Abstract
Acute alterations in skeletal muscle protein metabolism are a well-established event associated with the stress response to burns. Nevertheless, the long-lasting effects of burn injury on skeletal muscle protein turnover are incompletely understood. This study was undertaken to investigate fractional synthesis (FSR) and breakdown (FBR) rates in skeletal muscle of pediatric burn patients (N=42, >30% total body surface area burns) for up to 1 year after injury. Skeletal muscle protein kinetics were measured in the postprandial state following bolus injections of 13C6 and 15N phenylalanine stable isotopes. Plasma and muscle phenylalanine enrichments were quantified using gas chromatography-mass spectrometry. We found that the FSR in burn patients was 2- to 3-fold higher than values from healthy men previously reported in the literature (p≤0.05). The FBR was 4- to 6-fold higher than healthy values (p<0.01). Therefore, net protein balance was lower in burn patients compared to healthy men from 2 weeks to 12 months post injury (p<0.05). These findings show that skeletal muscle protein turnover stays elevated for up to 1 year after burn, an effect attributable to simultaneous increases in FBR and FSR. Muscle FBR exceeds FSR during this time, producing a persistent net negative protein balance, even in the postprandial state, which likely contributes to the prolonged cachexia seen in burned victims.
Keywords: protein turnover, metabolism, thermal injury, muscle breakdown, stable isotopes
INTRODUCTION
Severe burns (>30% of the total body surface area [TBSA]) result in a pathophysiological stress response that lasts at least 2 years post injury (1). Inflammation and chronic adrenergic stress following burn injury are thought to contribute to increased amino acid turnover in skeletal muscle (2–4). Increased skeletal muscle protein breakdown and synthesis are hallmarks of the acute stress response to burns. However, increased muscle protein synthesis does not match the increase in muscle protein breakdown after burn injury, leading to chronic amino acid loss (5–8). This discordance in skeletal muscle proteolysis and accretion is considered the principal culprit underlying skeletal muscle cachexia in burn survivors.
We have previously reported that amino acid loss across the leg remains elevated in burn survivors for as long as 9 months after the burn (9). In that study, the rates of protein synthesis and breakdown in skeletal muscle were determined indirectly via cross-leg arterial venous balance studies. Notably, the necessity for arterial and venous catheters, prolonged infusion times to reach isotopic steady states (>3 h), and the measurement of blood flow cross-leg makes these studies invasive and technically challenging to perform, particularly in the outpatient setting. In recent years, our group has developed methodologies to determine skeletal muscle fractional synthesis rate (FSR) and fractional breakdown rate (FBR) during non-steady state conditions using bolus injections of two isotopically labeled phenylalanine tracers (10). This permits the determination of FSR and FBR in a study lasting 60 min, without the need for central lines and blood flow measurements.
In the current study, we used this novel method for determining skeletal muscle FSR and FBR to investigate the long-term effect of burns on protein turnover in skeletal muscle. We hypothesized that elevated skeletal muscle protein turnover, primarily increased FBR, would persist in burn victims long into their convalescence.
MATERIALS AND METHODS
Patients
This study was approved by the Institutional Review Board at the University of Texas Medical Branch (Galveston, TX). Informed consent was obtained from each patient’s parent or guardian prior to enrollment in the study. Between July 2008 and March 2014, 42 subjects who met the inclusion criteria were enrolled in this study. Inclusion criteria were as follows: children over the age of 1 year but less than 18 years, children with burns covering more than 30% of the TBSA who were admitted to Shriners Hospitals for Children—Galveston for acute burn care within 3 days of injury, and children able to participate in stable isotopic studies.
Upon arrival at the hospital, all patients received standard burn care, which included fluid resuscitation and total burn wound excision within 48 h of admission. Sequential surgical procedures for wound grafting were performed as needed until the burn wounds were healed. A constant infusion of insulin was administered intravenously to patients when their blood glucose concentration exceeded 200 mg/dl, in accordance with standard clinical practice.
Patients were intubated for operations and immediately extubated after. Ventilator settings for those who remained intubated followed ARDS-NET recommendations (11). Any occurrence of sepsis was recorded and aggressively treated with the administration of antibiotic/antimicrobial agents. Patients received up to 5 days of bed rest after excision and grafting procedures. Afterward, patients ambulated daily and received occupational and physical therapy as part of their rehabilitation.
Nutrition
Each patient received enteral nutrition via nasoduodenal tube during their acute hospitalization until they were able to consume food voluntarily. Patients received Vivonex® TEN (Sandoz Nutritional Corp., Minneapolis, MN), composed of 82% carbohydrate, 15% protein, and 3% fat. Daily caloric intake was calculated using the Galveston formula, which delivers 1,500 kcal/m2 TBSA burned (for burn hypermetabolism) + 1,500 kcal/m2 TBSA (maintenance) (12, 13). The target caloric intake equaled 1.4 times the patients’ resting energy expenditure, as measured by indirect calorimetry. This feeding regimen was started at admission and continued at a constant rate until the wounds were healed. Total caloric intake remained constant during hospitalization, and this protocol was carried through the infusion studies. Once discharged, patients received nutritional drinks (Boost, Nestle Health Care Nutrition) to ensure that caloric intake was 1.4 times the measured resting energy expenditure.
For outpatient infusion studies, nutritional support was provided with 10% Travasol® (amino acid) solution (Clintec Nutrition, Deerfield, IL). A primed dose of 0.45 ml/kg was followed by a continuous infusion at 1.35 ml/kg/h. This primed dose was administered 1 h prior to the first bolus injection followed by the constant infusion given through the duration of the study. The 10% Travasol® solution contained 100 mg/ml of amino acids composed of leucine (7.3 mg/ml), isoleucine (6 mg/ml), lysine (5.8 mg/ml), valine (5.8 mg/ml), phenylalanine (5.6 mg/ml), methionine (4 mg/ml), tryptophan (1.8 mg/ml), alanine (20.7 mg/ml), arginine (11.5 mg/ml), glycine (10.3 mg/ml), proline (6.8 mg/ml), serine (5 mg/ml), and lysine (0.4 mg/ml).
Stable isotope infusion procedure
Skeletal muscle protein kinetics were measured using a bolus tracer injection method. This method was used because it is less invasive and time intensive for patients than arterial-venous dilution approaches. We have previously found that this method produces FSR and FBR values similar to the muscle protein synthesis and muscle protein breakdown values derived from arterial-venous balance methodologies (10, 14).
Blood samples were collected prior to the bolus tracer injection to determine background enrichment. A bolus injection of L-[ring-13C6]Phe (5.56 mg/kg) and L-[15N]Phe (5.40 mg/kg) in 3 ml of 0.45% saline were injected intravenously at 0 and 30 min, respectively. Blood samples (1.0 ml) were taken at 5, 10, 15, 20, 29, 35, 40, 50, and 60 min. Muscle samples (~100 mg total) were obtained from the m. vastus lateralis at 10 and 60 min using a suction-adapted Bergstrom needle. Muscle samples were washed in ice-cold saline to remove visible blood, frozen in liquid nitrogen, and stored at −80°C for later processing. Blood samples were centrifuged at 3,000 rpm for 20 min. Plasma (~0.5 ml) was aliquoted into cryotubes and stored at −20°C for later processing.
Sample analysis
For measurement of plasma enrichment, 500 µl of plasma was pipetted into a glass tube containing the same volume of 15% sulfasalicylic acid. These samples were centrifuged at 3,000 rpm for 10 min. Supernatants were loaded into solid phase extraction columns to separate phenylalanine. Next, phenylalanine was eluted using 1 M ammonia hydroxide and dried overnight in a speedvac. Phenylalanine enrichment was then determined in tert-butyldimethylsilyl derivatives by gas chromatography-mass spectrometry (15).
For measurement of phenylalanine concentration and enrichment in muscle samples, an internal standard containing 3 µmol/l of L-[ring-13C6,15N] Phe was added to ~30 mg of muscle. The muscle sample was homogenized twice in 10% perchloric acid. Samples were centrifuged at 3,000 rpm for 10 min. The supernatant was collected to measure free intracellular (unbound) amino acid (15). Enrichment of protein-bound phenylalanine was determined by thoroughly washing muscle pellets with saline and alcohol, drying them overnight at 50°C (16), and hydrolyzing the dry protein pellets by an overnight incubation in 6 N HCl at 110°C. The bound protein hydrolyzate and intracellular supernatant were processed by the same method as blood samples. Isotopic enrichments in blood and muscle samples were measured on an Agilent 6890 gas chromatograph-mass spectrometer. Ions were selectively monitored at mass-to-charge (m/z) ratios of 336, 337, 340, 342, and 346 for phenylalanine enrichment. Isotopic enrichments were expressed as tracer-to-tracee ratio. Muscle concentrations of free intracellular phenylalanine were calculated from the internal standard.
Calculations
Muscle FSR was calculated according to the precursor-product method. The value for the precursor was the area under the decay curve for intracellular phenylalanine enrichment, and the product was the change in bound enrichment over time (Eq. 1). FBR was calculated from the decay in intracellular and plasma enrichment E (10), where the ratio of bound to free intracellular phenylalanine is represented by QM/T. The amount of free phenylalanine in the muscle sample was measured and normalized to micromoles of free phenylalanine per gram of muscle. It has previously been shown that one gram of dry muscle protein contains 150 µmol of phenylalanine (17). The content of protein-bound phenylalanine in one gram of muscle was calculated by [(250 µmol/g) × (percent dry protein in muscle)].
| (1) |
| (2) |
Statistical analysis
A mixed multiple analysis of variance related each outcome (FSR, FBR, and net balance) to the time points (2 wk, 4 wk, 6 mo, 12 mo) and the potentially prognostic covariates of age and burned TBSA, blocking on subject. Differences among time points were assessed by Tukey-adjusted contrasts. An analysis of variance was performed with a compound symmetry correlation structure to compensate for repeated measures and with weighting per time point to compensate for heterogeneity of variance. Values for burned patient outcomes at each time point were compared to values from healthy subjects by student’s t-test. Statistical analyses were performed using R statistical software (R Core Team, 2013, version 3.1.1). A 95% level of confidence was assumed. Values are reported as mean ± SE. Statistical significance was accepted when p ≤ 0.05.
RESULTS
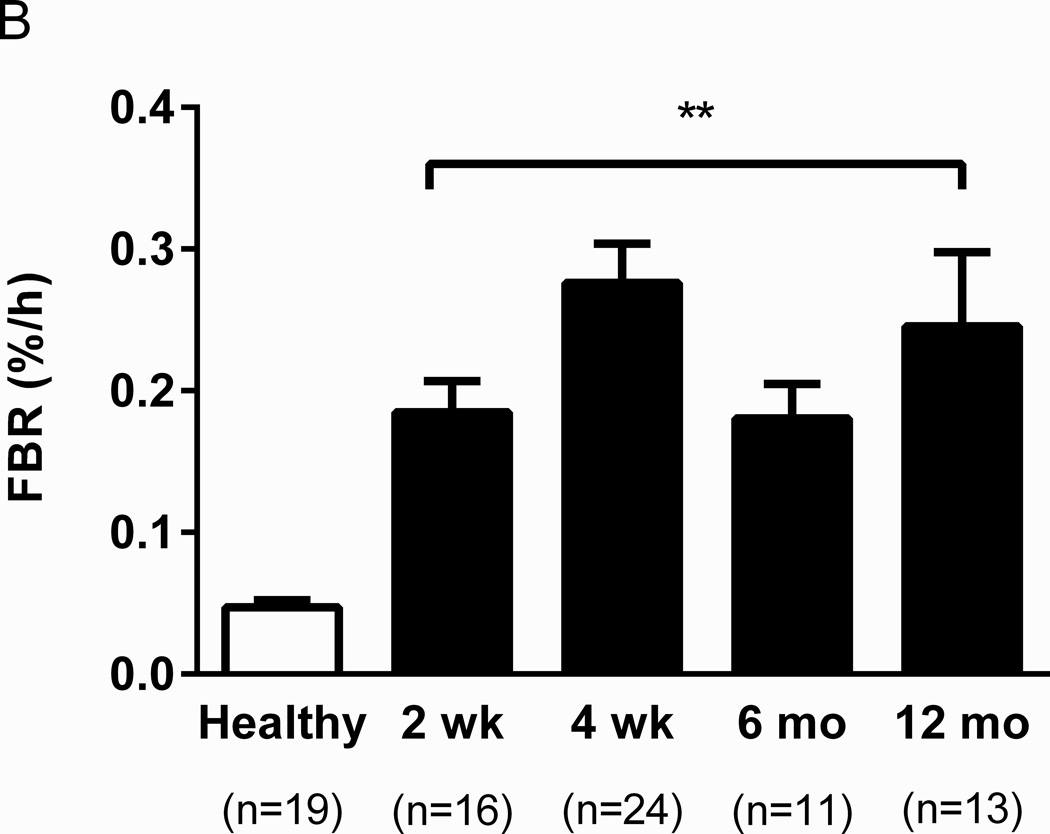
Muscle protein turnover was analyzed in 42 severely burned pediatric patients (30 male, 12 female) in the fed state. Their mean age was 7.1 ± 4.8 years with a burn injury of 50 ± 14% TBSA. Skeletal muscle FSR was 0.11 ± 0.02%/h at 2 weeks, 0.19 ± 0.03 %/h at 4 weeks, and 0.14 ± 0.02 %/h at 12 months. No significant differences were detected among any time points (Fig. 1A). However, all three values were significantly higher than muscle protein FSR previously reported in non-burned adults by Phillips et al. (0.07 ± 0.01 %/h, p ≤ 0.05) (18), who used labeled phenylalanine tracers to measure protein turnover in 19 healthy untrained young men who were also in the fed state. Similar to FSR, FBR values did not significantly differ at 2 weeks (0.18 ± 0.02 %/h), 4 weeks (0.28 ± 0.03 %/h), 6 weeks (0.18 ± 0.02 %/h), or 12 months (0.25 ± 0.05 %/h) (Fig. 1B). These values were 4- to 6-fold higher than those seen in healthy adults (0.05 ± 0.01 %/h, p < 0.01) as previously reported (18).
Fig. 1. Comparison of protein turnover in pediatric burn patients and healthy adults.
Fractional synthesis rate (FSR) (A) and fractional breakdown rate (FBR) (B) were elevated in burn patients. †p=0.05, *p<0.05, and **p<0.01 vs. healthy. Data from healthy men was from Phillips et al. (18).
Net protein balance was determined by calculating the difference between FSR and FBR. We observed a negative net protein balance from acute hospitalization to 12 months post burn, as elevation of muscle FBR was greater than that of FSR (Fig. 2). The net protein balance in burn patients was −0.07 ± 0.02 %/h at 2 weeks, −0.09 ± 0.04 %/h at 4 weeks, −0.06 ± 0.04 %/h at 6 months, and −0.11 ± 0.05 %/h at 12 months. In contrast, healthy, non-burned adults in the fed state showed a net positive protein balance (0.02 ± 0.01 %/h, p < 0.05 vs. burn all values).
Fig. 2. Comparison of net protein balance in pediatric burn patients and healthy adults.
Net balance was calculated as the difference of FBR from FSR. *p<0.05 and **p<0.01 vs. healthy.
DISCUSSION
Severe burns induce acute, concurrent elevations in muscle protein synthesis and breakdown, with the rate of breakdown outpacing synthesis to induce loss of skeletal muscle proteins. Whether this imbalance in skeletal muscle protein metabolism persists after closure of burn wounds remains unclear. Using a novel stable isotope approach, we found that, in skeletal muscle, derangements in protein metabolism persist for up to one year after burns. In particular, our results indicate that increased muscle protein turnover following burn injury is attributable to elevated FBR. What we found particularly interesting was the similar increasing and decreasing pattern seen for both FSR and FBR following burn injury. However, the magnitude of the increase in FBR was consistently greater than that of FSR, which likely leads to a chronic loss of skeletal muscle proteins over time in burn survivors.
Consumption of protein or intravenous amino acid infusion results in hyperaminoacidemia. This increases availability of amino acids within myocytes, where protein synthesis incorporates some of these amino acids into bound proteins such as cellular organelles and myofibrillar proteins. Free amino acids arising from protein catabolism also augment intracellular amino acid concentration. When breakdown occurs at greatly elevated rates, as seen in burned patients, the intracellular free amino acid pool becomes saturated, stimulating protein synthesis. Our group has previously shown that intravenous amino acid infusion does not further stimulate protein synthesis in children with massive burns (19), suggesting that the inward flow of amino acids from feeding does not significantly augment the availability of free intracellular amino acids. Perhaps this is because burn victims have intracellular amino acid concentrations in skeletal muscle that are several fold higher than those seen in healthy individuals.
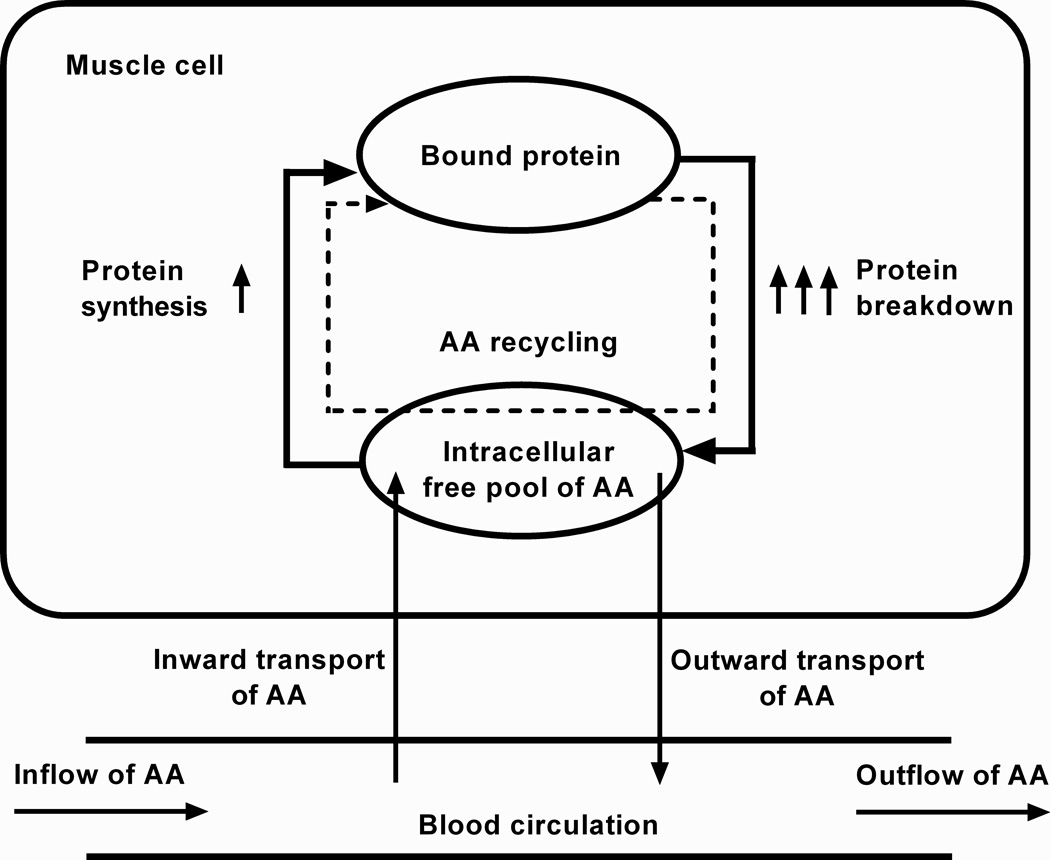
Intracellular amino acid cycling (see schematic of process in Fig. 3), where amino acids derived from breakdown are resynthesized into proteins, is known to increase in burn victims (4, 19). Therefore, we suggest that FBR drives FSR in skeletal muscle of burn vicitms. While this could be viewed as energetically wasteful and contributing to hypermetabolism in burn survivors, it may be somewhat protective by preventing unchecked amino acid loss from skeletal muscle.
Fig. 3. Schematic of amino acid (AA) recycling.
Elevated protein breakdown stimulates release of AAs from bound protein. This increases the availability free intracellular AAs, driving incorporation of the same AAs back into bound protein. The intracellular free AA pool is largely augmented by protein breakdown and not affected by influx of AA from exogenous sources. Diagram is adapted from Diaz et al. (24).
Our current findings are consistent with our previous protein kinetic data obtained from healthy and severely burned adults (20). In this previous study, muscle protein breakdown was 83% greater in burn victims than in healthy individuals. Similarly, muscle protein synthesis was 50% greater in burn patients than in healthy adults, most likely due to the significantly higher intracellular amino acid concentration in muscle of burn patients (20). Moreover, leucine and lycine kinetics were similarly perturbed as phenylalanine kinetics in burn victims. In this previous study, our group used the three-compartmental model measuring arterio-venous differences in amino acid concentrations to calculate muscle protein breakdown and synthesis. As in the current study, skeletal muscle amino acid losses after burn could be explained by an increase in muscle protein breakdown that was not accompanied by an elevation in muscle protein synthesis of similar magnitude. The ability of the bolus injection method to identify the same burn-related disruption in skeletal muscle amino acid kinetics as more contemporary constant infusion approaches suggests that this method is a valid means of determining protein turnover in skeletal muscle after burns.
We have previously determined skeletal muscle amino acid kinetics in pediatric patients for up to 1 year after burns using arterial-venous balance methods (9). We demonstrated that, consistent with our current findings, the rate of amino acid appearance in the femoral vein (a marker of skeletal muscle protein breakdown) was elevated for 9 months post injury. Moreover, amino acid appearance in the femoral vein occurred at a greater rate than amino acid disappearance from the femoral artery (an index of muscle protein synthesis), resulting in a negative protein balance across the leg. Our current study, which involves direct measurement of skeletal muscle FSR and FBR, provides further support for the notion that muscle cachexia in burn victims is the result of marked elevations in proteolysis.
In recent decades, advances in the standard of clinical care for burn victims have significantly increased survival. However, our current data show that these patients experience profound alterations in skeletal muscle protein metabolism for as long as 1 year following burn injury (Fig. 2). This chronic muscle proteolysis contributes to the profound cachexia seen in burn survivors, which delays recovery and negatively affects quality of life. Therapeutic strategies aimed at improving skeletal muscle protein balance and thus mass, will likely be efficacious in reducing morbidity in burn victims.
Previous studies have shown that a number of pharmacological interventions increase muscle protein synthesis in burn victims (21–23), leading to a better match between the rates of muscle protein breakdown and muscle protein synthesis. Diaz and colleagues recently reviewed the effects of oxandrolone and propranolol on skeletal muscle protein turnover in burn victims (24). It was noted that most pharmacological interventions increase the efficiency of muscle protein synthesis but have little effect on protein breakdown. Given that excessive muscle protein breakdown is the overriding phenotype of the massively burned patients, interventions to reduce catabolism of muscle protein may be more useful in preserving lean mass in burn victims.
One limitation of the current study is that muscle protein kinetics in burned children were unable to be compared to those in healthy non-burned children due to ethical issues involved in performing invasive studies in otherwise healthy children. However, we were able to compare data from burned children to data from healthy non-burned young men (18), which are likely similar to healthy children. This particular study was preferred for our healthy control comparison because their FSR and FBR were measured while subjects were in the fed state, which would eliminate any assumptions we would have to make if they were fasted. Although their methods were different, previous studies show that there were no significant differences between the bolus injection and constant infusion method (10, 14). Additionally, this was a cohort study. A total of 42 patients were included in the study, but each individual was not included in each time point. Some cases were deemed unsafe for patients to be studied, and some patients were lost to follow up at 6 and 12 months. Not all 42 patients were represented equally in all 4 groups. However, our mixed analyses of covariance were blocked on subjects to compensate for repeated measures, which should help compensate for this problem. Future prospective studies are warranted to study the same patients over time. With that said, the stable isotope bolus injection method used here allows skeletal muscle protein turnover to be determined in a study lasting less than 2 h, without requiring the catheterization of the femoral artery and vein or the measurement of blood flow. This provides a less invasive and time-consuming approach for quantifying skeletal muscle amino acid kinetics in clinical populations. Further, these future studies can investigate the cellular pathways responsible for this deviation in protein turnover we observed in severely burned patients.
In conclusion, we demonstrated that protein turnover remains elevated for up to 1 year following severe burn injury. Chronically elevated skeletal muscle catabolism is not matched by a similar increase in muscle protein synthesis, causing persistent loss of skeletal muscle amino acids. We suggest that chronically elevated FBR is responsible for muscle cachexia observed in burn survivors. Investigation of novel therapies that blunt skeletal muscle proteolysis to improve protein net balance will likely hasten the recovery of burn survivors.
Acknowledgments
This study was funded by the National Institutes of Health (P50 GM060338 [Project 9], R01 AR049877, P30 AG024832, T32 GM008256), the Institute for Translational Sciences at UTMB (supported in part by a Clinical and Translational Science Award [UL1TR000071] from the National Center for Advancing Translational Sciences, NIH), and Shriners Hospitals for Children (84090, 71006, 85310). CP is supported by an Interdisciplinary Rehabilitation Research Postdoctoral Training Grant from NIDRR (H133P110012).
Footnotes
Conflicts of Interest and Source of Funding: The authors declare no conflicts of interest.
REFERENCES
- 1.Jeschke MG, Gauglitz GG, Kulp GA, Finnerty CC, Williams FN, Kraft R, Suman OE, Mlcak RP, Herndon DN. Long-term persistance of the pathophysiologic response to severe burn injury. Plos One. 2011;6(7):e21245. doi: 10.1371/journal.pone.0021245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu YM, Tompkins RG, Ryan CM, Young VR. The metabolic basis of the increase of the increase in energy expenditure in severely burned patients. JPEN J Parenter Enteral Nutr. 1999;23(3):160–168. doi: 10.1177/0148607199023003160. [DOI] [PubMed] [Google Scholar]
- 3.Jahoor F, Desai M, Herndon DN, Wolfe RR. Dynamics of the protein metabolic response to burn injury. Metabolism: Clinical & Experimental. 1988;37(4):330–337. doi: 10.1016/0026-0495(88)90132-1. [DOI] [PubMed] [Google Scholar]
- 4.Wolfe RR, Jahoor F, Hartl WH. Protein and amino acid metabolism after injury. Diabetes Metab Rev. 1989;5(2):149–164. doi: 10.1002/dmr.5610050205. [DOI] [PubMed] [Google Scholar]
- 5.Herndon DN, Ramzy PI, DebRoy MA, Zheng M, Ferrando AA, Chinkes DL, Barret JP, Wolfe RR, Wolf SE. Muscle protein catabolism after severe burn: Effects of igf-1/igfbp-3 treatment. Ann Surg. 1999;229(5):713–720. doi: 10.1097/00000658-199905000-00014. discussion 720-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chai J, Wu Y, Sheng Z. The relationship between skeletal muscle proteolysis and ubiquitin–proteasome proteolytic pathway in burned rats. Burns. 2002;28(6):527–533. doi: 10.1016/s0305-4179(02)00049-9. [DOI] [PubMed] [Google Scholar]
- 7.Fang CH, Tiao G, James H, Ogle C, Fischer JE, Hasselgren PO. Burn injury stimulates multiple proteolytic pathways in skeletal muscle, including the ubiquitin-energy-dependent pathway. J Am Coll Surg. 1995;180(2):161–170. [PubMed] [Google Scholar]
- 8.Fang CH, Sun X, Li BG, Fischer DR, Pritts TA, Penner G, Hasselgren PO. Burn injuries in rats upregulate the gene expression of the ubiquitin-conjugating enzyme e2(14k) in skeletal muscle. J Burn Care Rehabil. 2000;21(6):528–534. doi: 10.1097/00004630-200021060-00010. [DOI] [PubMed] [Google Scholar]
- 9.Hart DW, Wolf SE, Mlcak R, Chinkes DL, Ramzy PI, Obeng MK, Ferrando AA, Wolfe RR, Herndon DN. Persistence of muscle catabolism after severe burn. Surgery. 2000;128(2):312–319. doi: 10.1067/msy.2000.108059. [DOI] [PubMed] [Google Scholar]
- 10.Zhang XJ, Chinkes DL, Wolfe RR. Measurement of muscle protein fractional synthesis and breakdown rates from a pulse tracer injection. Am J Physiol Endocrinol Metab. 2002;283(4):E753–E764. doi: 10.1152/ajpendo.00053.2002. [DOI] [PubMed] [Google Scholar]
- 11.Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A, Wiedemann HP, Arroliga AC, Fisher CJ, Komara JJ, Perez-Trepichio P, Parsons PE, Wolkin R, Welsh C, Fulkerson WJ, MacIntyre N, Mallatratt L, Sebastian M, McConnell R, Wilcox C, Govert J, Thompson D, Clemmer T, Davis R, Orme J, Weaver L, Grissom C, Eskelson M, Young M, Gooder V, McBride K, Lawton C, d'Hulst J, Peerless JR, Smith C, Brownlee J, Pluss W, Kallet R, Luce JM, Gottlieb J, Elmer M, Girod A, Park P, Daniel B, Gropper M, Abraham E, Piedalue F, Glodowski J, Lockrem J, McIntyre R, Reid K, Stevens C, Kalous D, Silverman HJ, Shanholtz C, Corral W, Toews GB, Arnoldi D, Bartlett RH, Dechert R, Watts C, Lanken PN, Anderson H, Finkel B, Hanson CW, Barton R, Mone M, Hudson LD, Lee C, Carter G, Maier RV, Steinberg KP, Bernard G, Stroud M, Swindell B, Stone L, Collins L, Mogan S, Ancukiewicz M, Hayden D, Molay F, Ringwood N, Wenzlow G, Kazeroonian AS, Gail DB, Bosken CH, Randall P, Waclawiw M, Spragg RG, Boyett J, Kelley J, Leeper K, Secundy MG, Slutsky A, Hyers TM, Emerson SS, Garcia JGN, Marini JJ, Pingleton SK, Shasby MD, Sibbald WJ, Acute Resp Distress Syndrome N. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. New England Journal of Medicine. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 12.Hart DW, Wolf SE, Chinkes DL, Beauford RB, Mlcak RP, Heggers JP, Wolfe RR, Herndon DN. Effects of early excision and aggressive enteral feeding on hypermetabolism, catabolism, and sepsis after severe burn. J Trauma. 2003;54(4):755–761. doi: 10.1097/01.TA.0000060260.61478.A7. discussion 761-754. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez NA, Jeschke MG, Williams FN, Kamolz LP, Herndon DN. Nutrition in burns: Galveston contributions. JPEN J Parenter Enteral Nutr. 2011;35(6):704–714. doi: 10.1177/0148607111417446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tuvdendorj D, Chinkes DL, Bahadorani J, Zhang XJ, Sheffield-Moore M, Killewich LA, Wolfe RR. Comparison of bolus injection and constant infusion methods for measuring muscle protein fractional synthesis rate in humans. Metabolism: Clinical & Experimental. 2014;63(12):1562–1567. doi: 10.1016/j.metabol.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol. 1997;273(1 Pt 1):E99–E107. doi: 10.1152/ajpendo.1997.273.1.E99. [DOI] [PubMed] [Google Scholar]
- 16.Zhang XJ, Chinkes DL, Sakurai Y, Wolfe RR. An isotopic method for measurement of muscle protein fractional breakdown rate in vivo. Am J Physiol. 1996;270(5 Pt 1):E759–E767. doi: 10.1152/ajpendo.1996.270.5.E759. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X-j, Cortiella J, Doyle D, Wolfe RR. Ketamine anesthesia causes greater muscle catabolism in rabbits than does propofol. The Journal of Nutritional Biochemistry. 1997;8(3):133–139. [Google Scholar]
- 18.Phillips SM, Parise G, Roy BD, Tipton KD, Wolfe RR, Tamopolsky MA. Resistance-training-induced adaptations in skeletal muscle protein turnover in the fed state. Can J Physiol Pharmacol. 2002;80(11):1045–1053. doi: 10.1139/y02-134. [DOI] [PubMed] [Google Scholar]
- 19.Porter C, Cotter M, Diaz EC, Jennings K, Herndon DN, Borsheim E. Amino acid infusion fails to stimulate skeletal muscle protein synthesis up to 1 year after injury in children with severe burns. J Trauma Acute Care Surg. 2013;74(6):1480–1485. doi: 10.1097/TA.0b013e3182921651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biolo G, Fleming RY, Maggi SP, Nguyen TT, Herndon DN, Wolfe RR. Inverse regulation of protein turnover and amino acid transport in skeletal muscle of hypercatabolic patients. J Clin Endocrinol Metab. 2002;87(7):3378–3384. doi: 10.1210/jcem.87.7.8699. [DOI] [PubMed] [Google Scholar]
- 21.Gore DC, Wolf SE, Herndon DN, Wolfe RR. Relative influence of glucose and insulin on peripheral amino acid metabolism in severely burned patients. JPEN J Parenter Enteral Nutr. 2002;26(5):271–277. doi: 10.1177/0148607102026005271. [DOI] [PubMed] [Google Scholar]
- 22.Herndon DN, Rodriguez NA, Diaz EC, Hegde S, Jennings K, Mlcak RP, Suri JS, Lee JO, Williams FN, Meyer W, Suman OE, Barrow RE, Jeschke MG, Finnerty CC. Long-term propranolol use in severely burned pediatric patients: A randomized controlled study. Ann Surg. 2012;256(3):402–411. doi: 10.1097/SLA.0b013e318265427e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy KD, Thomas S, Mlcak RP, Chinkes DL, Klein GL, Herndon DN. Effects of long-term oxandrolone administration in severely burned children. Surgery. 2004;136(2):219–224. doi: 10.1016/j.surg.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 24.Diaz EC, Herndon DN, Porter C, Sidossis LS, Suman OE, Borsheim E. Effects of pharmacological interventions on muscle protein synthesis and breakdown in recovery from burns. Burns. 2015;41(4):649–657. doi: 10.1016/j.burns.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]