RJ, Poulsom R, Wright NA, Goldenring JR. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab Invest. 1999;79:639–646. [PMC free article] [PubMed] [Google Scholar]
- 11.Bajtai A, Hidvégi J. The role of gastric mucosal dysplasia in the development of gastric carcinoma. Pathol Oncol Res. 1998;4:297–300. doi: 10.1007/BF02905220. [DOI] [PubMed] [Google Scholar]
- 12.Sakata K, Sakata A, Kong L, Dang H, Talal N. Role of Fas/FasL interaction in physiology and pathology: the good and the bad. Clin Immunol Immunopathol. 1998;87:1–7. doi: 10.1006/clin.1997.4504. [DOI] [PubMed] [Google Scholar]
- 13.O'Connell J, Bennett MW, O'Sullivan GC, Roche D, Kelly J, Collins JK, Shanahan F. Fas ligand expression in primary colon adenocarcinomas: evidence that the Fas counterattack is a prevalent mechanism of immune evasion in human colon cancer. J Pathol. 1998;186:240–246. doi: 10.1002/(SICI)1096-9896(199811)186:3<240::AID-PATH173>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 14.Mitsiades N, Yu WH, Poulaki V, Tsokos M, Stamenkovic I. Matrix metalloproteinase-7-mediated cleavage of Fas ligand protects tumor cells from chemotherapeutic drug cytotoxicity. Cancer Res. 2001;61:577–581. [PubMed] [Google Scholar]
- 15.Wilson MR. Apoptosis: unmasking the executioner. Cell Death Differ. 1998;5:646–652. doi: 10.1038/sj.cdd.4400394. [DOI] [PubMed] [Google Scholar]
- 16.Slee EA, Adrain C, Martin SJ. Serial killers: ordering caspase activation events in apoptosis. Cell Death Differ. 1999;6:1067–1074. doi: 10.1038/sj.cdd.4400601. [DOI] [PubMed] [Google Scholar]
- 17.Porter AG, Jänicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 18.Shaham S, Reddien PW, Davies B, Horvitz HR. Mutational analysis of the Caenorhabditis elegans cell-death gene ced-3. Genetics. 1999;153:1655–1671. doi: 10.1093/genetics/153.4.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tian Rh, Zhang GY, Yan CH, Dai YR. Involvement of poly(ADP-ribose) polymerase and activation of caspase-3-like protease in heat shock-induced apoptosis in tobacco suspension cells. FEBS Lett. 2000;474:11–15. doi: 10.1016/s0014-5793(00)01561-1. [DOI] [PubMed] [Google Scholar]
- 20.Kutsyi MP, Kuznetsova EA, Gaziev AI. Involvement of proteases in apoptosis. Biochemistry ( Mosc) 1999;64:115–126. [PubMed] [Google Scholar]
- 21.Truong-Tran AQ, Carter J, Ruffin RE, Zalewski PD. The role of zinc in caspase activation and apoptotic cell death. Biometals. 2001;14:315–330. doi: 10.1023/a:1012993017026. [DOI] [PubMed] [Google Scholar]
- 22.Fujii Y, Matsura T, Kai M, Matsui H, Kawasaki H, Yamada K. Mitochondrial cytochrome c release and caspase-3-like protease activation during indomethacin-induced apoptosis in rat gastric mucosal cells. Proc Soc Exp Biol Med. 2000;224:102–108. doi: 10.1046/j.1525-1373.2000.22407.x. [DOI] [PubMed] [Google Scholar]
- 23.Umeda T, Kouchi Z, Kawahara H, Tomioka S, Sasagawa N, Maeda T, Sorimachi H, Ishiura S, Suzuki K. Limited proteolysis of filamin is catalyzed by caspase-3 in U937 and Jurkat cells. J Biochem. 2001;130:535–542. doi: 10.1093/oxfordjournals.jbchem.a003016. [DOI] [PubMed] [Google Scholar]
- 24.Li H, Liu N, Guo L, Li JW, Liu J. Frequent expression of soluble Fas and Fas ligand in Chinese stomach cancer and its preneoplastic lesions. Int J Mol Med. 2000;5:473–476. doi: 10.3892/ijmm.5.5.473. [DOI] [PubMed] [Google Scholar]
- 25.Chang HY, Yang X. Proteases for cell suicide: functions and regulation of caspases. Microbiol Mol Biol Rev. 2000;64:821–846. doi: 10.1128/mmbr.64.4.821-846.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Connell J, Bennett MW, Nally K, Houston A, O'Sullivan GC, Shanahan F. Altered mechanisms of apoptosis in colon cancer: Fas resistance and counterattack in the tumor-immune conflict. Ann N Y Acad Sci. 2000;910:178–192; discussion 193-195. doi: 10.1111/j.1749-6632.2000.tb06708.x. [DOI] [PubMed] [Google Scholar]
- 27.Izban KF, Wrone-Smith T, Hsi ED, Schnitzer B, Quevedo ME, Alkan S. Characterization of the interleukin-1beta-converting enzyme/ced-3-family protease, caspase-3/CPP32, in Hodgkin's disease: lack of caspase-3 expression in nodular lymphocyte predominance Hodgkin's disease. Am J Pathol. 1999;154:1439–1447. doi: 10.1016/s0002-9440(10)65398-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belluco C, Esposito G, Bertorelle R, Alaggio R, Giacomelli L, Bianchi LC, Nitti D, Lise M. Fas ligand is up-regulated during the colorectal adenoma-carcinoma sequence. Eur J Surg Oncol. 2002;28:120–125. doi: 10.1053/ejso.2001.1223. [DOI] [PubMed] [Google Scholar]
- 29.Bennett MW, O'Connell J, Houston A, Kelly J, O'Sullivan GC, Collins JK, Shanahan F. Fas ligand upregulation is an early event in colonic carcinogenesis. J Clin Pathol. 2001;54:598–604. doi: 10.1136/jcp.54.8.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoshi T, Sasano H, Kato K, Yabuki N, Ohara S, Konno R, Asaki S, Toyota T, Tateno H, Nagura H. Immunohistochemistry of Caspase3/CPP32 in human stomach and its correlation with cell proliferation and apoptosis. Anticancer Res. 1998;18:4347–4353. [PubMed] [Google Scholar]
- 31.Bennett MW, O'Connell J, O'Sullivan GC, Brady C, Roche D, Collins JK, Shanahan F. The Fas counterattack in vivo: apoptotic depletion of tumor-infiltrating lymphocytes associated with Fas ligand expression by human esophageal carcinoma. J Immunol. 1998;160:5669–5675. [PubMed] [Google Scholar]
- 32.Tsutsumi S, Kuwano H, Shimura T, Morinaga N, Mochiki E, Asao T. Circulating soluble Fas ligand in patients with gastric carcinoma. Cancer. 2000;89:2560–2564. doi: 10.1002/1097-0142(20001215)89:12<2560::aid-cncr7>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 33.Torosian MH, Bartlett DL. Inhibition of tumor metastasis by a circulating suppressor factor. J Surg Res. 1993;55:74–79. doi: 10.1006/jsre.1993.1111. [DOI] [PubMed] [Google Scholar]


