Abstract
Wrist fractures are common in postmenopausal women and are associated with functional decline. Fracture patterns after wrist fracture are unclear. The goal of this study was to determine the frequency and types of fractures that occur after a wrist fracture among postmenopausal women. We carried out a post-hoc analysis of data from the Women’s Health Initiative Observational Study and Clinical Trials (1993–2010) carried out at 40 U.S. clinical centers. Participants were postmenopausal women aged 50–79 at baseline. Mean follow-up duration was 11.8 years. Main measures included incident wrist, clinical spine, humerus, upper extremity, lower extremity, hip, and total non-wrist fractures and bone mineral density (BMD) in a subset. Among women who experienced wrist fracture, 15.5% subsequently experienced non-wrist fracture. The hazard for non-wrist fractures was higher among women who had experienced previous wrist fracture than among women who had not experienced wrist fracture: non-wrist fracture overall (hazard ratio [HR] 1.40, 95% confidence interval [CI] 1.33–1.48), spine (HR 1.48, 95% CI 1.32–1.66), humerus (HR 1.78, 95% CI 1.57–2.02), upper extremity (non-wrist) (HR 1.88, 95% CI 1.70–2.07), lower extremity (non-hip) (HR 1.36, 95% CI 1.26–1.48), and hip (HR 1.50, 95% CI 1.32–1.71) fracture. Associations persisted after adjustment for BMD, physical activity, and other risk factors. Risk of non-wrist fracture was higher in women who were younger when they experienced wrist fracture (interaction p-value 0.02). Associations between incident wrist fracture and subsequent non-wrist fracture did not vary by baseline BMD category (normal, low bone density, osteoporosis). A wrist fracture is associated with increased risk of subsequent hip, vertebral, upper extremity, and lower extremity fractures. There may be substantial missed opportunity for intervention in the large number of women who present with wrist fractures.
Keywords: Fracture, osteoporosis
Introduction
The incidence of wrist and distal forearm fracture increases exponentially with age among women aged 50 years (1–6). Wrist fractures are the most common type of clinical fracture among U.S. women aged less than 65 years (7,8). Moreover, wrist fractures are associated with functional decline (9). In the 5 years following a distal forearm fracture, the risk of mortality ranges from 12% among women aged between 65 and 74 years to 43% for women aged 85+ (10). However, the current National Osteoporosis Foundation guidelines do not consider wrist fractures by themselves (in persons without prior hip or vertebral fracture or bone mineral density in the osteoporosis range) to be an indication for pharmacotherapy (11). In a recent position statement from the National Bone Health Alliance Working Group based on expert opinion, distal forearm fractures are characterized as osteoporotic fractures only if there is concomitant osteopenia (T-score between −1.0 and −2.5) on a lumbar spine or hip bone mineral density (BMD) measurement (12). Therefore, there is a lack of consensus among specialized bone societies regarding whether non-traumatic wrist fractures should be considered fragility fractures.
Prospective Canadian (13), Taiwanese (14), and Danish (15) studies have reported higher risk of future fractures among women with wrist fractures compared to expected background population rates, but there are few U.S. studies. One U.S. study found higher observed rates of fracture, compared with expected rates, subsequent to an initial wrist fracture among inhabitants of Rochester, MN (1,16). In the prospective National Osteoporosis Risk Assessment Program (NORA), wrist fractures were associated with increased risk of subsequent osteoporotic fracture (17,18), but the study duration was only 3 years, and detailed description of specific anatomic fracture sites of the subsequent fractures was not provided. Both studies showed increased risk of subsequent fracture in women who experienced an initial wrist fracture compared with women who did not experience initial wrist fracture.
Understanding the frequency, timing, and types of fractures that occur after an initial wrist fracture can help to address unmet opportunities for prevention of subsequent fractures and functional decline. The goal of the current study was to determine, among postmenopausal women, the associations between wrist fracture and subsequent fracture incidence, according to anatomical site and age and, in a subgroup of participants, femoral neck BMD. We hypothesized that wrist fracture would be strongly associated with increased incidence of subsequent fracture at each anatomical site examined.
Methods
Participants
For the current study, analyses of associations between incident wrist fracture and subsequent fractures were performed using data from participants of the Women’s Health Initiative Observational Study (WHI-OS) and WHI Clinical Trials (WHI-CT). The WHI, carried out at 40 U.S. clinical centers, is a study of postmenopausal women aged 50–79 years and free of serious medical conditions at baseline (19–22). The WHI-OS was designed to examine important causes of morbidity and mortality in postmenopausal women (20). The WHI-CTs examined the effects of menopausal hormone therapy (WHI Hormone Therapy Trials), calcium and vitamin D supplementation (WHI CaD Trial), and a low-fat eating pattern (WHI Dietary Modification Trial) (19). The WHI-OS and WHI-CT main studies were conducted between 1993 and 2005. Of 150,076 participants who were in active follow-up at the end of the main studies, 76.9% consented to participate in an extension study conducted between 2005 and 2010.
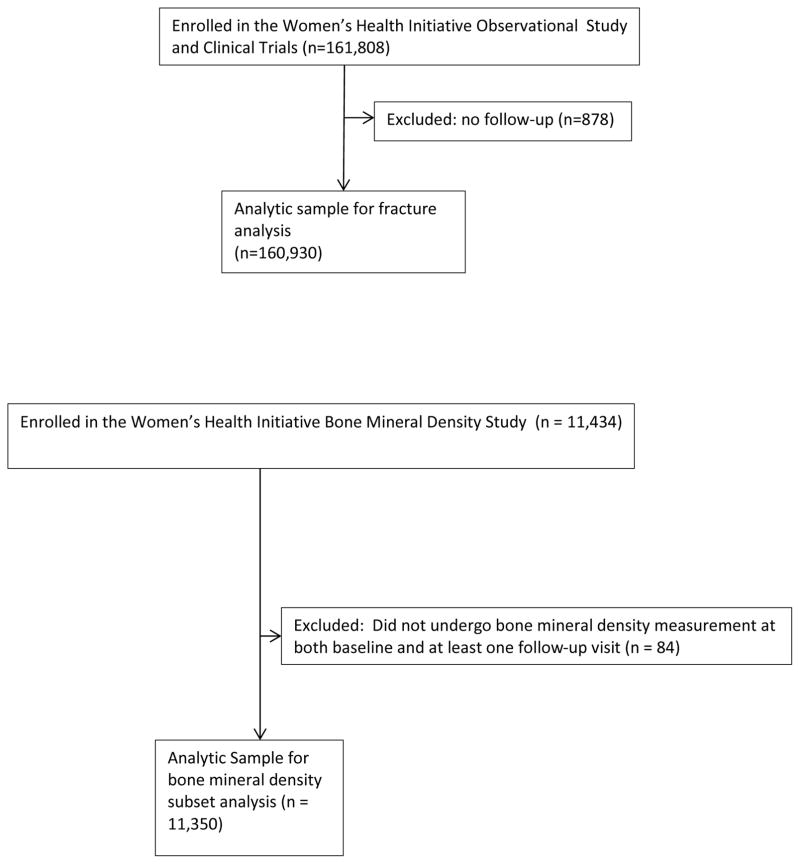
Of the 93,676 WHI-OS participants and 68,132 WHI-CT participants enrolled, we excluded data from 878 participants who provided no follow-up information, resulting in an analytic sample of 160,930 participants (93,049 WHI-OS, 67,881 WHI-CT participants) (Figure 1). Participants reporting a history of prior fractures were not excluded from the current study.
Figure 1.
STROBE flow diagram of the analytic sample
STROBE flow diagram: Wrist Fracture and Risk of Subsequent Fracture: Findings from the Women’s Health Initiative Study
To examine the influence of adjusting for BMD on the associations between initial wrist fracture and subsequent fracture, we used data from the WHI Bone Mineral Density (BMD) Cohort. At enrollment, participants at 3 of the 40 clinical centers (Tucson/Phoenix, Arizona; Pittsburgh, Pennsylvania; Birmingham, Alabama) underwent hip and lumbar spine dual-energy x-ray absorptiometry. Quality assurance methods included standard protocols for positioning and analysis, cross-clinic calibration phantoms, further evaluation of scans with specific problems, and review of a random sample of scans (23–25).
Of the 11,434 participants of the WHI BMD Cohort, 11,350 underwent at least one BMD measurement (lumbar spine and/or hip) at baseline and at least one follow-up assessment. Thus, the sample size for the BMD analysis was 11,350 participants.
Each institution obtained human subjects committee approval. All participants provided written informed consent.
Outcomes: Fracture incidence and BMD
Information regarding incident fractures was obtained semi-annually for the WHI-CT and annually for the WHI-OS. At each assessment, questionnaires asked whether participants had experienced fracture events since the previous visit: “Has a doctor told you for the first time that you have a new broken, fractured, or crushed bone? Which bone(s) did you break, fracture, or crush?” Response choices included: hip, upper leg (not hip), pelvis, knee (patella), lower leg or ankle, foot (not toe), tailbone (coccyx), spine or back (vertebra), upper arm or shoulder, elbow, lower arm or wrist, hand (not finger), finger or toe, jaw, nose, face, and/or skull, ribs and/or chest or breast bone, and “other”.
All hip fractures were adjudicated by trained staff using medical record review for both WHI-OS and WHI-CTs, but the adjudication of non-hip fractures was limited to a subset of participants during the main WHI study (26), including 1) fractures among participants of the WHI Clinical Trials and 2) fractures were among participants in the WHI BMD Cohort. Any fractures that occurred during the WHI Extension 1 phase in the WHI-OS and WHI-CTs were self-reported
We defined wrist fracture as first incident fracture of the forearm (radius or ulna) or carpal bones through the end of WHI Extension 1. We defined non-wrist fractures as first occurrence of clinical spine, humerus, upper extremity non-wrist (elbow, hand [except fingers], upper arm/humerus, shoulder), lower extremity non-hip (foot [except toes], knee/patella, upper leg, lower leg/ankle), or hip.
Other measures
We obtained information regarding age, race/ethnicity, education, family income, previous fracture, history of cancer, self-rated health, falls, alcohol consumption, smoking, physical activity, dietary supplement use, and medication use (including estrogen therapy and osteoporosis medications) from baseline self-assessment questionnaires. Baseline physical activity level was assessed using a validated scale (27). Food frequency questionnaires were used to assess dietary calcium and vitamin D intake (28). Baseline physical function was assessed by the 36-item Short-Form Health Survey (SF-36) (29,30).
The estimated 10-year risk of major osteoporotic fracture was calculated by the World Health Organization Collaborating Centre using the U.S. Fracture Risk Assessment tool (FRAX) without BMD (version 3.0)(31).
Participant weight and height were measured at baseline using standardized protocols.
Statistical Analysis
We examined baseline characteristics of participants overall and by subgroup of incident wrist fracture during WHI follow-up (yes vs. no). We calculated the annualized rate of non-wrist fracture (per 1000 person-years) overall and by 5 year intervals.
We determined the association between non-wrist fracture and prior wrist fracture using Cox proportional hazards models that included the occurrence of an initial incident wrist fracture as the time-varying binary exposure variable (yes vs. no [reference]), adjusting for baseline covariates selected a priori based on known fracture risk factors: age, race, BMI, education, income, cigarette smoking status (never, past, current), pack-years of cigarette smoking, physical activity (total metabolic equivalent of task hours/week), dietary calcium intake (mg/d), calcium supplement intake (mg/d), dietary vitamin D intake (IU/d), vitamin D supplement intake (IU/d), WHI-Hormone Therapy Trials treatment assignment, and WHI Dietary Modification Trial Treatment Assignment.
We included an interaction term in the Cox regression model described above to examine whether associations between wrist fracture and time to non-wrist fracture depended on age. We made the a priori decision to test whether associations varied by race/ethnicity, physical activity level, physical function, falls, FRAX score without BMD, and lowest femoral neck BMD category (T-score ≥ −1.0, T-score between −1.0 and −2.5, T-score ≤ −2.5) by including cross-product terms of these factors with wrist fracture in the regression models.
The interval between wrist fracture and subsequent fractures at other sites was estimated using cumulative incidence curves computed as complements of Kaplan-Meier survival estimates.
We performed a sensitivity analysis in which we excluded women who reported taking osteoporosis medication (alendronate, risedronate, zoledronic acid, calcitonin, selective estrogen receptor modulators, or denosumab) at any time during the study, as well as participants who reported taking self-assigned menopausal hormone therapy any time during the study period, participants assigned to menopausal hormone therapy, and participants assigned to the active arm of the WHI Ca/D trial (resulting sample size 37,931). In another sensitivity analysis, we defined a combined outcome as time to either fracture or death.
In a final sensitivity analysis, we examined associations between wrist fracture and subsequent fracture among participants in whom fractures were adjudicated by medical record review (i.e., WHI-OS participants in the BMD cohort and WHI-CT participants).
Using data from the WHI BMD Cohort (n = 11,350), we examined the influence of adjusting for baseline BMD on the magnitude of associations between non-wrist fracture and prior wrist fracture. We used Cox models as described above and included baseline femoral neck BMD as a covariate.
Data analysis was performed using SAS 9.3 (SAS Institute, Cary, NC).
Results
Participant characteristics and rates of fracture during follow-up
On average, participants were 63.2 years-old and 82.9% were White (Table 1). At baseline, mean BMI was 28.0, 40.2% were taking menopausal hormone therapy at baseline, 7.0% were current smokers, 48.0% were using supplemental vitamin D, and 8.3% of participants had fallen 2 times in the year prior to baseline. At baseline 2.0% of participants were taking bisphosphonates and the prevalence of the use of selective estrogen receptor modulators, calcitonin, aromatase inhibitors, tamoxifen, anti-depressants, proton pump inhibitors, oral corticosteroids, and thiazolidinediones was low (Supplemental Table 1). Mean follow-up duration (standard deviation) was 11.8 (3.4) years, during which 8,792 wrist fractures occurred.
Table 1.
Baseline characteristics, by wrist fracture, among WHI CT and OS participantsa
| Wrist Fractureb | ||||
|---|---|---|---|---|
|
| ||||
| Total | No | Yes | ||
|
| ||||
| 160,930 | 152,138 | 8,792 | ||
|
| ||||
| N (%) | N (%) | N (%) | p-value | |
| Age, years | ||||
| Mean ± SD | 63.2 (7.2) | 63.2 (7.2) | 64.6 (7.2) | <0.001 |
| <55 | 21,430 (13.3) | 20,570 (13.5) | 860 (9.8) | <0.001 |
| 55–59 | 31,804 (19.8) | 30,325 (19.9) | 1,479 (16.8) | |
| 60–64 | 37,016 (23.0) | 35,163 (23.1) | 1,853 (21.1) | |
| 65–69 | 35,227 (21.9) | 33,013 (21.7) | 2,214 (25.2) | |
| 70–74 | 24,781 (15.4) | 23,175 (15.2) | 1,606 (18.3) | |
| 75–79 | 10,672 (6.6) | 9,892 (6.5) | 780 (8.9) | |
|
| ||||
| Ethnicity | ||||
| White | 133,032 (82.9) | 125,059 (82.4) | 7,973 (90.9) | <0.001 |
| Black or African-American | 14,469 (9.0) | 14,159 (9.3) | 310 (3.5) | |
| Hispanic/Latino | 6,329 (3.9) | 6,108 (4.0) | 221 (2.5) | |
| Asian or Pacific Islander | 4,158 (2.6) | 4,014 (2.6) | 144 (1.6) | |
| American Indian or Alaskan Native | 703 (0.4) | 669 (0.4) | 34 (0.4) | |
| Unknown | 1,830 (1.1) | 1,741 (1.1) | 89 (1.0) | |
| Missing | 409 | 388 | 21 | |
|
| ||||
| Education | ||||
| ≤High school diploma | 35,962 (22.5) | 34,184 (22.6) | 1,778 (20.3) | <0.001 |
| Some college/vocational school | 60,610 (37.9) | 57,318 (38.0) | 3,292 (37.7) | |
| College degree or higher | 63,151 (39.5) | 59,483 (39.4) | 3,668 (42.0) | |
| Missing | 1,207 | 1,153 | 54 | |
|
| ||||
| Clinical Trial Participant | ||||
| No | 93,049 (57.8) | 87,981 (57.8) | 5,068 (57.6) | 0.731 |
| Yes | 67,881 (42.2) | 64,157 (42.2) | 3,724 (42.4) | |
|
| ||||
| Body Mass Index (kg/m2) | ||||
| Mean ± SD | 28.0 (5.9) | 28.0 (6.0) | 27.3 (5.5) | <0.001 |
| Underweight (< 18.5) | 1,390 (0.9) | 1,307 (0.9) | 83 (1.0) | <0.001 |
| Normal (18.5 – 24.9) | 54,697 (34.3) | 51,443 (34.1) | 3,254 (37.4) | |
| Overweight (25.0 – 29.9) | 55,419 (34.7) | 52,265 (34.7) | 3,154 (36.2) | |
| Obesity I (30.0 – 34.9) | 29,547 (18.5) | 28,110 (18.6) | 1,437 (16.5) | |
| Obesity II (35.0 – 39.9) | 12,089 (7.6) | 11,552 (7.7) | 537 (6.2) | |
| Extreme Obesity III (>= 40) | 6,377 (4.0) | 6,134 (4.1) | 243 (2.8) | |
| Missing | 1,411 | 1,327 | 84 | |
|
| ||||
| Use of estrogen alone or estrogen + progestogen | ||||
| Never used | 70,390 (43.8) | 66,076 (43.5) | 4,314 (49.1) | <0.001 |
| Past userc | 25,794 (16.0) | 24,189 (15.9) | 1,605 (18.3) | |
| Current user | 64,607 (40.2) | 61,743 (40.6) | 2,864 (32.6) | |
| Missing | 139 | 130 | 9 | |
|
| ||||
| Fracture at Age 55+ | ||||
| No | 102,551 (71.2) | 97,563 (71.5) | 4,988 (65.5) | <0.001 |
| Yes | 20,130 (14.0) | 18,366 (13.5) | 1,764 (23.2) | |
| Age <55 | 21,430 (14.9) | 20,570 (15.1) | 860 (11.3) | |
| Missing | 16,819 | 15,639 | 1,180 | |
|
| ||||
| Falls (last 12 months) | ||||
| None | 104,167 (67.5) | 99,060 (67.9) | 5,107 (60.8) | <0.001 |
| 1 time | 30,952 (20.1) | 29,067 (19.9) | 1,885 (22.4) | |
| 2 times | 12,810 (8.3) | 11,909 (8.2) | 901 (10.7) | |
| 3 or more times | 6,442 (4.2) | 5,934 (4.1) | 508 (6.0) | |
| Missing | 6,559 | 6,168 | 391 | |
|
| ||||
| Alcohol intake | ||||
| Non-drinker | 17,498 (11.0) | 16,535 (10.9) | 963 (11.1) | <0.001 |
| Past drinker | 29,884 (18.7) | 28,458 (18.8) | 1,426 (16.4) | |
| <1 drink per month | 19,838 (12.4) | 18,774 (12.4) | 1,064 (12.2) | |
| <1 drink per week | 32,782 (20.5) | 31,026 (20.5) | 1,756 (20.2) | |
| 1 to <7 drinks per week | 41,029 (25.7) | 38,634 (25.6) | 2,395 (27.5) | |
| 7+ drinks per week | 18,692 (11.7) | 17,583 (11.6) | 1,109 (12.7) | |
| Missing | 1,207 | 1,128 | 79 | |
|
| ||||
| Smoking status | ||||
| Never Smoked | 81,007 (51.0) | 76,612 (51.0) | 4,395 (50.7) | 0.004 |
| Past Smoker | 66,783 (42.0) | 63,037 (42.0) | 3,746 (43.2) | |
| Current Smoker | 11,048 (7.0) | 10,512 (7.0) | 536 (6.2) | |
| Missing | 2,092 | 1,977 | 115 | |
|
| ||||
| Total MET-hours per weekd | ||||
| Mean ± SD | 12.4 (13.7) | 12.4 (13.7) | 13.3 (14.0) | <0.001 |
| Quartile 1 | 38,858 (25.3) | 36,973 (25.5) | 1,885 (22.7) | <0.001 |
| Quartile 2 | 37,765 (24.6) | 35,807 (24.7) | 1,958 (23.6) | |
| Quartile 3 | 38,309 (25.0) | 36,128 (24.9) | 2,181 (26.2) | |
| Quartile 4 | 38,566 (25.1) | 36,281 (25.0) | 2,285 (27.5) | |
| Missing | 7,432 | 6,949 | 483 | |
|
| ||||
| Supplemental Calcium (mg) | ||||
| Mean ± SD | 354.9 (569.9) | 353.5 (571.4) | 379.4 (542.7) | <0.001 |
| Missing | 2 | 2 | 0 | |
|
| ||||
| Supplemental Vitamin D (IU) | ||||
| Mean ± SD | 196 (248) | 195 (247) | 209 (249) | <0.001 |
| None | 83,741 (52.0) | 79,404 (52.2) | 4,337 (49.3) | <0.001 |
| <400 IU | 16,227 (10.1) | 15,304 (10.1) | 923 (10.5) | |
| 400 IU | 45,427 (28.2) | 42,853 (28.2) | 2,574 (29.3) | |
| >400 IU | 15,533 (9.7) | 14,575 (9.6) | 958 (10.9) | |
| Missing | 2 | 2 | 0 | |
|
| ||||
| Bisphosphonates | ||||
| No | 157,773 (98.0) | 149,252 (98.1) | 8,521 (96.9) | <0.001 |
| Yes | 3,155 (2.0) | 2,884 (1.9) | 271 (3.1) | |
| Missing | 2 | 2 | 0 | |
Values expressed as n (%) unless otherwise noted.
Includes wrist fractures (radius, ulna, carpal bones) through the end of WHI extension phase
Past hormone therapy use was defined as the use of an estrogen- or progestogen-containing pill or transdermal patch for 3 months or longer following menopause
MET denotes metabolic equivalent of task
Baseline characteristics of the analytic sample were similar to those of excluded participants, but a lower proportion of the included participants were Black (9% vs. 17%) or Hispanic (4% vs. 18%), had less than high school education (23% vs. 35%), were nonusers of menopausal hormone therapy (40% vs. 28%), did not consume alcohol (11% vs. 18%), did not regularly perform moderate-strenuous activity (16% vs. 24%), or had family income less than $10,000/year (4% vs. 15%) (data not shown).
Absolute (unadjusted) risks of fracture (rates per 1,000 person-years) during the follow-up period, stratified by age, are displayed in Table 2. The rate of any incident non-wrist fracture was higher among women who had previously experienced incident wrist fracture (36.0 per 1000 person-years) than among women who had not previously experienced wrist fracture (19.5 per 1000 person-years) during the follow-up period. The rates of clinical spine fracture, humerus fracture, upper extremity (non-wrist) fracture, lower extremity fracture, and hip fracture were each higher among women who had experienced previous wrist fracture than among women who did not experience previous wrist fracture. For all fracture types, fracture rates were higher in older than younger age groups.
Table 2.
Absolute risks of fracture overall, before wrist fracture, and after wrist fracture (rates per 1000 person years [95% confidence interval]), stratified by current age
| Non-wrist Fractures | Spine Fractures | Humerus Fractures | Upper Extremity Fractures | Lower Extremity Fractures | Hip Fractures | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||
| Age | Overall** | Prior Wrist Fracture | Overall | Prior Wrist Fracture | Overall | Prior Wrist Fracture | Overall | Prior Wrist Fracture | Overall | Prior Wrist Fracture | Overall | Prior Wrist Fracture | ||||||
| No†† | Yes‡‡ | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | |||||||
| All | 19.9 (19.6, 20.1) |
19.5 (19.3,19.7) |
36.0 (34.0, 37.9) |
2.9 (2.8–2.9) |
2.8 (2.7,2.8) |
6.5 (5.8,7.2) |
2.3 (2.3,2.4) |
2.2 (2.2,2.3) |
5.5 (4.8,6.1) |
3.9 (3.8,4.0) |
3.8 (3.7,3.9) |
9.3 (8.4,10.1) |
8.3 (8.2,8.4) |
8.2 (8.1,8.3) |
12.8 (11.8,13.8) |
2.0 (2.0,2.1) |
2.0 (1.9,2.0) |
4.9 (4.3,5.5) |
| < 60 | 11.9 (11.4,12.3) |
11.8 (11.3,12.2) |
26.6 (19.0, 34.1) |
0.7 (0.6,0.8) |
0.7 (0.6,0.8) |
0.9 (−0.4,2.2) |
0.9 (0.8,1.0) |
0.9 (0.8,1.0) |
1.4 (−0.2,3.0) |
2.0 (1.8,2.1) |
1.9 (1.8,2.1) |
5.4 (2.2,8.7) |
7.3 (7.0,7.6) |
7.2 (6.9,7.6) |
13.0 (8.0,17.9) |
0.3 (0.2,0.3) |
0.3 (0.2,0.3) |
0.5 (0,1.4) |
| 60–69 | 16.1 (15.8,16.4) |
15.9 (15.6,16.2) |
28.1 (25.1, 31.1) |
1.6 (1.6,1.7) |
1.6 (1.5,1.7) |
3.6 (2.7,4.6) |
1.6 (1.6,1.7) |
1.6 (1.5,1.7) |
4.5 (3.5,5.6) |
3.2 (3.0,3.3) |
3.0 (2.9,3.2) |
8.5 (7.0,10.0) |
8.0 (7.8,8.2) |
7.9 (7.7,8.1) |
12.3 (10.5,14.1) |
0.7 (0.6,0.7) |
0.7 (0.6,0.7) |
1.2 (0.7,1.8) |
| 70–79 | 23.2 (22.9,23.6) |
22.8 (22.5, 23.2) |
37.1 (34.2, 39.9) |
3.8 (3.6,3.9) |
3.7 (3.5,3.8) |
6.3 (5.2,7.3) |
3.0 (2.8,3.1) |
2.9 (2.8,3.0) |
5.4 (4.4,6.3) |
4.7 (4.6,4.9) |
4.6 (4.4,4.8) |
9.2 (7.9,10.5) |
8.6 (8.4,8.8) |
8.4 (8.2,8.7) |
13.0 (11.4,14.5) |
2.6 (2.5,2.7) |
2.5 (2.4,2.6) |
4.7 (3.8,5.6) |
| 80 or older | 38.1 (37.1, 39.1) |
37.5 (36.5, 38.6) |
49.8 (44.4, 55.1) |
7.9 (7.5,8.3) |
7.6 (7.2,8.0) |
12.5 (10.3,14.7) |
4.8 (4.5,5.1) |
4.6 (4.3,5.0) |
7.9 (6.2,9.6) |
7.0 (6.7,7.4) |
6.8 (6.4,7.2) |
11.4 (9.2,13.5) |
10.2 (9.7,10.7) |
10.0 (9.5,10.5) |
13.2 (10.9,15.6) |
8.5 (8.0,8.9) |
8.3 (7.8,8.7) |
11.9 (9.8,14.0) |
Age-adjusted rate of fractures during follow-up (all participants).
Age-adjusted rate of fractures among women prior to incident wrist fracture during follow-up
Age-adjusted rate of fractures among women after an incident wrist fracture during follow-up
Within 10 years of initial wrist fracture, the proportion of participants who subsequently experienced non-wrist fracture were: clinical spine 6.8%, humerus 6.0%, upper extremity non-wrist fracture 9.4%, lower extremity non-hip 12.6%, and hip 4.9% (Table 3).
Table 3.
Proportion of women with subsequent fracture within 10 years of wrist fracture, by site, with 95% confidence interval
| Spine fracture | 0.068 (0.059–0.076) |
| Humerus fracture | 0.060 (0.052–0.068) |
| Upper extremity (non-wrist) fracture | 0.094 (0.084–0.103) |
| Lower extremity fracture | 0.126 (0.115–0.137) |
| Hip fracture | 0.049 (0.042–0.056) |
Adjusted associations between initial wrist fracture and subsequent non-wrist fracture
After adjustment for age, race/ethnicity, and BMI, the hazard ratio (HR) for non-wrist fractures was higher among participants who had experienced initial wrist fracture than among participants who had not experienced an initial wrist fracture (Table 4). This was true for non-wrist fracture overall (HR 1.40, 95% confidence interval [CI] 1.33–1.48), spine (HR 1.48, 95% CI 1.32–1.66), humerus (HR 1.78, 95% CI 1.57–2.02), upper extremity (non-wrist) (HR 1.88, 95% CI 1.70–2.07), lower extremity (non-hip) (HR 1.36, 95% CI 1.26–1.48), and hip (HR 1.50, 95% CI 1.32–1.71) fracture. The HR values remained nearly identical after additional adjustment for other covariates.
Table 4.
Associations between Incident Wrist Fracture and Subsequent Fracture
| Wrist Fracture | ||||
|---|---|---|---|---|
|
| ||||
| No | Yes | |||
|
| ||||
| Total N | Event | HR (95% CI) | ||
|
| ||||
| Any non-wrist fracture | ||||
| Crude | 160,930 | 33,979 | 1 (ref) | 1.54 (1.46–1.62) |
| Model 1§§ | 159,118 | 33,596 | 1 (ref) | 1.40 (1.33–1.48) |
| Model 2*** | 139,790 | 29,540 | 1 (ref) | 1.40 (1.32–1.49) |
| Model 3††† | 136,017 | 28,790 | 1 (ref) | 1.37 (1.29–1.46) |
|
| ||||
| Spine Fracture | ||||
| Crude | 160,930 | 5,373 | 1 (ref) | 1.75 (1.57–1.96) |
| Model 1 | 159,118 | 5,301 | 1 (ref) | 1.48 (1.32–1.66) |
| Model 2 | 139,790 | 4,658 | 1 (ref) | 1.51 (1.34–1.70) |
| Model 3 | 136,017 | 4,544 | 1 (ref) | 1.46 (1.29–1.65) |
|
| ||||
| Humerus Fracture | ||||
| Crude | 160,930 | 4,361 | 1 (ref) | 1.99 (1.76–2.26) |
| Model 1 | 159,118 | 4,309 | 1 (ref) | 1.78 (1.57–2.02) |
| Model 2 | 139,790 | 3,793 | 1 (ref) | 1.72 (1.50–1.96) |
| Model 3 | 136,017 | 3,676 | 1 (ref) | 1.67 (1.46–1.92) |
|
| ||||
| Upper extremity (non-wrist) fracture‡‡‡ | ||||
| Crude | 160,930 | 7,312 | 1 (ref) | 2.06 (1.87–2.27) |
| Model 1 | 159,118 | 7,228 | 1 (ref) | 1.88 (1.70–2.07) |
| Model 2 | 139,790 | 6,360 | 1 (ref) | 1.85 (1.67–2.06) |
| Model 3 | 136,017 | 6,184 | 1 (ref) | 1.80 (1.62–2.01) |
|
| ||||
| Lower extremity Fracture§§§ | ||||
| Crude | 160,930 | 15,034 | 1 (ref) | 1.41 (1.30–1.53) |
| Model 1 | 159,118 | 14,867 | 1 (ref) | 1.36 (1.26–1.48) |
| Model 2 | 139,790 | 13,051 | 1 (ref) | 1.35 (1.24–1.48) |
| Model 3 | 136,017 | 12,718 | 1 (ref) | 1.30 (1.19–1.43) |
|
| ||||
| Hip fracture | ||||
| Crude | 160,930 | 3,836 | 1 (ref) | 1.97 (1.73–2.24) |
| Model 1 | 159,118 | 3,801 | 1 (ref) | 1.50 (1.32–1.71) |
| Model 2 | 139,790 | 3,291 | 1 (ref) | 1.51 (1.31–1.74) |
| Model 3 | 136,017 | 3,186 | 1 (ref) | 1.48 (1.28–1.71) |
Model 1 adjusted for age, race, and BMI
Model 2 is adjusted for covariates in Model 1 plus education, income, cigarette smoking status, pack-years of cigarette smoking, total metabolic equivalent of task hours/week, dietary calcium intake, calcium supplement intake, dietary vitamin D intake, vitamin D supplement intake, WHI-Hormone Therapy Trials treatment assignment, and WHI Dietary Modification Trial treatment assignment
Model 3 is adjusted for covariates in Model 2 plus number of falls, alcohol intake, history of cancer, and physical function score
Includes elbow, hand, upper arm/humerus, and shoulder fractures, excludes finger fractures
Includes foot, knee/patella, upper leg, and lower leg/ankle fractures, excludes hip fractures
Associations between initial wrist fracture and subsequent non-wrist fracture varied according to participant race/ethnicity (interaction p-value = 0.03), with stronger magnitudes of associations in Hispanic/Latino women than in non-Hispanic White or Black women (Supplemental Table 2). Associations between initial wrist fracture and subsequent non-wrist fracture also differed by age at the time of wrist fracture, with stronger associations among younger than among older women (interaction p-value = 0.02). HRs ranged from 1.24 (1.11–1.39) among women aged 80 and older to 2.49 (1.18–5.24) among women aged <55 years.
Figure 2 illustrates the time to non-wrist fracture (Fig. 2a), humerus fracture (Fig. 2b), hip fracture (Fig. 2c), and spine fracture (Fig. 2d) according to presence and absence of initial wrist fracture. The difference in the cumulative incidence of fractures over time in women with vs. without initial wrist fracture is evident for each fracture type.
Figure 2.
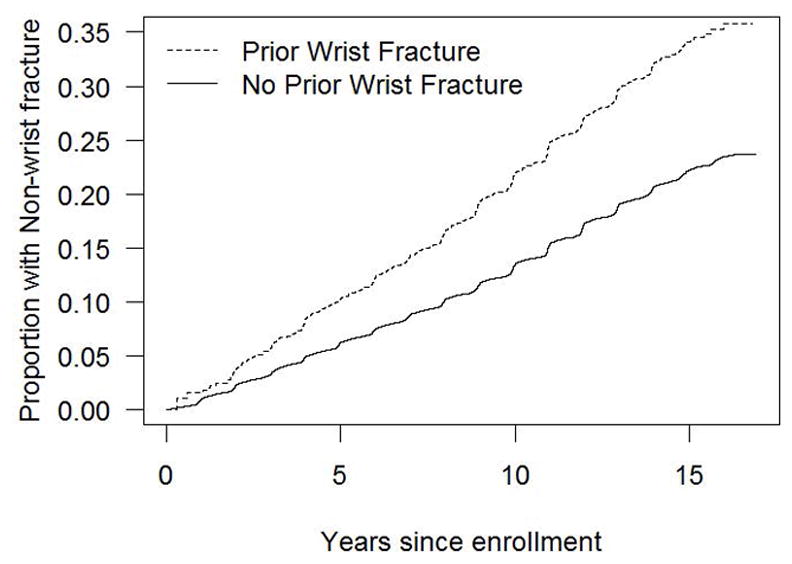
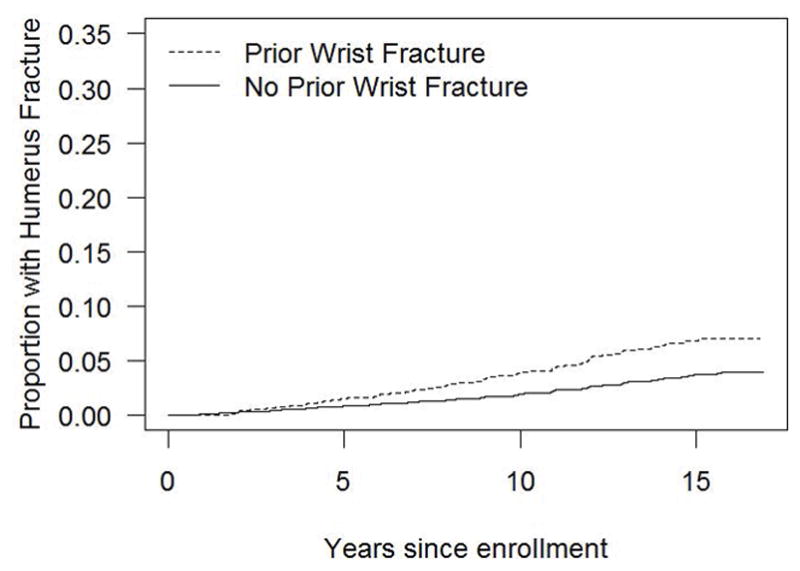
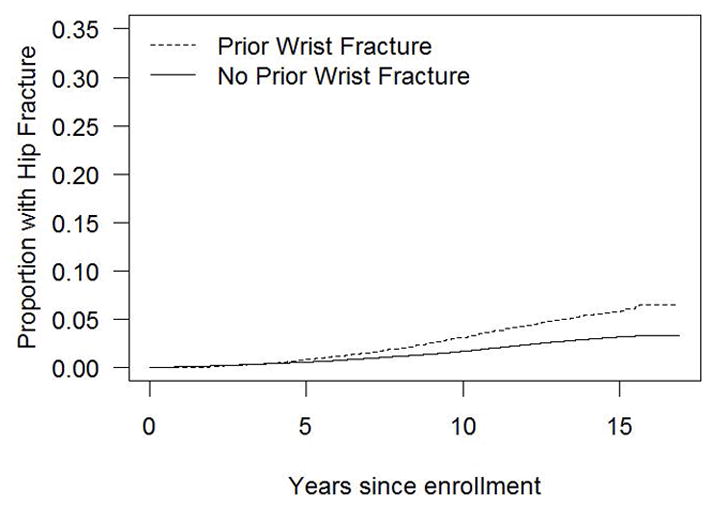
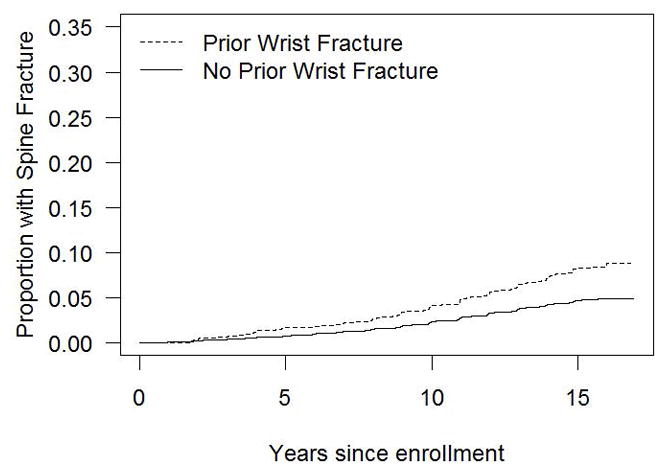
Figure 2a. Cumulative incidence of non-wrist fracture in the presence and absence of initial wrist fracture during the WHI follow-up period (non-parametric estimate)
Figure 2b. Cumulative incidence of humerus fracture in the presence and absence of initial wrist fracture during the WHI follow-up period (non-parametric estimate)
Figure 2c. Cumulative incidence of hip fracture in the presence and absence of initial wrist fracture during the WHI follow-up period (non-parametric estimate)
Figure 2d. Cumulative incidence of spine fracture in the presence and absence of initial wrist fracture during the WHI follow-up period (non-parametric estimate)
In a sensitivity analysis excluding data from women who reported use of osteoporosis medication any time during follow-up, participants who self-initiated menopausal hormone therapy at any time during the study period, as well as participants assigned to the active arms of the WHI Hormone Therapy and WHI/CaD Trials, hazard ratios were slightly attenuated in magnitude, but were similar to those in the primary analysis (data not shown).
When we defined the outcome as time to fracture or death, results were similar to those of the primary analyses; associations between incident wrist fracture and subsequent non-wrist fracture showed a pattern of higher hazard ratios among younger age groups (Supplemental Table 3).
In a final sensitivity analysis in which we examined associations between wrist fracture and subsequent fracture among the participants whose fractures had been confirmed by medical record review. Magnitudes of associations were very similar to those of the primary analyses (data not shown).
Secondary analyses
Associations between initial wrist fracture and subsequent non-wrist fracture after adjustment for BMD
Using Cox proportional hazards regression, we examined the influence of adjustment for baseline femoral neck BMD on the associations between wrist fracture and subsequent non-wrist fracture (Supplemental Table 4). HRs for associations between wrist fracture and subsequent non-wrist fracture in the BMD sample were similar to those in the overall analytic sample. After adjustment for age, race, and BMI, the incidence of any non-wrist fracture was higher for participants who experienced initial wrist fracture than for participants who did not experience initial wrist fracture (HR 1.42, 95% CI 1.16–1.74). After additional adjustment for baseline femoral neck BMD, the associations between wrist fracture and subsequent non-wrist fracture remained significant (HR 1.30, 95% CI 1.06–1.59), although slightly decreased in magnitude. Associations between initial wrist fracture and subsequent non-wrist fracture did not significantly vary by baseline femoral neck BMD category.
Discussion
In this cohort, compared with postmenopausal women who did not experience a wrist fracture during 11.8 years of follow-up, those who experienced a wrist fracture during follow-up had a markedly elevated risk of subsequent vertebral, humerus, upper extremity (non-wrist), lower extremity (non-hip), and hip fractures, with hazards ratios ranging from 1.36 (for lower extremity non-hip fracture) to 1.88 (for upper extremity non-wrist fracture). Participants who experienced wrist fracture during follow-up were at 1.5-fold higher risk of subsequent hip fracture. The association between initial wrist fracture and any subsequent non-wrist fracture persisted after adjustment for other osteoporosis risk factors and baseline femoral neck BMD.
To our knowledge, this study is the first large multisite prospective U.S. study that has focused on associations between wrist fracture and subsequent incidence of upper extremity, lower extremity, and spine fracture. In a study of residents of Rochester, Minnesota, over a 7.5-year follow-up, women who had initial distal forearm fracture had approximately a 5- to 6-fold increase in subsequent vertebral fracture and a doubling of risk of subsequent proximal femur fracture (16). In the National Osteoporosis Risk Assessment (NORA) study of women aged ≥ 50 years old, during 3 years of follow-up, the risk of subsequent hip fracture was higher among women with initial wrist fracture (17,18). As far as we are aware, patterns of other specific types of fractures after initial wrist fracture have not yet been reported in the NORA cohort.
The association between wrist fracture and increased risk of subsequent non-wrist fracture persisted after adjustment for BMD. This finding, combined with the observation that the associations persisted despite adjustment for all known major fracture risk factors, suggest that aberrations in bone structure and/or strength are at least partly responsible for placing women with wrist fracture at increased risk of subsequent fracture. Frequency of falls did not account for the increased risk of non-wrist fractures following a wrist fracture. Treatment guided by spine and/or hip BMD measurements alone may underestimate the increased risk of subsequent fracture risk in the setting of an initial wrist fracture.
Clinical trials have not specifically tested fracture reduction strategies that are tailored to women with wrist fracture who have BMD T-scores between −1 and −2.5. A subgroup analysis from the Fracture Intervention Trial focused on older women with BMD T-scores between −1 and −2.5. In that subgroup of women, the reduction in fracture risk after treatment with a bisphosphonate (alendronate) was no greater in women with a previous non-vertebral fracture (26% of which were wrist fractures) than in women without a previous non-vertebral fracture (32).
Our results have clinical and public health implications. First, clinicians should identify postmenopausal women with wrist fractures as being at significantly elevated risk for multiple types of future fracture, including hip fracture. Also, clinicians should be aware the younger the woman is when she experiences wrist fracture, the higher the relative risk of subsequent fracture. In fully adjusted models, wrist fracture was associated with a 37% higher relative risk of subsequent non-wrist fracture, which was similar in magnitude to being 10 years older (35% higher). Fourth, the increased incidence of non-wrist fractures following a wrist fracture highlight the need for future studies that focus on developing and testing interventions specifically to prevent subsequent fractures after an initial wrist fracture. There is currently no proven intervention that specifically targets women with wrist fracture who have normal lumbar spine and femoral neck BMD. Finally, the elevated risk of subsequent fracture among postmenopausal women with wrist fracture persisted even after we adjusted for BMD, suggesting that the increased risk of subsequent fractures not entirely explained by spine and/or hip BMD measurements.
Strengths of our study include the large sample size, the prospective follow-up, and availability of detailed information regarding major osteoporotic risk factors.
Our study has limitations. First, self-reported information regarding fractures is not as accurate as medical record-verified fractures. However, misclassification of fractures in WHI is low. In a validation study in WHI, Chen and colleagues found that the agreement between self-reports for single-site fractures and medical records within the WHI was high for hip (78%) and forearm/wrist (81%), but low for clinical spine fracture (51%), and the average confirmation rate for all single-site fractures was 71% (26). Second, WHI participants are likely healthier than postmenopausal women in the general population, and may not be representative of the general population of postmenopausal women. Thus, associations between wrist fracture and subsequent fracture may be stronger in the general population than in our study participants. Third, the number of women with wrist fractures and normal BMD was small. Fourth, although we adjusted for multiple lifestyle-related risk factors (smoking, total metabolic equivalent of task hours/week, calcium and vitamin D intake, falls, alcohol intake), there may exist other lifestyle-related causes of repeat fractures for which we lacked information. Finally, we did not adjust for multiple comparisons, so the probability of at least one of the reported confidence intervals will exclude unity under an overall null hypothesis is greater than 0.05.
Conclusions
In conclusion, nearly one in five women with initial wrist fracture went on to experience a subsequent non-wrist fracture over 11 years of follow-up. Our results suggest substantial missed opportunity for intervention in the large number of women who present with wrist fractures to prevent subsequent fractures. Our findings support the approach of the recent position statement advocating that women with wrist fracture should undergo BMD testing, and that those with BMD T-score ≤ −1.0 should receive a diagnosis of osteoporosis (12). Studies should develop and test interventions specifically targeted to women with sentinel forearm fracture. Increased attention to wrist fracture as a fragility fracture is important to allow the early identification of women at risk for future fracture for preventive measures.
Supplementary Material
Acknowledgments
The authors are grateful to the study participants for their dedication to the study.
Funding
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
Role of the sponsor: The Women’s Health Initiative (WHI) project office at the National Heart, Lung, and Blood Institute (NHLBI), which was the sponsor, had a role in the design and conduct of the study and in the collection and management of the data. The sponsor did not have a role in analysis and interpretation of the data, the preparation of the manuscript, or the decision to submit the manuscript for publication. Review and approval of the manuscript was carried out by committees composed of WHI investigators and NHLBI representatives.
SHORT LIST OF WHI INVESTIGATORS
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller
Clinical Coordinating Center: Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg
Investigators and Academic Centers: (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker
Women’s Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker
For a list of all the investigators who have contributed to WHI science, please visit: https://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf
Footnotes
Financial disclosures
None: CJC, WL, KMH, CAA, JAC, JW-W, MSL
NCW, JRC: Research contracts with Amgen, Inc.
Author contributions
Study conception and design: CJC, MSL
Acquisition of data: JWW
Statistical analysis: CA, KH
Data interpretation: all authors
Drafting of manuscript: CJC
Critical revision of manuscript for important intellectual content: all authors
Resource Sharing:
The Women’s Health Initiative Study data are available via the BioLINCC website of the National Heart, Lung, and Blood Institute at https://biolincc.nhlbi.nih.gov/home/
Contributor Information
Carolyn J. Crandall, Email: ccrandall@mednet.ucla.edu, Professor of Medicine, Dept. of Medicine, David Geffen School of Medicine at the University of California at Los Angeles, UCLA Medicine/GIM, 911 Broxton Ave., 1st floor, Los Angeles, CA, 90024.
Kathleen M. Hovey, Email: koreilly@buffalo.edu, Statistician, Dept. of Epidemiology and Environmental Health, State University of NY at Buffalo, 235 Farber Hall, Buffalo, NY, 14214.
Jane A. Cauley, Email: jcauley@edc.pitt.edu, Vice Chair for Research, Department of Epidemiology, University of Pittsburgh, Graduate School of Public Health, Crabtree Hall A547 - 130 DeSoto Street, Pittsburgh, PA, 15213.
Christopher A. Andrews, Email: chrisaa@umich.edu, Statistician Expert, Department of Ophthalmology and Visual Sciences, University of Michigan, Ann Arbor, MI, 48105.
Jeffrey R. Curtis, Email: jcurtis@uab.edu, William J. Koopman Endowed Professor in Rheumatology and Immunology, Dept. of Clinical Immunology and Rheumatology, University of Alabama at Birmingham, Birmingham AL, 35294.
Jean Wactawski-Wende, Email: jww@buffalo.edu, Department of Epidemiology and Environmental Health, State University of NY at Buffalo, Buffalo, NY, 14214.
Nicole C. Wright, Email: ncwright@uab.edu, Assistant Professor, Dept. of Epidemiology, University of Alabama at Birmingham, RPHB 523C, Birmingham, Alabama, 35294.
Wenjun Li, Email: Wenjun.Li@umassmed.edu, Associate Professor of Medicine, Division of Preventive and Behavioral Medicine, University of Massachusetts Medical School, 419 Belmont Street, Worcester, MA, 01605.
Meryl S. LeBoff, Email: mleboff@partners.org, Professor Medicine, Distinguished Chair in Skeletal Health and Osteoporosis, Dept. of Medicine, Endocrine, Diabetes and Hypertension Division, Brigham and Women’s Hospital, Harvard Medical School, 221 Longwood Avenue, Boston, Massachusetts, 02115.
References
- 1.Owen RA, Melton LJ, 3rd, Ilstrup DM, Johnson KA, Riggs BL. Colles’ fracture and subsequent hip fracture risk. Clin Orthop Relat Res. 1982;(171):37–43. [PubMed] [Google Scholar]
- 2.Melton LJ, 3rd, Christen D, Riggs BL, et al. Assessing forearm fracture risk in postmenopausal women. Osteoporos Int. 2010;21(7):1161–9. doi: 10.1007/s00198-009-1047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orces CH, Martinez FJ. Epidemiology of fall related forearm and wrist fractures among adults treated in US hospital emergency departments. Inj Prev. 2011;17(1):33–6. doi: 10.1136/ip.2010.026799. [DOI] [PubMed] [Google Scholar]
- 4.Jones G, Nguyen T, Sambrook PN, Kelly PJ, Gilbert C, Eisman JA. Symptomatic fracture incidence in elderly men and women: the Dubbo Osteoporosis Epidemiology Study (DOES) Osteoporos Int. 1994;4(5):277–82. doi: 10.1007/BF01623352. [DOI] [PubMed] [Google Scholar]
- 5.Amin S, Achenbach SJ, Atkinson EJ, Khosla S, Melton LJ., 3rd Trends in fracture incidence: a population-based study over 20 years. J Bone Miner Res. 2014;29(3):581–9. doi: 10.1002/jbmr.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosengren BE, Karlsson M, Petersson I, Englund M. The 21st-century landscape of adult fractures: cohort study of a complete adult regional population. J Bone Miner Res. 2015;30(3):535–42. doi: 10.1002/jbmr.2370. [DOI] [PubMed] [Google Scholar]
- 7.Black DM, Cooper C. Epidemiology of fractures and assessment of fracture risk. Clin Lab Med. 2000;20(3):439–53. [PubMed] [Google Scholar]
- 8.Cerocchi I, Ghera S, Gasbarra E, Feola M, Tarantino U. The clinical significance of wrist fracture in osteoporosis. Aging clinical and experimental research. 2013;25 (Suppl 1):S81–2. doi: 10.1007/s40520-013-0083-0. [DOI] [PubMed] [Google Scholar]
- 9.Edwards BJ, Song J, Dunlop DD, Fink HA, Cauley JA. Functional decline after incident wrist fractures--Study of Osteoporotic Fractures: prospective cohort study. BMJ. 2010;341:c3324. doi: 10.1136/bmj.c3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curtis JR, Arora T, Matthews RS, et al. Is withholding osteoporosis medication after fracture sometimes rational? A comparison of the risk for second fracture versus death. J Am Med Dir Assoc. 2010;11(8):584–91. doi: 10.1016/j.jamda.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siris ES, Adler R, Bilezikian J, et al. The clinical diagnosis of osteoporosis: a position statement from the National Bone Health Alliance Working Group. Osteoporos Int. 2014;25(5):1439–43. doi: 10.1007/s00198-014-2655-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gay JD. Radial fracture as an indicator of osteoporosis: a 10-year follow-up study. Can Med Assoc J. 1974;111(2):156–7. [PMC free article] [PubMed] [Google Scholar]
- 14.Chen CW, Huang TL, Su LT, et al. Incidence of subsequent hip fractures is significantly increased within the first month after distal radius fracture in patients older than 60 years. J Trauma Acute Care Surg. 2013;74(1):317–21. doi: 10.1097/ta.0b013e31824bb325. [DOI] [PubMed] [Google Scholar]
- 15.Lauritzen JB, Schwarz P, McNair P, Lund B, Transbol I. Radial and humeral fractures as predictors of subsequent hip, radial or humeral fractures in women, and their seasonal variation. Osteoporos Int. 1993;3(3):133–7. doi: 10.1007/BF01623274. [DOI] [PubMed] [Google Scholar]
- 16.Cuddihy MT, Gabriel SE, Crowson CS, O’Fallon WM, Melton LJ., 3rd Forearm fractures as predictors of subsequent osteoporotic fractures. Osteoporos Int. 1999;9(6):469–75. doi: 10.1007/s001980050172. [DOI] [PubMed] [Google Scholar]
- 17.Barrett-Connor E, Sajjan SG, Siris ES, Miller PD, Chen YT, Markson LE. Wrist fracture as a predictor of future fractures in younger versus older postmenopausal women: results from the National Osteoporosis Risk Assessment (NORA) Osteoporos Int. 2008;19(5):607–13. doi: 10.1007/s00198-007-0508-8. [DOI] [PubMed] [Google Scholar]
- 18.Siris ES, Miller PD, Barrett-Connor E, et al. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. JAMA. 2001;286(22):2815–22. doi: 10.1001/jama.286.22.2815. [DOI] [PubMed] [Google Scholar]
- 19.Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials. 1998;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 20.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13(9 Suppl):S107–21. doi: 10.1016/s1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 21.Cauley JA, Wampler NS, Barnhart JM, et al. Incidence of fractures compared to cardiovascular disease and breast cancer: the Women’s Health Initiative Observational Study. Osteoporos Int. 2008;19(12):1717–23. doi: 10.1007/s00198-008-0634-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson GL, Manson J, Wallace R, et al. Implementation of the Women’s Health Initiative study design. Ann Epidemiol. 2003;13(9 Suppl):S5–17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 23.Beck TJ, Petit MA, Wu G, LeBoff MS, Cauley JA, Chen Z. Does obesity really make the femur stronger? BMD, geometry, and fracture incidence in the women’s health initiative-observational study. J Bone Miner Res. 2009;24(8):1369–79. doi: 10.1359/JBMR.090307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LaCroix AZ, Beck TJ, Cauley JA, et al. Hip structural geometry and incidence of hip fracture in postmenopausal women: what does it add to conventional bone mineral density? Osteoporos Int. 2010;21(6):919–29. doi: 10.1007/s00198-009-1056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Z, Arendell L, Aickin M, et al. Hip bone density predicts breast cancer risk independently of Gail score: results from the Women’s Health Initiative. Cancer. 2008;113(5):907–15. doi: 10.1002/cncr.23674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Z, Kooperberg C, Pettinger MB, et al. Validity of self-report for fractures among a multiethnic cohort of postmenopausal women: results from the Women’s Health Initiative observational study and clinical trials. Menopause. 2004;11(3):264–74. doi: 10.1097/01.gme.0000094210.15096.fd. [DOI] [PubMed] [Google Scholar]
- 27.Manson JE, Greenland P, LaCroix AZ, et al. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. The New England journal of medicine. 2002;347(10):716–25. doi: 10.1056/NEJMoa021067. [DOI] [PubMed] [Google Scholar]
- 28.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9(3):178–87. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 29.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 30.Hays RD, Sherbourne CD, Mazel RM. The RAND 36-Item Health Survey 1.0. Health economics. 1993;2(3):217–27. doi: 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- 31.Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19(4):385–97. doi: 10.1007/s00198-007-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryder KM, Cummings SR, Palermo L, et al. Does a history of non-vertebral fracture identify women without osteoporosis for treatment? J Gen Intern Med. 2008;23(8):1177–81. doi: 10.1007/s11606-008-0622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.