Abstract
AIM: To assess the putative involvement of NF-κB and pro-inflammatory cytokines in the pathogenesis of cancer cachexia and the therapeutic efficacy of indomethacin (IND) on cachexia.
METHODS: Thirty young male BABL/c mice were divided randomly into five groups: (a) control, (b) tumor-bearing plus saline, (c) tumor-bearing plus IND (0.25 mg•kg-1), (d) tumor-bearing plus IND (0.5 mg•kg-1), and (e) tumor-bearing plus IND (2 mg•kg-1). Colon 26 adenocarcinoma cells of murine were inoculated subcutaneously to induce cachexia. Saline and IND were given intraperitoneally daily for 7 d from the onset of cachexia to sacrifice. Food intake and body composition were documented, serum levels of TNF-α and IL-6 and activity of NF-κB in the spleen were investigated in all animals.
RESULTS: Weight loss was observed in all tumor-bearing mice. By day 16, body weights of non-tumor mice were about 72% of healthy controls (P < 0.01), and the weight of gastrocnemius was decreased by 28.7% (P < 0.01). No difference was found between groups in food intake (P > 0.05). Gastrocnemius weight was increased markedly (P < 0.01) after treatment of IND (0.5 mg•kg-1), while the non-tumor body weights were not significantly elevated. Tumor-bearing caused a 2-3 fold increase in serum levels of both TNF-α and IL-6 (P < 0.01). The concentration of TNF-α (P < 0.05) and IL-6 (P < 0.01) in tumor-bearing mice was reduced after administration of 0.5 mg•kg-1 IND for 7 d. But the level of IL-6 was slightly elevated following treatment of IND 2.0 mg•kg-1. NF-κB activation in the spleen was increased in tumor-bearing mice in comparison with controls in electrophoretic mobility shift assay (EMSA). NF-κB activity was reduced in mice treated with 0.5 mg•kg-1 of IND, whereas a higher NF-κB activity was observed in mice treated with 2.0 mg•kg-1 of IND.
CONCLUSION: Colon 26 adenocarcinoma cells can induce severe cancer cachexia experimentally, and the mechanism may be partially due to the enhanced TNF-α and IL-6 in tumor-bearing animals, which is controlled by NF-κB. Low dose of indomethacin alleviates the cachexia, decreases the activation of NF-κB and the serum levels of TNF-α and IL-6, and prevents body weight loss and muscle atrophy, while no further effect is gained by a higher dosage.
INTRODUCTION
Cancer cachexia is characterized by significant weight loss even at an early course of malignancy, and reduces the quality of life of patients as well as responsiveness to chemotherapy. It is the most debilitating and life-threatening aspect of cancer and is associated with psychological distress and a lower quality of life. Thus, it is necessary to clarify the cellular and molecular biological mechanisms of cancer cachexia for the improvement of cancer treatment.
It has been well established that pro-inflammatory cytokines are important in inducing and promoting the development of experimental cancer cachexia. Most research efforts have focused on the role of cytokines of tumor necrosis factor α (TNF-α), interleukin 6 (IL-6) and interleukin 1 (IL-1)[1-4]. Many of these cytokines are responsible for weight loss, an acute phase of protein response, fat and skeletal muscle protein breakdown and elevated energy expenditure in animals and patients[5]. The nuclear factor-kappaB proteins are ubiquitous transcription factors that mediate cellular responses to a diverse array of stimuli, including lipopolysaccharide, reactive oxygen species (ROS) and several cytokines. Over the past decade, significant advances have been made in elucidation of the molecular signals leading to NF-κB activation, as well as in identification of gene regulation by NF-κB. The role of NF-κB proteins in regulating genes associated with immune system and inflammation has been extensively studied. Activation of NF-κB in immune cells upregulates the expression of cytokines, and growth factors that are essential to immune response contribute to inflammation. An autoregulatory feedback loop has been generated with production of cytokines TNF-α and IL-1. NF-κB represents an upstream element of a common pathway that produces catabolic cytokines[6-8]. Such a pathway has an obvious clinical importance in providing potential targets for therapeutic intervention to inhibit or reverse cancer cachexia.
In the present study, a cachectic model was established in mouse bearing colon 26 adenocarcinoma cells for studying the mechanism and therapies of cancer cachexia. As with human cachexia, there was a significant loss of body weight in this model, and the animals had substantial hypoglycemia and an increase of circulating pro-inflammatory cytokines that were thought to be correlative to the onset of cancer cachexia[9-12]. Early researches showed that indomethacin (IND) could prolong the mean survival time of patients with advanced solid cancer[13]. In this study, we chose IND as an anti-inflammatory agent to study its effect on peripheral blood cytokine levels and NF-κB activation in spleen. The purpose of this study was to assess the relationship of NF-κB and cancer cachexia and the role of IND in the treatment of cachexia.
MATERIALS AND METHODS
Animals and tumor implantation
BABL/c male mice aged 6-8 wk (weighing 19-22 g) were purchased from the Animal Center of Chinese Academy of Sciences, Shanghai, China. They had free access to standard laboratory chow and tap water, and were maintained in a temperature-controlled room (22 ± 1 °C) on a 12-h light-dark cycle. Thirty animals were evenly divided into five groups randomly: (a) control, (b) tumor-bearing plus saline, (c) tumor-bearing plus IND (0.25 mg•kg-1, ICN, Biomedicals Inc. Ohio, USA), (d) tumor-bearing plus IND (0.5 mg•kg-1), and (e) tumor-bearing plus IND (2 mg•kg-1). The mice were allowed to adjust to new environment and diet for at least 1 wk before experiment.
Colon 26 adenocarcinoma is appropriate for investigating cancer cachexia. This murine tumor is responsible for inducing weakness, abnormal carbohydrate metabolism, hypercorticism, and elevated levels of pro-inflammatory cytokines. Colon 26 adenocarcinoma cells were kindly supplied by Institute Materia Medica, Chinese Academy of Medical Sciences. Stock cells were passed on the BABL/c mice. Mice of passages three or four were used in the study. On day 0, a homogenate of murine colon 26 adenocarcinoma (50 mg of solid tumor tissue in 0.1 mL of sterile 0.9% NaCl) was injected s.c. into armpits of the tumor-bearing groups. All procedures in the study were approved by the Institutional Animal Care Committee.
Experimental procedures
After inoculation of tumor cells, physical activity, fur condition, and other signs of general well being of the animals were registered. The tumor inoculation site and the tumor size were inspected. The tumor volume was estimated by using the equation ab2/2, where a and b are length and width (cm) of the tumor. Body weight was monitored and food intake was measured at 10 a.m. everyday.
Significant loss of body weight was observed in tumor-bearing groups beginning on day 9 to day 16, 0.1 mL saline and variable dosages of indomethacin were given intraperitoneally to groups b, c, d and e daily for 7 d, respectively. On day 16, carcass weight was measured after removing the entire tumor. Blood samples were collected from orbital veins by removal of one side eyeballs of the animals and stored at -20 °C for determination of TNF-α and IL-6 by ELISA kit (Bender Medsystems, Vienna, Austria). Weight of the gastrocnemius of left hind leg was measured. The spleens were frozen in liquid nitrogen, and stored at -70 °C for nuclear protein extraction and electrophoretic mobility shift assay.
Nuclear protein extraction and electrophoretic mobility shift assay (EMSA)
Nuclear extracts of spleen tissues were prepared by hypotonic lyses followed by high salt extraction as references[14,15]. EMSA was performed using a commercial kit (Gel Shift Assay System; Promega, Madison, WI) as previously described. The NF-κB oligonucleotide probe, (5-AGTTGAGGGGACTTTCCCAGGC-3), was end-labeled with [γ-32P] ATP (Free Biotech, Beijing, China) with T4-polynucleotide kinase. Nuclear protein (20 μL) was preincubated in 9 μL of a binding buffer, consisting of 10 mM Tris -Cl, pH7.5, 1 mM MgCl2, 50 mM NaCl, 0.5 mM EDTA, 0.5 mM DTT, 4% glycerol, and 0.05 g•l-1 of poly-(deoxyinosinic deoxycytidylic acid) for 15 min at room temperature. After addition of 1 μL 32P-labeled oligonuleotide probe, the incubation was continued for 30 min at room temperature. Reaction was stopped by adding 1 μL of gel loading buffer, and the mixture was subjected to non-denaturing 4% polyacrylamide gel electrophoresis in 0.5 × TBE buffer. The gel was vacuum-dried and exposed to X-ray film (Fuji Hyperfilm) at -70 °C with an intensifying screen. NF-κB was quantitated with densitometry.
Statistical analysis
Data were expressed as means ± SE. Statistical significance was determined by one-way ANOVA using SPSS 10.0. P < 0.05 was considered statistically significant.
RESULTS
Tumors were palpable in mice initially on day 5 after inoculation of tumor cells, symptoms of cachexia began 3-4 d later. Weight loss began once a tumor grew to 1 cm3 and a rapid loss of body weight occurred. Initial body weights of mice before experiment had no difference. By day 16, non-tumor body weights of tumor-bearing mice were about 72% of healthy controls (P < 0.01), and the weights of gastrocnemius were lowered by 28.7% (P < 0.01), though the final whole body weights were elevated because of tumor growth. Food intake between groups was not different (P > 0.05, Table 1).
Table 1.
Clinical features of tumor-bearing and non-tumor-bearing mice
| Group | Initial body wt(g) | Final body wt (g) | Nontumor body wt (g) | Gastrocnemius muscle wt (mg) | Dry food intake (g ··day -1) |
| Control | 20.93 ± 1.40 | 25.50 ± 1.71 | 25.50 ± 1.71 | 136.8 ± 6.11 | 6.65 ± 0.24 |
| Tumor + saline | 20.91 ± 1.29 | 25.95 ± 1.61 | 20.92 ± 1.52a | 97.5 ± 8.32a | 6.53 ± 0.31 |
| Tumor+IND(0.25 mg·kg-1) | 20.98 ± 1.42 | 27.75 ± 2.20 | 21.75 ± 1.64 | 95.65 ± 13.5 | 6.56 ± 0.27 |
| Tumor +IND(0.5 mg·kg-1) | 20.92 ± 1.14 | 27.83 ± 1.88 | 22.48 ± 1.57 | 115.82 ± 9.63b | 6.70 ± 0.32 |
| Tumor +IND(2.0 mg·kg-1) | 21.38 ± 1.00 | 28.00 ± 1.24 | 21.90 ± 1.38 | 93.15 ± 10.83 | 6.53 ± 0.19 |
P < 0.01, vs control;
P < 0.01, vs tumor + saline.
After administration of indomethacin for 1 wk, the non-tumor body weights of tumor-bearing mice were increased, but had no significant difference from that in group b. The gastrocnemius weights in animals treated with 0.5 mg•kg-1 of IND increased significantly (P < 0.01). But no weight gain of gastrocnemius in animals treated with IND 2.0 mg•kg-1 was observed. On the contrary, the tumor weight of this group was increased compared with that in saline group (P < 0.01, data not shown). There was no evidence that IND had additional effects on appetite because quantity of food intake of mice treated with IND did not increase.
Tumor-bearing caused a 2-3 fold increase in serum levels of both TNF-α and IL-6 (P < 0.01). Administration of IND 0.5 mg•kg-1 for 7 d reduced the concentrations of TNF-α (P < 0.05) and IL-6 (P < 0.01) in tumor-bearing mice (Table 2). But level of IL-6 slightly increased after administration of 2.0 mg•kg-1 of IND.
Table 2.
Serum levels of TNF-α and IL-6 in mice
| Parameter | Control | Tumor + saline | Tumor + IND(0.25mg ··kg-1) | Tumor+IND(0.5mg ··kg-1) | Tumor+IND(2.0mg ··kg-1) |
| TNF-α(pg/ml) | 43.24 ± 13.37 | 113.83 ± 16.91a | 89.9 ± 4.5 | 71.68 ± 16.70 b | 110.96 ± 20.93 |
| IL-6 (pg/ml) | 1445.82 ± 244.20 | 2646.05 ± 93.39a | 2527.78 ± 266.43 | 1983.07 ± 219.19 c | 2733.33 ± 201.84 |
P < 0.01, vs control;
P < 0.05, vs tumor + saline;
P < 0.01, vs tumor + saline.
EMSA experiments were performed to examine the effect of indomethacin on the activation of NF-κB induced by tumor bearing. As shown in Figure 1, NF-κB activation in spleen was increased in tumor-bearing mice, compared to controls. The tumor-bearing mice treated with 0.5 mg•kg-1 of indomethacin had a lower level of NF-κB than the cachectic mice without treatment. No dose-dependent response of NF-κB activity to different dose of IND treatment was observed. Instead, a higher NF-κB activity was found in the mice treated with IND 2.0 mg•kg-1.
Figure 1.
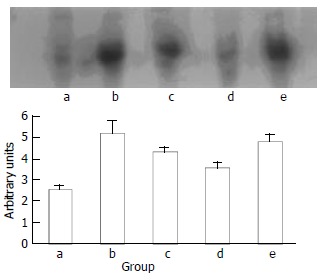
Activation of NF-κB in spleen. Lane a represents the control group, Lane b, c, d, e represents the group of tumor-bearing plus saline, indomethacin 0.25 mg•kg-1, 0.5 mg•kg-1, 2 mg•kg-1, respectively.
DISCUSSION
The syndrome of cancer cachexia including progressive weight loss, particularly in skeletal muscle and adipose tissue, weakness, anemia, and often accompanied by anorexia is an important problem in the management of cancer patients. Nearly all cancer patients have significant weight loss by the time of death. Progressive wasting is an acknowledged clinical problem, it contributes to cancer mortality and may reduce the ability of patients to tolerate aggressive chemotherapy and radiotherapy. Although cachexia is frequently accompanied by anorexia, the decrease of food intake alone may be insufficient to account for cachexia because the body composition change in cachexia differs from that of starvation. Loss of skeletal muscle is more prominent in cachectic patients, leading to weakness and immobility of cancer patients eventually to death due to dysfunction of respiratory muscle. The fact that calories provided by total parenteral nutrition cannot maintain body weight of cancer patients suggests that weight loss is resulted from complex metabolic events rather than simple nutrition insufficiency[16].
Some possible mechanisms can be considered for the wasting in cachexia. It has been reported that weight loss is associated with anorexia in some tumor cachexia model[17]. But it is unlikely that the cachexia in our model was due to anorexia, because food intake was not significantly reduced while the mice were losing weight. Several cytokines are suggested to be involved in the development of cancer cachexia. The last decade has witnessed the discovery of multiple actions of cytokines on the regulation of metabolism in normal and pathophysiological conditions. Some of them are clearly involved in the wasting process that is often accompanied by chronic infection or cancer. We found that TNF-α and IL-6 levels in peripheral blood of animals were elevated while body and muscle weight decreased, which suggested the two cytokines were involved in our cachectic model. TNF-α levels were detectable in serum of pancreatic cancer patients, particularly in those with advanced disease, and these levels correlated with poor nutritional status[18]. Muscle wasting during tumor growth may be associated with the activation of non-lysosomal ubiquitin-dependent proteases associated with enhanced skeletal muscle proteolysis. This activation seems to be mediated by these cytokines, especially TNF-α, IL-6 and some other catabolic factors produced by tumors and hosts[19-22]. It was previously reported that TNF-α was partially mediated DNA fragmentation in skeletal muscle, suggesting an apoptotic phenomenon during experimental cancer-associated cachexia[23]. Production of pro-inflammatory cytokines also induces production of corresponding anti-inflammatory cytokines such as IL-15, IL-1 and IL-6 receptor antagonist, which may significantly reduce the severity of key parameters of cachexia[24-26]. Clearly the balance between pro-inflammatory and anti-inflammatory mediators may be crucial in determining the net clinical effects.
Several experiments suggested that cytokines (IL-1, IL-6, and TNF-α) induced anorexia in tumor-bearing and infected animals, and the appetite was improved in indomethacin-treated animals[17]. However, tumor-bearing mice in our experiments had no significant appetite change, and indomethacin had no obvious effect on diet consumption. So, the wasting condition in this model may have no relation with food consumption, but may be attributed to metabolic disorders. The present study confirmed previous reports demonstrating that a low dose of NASID, particularly indomethacin could inhibit cancer cachexia, whereas a high dose might be toxic[17,27,28]. We found a low dose of indomethacin treatment could decrease TNF-α and IL-6 productions which were very important in the process of immune regulation and inflammation in this experimental model. Indomethacin also protected the host from deterioration in body composition, particularly lean body mass. It is likely that the inhibition was confined to host cell, since indomethacin caused no growth inhibition of tumors. However, contrary evidence also exists. Other reports suggested that IND appeared to suppress the growth of colon 26 as long as the tumor burden was small, whereas it facilitated the tumor growth when the tumor burden was large[29].
NF-κB is normally sequestered in the cytoplasm of nonstimulated cells and must translocate into nuclei to regulate effector gene expression. A family of inhibitory proteins, IkBs, binds to NF-κB and masks its nuclear localization signal domain and therefore controls the translocation of NF-κB. Exposure of cells to extracellular stimuli that perturb redox balance results in rapid phosphorylation, ubiquitination, and proteolytic degradation of IkBs. This process frees NF-κB from the NF-κB/IkBs complexes and enables NF-κB to translocate to the nuclei where they regulate gene transcription. Many effector genes such as those encoding cytokines and adhesion molecules are in turn regulated by NF-κB[6]. Some anti-inflammatory agents (e.g. salicylates, dexamethasone) can inhibit NF-κB, which also indicates that NF-κB is an important molecular target for modulation of inflammatory disease[30-33]. However, the identity of NF-κB, and its role in cancer cachexia still remain to be investigated. In the present study, a low base-line activity of spleen NF-κB was observed in controls, while tumor bearing increased NF-κB activities markedly. A low dose of indomethacin decreased NF-κB activities variably.
In summary, colon 26 adenocarcinoma cells could produce severe cancer cachexia, including loss of non-tumor whole body and gastrocnemius muscle weight. The wasting condition may be partially due to the enhanced TNF-α and IL-6 production in the tumor-bearing animals, which is controlled by NF-κB. A low dose of indomethacin alleviates the wasting, decreases the activation of NF-κB, and serum level of TNF-α and IL-6, delays body weight loss and muscle atrophy. These results suggest that activation of NF-κB may play a critical role in inflammatory response of cancer cachexia. However, other possible mechanisms cannot be excluded. Indomethacin can suppress activation of NF-κB, and can be used as a reagent to improve the catabolic status in cancer cachexia. Although the present study could not clarify the definite mode of the anticachectic action of IND, the ability of IND to reverse cachexia should be considered as one of the various actions of this drug. Further studies are required to evaluate its clinical effects and mechanism in patients with cancer cachexia.
ACKNOWLEDGMENTS
We thank Dr. Genbao Feng for his technical assistance.
Footnotes
Edited by Ren SY and Wang XL
References
- 1.Kurzrock R. The role of cytokines in cancer-related fatigue. Cancer. 2001;92:1684–1688. doi: 10.1002/1097-0142(20010915)92:6+<1684::aid-cncr1497>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 2.Tisdale MJ. Wasting in cancer. J Nutr. 1999;129:243S–246S. doi: 10.1093/jn/129.1.243S. [DOI] [PubMed] [Google Scholar]
- 3.Barton BE, Murphy TF. Cancer cachexia is mediated in part by the induction of IL-6-like cytokines from the spleen. Cytokine. 2001;16:251–257. doi: 10.1006/cyto.2001.0968. [DOI] [PubMed] [Google Scholar]
- 4.Barton BE. IL-6-like cytokines and cancer cachexia: consequences of chronic inflammation. Immunol Res. 2001;23:41–58. doi: 10.1385/IR:23:1:41. [DOI] [PubMed] [Google Scholar]
- 5.O'Riordain MG, Falconer JS, Maingay J, Fearon KC, Ross JA. Peripheral blood cells from weight-losing cancer patients control the hepatic acute phase response by a primarily interleukin-6 dependent mechanism. Int J Oncol. 1999;15:823–827. doi: 10.3892/ijo.15.4.823. [DOI] [PubMed] [Google Scholar]
- 6.Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz SA, Hernandez A, Mark Evers B. The role of NF-kappaB/IkappaB proteins in cancer: implications for novel treatment strategies. Surg Oncol. 1999;8:143–153. doi: 10.1016/s0960-7404(00)00012-8. [DOI] [PubMed] [Google Scholar]
- 8.Wang T, Zhang X, Li JJ. The role of NF-kappaB in the regulation of cell stress responses. Int Immunopharmacol. 2002;2:1509–1520. doi: 10.1016/s1567-5769(02)00058-9. [DOI] [PubMed] [Google Scholar]
- 9.Strassmann G, Jacob CO, Fong M, Bertolini DR. Mechanisms of paraneoplastic syndromes of colon-26: involvement of interleukin 6 in hypercalcemia. Cytokine. 1993;5:463–468. doi: 10.1016/1043-4666(93)90037-6. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka M, Miyazaki H, Takeda Y, Takeo S. Detection of serum cytokine levels in experimental cancer cachexia of colon 26 adenocarcinoma-bearing mice. Cancer Lett. 1993;72:65–70. doi: 10.1016/0304-3835(93)90012-x. [DOI] [PubMed] [Google Scholar]
- 11.Strassmann G, Fong M, Kenney JS, Jacob CO. Evidence for the involvement of interleukin 6 in experimental cancer cachexia. J Clin Invest. 1992;89:1681–1684. doi: 10.1172/JCI115767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka Y, Eda H, Tanaka T, Udagawa T, Ishikawa T, Horii I, Ishitsuka H, Kataoka T, Taguchi T. Experimental cancer cachexia induced by transplantable colon 26 adenocarcinoma in mice. Cancer Res. 1990;50:2290–2295. [PubMed] [Google Scholar]
- 13.Lundholm K, Gelin J, Hyltander A, Lönnroth C, Sandström R, Svaninger G, Körner U, Gülich M, Kärrefors I, Norli B. Anti-inflammatory treatment may prolong survival in undernourished patients with metastatic solid tumors. Cancer Res. 1994;54:5602–5606. [PubMed] [Google Scholar]
- 14.Gong JP, Liu CA, Wu CX, Li SW, Shi YJ, Li XH. Nuclear factor kB activity in patients with acute severe cholangitis. World J Gastroenterol. 2002;8:346–349. doi: 10.3748/wjg.v8.i2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Z, Yu Y, Jiang Y, Li J. Growth hormone increases lung NF-kappaB activation and lung microvascular injury induced by lipopolysaccharide in rats. Ann Clin Lab Sci. 2002;32:164–170. [PubMed] [Google Scholar]
- 16.Tisdale MJ. Cancer anorexia and cachexia. Nutrition. 2001;17:438–442. doi: 10.1016/s0899-9007(01)00506-8. [DOI] [PubMed] [Google Scholar]
- 17.Cahlin C, Körner A, Axelsson H, Wang W, Lundholm K, Svanberg E. Experimental cancer cachexia: the role of host-derived cytokines interleukin (IL)-6, IL-12, interferon-gamma, and tumor necrosis factor alpha evaluated in gene knockout, tumor-bearing mice on C57 Bl background and eicosanoid-dependent cachexia. Cancer Res. 2000;60:5488–5493. [PubMed] [Google Scholar]
- 18.Karayiannakis AJ, Syrigos KN, Polychronidis A, Pitiakoudis M, Bounovas A, Simopoulos K. Serum levels of tumor necrosis factor-alpha and nutritional status in pancreatic cancer patients. Anticancer Res. 2001;21:1355–1358. [PubMed] [Google Scholar]
- 19.Llovera M, Carbó N, López-Soriano J, García-Martínez C, Busquets S, Alvarez B, Agell N, Costelli P, López-Soriano FJ, Celada A, et al. Different cytokines modulate ubiquitin gene expression in rat skeletal muscle. Cancer Lett. 1998;133:83–87. doi: 10.1016/s0304-3835(98)00216-x. [DOI] [PubMed] [Google Scholar]
- 20.Costelli P, Bossola M, Muscaritoli M, Grieco G, Bonelli G, Bellantone R, Doglietto GB, Baccino FM, Rossi Fanelli F. Anticytokine treatment prevents the increase in the activity of ATP-ubiquitin- and Ca(2+)-dependent proteolytic systems in the muscle of tumour-bearing rats. Cytokine. 2002;19:1–5. doi: 10.1006/cyto.2002.1036. [DOI] [PubMed] [Google Scholar]
- 21.Tisdale MJ. The 'cancer cachectic factor'. Support Care Cancer. 2003;11:73–78. doi: 10.1007/s00520-002-0408-6. [DOI] [PubMed] [Google Scholar]
- 22.Llovera M, García-Martínez C, Agell N, López-Soriano FJ, Argilés JM. TNF can directly induce the expression of ubiquitin-dependent proteolytic system in rat soleus muscles. Biochem Biophys Res Commun. 1997;230:238–241. doi: 10.1006/bbrc.1996.5827. [DOI] [PubMed] [Google Scholar]
- 23.Carbó N, Busquets S, van Royen M, Alvarez B, López-Soriano FJ, Argilés JM. TNF-alpha is involved in activating DNA fragmentation in skeletal muscle. Br J Cancer. 2002;86:1012–1016. doi: 10.1038/sj.bjc.6600167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strassmann G, Kambayashi T. Inhibition of experimental cancer cachexia by anti-cytokine and anti-cytokine-receptor therapy. Cytokines Mol Ther. 1995;1:107–113. [PubMed] [Google Scholar]
- 25.Carbó N, López-Soriano J, Costelli P, Busquets S, Alvarez B, Baccino FM, Quinn LS, López-Soriano FJ, Argilés JM. Interleukin-15 antagonizes muscle protein waste in tumour-bearing rats. Br J Cancer. 2000;83:526–531. doi: 10.1054/bjoc.2000.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strassmann G, Masui Y, Chizzonite R, Fong M. Mechanisms of experimental cancer cachexia. Local involvement of IL-1 in colon-26 tumor. J Immunol. 1993;150:2341–2345. [PubMed] [Google Scholar]
- 27.Gelin J, Andersson C, Lundholm K. Effects of indomethacin, cytokines, and cyclosporin A on tumor growth and the subsequent development of cancer cachexia. Cancer Res. 1991;51:880–885. [PubMed] [Google Scholar]
- 28.Niu Q, Li T, Liu A. [Cytokines in experimental cancer cachexia] Zhonghua Zhongliu Zazhi. 2001;23:382–384. [PubMed] [Google Scholar]
- 29.Tanaka Y, Tanaka T, Ishitsuka H. Antitumor activity of indomethacin in mice bearing advanced colon 26 carcinoma compared with those with early transplants. Cancer Res. 1989;49:5935–5939. [PubMed] [Google Scholar]
- 30.Cai E, Chen Z, Wu W. [The effects of lipopolysaccharide and anti-inflammatory drugs on nuclear factor-kappa B in pulmonary intravascular macrophage] Zhonghua Jiehe He Huxi Zazhi. 1999;22:283–286. [PubMed] [Google Scholar]
- 31.Crinelli R, Antonelli A, Bianchi M, Gentilini L, Scaramucci S, Magnani M. Selective inhibition of NF-κB activation and TNF-alpha production in macrophages by red blood cell-mediated delivery of dexamethasone. Blood Cells Mol Dis. 2000;26:211–222. doi: 10.1006/bcmd.2000.0298. [DOI] [PubMed] [Google Scholar]
- 32.Chang CK, Llanes S, Schumer W. Effect of dexamethasone on NF-κB activation, tumor necrosis factor formation, and glucose dyshomeostasis in septic rats. J Surg Res. 1997;72:141–145. doi: 10.1006/jsre.1997.5173. [DOI] [PubMed] [Google Scholar]
- 33.Amann R, Peskar BA. Anti-inflammatory effects of aspirin and sodium salicylate. Eur J Pharmacol. 2002;447:1–9. doi: 10.1016/s0014-2999(02)01828-9. [DOI] [PubMed] [Google Scholar]


