Abstract
Between 6 and 12 months, typically developing infants undergo a socio-cognitive ‘revolution’. The Interactive Specialization (IS) theory of brain development predicts that these behavioral changes will be underpinned by developmental increases in the power and topographic extent of socially selective cortical responses. To test this hypothesis, we used EEG to examine developmental changes in cortical selectivity for ecologically valid dynamic social versus non-social stimuli in a large cohort of 6- and 12-month-old infants. Consistent with the Interactive Specialization model, results showed that differences in EEG theta activity between social and non-social stimuli became more pronounced and widespread with age. Differences in EEG activity were most clearly elicited by a live naturalistic interaction, suggesting that measuring brain activity in ecologically valid contexts is central to mapping social brain development in infancy.
Keywords: Interactive Specialization, Infant, EEG, social brain, video deficit effect, alpha power, theta power
Introduction
Between 6 and 12 months, typically developing infants undergo a socio-cognitive ‘revolution’ (Tomasello, 1995). During this period, infants begin to engage in joint attention, early verbal communication emerges, and infants begin to actively participate in cultural scripts, rituals and games. Whilst there is substantial understanding of the behavioral changes that occur in this period, our understanding of the changes in the brain that underlie these behavioral changes remains limited. Work in this area is important not only for testing theories of typical social development, but for identifying new ways to study the mechanisms underlying the emergence of social and communication difficulties in infants who later develop Autism Spectrum Disorder (ASD, Jones et al., 2014).
Understanding social brain development requires an organizing theoretical framework. Traditional views of brain development contrast maturational accounts (based on particular brain regions coming ‘online’ at certain developmental stages, supporting the development of new abilities) with expertise-based frameworks (in which an infant's mounting experience with social stimuli drives the specialization of relevant neural regions). However, both accounts have limitations in explaining available evidence (Johnson, 2010). Interactive Specialization (IS) is an alternative new framework for understanding brain development (Johnson, 2010) that has received significant empirical support, and makes clear and testable predictions. Under this framework, progressive specialization is driven by competition between brain regions with different pre-existing neurocomputational biases. New behavioral and cognitive competences emerge through coordinated changes in the activity of networks of brain regions, rather than the developmental ‘maturation’ of particular brain areas. By this view, the socio-cognitive revolution in infancy should be accompanied by large-scale changes in networks of brain regions that will become the ‘social brain’ (Insel & Fernald, 2004). Empirical tests of the IS framework thus hold the potential to accelerate our understanding of the mechanisms that underlie early social brain development.
According to the IS framework, during development the responses of particular regions or networks become increasingly tuned to respond to particular dimensions of social stimuli. For example, a brain region may initially respond to both upright and inverted faces, but over developmental time responses may become specific to upright faces. Such tuning of neural responses to particular social dimensions in the first year of life has been relatively well studied using event-related potential (ERPs), which represent the averaged time-locked neural response to a particular stimulus class. Broadly, work with ERPs has supported the prediction that processing of faces (a key source of social information) become increasingly specialized over the first year of life. For example, the N290 (a negative-going deflection over occipital electrodes that peaks 290ms after stimulus onset) begins to show differential responses to upright and inverted faces between 6 and 12 months of age (de Haan et al., 2002; Halit et al., 2003). The P400 response to face inversion (a positive-going deflection over occipital electrodes that peaks around 400ms after stimulus onset) becomes specific to human (versus monkey) faces over the same age range (de Haan et al., 2002; Halit et al., 2003). Finally, the Nc (a negative-going deflection over fronto-central electrodes reflecting attentional allocation) responds differently to fearful and neutral faces at 6 but not 3-months (Hoehl & Striano, 2010). Thus, ERP evidence supports the proposition that there are age-related increases in the specificity of particular neural responses to critical facial dimensions over the first year of life.
Other key predictions of the IS theory have been less well studied in the infancy period. As cortical regions become tuned to particular stimulus dimensions (and stop responding to ‘non-preferred’ stimuli), the IS theory predicts that there should be a developmental decrease in the amount of cortical tissue that is sensitive to particular stimuli (e.g. responds to faces more than a neutral baseline stimulus; Johnson, 2010, Hypothesis S2a). Empirically, this proposal can be tested by comparing neural responses to a stimulus with neural responses to an unrelated baseline condition (such as a fixation cross). Second, as regions become increasingly specialized there should be a developmental increase in the extent of activation of cortical tissue that is selective for a particular stimulus type (e.g. responds to faces more than closely matched objects; Hypothesis S2b). Since such hypotheses relate to the spatial extent of activity in networks of brain regions, one way to address these questions is examining the oscillatory rhythms of the ongoing electroencephalogram (EEG). Oscillatory rhythms in the EEG are typically driven by the synchronized activity of large networks of neurons. Although EEG has relatively poor spatial resolution, changes in the selectivity and sensitivity of brain activity should be reflected in the magnitude and scalp location of changes in oscillatory power.
Spontaneous EEG can be decomposed into different frequency bands, which have been associated with different functional correlates. There is preliminary evidence that both the theta (4 to 6Hz) and alpha (6 to 9hz) bands may be sensitive to aspects of social brain activity in infancy. Theta rhythm (4 to 6Hz) is thought to support species-relevant behaviors (Miller, 1991; Orekhova et al., 2006); in human infants, theta power is often greater during social interaction and exploratory activity. For example, theta power increases when 5-month-old infants look at a face with a neutral expression versus a smiling face during a period of interaction (Bazhenova et al., 2007), possibly related to the increase in social initiation attempts seen during this period. As well, infants show greater theta power in response to child-directed speech and toy play (Orekhova et al., 2006) or peek-a-boo (Stroganova et al., 1998) than an experimenter blowing bubbles. Activity in the alpha band (6 to 9Hz) is often considered to reflect cortical ‘idling’. Thus, alpha suppression is often equated with increased processing in a particular brain region. Alpha suppression may also reflect the release of inhibition of non-essential activity in association with the emergence of complex spreading activation processes (Klimesch et al., 2007). Consistent with this view, observing or executing goal-directed actions elicits suppression of alpha activity (relative to non-goal-directed actions) over central regions in 8 to 16-month-old infants (e.g. Nystrom et al., 2010; Southgate et al., 2009, 2010). Further, making eye contact with an adult before jointly viewing a toy elicits alpha suppression in 9-month-old infants, whilst viewing a toy in the absence of eye contact does not (Hoehl, Michel, Reid, Parise & Striano, 2014). Taken together, previous work shows that activity in the theta and alpha bands may be sensitive to core dimensions of social processing in infancy. However, no studies have used theta and alpha power to test whether there are developmental increases in the strength and topographic extent of socially-selective brain activity.
In the present study, we examined the development of socially-selective brain activity in the first year of life. Specifically, we examined theta and alpha EEG power during the presentation of naturalistic social and nonsocial stimuli in 6- and 12-month-old infants. This age range was chosen to allow us to examine brain activity around the time of the ‘socio-cognitive revolution’ in social behavior. We chose to present naturalistic stimuli (rather than static pictures of faces or specific social actions) in order to examine social brain function in a context that was as close as possible to the child's normative experiences. Thus, we examined brain responses during both social and non-social movies (Movie format), and during a naturalistic live interaction (Live Action format) in which infants could pay attention to either social or nonsocial features (woman singing while holding a toy). We chose to employ both Movie and Live Action formats to identify converging evidence of social selectivity across the Movie and Live Action formats, which would minimize potential confounds of specific stimulus classes. Of note, our formats were not designed to allow us to ask specific questions about the source of format effects (e.g. live vs video presentation). Rather, we sought to maximize the ecological validity of the Live Action condition. We compared it to a highly standardized video-based presentation (Movie condition) that has been used in several previous studies, including work with populations at high risk for developing social problems (e.g. Orekhova et al., 2014; Tomalski et al., 2013). This was designed to allow us to determine how the responses elicited by more standardized video-based stimuli relate to those obtained in a more natural setting. We asked which of the two contexts appeared to provide the clearest evidence of social selectivity, because this may be of use to researchers examining the development of social selectivity in clinical populations. We predicted that we would observe increased selectivity (greater differences in power between social and nonsocial stimuli over more scalp regions) at 12 months than at 6 months. We expected this to be reflected in greater theta power and greater alpha suppression to social versus nonsocial stimuli, indicating increasing engagement of key brain networks by social stimuli. Finally, we expected that the Live Action context may provide the clearest evidence of selectivity, being maximally ecologically valid.
Methods
Participants were 117 6-month-old (51 female) and 106 12-month-old (51 female) typically developing full-term infants. Of this group, 88 6-month-old (39 female) and 80 12-month-old infants (40 female) provided sufficient artifact-free data in both Movie and Live Action formats. Parents and their infants were recruited using a University Infant Participant Pool. Table 1 shows demographic and descriptive data for the full sample.
Table 1.
Participant demographics.
| Participant Demographics | 6 months | 12 months |
|---|---|---|
| Age (days) M (SD) and Range | 192.9 (9.5) 166-220 |
378.2 (12.0) 361-448 |
| First Child | 68% | 58% |
| Primary (Mother | Employed) | 94% | 94% | 95% | 95% |
| Participant Race (Caucasian, Asian, More than one race) | 76%, 4%, 19% | 79%, 4%, 17% |
| Primary: College Education (None, Some, Graduate) | 0%, 12%, 87% | 1%, 9%, 89% |
| Family Income (<$35k, $35-75k, >$75) | 5%, 23%, 67%, | 6%, 32%, 65%, |
EEG recording
EEG was recorded in a shielded room from 128-channel Geodesic sensor nets; recorded online with reference to the vertex; digitization at 500Hz; amplification at 1000x and band-pass filtering at 0.1 to 100Hz. Children were presented with two movies of 1-minute duration repeated twice during the session; order of presentation was counterbalanced. Movies were (a) Social: two women telling nursery rhymes with gestures; (b) Non-Social: child-appropriate dynamic toys (e.g. balls dropping down a chute). Similar stimuli have been used in previous studies of the effects of socioeconomic status on brain activity (Tomalski et al., 2013) and connectivity in infants with older siblings with ASD (Orekhova et al., 2014). After the movies were presented, children faced an experimenter while she sang for two one-minute periods (Live Action format). During this period, the experimenter held plain infant toys in her hands (e.g. teething rings); infants were free to look at either the experimenter's face (Social) or the toys in her hands (Non-Social).
EEG processing
EEG data was segmented into 1-second segments. Segments during which infants were not visually attending to the movie were dropped from analysis; for the Live Action format, segments were divided (based on offline coding from video) by whether infants were predominantly looking at social aspects of the display (e.g. the experimenter's face) or at non-social aspects of the display (e.g. the toys in her hand). Artifact detection of EEG data was accomplished with both automatic artifact-detection software (NetStation 4.3) and through hand-editing (EJ). Segments were rejected if the signal amplitude exceeded 250 μV, or if electro-ocular, movement or muscular artifact occurred. Channels with bad data were interpolated by an algorithm incorporated within NetStation 4.3 (segments were excluded from analysis if more than 20% of channels were subject to interpolation, or if there were more than 5 interpolated channels within a scalp region). Data was then re-referenced to the average reference, and the resulting segmented data was imported into Matlab.
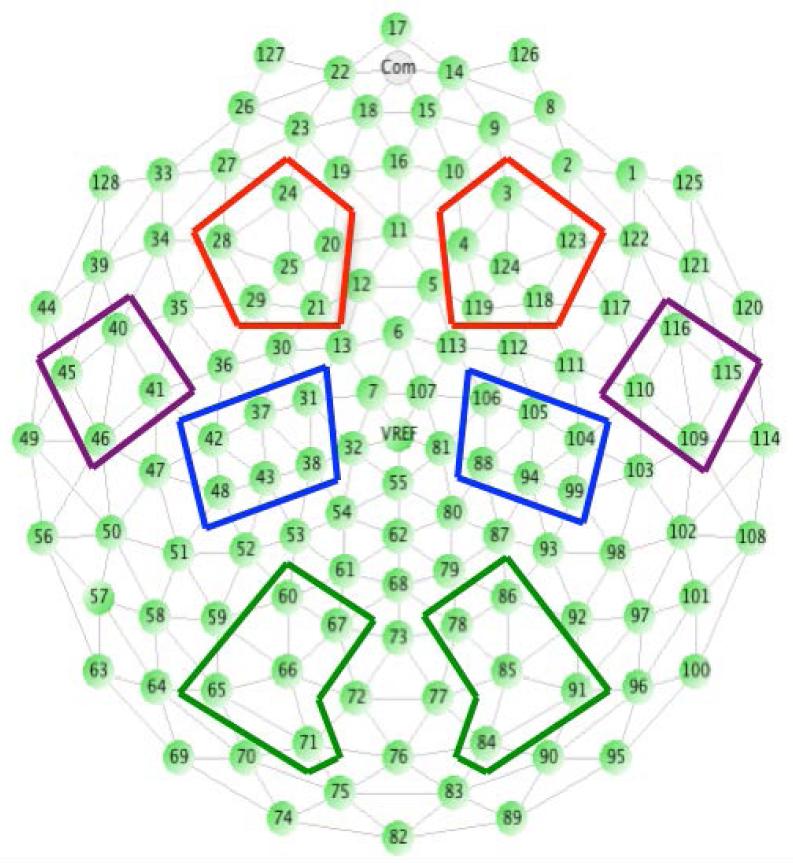
Within Matlab (using in-house algorithms) segments were detrended and subjected to an FFT, producing power spectra for electrodes grouped within a priori regions (Figure 1). For each segment, data from electrodes with a power value of more than 3 standard deviations from the mean of the remaining electrodes in a topographical group in the frequency bands of interest were dropped. Power values were then averaged across artifact-free segments and electrodes within topographical groups; natural logs were calculated to reduce skew. Finally, logged power values were averaged across the theta (3 to 6 Hz) and alpha (6 to 9 Hz) frequency ranges. Participants were only included in analyses if they provided at least 5 artifact-free trials per condition (e.g. Live Action Social condition; Live Action Nonsocial condition). This figure was chosen based on previous work, and from examining scatter plots of the distribution of EEG power relative to artifact-free trial numbers, which showed that power values from infants with under 5 trials/condition appeared less reliable (e.g. more variable across nearby electrodes).
Figure 1.
Electrode groups selected for analyses: Occipital (green), Parietal (blue), Temporal (purple), Frontal (red).
Analyses
We used repeated-measures ANOVA with Greenhouse-Geisser corrections to compare brain activity during the Social and Non-Social Movies, and during episodes of looking to Social and Non-Social aspects of the Live Action display. Within subject variables for both analyses included region (frontal, temporal, parietal, occipital), hemisphere (left, right), and stimulus (Social, Non-Social). Between subject variables were age (6- and 12-months) and sex (male, female). Within each section, overall power effects are reported first (e.g. region, laterality, age and sex effects), followed by main effects and interactions with stimulus (Social, Non-Social) since these were the focus of our a priori hypotheses. We used follow-up ANOVAs to explore any significant interactions; splitting analyses by region and/or age where indicated.
Results
Visual behavior
Infants looked more at the Nonsocial than the Social Movies (F(1,194) = 9.77, p =0.002) and more at Nonsocial than Social elements of the Live Action display (F(1,184) = 103.75, p <0.001). The latter was more pronounced at 12-months (Age by Stimulus interaction; F(1,184) = 19.11, p < 0.001). These differences in visual attention produced differences in the average number of artifact-free segments available for each condition (see Table 2). Rather than artificially removing segments of artifact-free data to match the number of trials per condition, we conducted preliminary analyses to determine whether trial numbers present a significant confound to our results. Across both alpha and theta bands, when including trial number as a covariate in all analyses the pattern of results presented below remained the same. Further, all major effects reported below were replicated in a subset of infants who had matched amounts of artifact-free data in the Social and Nonsocial conditions (n=32 6-month-olds and n=30 12-month-olds for Movies and n=27 6-month-olds and n=17 12-month-olds for the Live Action). Thus, differences in the number of artifact-free segments between the Social and Nonsocial stimuli did not account for differences in EEG power reported below.
Table 2.
Variables contributing to attrition in the Video and Live Formats.
| 6 months | 12 months | |
|---|---|---|
| N (female) | 117 (51) | 106 (51) |
| Fail to wear net | 0 | 1 |
| Fail to meet data criteria | 29 (11) | 25 (11) |
| EEG Data | 88 (39) | 80 (46) |
| Movies | Live | Movies | Live | |||||
|---|---|---|---|---|---|---|---|---|
| S | NS | S | NS | S | NS | S | NS | |
| # sec. attended | 91.9 (17.6) | 96.8 (15.9) | 48.0 (25.2) | 67.8 (25.6) | 104.1 (12.6) | 105.4 (12.6) | 35.2 (19.3) | 83.1 (20.1) |
| # trials good EEG | 53.9 (24.19) | 58.3 (19.9) | 23.7 (16.12) | 35.9 (21.7) | 68.6 (26.35) | 60.4 (23.97) | 18.7 (11.9) | 45.9 (21.4) |
Numbers in brackets within the first four lines of the table indicate the number of female infants within each category. In the second half of the table, figures are mean (standard deviation).
EEG Theta Power
Movies
We first examined theta power during periods of visual attention to the Social and Non-Social movies.
Overall power
Overall theta power was greater at 12-months than 6-months (Age F(1,164) = 18.7, p < 0.001; Age x Region F(3,492) = 39.22, p < 0.001; Age x Hemisphere F(1,164) = 8.47, p = 0.004); greater in male than female infants (F(1,164) = 5.35, p = 0.022); greater in left than right hemisphere (F(1,164) = 81.08, p < 0.001; Hemisphere x Region F(3,492) = 6.95, p < 0.001); and varied significantly by region (F(3,492) = 47.62, p < 0.001). These effects are illustrated in the left and central panels of Figure 2A. Examining scalp regions separately, age-related increases in theta power were observed over frontal (F(1,164) = 53.45, p < 0.001), parietal (F(1,164) = 12.50, p = 0.001) and temporal (F(1,164) =25.71, p < 0.001) but not occipital (F(1,164) = 0.74, p =0.39) regions. Hemisphere effects (left>right) were significant across all regions (Fs > 15, ps < 0.001) but stronger frontally (η2 = .37) and weaker occipitally (η2= .09). Hemisphere effects were also significant at both ages (Fs > 10, ps < 0.001) but stronger at 12 months (η2 = 0.45) than 6 months (η2 = 0.19).
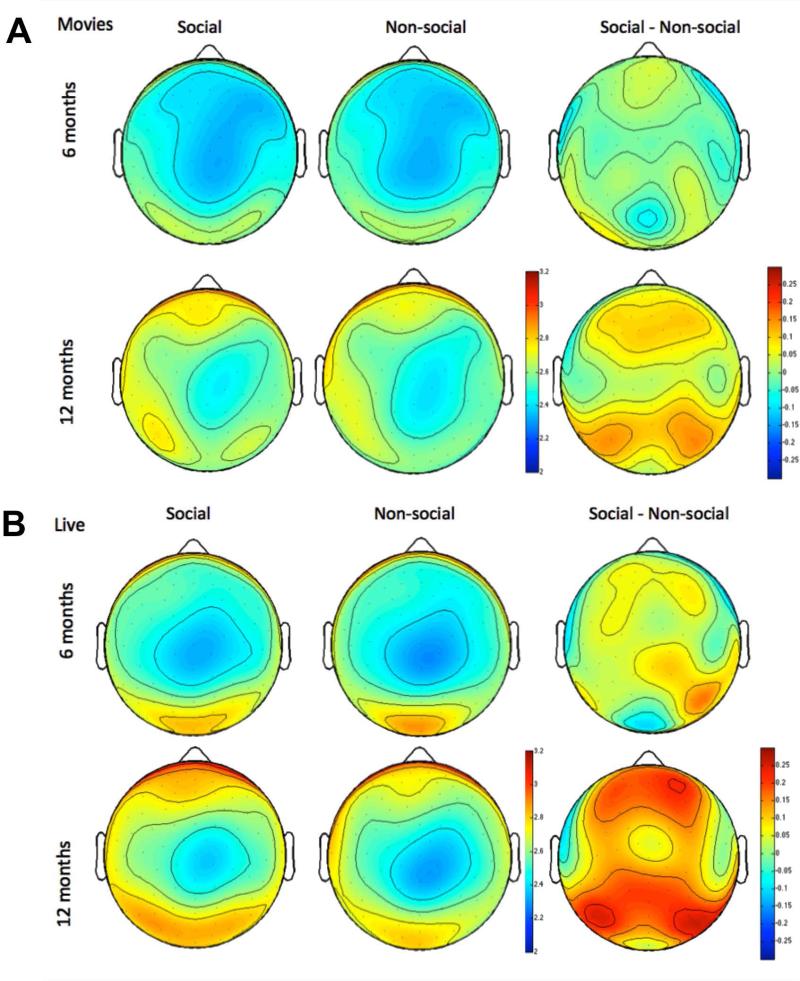
Figure 2.
Scalp topography of log theta power during Movie (top) and Live Action (bottom) social (left) and nonsocial (right) epochs, and topography of stimulus differences (right panel). Scales are in uV.
Social versus Non-Social effects
Stimulus effects varied by age (F(1,164) = 5.18, p = 0.02) and scalp region (F(3,492) = 17.62, p < 0.001; see Figure 2A right panel). No significant differentiation between social and non-social movies was observed over any region at 6-months (Fs < 2, ps > 0.2). At 12 months, greater theta power to social versus non-social stimuli was seen over occipital (F(1,78) = 18.58, p < 0.001) and frontal regions (F(1,78) = 8.71, p =0.004) . No significant differentiation was observed over temporal and parietal regions at 12 months (Fs < 2, ps > 0.2). Thus, the Movie condition revealed age-related increases in the strength and topographical extent of socially-selective theta activity.
Live Action
We next examined theta power during the Live Action episode, divided into periods of attention to Social or Non-Social aspects of the display.
Overall power
Overall theta power was greater at 12-months than 6-months (Age F(1,164) = 10.88, p = 0.001; Age x Region F(3,492) = 20.73, p < 0.001; Age x Hemisphere F(1,164) = 5.99, p = 0.015); greater in male than female infants (Sex F(1,164) = 8.15, p = 0.005; Sex x Region F(3,492) = 3.71, p = 0.016); greater in left than right hemisphere (Hemi F(1,164) = 46.5, p < 0.001; Hemisphere x Region F(3,492) = 2.99, p=0.035); and varied significantly by region (F(3,492) =177.21, p < 0.001). These effects are illustrated in the left and central panels of Figure 2B. Examining scalp regions separately, age-related increases in theta power were observed over frontal (F(1,164) = 33.48, p < 0.001), parietal (F(1,164) = 12.91, p = 0.001) and temporal (F(1,164) =5.45, p =0.021) but not occipital (F(1,164) = 1.09, p =0.30) regions. Sex differences in theta power were significant over all regions (Fs > 3, ps < 0.05) but strongest over temporal and parietal regions (η2 = 0.058, 0.067) and weakest over the occipital region (η2 = 0.026). Hemisphere effects were significant across all regions (Fs > 4, ps < 0.05) but stronger frontally (η2 = 0.23) and weaker occipitally (η2= 0.03). Hemisphere effects were also significant at both ages (Fs > 8, ps < 0.001) but stronger at 12 months (η2 = 0.39) than 6 months (η2 = 0.09). These patterns of theta modulation by age, gender, laterality and region during the Live Action format are very similar to those observed during the Movie format.
Social versus Nonsocial effects
Theta power was generally greater during social versus non-social attention (F(1,164) = 20.32, p < 0.001), but stimulus effects were greater at 12-months than 6-months (F(1,164) = 8.66, p = 0.004), greater over the right than left hemisphere (F(1,164) = 4.05, p = 0.046) and varied by scalp region (F(1,164) = 19.36, p < 0.001), and age and scalp region (F(1,164) = 3.03, p =0.046). These effects are illustrated in Figure 2B. Greater theta power during social versus non-social attention was seen over frontal regions in both age groups, though effects were strongest in the older infants (6-months η2 = 0.06; 12-months η2 = 0.32). In addition, in 12-month-olds only, significant effects of stimulus were observed over parietal (F(1,78) = 25.50, p < 0.001) and occipital regions (F(1,78) = 24.02, p < 0.001). There was no differentiation between social and non-social stimuli over temporal regions at either age (Fs < 1.5, ps > 0.3). Thus, the Live Action condition also revealed age-related increases in the strength and topographical extent of socially-selective theta activity.
Summary
In both Movie and Live Action formats, we observed increases in the power and topographical extent of socially selective theta responses between 6 and 12 months (see Figure 2 and Table 3). Further, greater levels of socially-selective theta power in the Live Action context correlated with better communication skills at 12 months. Thus, these results support the prediction derived from the IS framework that there will be developmental increases in the degree and extent of socially selective brain activity that relate to changes in social behavior.
Table 3.
Summary of Social versus Nonsocial power differences across age and region.
| Frontal | Temporal | Parietal | Occipital | |
|---|---|---|---|---|
| 6 months | ||||
| Theta | [L] Soc>Non | |||
| Alpha | [L] Non>Soc | [L] Non>Soc | ||
| 12 months | ||||
| Theta | [M] Soc> Non$ [L] Soc>Non$ |
[L] Soc>Non$ | [M] Soc>Non$ [L] Soc>Non$ |
|
| Alpha | [L] Non>Soc | [L] Non>Soc | ||
Key: [L] Live; [M] Movie; > greater than; $ significantly stronger at 12m.
We did not make direct statistical comparisons between the Live Action and Movie stimuli because they differed on multiple dimensions, including order of presentation (Movie stimuli were always presented before Live Stimuli), format (live versus screen-based presentation) and sequential (Movie) versus interspersed (Live) social and non-social segments. However, it is interesting to note that inspection of Figure 2 suggests that the Live Action format elicited more socially selective brain activity than the Movies in both age groups (see Figure 2 right panel). Specifically, socially-selective frontal theta responses were observed in the Live Action but not Movie format for 6-month-olds. At 12-months, socially selective frontal theta responses were detected in both the Live Action and Movie formats, but were more widespread in the Live Action context. Our current design did not allow us to determine which difference between the Live Action and Movie-based stimuli was the most critical in driving these effects. However, researchers seeking to examine social brain activity in (for example) high-risk populations may find it useful to note that following our Live Action design may be likely to elicit stronger evidence of social selectivity in typically developing infants.
EEG Alpha Power
Power in the alpha frequency band is reduced during active attention to a stimulus and/or during cognitive tasks such that magnitude of the band is inversely proportional to cortical activation (Stroganova & Orekhova, 2007). Thus, greater alpha suppression is associated with greater cortical activation and potentially increased attention engagement. In the present analyses, we thus expected social brain activity to be indicated by greater suppression of alpha power to the social stimuli.
Movies
Overall power
Overall alpha power was greater at 12-months than 6-months (F(1,164) = 85.82, p < 0.001; Age x Region F(3,492) = 16.50, p < 0.001); greater in left than right hemisphere (F(1,164) = 57.70, p < 0.001; Hemisphere x Region F(3,492) = 12.61, p < 0.001); and varied significantly by region (F(3,492) =48.93, p < 0.001). These effects are illustrated in the left and central panels of Figure 3A. Examining scalp regions separately, age-related increases in alpha power were observed over all scalp regions (Fs> 40, ps < 0.001), but were strongest frontally (η2 = 0.42) and weakest occipitally (η2 = 0.21). Hemisphere effects were significant across all regions (Fs > 10, ps < 0.002) but stronger parietally (η2 = 0.26) and weaker occipitally (η2= 0.058).
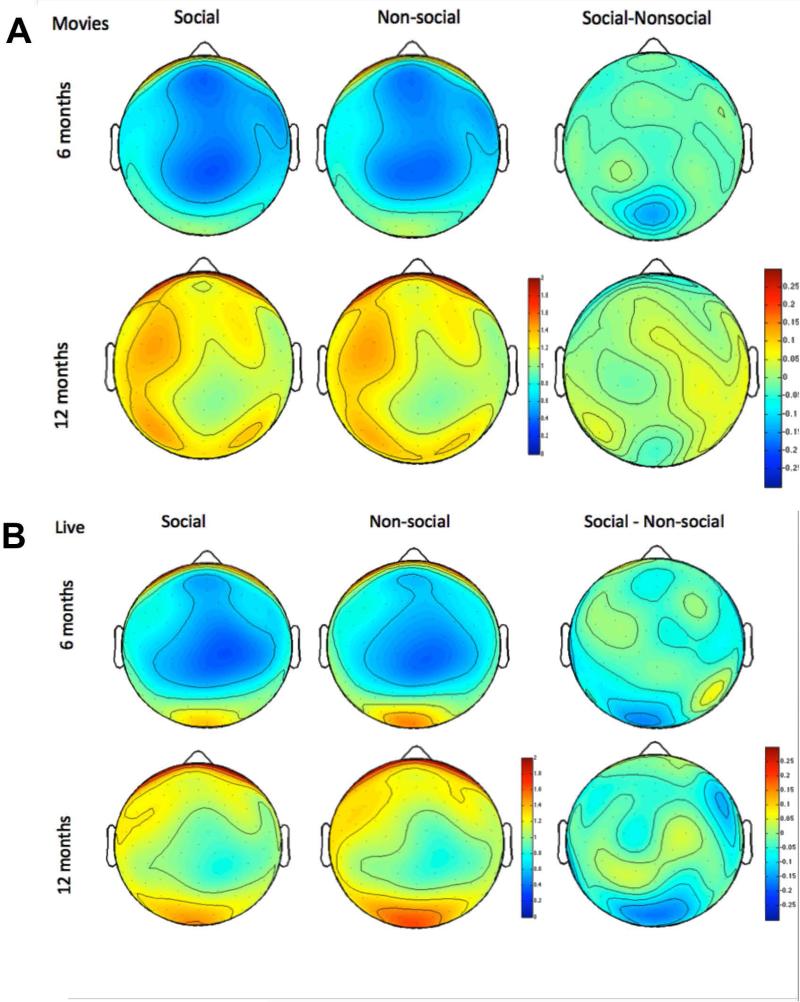
Figure 3.
Scalp topography of log alpha power during Movie (top) and Live Action (bottom) social (left) and nonsocial (right) epochs, and topography of stimulus differences (right panel). Scales are in uV.
Social versus Nonsocial effects
There were no significant effects or interactions with stimulus in the alpha band for the Movie stimuli (Fs < 2, ps > 0.2).
Live Action
Overall power
Overall alpha power was greater at 12-months than 6-months (F(1,164) = 56.75, p < 0.001; Age x Region F(3,492) = 18.60, p < 0.001; Age x Region x Hemisphere F(3,492) = 3.12, p =0.028); greater in left than right hemisphere (F(1,164) = 44.97, p < 0.001; Hemisphere x Region F(3,492) = 4.88, p=0.003); and varied significantly by region (F(3,492) =114.77, p < 0.001). These effects are illustrated in the left and central panels of Figure 3B. Examining scalp regions separately, hemisphere effects (left > right) were significant across all regions (Fs > 9, ps < 0.003) but stronger parietally (η2 = 0.18) and weaker occipitally (η2= 0.052). Hemisphere effects were greater at 12 compared to 6 months (F(1,164) = 3.98, p = 0.048). Age-related differences in alpha power were observed over all scalp regions (Fs> 17, ps < 0.001), but were strongest frontally (η2 = 0.40) and weakest occipitally (η2 = 0.096).
Social versus Non-Social effects
Alpha power was suppressed more during social than nonsocial epochs of attention (F(1,164) = 8.35, p = 0.004). However, this varied by scalp region (F(3,492) = 7.32, p < 0.001). Alpha power was generally more suppressed during social than non-social attention over temporal (F(1,164) = 5.40, p = 0.021) and occipital regions (F(1,164) = 23.28, p < 0.001) but not over the parietal region (F(1,164) = 0.006, p = 0.94) or the frontal region (F(1,164) = 0.31, p = 0.58).
Summary
Within the Live Action condition, suppression of alpha power during social attention was observed as expected. However, across both Movie and Live Action stimuli, results indicated that the strength and topographical extent of alpha power responses to social versus non-social stimuli did not change over the age-ranged studied (see Figure 3). Possibly, alpha power is a less sensitive measure of social brain development in the first year of life.
Discussion
In this study we used EEG to investigate age-related changes in naturalistic social brain activity in a large cohort of 6- and 12-month-old infants. We measured brain activity in response to highly naturalistic Movie-based stimuli, and an ecologically valid Live Action condition. Our pattern of results (summarized in Table 3) yielded two main conclusions. First, in the theta band there were significant differences in power between social and nonsocial epochs of attention that were stronger and more widespread at 12 months than at 6 months. In contrast, although alpha power differed by social context (with greater alpha suppression during social stimuli, particularly in the Live Action format), there were no age-related changes in the magnitude or extent of this effect. Of note, this difference between the two frequency bands makes it unlikely that domain-general changes in skull thickness, brain size, or the differing number of segments between social and non-social conditions could account for our findings. Possibly, theta (compared to alpha) power may a more sensitive measure of social brain development in the first year of life. Second, across both frequency bands, socially selective activity appeared stronger and more widespread during a naturalistic episode of live singing than a more standardized video-based presentation. These results may indicate that naturalistic live experiences engage the developing brain more effectively than video-based stimuli, although the multiple differences between our Live Action and Movie stimuli mean that the precise reason for differences cannot be determined. These results are consistent with the IS hypothesis that there are age-related increases in the strength and topographical extent of social brain activity in early development, and suggest that EEG theta power measured in live interactive contexts may be particularly sensitive to these changes.
Social Selectivity
Further work is required to identify which specific aspects of the social versus nonsocial stimuli used in the current study elicit particular patterns of brain activity. Both our live action and movie presentations included the presence of a face, direct gaze, biological motion, and language (for review, Grossman & Johnson, 2007). Previous work suggests that neural responses to many of these more specific features become more localized and specialized over time (Johnson et al., 2009). The present data extends this picture by indicating that the combination of those features (as is the case in complex naturalistic interactions) leads to an age-related increase in the spread of specialized neural activity. The social brain includes regions that span frontal, temporal and parietal cortices (e.g. Frith & Frith, 2007), indicating that engagement of the full system would likely produce the kind of widespread brain activity noted in the present study. One possibility to be tested in future work is that increased functional connectivity between brain regions underlies this more robust and widespread activation of the social brain network in older infants (e.g. Pandit et al., 2013).
Interestingly, whilst theta power (which is generally associated with attention) was greater during epochs of social than nonsocial attention, behaviorally infants spent more time looking at the nonsocial than social movie, and more time looking at the toy than the experimenter's face during the Live Action condition. These effects were strongest at 12 months. Although studies with screen-based and static stimuli generally find increased attention to faces versus other objects in infants (e.g. Elsabbagh et al., 2013), our results are consistent with behavioral studies showing that infants actually spend relatively little time looking at people when tested in naturalistic environments (e.g. de Barbaro et al., 2012; Yoshida & Smith, 2008). Thus, studying patterns of social attention in naturalistic dynamic contexts is critical. Our findings further illustrate that to understand social attention in early infancy, we must not only examine what infants choose to look at, but also what their brains are doing whilst they look at it. One population to which this may be particularly relevant is infants at high risk for developing Autism Spectrum Disorder (ASD). This group of infants are at heightened risk of developing ASD themselves, and so can be followed prospectively to identify early markers of later diagnosis. Several recent such studies have revealed that infants with later ASD show remarkably typical patterns of looking at social stimuli in early infancy (e.g. Ozonoff et al., 2010; Elsabbagh et al., 2013). However, very few studies have examined brain activity during visual attention to social and nonsocial stimuli. Our present results suggest that this may be critical to understanding what may be different about social processing in the very early development of infants with emerging ASD.
Specificity of EEG Frequency response
Effects seen in specific frequency bands provide further insight into social brain development in infancy. First, frontal theta power was greater during social vs non-social attention in the Live Action format for 6-month-olds and in both Live and Movie-based stimuli for 12-month-olds. This is consistent with a range of evidence to suggest that frontal theta is related to social attention (Bazhenova et al., 2007), or responds more generally to child engagement (Orekhova et al., 2006). The present findings extend previous work by showing significant differences in the theta response to engaging social and nonsocial stimuli, and significant age-related increases in this differential response. Given that infants generally showed more looking to the nonsocial stimuli, the present results suggest that frontal theta in infancy is particularly engaged by social stimuli, and not just any stimulus that engages visual attention. Significant increases in theta in response to social versus non-social stimuli were also observed over parietal and occipital regions for 12-month-old infants, which is again consistent with findings reported by Orekhova and colleagues (2006). However, these were not apparent at 6 months, suggesting age-related changes in spatial extent of theta responses.
In contrast to effects in the theta band, there were no age-related changes in differential alpha suppression by the social and nonsocial stimuli. Of note, general age-related increases in alpha and theta power were observed that are consistent with previous work and have been attributed to a number of possible factors including general neuronal maturation, the development of myelination across the cortex, changes in the orientation and density of neuronal assemblies, and changes in the skull and supportive tissue (Marshall et al., 2002). In the present study, epochs of social attention were associated with suppression of alpha activity over temporal and occipital cortices at both 6- and 12-months that were observed in the Live Action format only. The topography of this response is not consistent with ‘mu’ activity (alpha over central regions). Further, we did not observe differential suppression of frontal alpha, which is commonly associated with individual differences in approach and avoidance responses (Davidson & Fox, 1989; Hane et al., 2008). Rather, since alpha is commonly interpreted as an idling rhythm (Klimesch et al., 2007) the observed patterns may represent an increase in activity in visual processing and language processing areas. Interestingly, it is known that language learning between 6 and 12 months is facilitated by live versus movie-based presentation (Kuhl, Tsao & Liu, 2003). Possibly, live presentations more effectively engage brain regions required to integrate language and visual input. Studying how these patterns of brain activity relate to language learning is an important step for future work.
Effects of stimulus format
The present study highlights the importance of considering the context in which social and non-social responses are studied. We chose two very different formats to maximize standardization (the Movie format) and ecological validity (Live Action). Broadly, our present results suggest that the Live Action format was more successful at eliciting socially-selective brain activity than the Movie-based stimuli. For example, socially selective effects were seen in the Live Action format for 6-month-old infants, but none of these were elicited by the Movie format. 12-month-old infants did show some socially selective patterns of brain activity in response to the Movie stimuli, but effects were stronger and more widespread during Live Action. Because they were designed to maximize standardization (Movie stimulus) or ecological validity (Live stimulus) rather than be matched on multiple variables, the Live and Movie stimuli differed on several parameters that may be of relevance to the observed findings. First, a range of other studies have also found that infants learn more readily from live than video-based presentations, particularly at 12 months and above (e.g. Cleveland & Striano, 2008; Barr et al., 2007), even though infants scan the relevant parts of video-based presentations appropriately (Taylor & Herbert, 2013). Action processing may also differ: suppression of the mu EEG rhythm is greater in response to a live demonstration of a person producing goal-directed actions than a video-taped stimulus at 6- to 7-months (Shimada & Hiraki, 2006) and 18- to 36-months (Ruysschaert et al., 2013). Taken together, previous work suggests that differences in the way that infants process live and video-based stimuli could have contributed to the differences between the Live Action and Movie formats examined in the present report. However, there were also other differences between the Live Action and Movie formats that could have contributed to effects. For example, attention was possibly more endogenously driven in the Live Action format than in the Movie format, because the child had a more direct choice between paying attention to the experimenter's face and the presented toy. This may lead to more effective engagement of specialized brain regions because making a choice may elicit more ‘active’ versus passive attention to the object of that choice (e.g. Orekhova et al., 2006; Kida et al., 2006), or may have required greater use of cognitive control (also associated with increased theta power; Orekhova et al., in review). In contrast, segments of social and nonsocial attention during the movie presentations were sequential. Further work could examine the differences between presenting both live and more naturalistic movie-based stimuli in social and nonsocial blocks verses allowing naturalistic attention switching between competing social and nonsocial elements. Further, the Movie format was always administered prior to the Live Action format during testing. Systematic differences in state over time may thus confound interpretation of the overall differences between the Live Action and Movie formats. The Movie format also featured two women, whilst only one was present in the Live Action context; this would have affected the relatively familiarity of the social stimulus across conditions. Other differences were that whilst the soundtrack of the movies differed (toy noises in the nonsocial movie and women singing in the social movie), the experimenter was always singing in the Live Action format. Further, the Live Action format could have elicited episodes of triadic joint attention (rendering the Non-Social condition more ‘social’ in nature), which were rare in the Movie context. However, these latter differences would appear if anything to increase the similarly of social and nonsocial epochs in the live episode, reducing the likelihood of finding effects in the Live Action format.
Although the theoretical interpretation is unclear, the difference between results from the Live Action and Movie formats does have several implications for future work. First, needs for standardization versus ecological validity are often in opposition, but must be carefully considered. Video-based sequentially presented stimuli enable the same data to be collected across labs and processed relatively easily, a significant advantage in fields performing high-cost longitudinal work with low-incidence participants. However, these stimuli may be less effective at engaging relevant brain regions than more naturalistic social experiences, particularly in early development. Using video-based stimuli to identify atypical social processing in risk groups may be futile in early infancy, since such effects are not clearly seen in typically developing infants. This study provides strong motivation for studying social brain activity in more naturalistic settings in early infancy (see also Ruysschaerts et al., 2013). Further, the present data also suggest that using live periods of social interaction to elicit recordings of ‘resting’ or ‘baseline’ EEG is also potentially confounded by individual differences in the degree to which infants look at the experimenter during recording, as direction of visual attention strongly influenced the spectral power EEG.
In summary, we demonstrated that socially-selective oscillatory theta responses increase in power and topographic extent over the second half of the first year of life. These results are consistent with Interactive Specialization model of brain development, although further work is required to understand why effects were specific to the theta band and to replicate findings in a longitudinal sample. Our work forms an important normative profile to which work with risk groups (such as infants with later emerging autism) can be compared. Further, our results suggest that examining brain activity during naturalistic live social episodes is critical to studying social brain development in infancy. This has widespread implications for studies of infant brain function, which commonly rely on video or static- stimuli.
Acknowledgments
Support for this project was provided by an Autism Speaks Postdoctoral Fellowship and a L'Oreal For Women in Science Fellowship (Jones), the Eunice Kennedy Shriver National Institute of Child Health and Development (P50 HD055782 and R01 HD064820, Webb). We thank the families who participated in the University of Washington Early Attention Study.
Footnotes
The authors report no conflicts of interest.
References
- Barr R, Muentener P, Garcia A, Fujimoto M, Chavez V. The effect of repetition on imitation from television during infancy. Infancy. 2007;49:196–207. doi: 10.1002/dev.20208. [DOI] [PubMed] [Google Scholar]
- Bazhenova OV, Stroganova TA, Doussard-Roosevelt JA, Posikera IA, Porges SW. Physiological responses of 5-month-old infants to smiling and blank faces. International journal of psychophysiology. 2007;63(1):64–76. doi: 10.1016/j.ijpsycho.2006.08.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benasich AA, Gou Z, Choudhury N, Harris KD. Early cognitive and language skills are linked to resting frontal gamma power across the first 3 years. Behavioural brain research. 2008;195(2):215–222. doi: 10.1016/j.bbr.2008.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver LJ, Meltzoff AN, Dawson G. Event-related potential (ERP) indices of infants’ recognition of familiar and unfamiliar objects in two and three dimensions. Developmental Science. 2006;9(1):51–62. doi: 10.1111/j.1467-7687.2005.00463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland A, Striano T. Televised social interaction and object learning in 14-and 18-month-old infants. Infant Behavior and Development. 2008;31(2):326–331. doi: 10.1016/j.infbeh.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Csibra G, Davis G, Spratling MW, Johnson MH. Gamma oscillations and object processing in the infant brain. Science. 2000;290(5496):1582–1585. doi: 10.1126/science.290.5496.1582. [DOI] [PubMed] [Google Scholar]
- Cuevas K, Bell MA. EEG and ECG from 5 to 10months of age: Developmental changes in baseline activation and cognitive processing during a working memory task. International Journal of Psychophysiology. 2011;80(2):119–128. doi: 10.1016/j.ijpsycho.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, Fox NA. Frontal brain asymmetry predicts infants’ response to maternal separation. Journal of abnormal psychology. 1989;98(2):127–131. doi: 10.1037//0021-843x.98.2.127. [DOI] [PubMed] [Google Scholar]
- de Barbaro K, Johnson CM, Forster D, Littlewort G, Deak G. Sensory-motor dynamics of mother-infant-object interactions: Longitudinal changes in micro-behavioral patterns across the first year.. Development and Learning and Epigenetic Robotics (ICDL), 2012 IEEE International Conference on; IEEE; Nov, 2012. pp. 1–2. [Google Scholar]
- De Haan M, Pascalis O, Johnson MH. Specialization of neural mechanisms underlying face recognition in human infants. Journal of cognitive neuroscience. 2002;14(2):199–209. doi: 10.1162/089892902317236849. [DOI] [PubMed] [Google Scholar]
- Elsabbagh M, Gliga T, Pickles A, Hudry K, Charman T, Johnson MH, BASIS Team The development of face orienting mechanisms in infants at-risk for autism. Behavioural brain research. 2013;251:147–154. doi: 10.1016/j.bbr.2012.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, Frith U. Social cognition in humans. Current Biology. 2007;17(16):724–732. doi: 10.1016/j.cub.2007.05.068. [DOI] [PubMed] [Google Scholar]
- Gliga T, Volein A, Csibra G. Verbal labels modulate perceptual object processing in 1-year-old children. Journal of Cognitive Neuroscience. 2010;22(12):2781–2789. doi: 10.1162/jocn.2010.21427. [DOI] [PubMed] [Google Scholar]
- Gou Z, Choudhury N, Benasich AA. Resting frontal gamma power at 16, 24 and 36 months predicts individual differences in language and cognition at 4 and 5 years. Behavioural brain research. 2011;220(2):263–270. doi: 10.1016/j.bbr.2011.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann T, Johnson MH. The development of the social brain in human infancy. European Journal of Neuroscience. 2007;25(4):909–919. doi: 10.1111/j.1460-9568.2007.05379.x. [DOI] [PubMed] [Google Scholar]
- Halit H, De Haan M, Johnson MH. Cortical specialisation for face processing: face-sensitive event-related potential components in 3-and 12-month-old infants. Neuroimage. 2003;19(3):1180–1193. doi: 10.1016/s1053-8119(03)00076-4. [DOI] [PubMed] [Google Scholar]
- Hane AA, Fox NA, Henderson HA, Marshall PJ. Behavioral reactivity and approach-withdrawal bias in infancy. Developmental Psychology. 2008;44(5):1491–1496. doi: 10.1037/a0012855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH, Griffin R, Csibra G, Halit H, Farroni T, De Haan M, Richards J. The emergence of the social brain network: Evidence from typical and atypical development. Development and psychopathology. 2005;17(3):599–619. doi: 10.1017/S0954579405050297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH, Grossmann T, Kadosh KC. Mapping functional brain development: Building a social brain through interactive specialization. Developmental psychology. 2009;45(1):151–159. doi: 10.1037/a0014548. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Csibra G, Johnson MH. Representing occluded objects in the human infant brain. Proceedings of the Royal Society of London. Series B: Biological Sciences. 2003;270(2):140–143. doi: 10.1098/rsbl.2003.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida T, Wasaka T, Nakata H, Akatsuka K, Kakigi R. Active attention modulates passive attention-related neural responses to sudden somatosensory input against a silent background. Experimental brain research. 2006;175(4):609–617. doi: 10.1007/s00221-006-0578-4. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: the inhibition–timing hypothesis. Brain research reviews. 2007;53(1):63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Kuhl PK, Tsao FM, Liu HM. Foreign-language experience in infancy: Effects of short-term exposure and social interaction on phonetic learning. Proceedings of the National Academy of Sciences. 2003;100(15):9096–9101. doi: 10.1073/pnas.1532872100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Fox S, Blasi A, Elwell CE. Illuminating the developing brain: the past, present and future of functional near infrared spectroscopy. Neuroscience & Biobehavioral Reviews. 2010;34(3):269–284. doi: 10.1016/j.neubiorev.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Bar-Haim JY, Fox NA. Development of the EEG from 5 months to 4 years of age. Clinical Neurophysiology. 2002;113:1199–1208. doi: 10.1016/s1388-2457(02)00163-3. [DOI] [PubMed] [Google Scholar]
- Miller R. Cortico-hippocampal interplay and the representation of contexts in the brain. Vol. 37. Springer-Verlag; New York: 1991. [Google Scholar]
- Mullen EM. Mullen scales of early learning. American Guidance Service, Inc; Circle Pines, MN: 1997. [Google Scholar]
- Müller MM, Gruber T, Keil A. Modulation of induced gamma band activity in the human EEG by attention and visual information processing. International Journal of Psychophysiology. 2000;38(3):283–299. doi: 10.1016/s0167-8760(00)00171-9. [DOI] [PubMed] [Google Scholar]
- Mundy P, Card J, Fox N. EEG correlates of the development of infant joint attention skills. Developmental psychobiology. 2000;36(4):325–338. [PubMed] [Google Scholar]
- Mundy P, Fox N, Card J. EEG coherence, joint attention and language development in the second year. Developmental Science. 2003;6(1):48–54. [Google Scholar]
- Nyström P, Ljunghammar T, Rosander K, von Hofsten C. Using mu rhythm desynchronization to measure mirror neuron activity in infants. Developmental science. 2011;14(2):327–335. doi: 10.1111/j.1467-7687.2010.00979.x. [DOI] [PubMed] [Google Scholar]
- Orekhova EV, Stroganova TA, Posikera IN, Elam M. EEG theta rhythm in infants and preschool children. Clinical neurophysiology. 2006;117(5):1047–1062. doi: 10.1016/j.clinph.2005.12.027. [DOI] [PubMed] [Google Scholar]
- Orekhova EV, Elsabbagh M, Jones EJ, Dawson G, Charman T, Johnson MH, BASIS Team EEG hyper-connectivity in high-risk infants is associated with later autism. Journal of neurodevelopmental disorders. 2014;6(1):1–11. doi: 10.1186/1866-1955-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orekhova EV, Jones EJH, Johnson MH. Dynamic change in EEG theta oscillations during sustained attention in infants. in review. [Google Scholar]
- Ozonoff S, Iosif AM, Baguio F, Cook IC, Hill MM, Hutman T, Young GS. A prospective study of the emergence of early behavioral signs of autism. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49(3):256–266. [PMC free article] [PubMed] [Google Scholar]
- Pandit AS, Robinson E, Aljabar P, Ball G, Gousias IS, Wang Z, Edwards AD. Whole-Brain Mapping of Structural Connectivity in Infants Reveals Altered Connection Strength Associated with Growth and Preterm Birth. Cerebral Cortex. 2013;23(10):1–10. doi: 10.1093/cercor/bht086. [DOI] [PubMed] [Google Scholar]
- Reid VM, Csibra G, Belsky J, Johnson MH. Neural correlates of the perception of goal-directed action in infants. Acta Psychologica. 2007;124(1):129–138. doi: 10.1016/j.actpsy.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Ribary U, Ioannides AA, Singh KD, Hasson R, Bolton JP, Lado F, Llinas R. Magnetic field tomography of coherent thalamocortical 40-Hz oscillations in humans. Proceedings of the National Academy of Sciences. 1991;88(24):11037–11041. doi: 10.1073/pnas.88.24.11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum LD, Schmuckler MA, Johnson JA. The McGurk effect in infants. Perception & Psychophysics. 1997;59(3):347–357. doi: 10.3758/bf03211902. [DOI] [PubMed] [Google Scholar]
- Ruysschaert L, Warreyn P, Wiersema JR, Metin B, Roeyers H. Neural mirroring during the observation of live and video actions in infants. Clinical Neurophysiology. 2013;124(9):1765–1770. doi: 10.1016/j.clinph.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Shimada S, Hiraki K. Infant's brain responses to live and televised action. Neuroimage. 2006;32(2):930–939. doi: 10.1016/j.neuroimage.2006.03.044. [DOI] [PubMed] [Google Scholar]
- Southgate V, Johnson MH, Osborne T, Csibra G. Predictive motor activation during action observation in human infants. Biology Letters. 2009;5(6):769–772. doi: 10.1098/rsbl.2009.0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southgate V, Johnson MH, El Karoui I, Csibra G. Motor system activation reveals infants’ on-line prediction of others’ goals. Psychological Science. 2010;21(3):355–359. doi: 10.1177/0956797610362058. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Balla DA, Cicchetti DV. Vineland-II adaptive behavior scales. AGS Publishing; 2005. [Google Scholar]
- Stroganova TA, V Orekhova E, Posikera IN. Externally and internally controlled attention in infants: an EEG study. International Journal of Psychophysiology. 1998;30(3):339–351. doi: 10.1016/s0167-8760(98)00026-9. [DOI] [PubMed] [Google Scholar]
- Stroganova TA, Posikera IN, Prokofiev AO, Morozov AA, Obukhov YV, Morozov VA. EEG alpha activity in the human brain during perception of an illusory kanizsa square. Neuroscience and behavioral physiology. 2011;41(2):130–139. [Google Scholar]
- Tomalski P, Moore DG, Ribeiro H, Axelsson EL, Murphy E, Karmiloff-Smith A, Kushnerenko E. Socioeconomic status and functional brain development–associations in early infancy. Developmental Science. 2013;16(5):676–687. doi: 10.1111/desc.12079. [DOI] [PubMed] [Google Scholar]
- Webb SJ, Jones EJH, Merkle K, Venema K, Greenson J, Murias M, Dawson G. Developmental change in the ERP responses to familiar faces in toddlers with Autism Spectrum Disorders versus typical development. Child Development. 2012;82:1868–1886. doi: 10.1111/j.1467-8624.2011.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitham EM, Pope KJ, Fitzgibbon SP, Lewis T, Clark CR, Loveless S, Willoughby JO. Scalp electrical recording during paralysis: quantitative evidence that EEG frequencies above 20Hz are contaminated by EMG. Clinical Neurophysiology. 2007;118(8):1877–1888. doi: 10.1016/j.clinph.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Smith LB. What's in view for toddlers? Using a head camera to study visual experience. Infancy. 2008;13(3):229–248. doi: 10.1080/15250000802004437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuval-Greenberg S, Tomer O, Keren AS, Nelken I, Deouell LY. Transient induced gamma-band response in EEG as a manifestation of miniature saccades. Neuron. 2008;58(3):429–441. doi: 10.1016/j.neuron.2008.03.027. [DOI] [PubMed] [Google Scholar]