Abstract
Objectives
Electrical stimulation at the dorsal column (DC) and dorsal root (DR) may inhibit spinal wide-dynamic-range (WDR) neuronal activity in nerve-injured rats. The objective of this study was to determine if applying electrical conditioning stimulation (CS) at both sites provides additive or synergistic benefits.
Materials and Methods
By conducting in vivo extracellular recordings of WDR neurons in rats that had undergone L5 spinal nerve ligation, we tested whether combining 50 Hz CS at the two sites in either a concurrent (2.5 minutes) or alternate (5 minutes) pattern inhibits WDR neuronal activity better than CS at DC alone (5 minutes). The intensities of CS were determined by recording antidromic compound action potentials to graded stimulation at the DC and DR. We measured the current thresholds that resulted in the first detectable Aα/β waveform (Ab0) and the peak Aα/β waveform (Ab1) to select CS intensity at each site. The same number of electrical pulses and amount of current were delivered in different patterns to allow comparison.
Results
At a moderate intensity of 50%(Ab0+Ab1), different patterns of CS all attenuated the C-component of WDR neurons in response to graded intracutaneous electrical stimuli (0.1-10 mA, 2 ms), and inhibited windup in response to repetitive noxious stimuli (0.5 Hz). However, the inhibitory effects did not differ significantly between different patterns. At the lower intensity (Ab0), no CS inhibited WDR neurons.
Conclusions
These findings suggest that combined stimulation of DC and DR may not be superior to DC stimulation alone for inhibition of WDR neurons.
Keywords: Neurostimulation, dorsal root, dorsal column, wide-dynamic-range neurons, nerve injury
Introduction
Spinal cord stimulation (SCS) and peripheral nerve stimulation are important clinical strategies for treating certain debilitating pain conditions when drug treatments have failed or when drug side effects have impaired a patient's quality of life (3). These electrical neurostimulation therapies have potential for long-term pain treatment because they are adjustable, reversible, and minimally invasive (4, 5), but their mechanisms of action are not fully understood. SCS primarily activates the dorsal column (DC) and uses both spinal and supraspinal mechanisms to induce pain inhibition(5, 6). Recent preclinical studies suggested that electrical conditioning stimulation (CS) at the DC (a site proximal to SCS) and at the dorsal roots (DR, a site distal to SCS), attenuates the hyperexcitability of wide-dynamic-range (WDR) neurons in the dorsal horn of nerve-injured rats (10, 23). WDR neurons are important to the transmission and modulation of nociceptive information at the spinal level. Like nociceptive-specific neurons, they show increased excitability and enhanced response to peripheral stimuli after tissue inflammation and nerve injury. Further, they display a progressive increase in response to repeated noxious inputs of the same stimulus intensity (7, 13). This “windup” phenomenon is an important experimental tool with which to study mechanisms that might trigger short-term neuronal sensitization and the development of pain hypersensitivity (2, 11, 17).
Attenuation of WDR neuronal hyperexcitability by stimulation at the DC may contribute to SCS-induced pain relief (10, 18, 21). However, recent studies suggested that the DRs may also be a useful target at which neurostimulation might induce pain inhibition (10, 24). We hypothesized that applying combined CS at both sites might produce beneficial additive or synergistic effects, as DC and DR stimulation have different modes of action for inhibiting pain. By conducting in vivo electrophysiology recording, our goal was to determine whether combining 50 Hz CS at the two sites in either a concurrent (DCIIDR) or alternate (DC-DR) pattern produces greater inhibition of spinal nociceptive transmission in rats after an L5 spinal nerve ligation (SNL). Specifically, we compared the evoked responses to graded electrical stimuli and windup-inducing stimulation in WDR neurons before and after different patterns of CS. We also postulated that the pattern of combined CS might affect its efficacy to inhibit WDR neuronal response in nerve-injured rats.
Materials And Methods
The Johns Hopkins University Animal Care and Use Committee approved all procedures as consistent with the National Institutes of Health Guide for the Use of Experimental Animals. All animals were euthanized at the end of the experiment by an intraperitoneal (i.p.) injection of sodium pentobarbital (100–300 mg).
Spinal Nerve Ligation
The L5 spinal nerve of adult male Sprague-Dawley rats (300–400 g, Harlan Bioproducts for Science, Indianapolis, IN) was ligated as described previously(10). Briefly, the animals were anesthetized with isoflurane (2%, Abbott Laboratories, North Chicago, IL). The left L5 spinal nerve was tightly ligated with a 6-0 silk suture and cut distally, with care being taken not to pull the nerve or touch the L4 spinal nerve. The animals were monitored after surgery for signs of wound infection, inadequate food and water intake, or weight loss until the surgical site was healed. In light of possible differences in WDR neuronal excitability changes associated with “allodynic” and “non-allodynic” animals, and because SCS only normalizes the WDR neuronal hyperexcitability in allodynic rats after nerve injury (21), we excluded SNL rats from the electrophysiologic studies if they did not show mechanical hypersensitivity (paw withdrawal threshold decrease of >50% from the pre-injury level) at day 5 post-injury and also at 2–3 days before the electrophysiologic recording dates (day 30-40 post-SNL) (1, 10, 19). Because neurostimulation pain therapies are applied mostly as post-injury treatments in clinic, the electrophysiology experiments were conducted in rats at day 30-40 post-SNL, which corresponds to the maintenance phase of neuropathic pain in this model (8).
Tracheotomy and mechanical ventilation
The rats were anesthetized initially with pentobarbital (45–50 mg/kg, i.p.). Then a tracheotomy was performed and mechanical ventilation (Kent Scientific Corporation, Litchfield, CT) was initiated at 50–70 cycles/minute with inspiratory pressures of 10–14 cm H2O. During the neurophysiologic experiments, anesthesia was maintained with 1.5% isoflurane. To facilitate controlled ventilation during electrophysiology recording, we paralyzed animals with pancuronium bromide (1–2 mg/kg, i.p., Elkins-Sinn Inc., Cherry Hill, NJ). Sufficient depth of anesthesia was judged from areflexia to sensory stimuli (e.g., no withdrawal reflexes, corneal reflex) when rats were in the unparalyzed state and by the absence of gross fluctuations of heart rate (300–350 beats per minute) during paralysis. Core body temperature was kept in the normal range (36.0–37.0°C).
Recording of sciatic compound action potentials evoked by CS
In each experiment, we first determined the intensities of CS at each site by recording the antidromic sciatic compound action potential (AP) evoked by graded electrical stimulation (0.001–5.0 mA, 0.2 ms, biphasic) at the DC and DR. Two tungsten needle stimulating electrodes were inserted into the ipsilateral DC at T13–L1 level for DC stimulation. The DR stimulation was applied through a pair of platinum hook electrodes placed underneath the L4 and L5 dorsal roots. A monopolar silver hook electrode was placed on the left sciatic nerve at mid-thigh level for recording compound APs (10). The reference electrode was placed in the nearby muscle. Both stimulating and recording areas were covered with mineral oil. Similar techniques have been used in previous studies (10, 14, 18). We measured the current thresholds that resulted in the first detectable Aα/β waveform (Ab0) and the peak Aα/β waveform (Ab1, Fig. 1A), without inducing an Aδ waveform, in order to apply CS at DR (e.g., DR:Ab0, DR:Ab1) and DC structures (e.g., DC:Ab0, DC:Ab1, Table 1).
Figure 1.
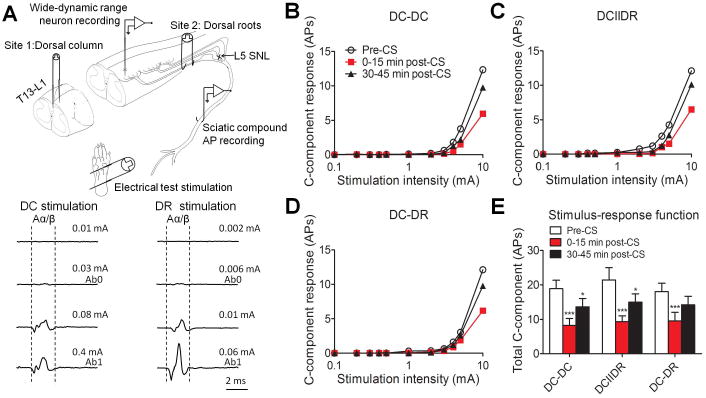
Effects of different patterns of conditioning stimulation (CS) at moderate intensity on WDR neuronal response to graded electrical stimuli. (A) Upper: A schematic diagram shows the experimental paradigm used in the neurophysiologic studies. Antidromic sciatic compound action potential (AP) was evoked by graded electrical stimulation (0.001-5.0 mA, 0.2 ms, biphasic) applied at the dorsal column (DC) and dorsal root (DR). Lower: The intensities that produced the first detectable Aα/β waveform (Ab0) and the peak Aα/β waveform (Ab1) were determined. (B-D) The stimulus–response functions of C-component of WDR neuronal response to graded intra-cutaneous electrical stimulation (0.1-10 mA, 2 ms) before and after different patterns of CS (DC-DC: n = 21; DCIIDR: n = 21; DC-DR: n = 20 neurons) at the moderate stimulus intensity of 50%(Ab0+Ab1) in SNL rats. For clarity, error bars are not shown. (E) The total number of action potentials in the C-component before and after CS. Data are expressed as mean + SEM. *P<0.05, ***P<0.001 as compared with the pre-CS baseline.
Table 1. Protocols of the conditioning stimulation patterns.
| Pattern | Duration | Protocol |
|---|---|---|
|
DC–DC Alternate intensity at DC DC:Ab0(0.029±0.006 mA) Ab1(0.48±0.06 mA) DR:Ab0(0.0058±0.0003 mA) Ab1(0.066±0.015 mA) |
5 min |
Moderate intensity: 50 sec of DC stimulation at 50% DC:(Ab0+Ab1) intensity followed by 50 sec of DC stimulation at 50%DR:(Ab0+Ab1) intensity in each session, repeat 3 sessions. Low intensity: 50 sec of DC stimulation at DC:Ab0 intensity followed by 50 sec of DC stimulation at DR:Ab0 intensity in each session, repeat 3 sessions. |
|
DC–DR Alternate sites between DC and DR DC:Ab0(0.026±0.004 mA) Ab1(0.45±0.06 mA) DR:Ab0(0.0056±0.0003 mA) Ab1(0.063±0.014 mA) |
5 min |
Moderate intensity: 50 sec of DC stimulation at 50% DC:(Ab0+Ab1) intensity followed by 50 sec of DR stimulation at 50%DR:(Ab0+Ab1) intensity in each session, repeat 3 sessions. Low intensity: 50 sec of DC stimulation at DC:Ab0 intensity followed by 50 sec of DR stimulation at DR:Ab0 intensity in each session, repeat 3 sessions. |
|
DC ‖ DR Concurrently at DC and DR DC:Ab0(0.03±0.006 mA) Ab1(0.46±0.05 mA) DR:Ab0(0.0057±0.0003 mA) Ab1(0.066±0.014 mA) |
2.5 min |
Moderate intensity: 150 sec of simultaneous DC stimulation at 50%DC:(Ab0+Ab1) intensity and 150 sec of DR stimulation at 50%DR:(Ab0+Ab1) intensity. Low intensity: 150 sec of simultaneous DC stimulation at DC:Ab0 intensity and 150 sec of DR stimulation at DR:Ab0 intensity. |
Spinal dorsal horn recordings
We recorded neuronal activity in the lumbar dorsal horn with rats under inhalation anesthesia (isoflurane, 1.5%), as described in our previous studies (10, 22). The experimental setup is illustrated as a schematic diagram in Fig. 1A. The cutaneous receptive fields of WDR neurons in the plantar region of the hindpaw were mapped, and a single site (most sensitive site) near the center of the receptive field was chosen for application of test stimuli. Test stimuli include graded intracutaneous electrical stimuli (0.1-10 mA, 2 ms), and a train of 0.5-Hz electrical stimulation (16 pulses, 0.2 ms, 1.5 × C-threshold) that induces windup. A train of 0.1-Hz stimulation (12 pulses, 0.2 ms, 1.5 × C-threshold) which does not induce windup was applied 30 seconds after 0.5 Hz train as a control.
A real-time, computer-based data acquisition and processing system (CED Spike 2, Cambridge, UK) was used to collect analog data. WDR cells were identified by their characteristic responses (9). Their responses to a suprathreshold electrical stimulus consists of an early A-fiber component (0–75 ms) and a later C-fiber component (75–500 ms) (10). We examined WDR neurons located at deep laminae (III–V, 400–1200 μm below the dorsal surface) in the ipsilateral uninjured L4 spinal segment.
Conditioning stimulation
The “combined CS” can be achieved by alternating the active CS between the DC and DR (alternate pattern, DC-DR) or by concurrent CS at both sites (concurrent pattern, DCIIDR). The DC-DR pattern consisted of three repeated sessions, which each consisted of 50 seconds of DC stimulation followed by 50 seconds of DR stimulation for a total of 5 minutes (Table 1). For the DCIIDR pattern, 2.5 minutes of CS was applied at the DC and DR simultaneously. To allow comparison with a single-site CS, we also tested 5 minutes of CS at the DC alone (DC-DC). Delivering 5 minutes of stimulation in concurrent group will double the amount of stimulation, and not be an equal comparison to the alternating groups. Therefore, we proposed to apply 2.5minutes of concurrent DCIIDR stimulation so we can compare it to the same total number of electrical pulses delivered in the 5 min DC-DR (alternate stimulation site) and DC-DC (alternate stimulation intensity) groups. Bipolar CS (50 Hz, 0.2 ms, biphasic, constant current) was applied at 10 minutes after the baseline test with a method similar to that used in our previous study (10, 22). CS was applied at a moderate stimulus intensity of 50%(Ab0+Ab1) and at a lower intensity of Ab0 at the corresponding site (Table 1). Bipolar CS (50 Hz, 0.2 ms, biphasic, constant current) was applied at 10 minutes after the baseline test with a method similar to that used in our previous study (10, 22). CS was applied at a moderate stimulus intensity of 50%(Ab0+Ab1) and at a lower intensity of Ab0 at the corresponding site (Table 1).
Because of the difficulty of long-term in vivo recording from the same neuron, and the potential for dorsal horn neurons to become sensitized after repetitive test stimuli and carryover effects with multiple CS, no more than two patterns of CS were tested in each experiment. We used a crossover design in which the order of two patterns in three different sets (DC-DC and DCIIDR, DC-DR and DCIIDR, DC-DC and DC-DR) was altered from experiment to experiment and a 60 interval between CS to minimize order effect and to avoid potential residue/carryover effect from the previous CS. Baseline was always measured before each CS to ensure the recovery. Only one neuron was tested in each animal. Accordingly, different patterns were not done in same set of neurons. Animals were randomly selected (by animal number) for each experiment. Different intensities of CS were examined in different groups of animals.
Data analysis
We compared the total number of APs in the C-component evoked by graded electrical stimulation between pre- and post-CS conditions using a one-way repeated measures ANOVA. To analyze windup, we used the C-component evoked by each stimulus in the train to plot windup functions against the stimulation number of the train. The total C-component evoked by 0.5 Hz train of stimulation (i.e., the area under the windup function) during pre- and post-CS conditions was compared with a one-way repeated measures ANOVA. The Tukey honestly significant difference post-hoc test was used to compare specific data points. STATISTICA 6.0 software (StatSoft, Inc., Tulsa, OK, USA) was used for all statistical analyses. Two-tailed tests were performed, and data are expressed as mean ± SEM.
Results
Different patterns of CS at moderate stimulus intensity induced comparable inhibition of WDR neurons
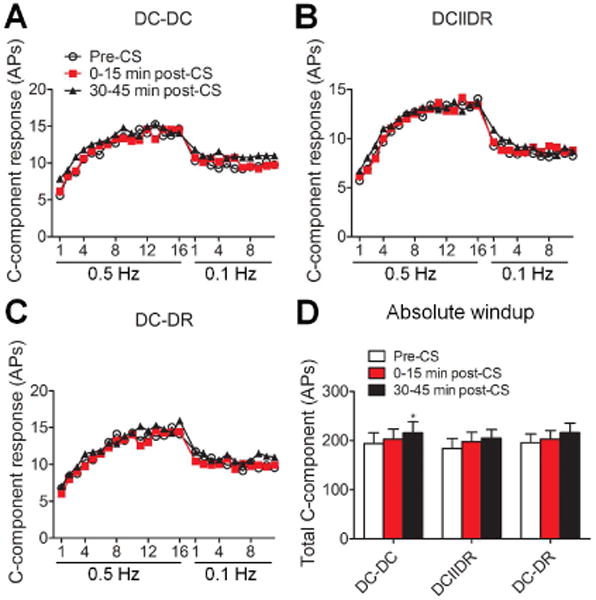
We first determined if combining a moderate stimulus intensity of CS at DC and DR has added value for inhibiting WDR neurons, as compared to CS at DC alone at 30-40 days post-SNL in rats. At 0–15 minutes after CS, stimulus-response (S-R) functions (Fig. 1B-D) and the total C-component of WDR neurons to graded intracutaneous stimuli (0.1–10 mA, 2 ms, Fig. 1E) applied to the skin receptive fields were significantly decreased in all three pattern groups (DC-DC: n = 21; DCIIDR: n = 21; DC-DR: n = 20 neurons,11-12 rats/pattern), as compared to those at pre-CS baseline. The decrease in C-component remained significant at 30-45 minutes after DC-DC and DCIIDR CS patterns.
Before CS, windup was induced by 0.5-Hz electrical stimulation (16 pulses, 0.2 ms, 1.5 × C-threshold), but not by 0.1-Hz stimulation (12 pulses, 0.2 ms, 1.5 × C-threshold) applied 30 seconds later, in all of the three groups. At 0–15 minutes post-CS, windup functions to 0.5-Hz train of stimulation were depressed in all groups (DC-DC: n = 22; DCIIDR: n = 23; DC-DR: n = 22 neurons, 11-12 rats/pattern, Fig. 2A-C). The total C-component evoked by 0.5 Hz train of stimulation (i.e., the area under the windup function) was significantly decreased from the respective pre-CS value in each pattern group (Fig. 2D), and remained significantly reduced at 30-45 minutes after DC-DC and DC-DR patterns.
Figure 2.
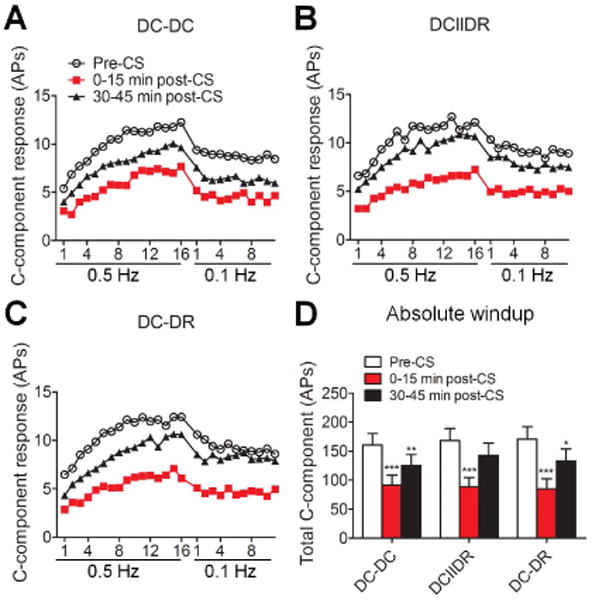
Effects of different patterns of conditioning stimulation (CS) at moderate intensity on windup in SNL rats. (A-C) C-components of WDR neuronal responses to a train of electrical stimuli at a frequency of 0.5 Hz (16 pulses, 2.0 msec, 1.5 × C-threshold) and then at 0.1 Hz (12 pulses, applied 30 seconds after the cessation of 0.5-Hz stimulation) were plotted against the stimulation sequence number in each pattern group (DC-DC: n = 22; DCIIDR: n = 23; DC-DR: n = 22 neurons). For clarity, error bars are not shown. (D) The area under the windup function to a 0.5 Hz train pre-CS, 0-15 minutes post-CS, and 30-45 minutes post-CS at the moderate intensity of 50%(Ab0+Ab1) was compared between different pattern groups. Data are expressed as mean + SEM. *P<0.05, **P<0.01, ***P<0.001 as compared with the pre-CS baseline. AP: action potential, DC: dorsal column, DR: dorsal root.
To compare the inhibitory effects across different patterns, we normalized the areas under the S-R function and windup function at 0–15 minutes and 30–45 minutes after CS to the pre-CS baseline. We found no significant difference in the inhibitory effect on S-R function (Fig. 3A) or windup (Fig. 3B) among the different patterns of CS.
Figure 3.
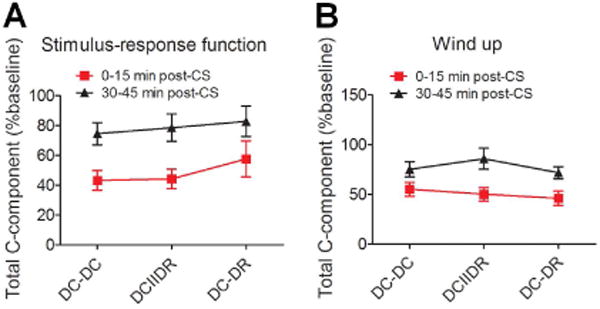
Comparison of the inhibitory effects of the different patterns of conditioning stimulation (CS) at moderate intensity. (A) The total C-component of WDR neuronal response to graded electrical stimulation after CS at the moderate intensity of 50%(Ab0+Ab1) was normalized to the pre-CS baseline in each pattern group (DC-DC: n = 21; DCIIDR: n = 21; DC-DR: n = 20 neurons). (B) The areas under the windup function to a 0.5 Hz train after CS were also normalized to the baseline value in each pattern group (DC-DC: n = 22; DCIIDR: n = 23; DC-DR: n = 22 neurons). Data are expressed as mean ± SEM. DC: dorsal column, DR: dorsal root.
No CS pattern inhibited WDR neurons at the lower stimulus intensity
No CS pattern (DC-DC: n = 17; DCIIDR: n = 17; DC-DR: n = 16 neurons, 8-9 rats/pattern) at the Ab0 intensity significantly inhibited the S-R functions to graded intracutaneous stimuli (0.1–10 mA, 2 ms) or windup at 0–15 minutes after application (Fig. 4A-C). Rather, the total number of APs in the C-component at 30-45 minutes post-CS was significantly increased from the DC-DC pre-CS baseline (Fig. 4D). The area under the windup function was also significantly increased from the DC-DC pre-CS level at 30-45 minutes after CS (Fig. 5A-D).
Figure 4.
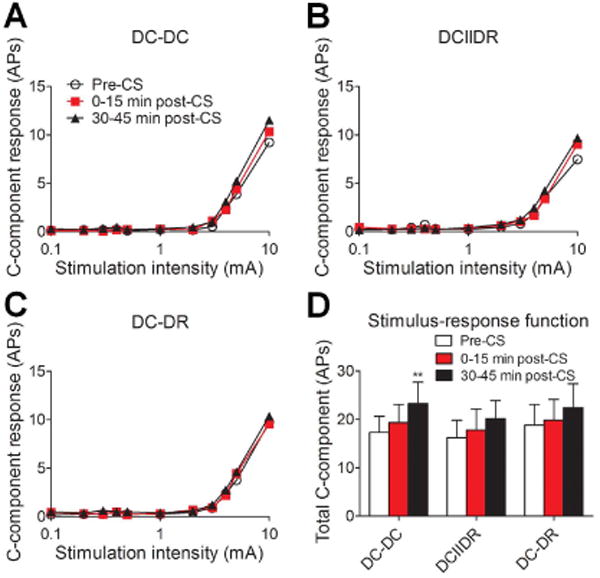
Effects of different patterns of low-intensity conditioning stimulation (CS) on WDR neuronal response to graded electrical stimuli. (A-C) The stimulus–response functions of the C-component of WDR neuronal response to graded intracutaneous electrical stimulation (0.1-10 mA, 2 ms) before and after different patterns of CS (DC-DC: n = 17; DCIIDR: n = 17; DC-DR: n = 16 neurons) at the low intensity of Ab0. For clarity, error bars are not shown. (D) The total number of action potentials in the C-component before and after CS in each pattern group. Data are expressed as mean + SEM. **P<0.01 as compared with the pre-CS baseline. AP: action potential, DC: dorsal column, DR: dorsal root.
Figure 5.
Effects of different patterns of low-intensity conditioning stimulation (CS) on windup. (A-C) C-component of WDR neuronal responses to a train of electrical stimuli at a frequency of 0.5 Hz (16 pulses, 2.0 msec, 1.5 × C-th) and then at 0.1 Hz (12 pulses, applied 30 seconds after the cessation of 0.5-Hz stimulation) were plotted against the stimulation sequence number (DC-DC: n = 17; DCIIDR: n = 17; DC-DR: n = 16 neurons). For clarity, error bars are not shown. (D) The area under the windup function to a 0.5 Hz train pre-CS, 0-15 minutes post-CS, and 30-45 minutes post-CS at the low intensity of Ab0 was compared in each group. Data are expressed as mean + SEM. *P<0.05 as compared with the pre-CS baseline. AP: action potential, DC: dorsal column, DR: dorsal root.
Discussion
The dorsal horn is the first central relay station for ascending pain signaling and an important site for nociceptive modulation. Inhibition of WDR neurons that are important to the spinal transmission of nociceptive information may contribute to pain inhibition from neurostimulation techniques, such as SCS and peripheral nerve stimulation (10, 18, 21). Both DC and DR may be useful targets for neurostimulation (10, 24), but we found that combining CS at DC and DR did not accentuate inhibition of WDR neuronal activity in nerve-injured rats.
We have shown previously that both DC-CS and DR-CS at the high intensity (e.g., Ab1) inhibit the C-fiber-mediated responses to graded electrical stimulation of WDR neurons in nerve-injured rats (10). Although both DC-CS and DR-CS are likely to inhibit pain through shared spinal segmental mechanisms, such as the pain “gate-control”, we postulate that each may also involve another mode of action, in part because the afferent fibers being activated by DC-CS may differ from those activated by DR-CS. For example, as only a subset of the A-fibers from the peripheral nerve and DR travel in the DC and send collateral branches to spinal cord, the inhibition of WDR neurons by DC-CS may be attributable to orthodromic and antidromic activation of these fibers. Unlike DC-CS, DR-CS would activate most A-fibers within the root, including those that connect in the DC and others that terminate only at the spinal level. It is unclear if the two populations of A-fibers engage with different neuronal components in the spinal cord (e.g., interneurons) and activate different pain modulatory mechanisms. In addition, DC-CS may activate both DC and other dorsal tracts (e.g., dorsolateral funiculus) that are in close proximity to the stimulating electrodes. Hence, the mechanism by which DC-CS inhibits spinal nociceptive transmission may involve other targets and modes of action.
We hypothesized that if CS at DC and DR attenuates spinal nociceptive transmission through different mechanisms, activating these mechanisms together might lead to faster or greater pain relief. Additive and synergistic effects in humans, similar to that in experimental animals, may have considerable translational relevance in the development of novel “combined CS” paradigms. However, combined CS at DC and DR sites with either a concurrent (DCIIDR) or alternate (DC-DR) pattern had no significant additive or synergistic effects on neuronal inhibition, as compared to CS at DC only. Importantly, we applied the same number of electric pulses and presumably delivered the same amount of electrical current in each CS pattern. This allows us to make a comparison among different pattern groups. We also used a moderate stimulus intensity of 50%(Ab0+Ab1) and a low intensity of Ab0 for CS at DC and DR, and hence may avoid a ceiling effect which prevents us from detecting additive and synergistic effects.
The reason for a lack of additive or synergistic effect of combined CS is unclear. Although this study did not provide a potential neuronal mechanism that would support clinical application of combined DC and DR stimulation for pain treatment, we cannot exclude the possibility that CS at other frequencies or intensities might work. At the spinal cord level, DC contains A-fibers that ascend from multiple caudal spinal segments. Thus, compared to DR-CS, DC-CS may induce a “broader” coverage of pain, and may be better suited for treating pain symptoms from large or diffuse areas. On the other hand, DR-CS may provide added or better relief from pain that is not controlled well by DC-CS alone or when severe pain arises from a discrete area (e.g., distal limb or digit). Thus, “combined CS” may still have potential advantages to DC-CS (broad pain coverage) and DR-CS (focalized or segmental pain inhibition in a particular area).
Previous studies showed that CS at the dorsal column and peripheral nerve inhibit WDR neurons in an intensity-dependent manner (18, 23). Different patterns of CS at moderate stimulus intensity induced significant inhibition of WDR neuronal activity, including windup, in SNL rats. Windup of WDR neuronal responses to repetitive C-fiber inputs is a form of short-term neuronal sensitization that may contribute to the development of persistent pain (7, 11, 13). A windup-like phenomenon also occurs in humans during natural stimulation of C-fibers (12), such as by heat stimuli and repeated noxious mechanical stimuli (15, 16, 20). The reduction of windup after CS at the moderate stimulus intensity suggests that CS inhibits the progress of neuronal sensitization to repetitive noxious inputs (10, 21). It remains unclear why DC-DC stimulation at the lower intensity of Ab0 did not inhibit, but rather increased the C-component at 30-45 minutes after stimulation. Nevertheless, a previous study suggested that a small subset of WDR neurons may increase excitability after SCS(21).
In summary, our findings suggest that combined stimulation of DC and DR may not be superior to DC stimulation alone for inhibition of WDR neurons. It remains unclear if DC-CS and DR-CS are suited for treating particular pain symptoms or modalities (e.g., mechanical, cold, heat, spontaneous pain). If so, combined CS may represent a unique case of hybrid CS that can treat various symptoms at the same time. Therefore, it will be important in the future to correlate the electrophysiologic findings with the putative pain inhibitory effect of combined DC and DR stimulation in animal behavioral studies.
Acknowledgments
The authors thank Claire F. Levine, MS (scientific editor, Department of Anesthesiology/CCM, Johns Hopkins University), for editing the manuscript.
Funding sources: This study was supported by grants from Medtronic and was subsidized by grants from the NIH (NS70814, NS26363).
Footnotes
Disclosures: Drs. Yun Guan and Srinivasa N. Raja received research grant support from Medtronic, Inc. Louis P. Vera-Portocarrero is employed by Medtronic, Inc. However, none of the authors has a commercial interest in the material presented in this paper. No other relationships might lead to a conflict of interest in the current study.
Reference List
- 1.Cui JG, O'Connor WT, Ungerstedt U, Linderoth B, Meyerson BA. Spinal cord stimulation attenuates augmented dorsal horn release of excitatory amino acids in mononeuropathy via a GABAergic mechanism. Pain. 1997;73:87–95. doi: 10.1016/s0304-3959(97)00077-8. [DOI] [PubMed] [Google Scholar]
- 2.Eide PK. Wind-up and the NMDA receptor complex from a clinical perspective. Eur J Pain. 2000;4:5–15. doi: 10.1053/eujp.1999.0154. [DOI] [PubMed] [Google Scholar]
- 3.Epstein LJ, Palmieri M. Managing chronic pain with spinal cord stimulation. Mt Sinai J Med. 2012;79:123–132. doi: 10.1002/msj.21289. [DOI] [PubMed] [Google Scholar]
- 4.Falowski S, Sharan A. A review on spinal cord stimulation. J Neurosurg Sci. 2012;56:287–298. [PubMed] [Google Scholar]
- 5.Foreman RD, Linderoth B. Neural mechanisms of spinal cord stimulation. Int Rev Neurobiol. 2012;107:87–119. doi: 10.1016/B978-0-12-404706-8.00006-1. [DOI] [PubMed] [Google Scholar]
- 6.Guan Y. Spinal cord stimulation: neurophysiological and neurochemical mechanisms of action. Curr Pain Headache Rep. 2012;16:217–225. doi: 10.1007/s11916-012-0260-4. [DOI] [PubMed] [Google Scholar]
- 7.Guan Y, Borzan J, Meyer RA, Raja SN. Windup in dorsal horn neurons is modulated by endogenous spinal mu-opioid mechanisms. J Neurosci. 2006;26:4298–4307. doi: 10.1523/JNEUROSCI.0960-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan Y, Johanek LM, Hartke TV, Shim B, Tao YX, Ringkamp M, Meyer RA, Raja SN. Peripherally acting mu-opioid receptor agonist attenuates neuropathic pain in rats after L5 spinal nerve injury. Pain. 2008;138:318–329. doi: 10.1016/j.pain.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan Y, Liu Q, Tang Z, Raja SN, Anderson DJ, Dong X. Mas-related G-protein-coupled receptors inhibit pathological pain in mice. Proc Natl Acad Sci U S A. 2010;107:15933–15938. doi: 10.1073/pnas.1011221107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan Y, Wacnik PW, Yang F, Carteret AF, Chung CY, Meyer RA, Raja SN. Spinal cord stimulation-induced analgesia: electrical stimulation of dorsal column and dorsal roots attenuates dorsal horn neuronal excitability in neuropathic rats. Anesthesiology. 2010;113:1392–1405. doi: 10.1097/ALN.0b013e3181fcd95c. [DOI] [PubMed] [Google Scholar]
- 11.Herrero JF, Laird JM, Lopez-Garcia JA. Wind-up of spinal cord neurones and pain sensation: much ado about something? Prog Neurobiol. 2000;61:169–203. doi: 10.1016/s0301-0082(99)00051-9. [DOI] [PubMed] [Google Scholar]
- 12.Hughes AM, Rhodes J, Fisher G, Sellers M, Growcott JW. Assessment of the effect of dextromethorphan and ketamine on the acute nociceptive threshold and wind-up of the second pain response in healthy male volunteers. Br J Clin Pharmacol. 2002;53:604–612. doi: 10.1046/j.1365-2125.2002.01602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Simone DA, Larson AA. Windup leads to characteristics of central sensitization. Pain. 1999;79:75–82. doi: 10.1016/S0304-3959(98)00154-7. [DOI] [PubMed] [Google Scholar]
- 14.Linderoth B, Gazelius B, Franck J, Brodin E. Dorsal column stimulation induces release of serotonin and substance P in the cat dorsal horn. Neurosurgery. 1992;31:289–296. doi: 10.1227/00006123-199208000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Magerl W, Wilk SH, Treede RD. Secondary hyperalgesia and perceptual wind-up following intradermal injection of capsaicin in humans. Pain. 1998;74:257–268. doi: 10.1016/s0304-3959(97)00177-2. [DOI] [PubMed] [Google Scholar]
- 16.Ramer MS. Anatomical and functional characterization of neuropil in the gracile fasciculus. J Comp Neurol. 2008;510:283–296. doi: 10.1002/cne.21785. [DOI] [PubMed] [Google Scholar]
- 17.Ren K. Wind-up and the NMDA receptor: from animal studies to humans. Pain. 1994;59:157–158. doi: 10.1016/0304-3959(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 18.Shechter R, Yang F, Xu Q, Cheong YK, He SQ, Sdrulla A, Carteret AF, Wacnik PW, Dong X, Meyer RA, Raja SN, Guan Y. Conventional and Kilohertz-frequency Spinal Cord Stimulation Produces Intensity- and Frequency-dependent Inhibition of Mechanical Hypersensitivity in a Rat Model of Neuropathic Pain. Anesthesiology. 2013;119:422–432. doi: 10.1097/ALN.0b013e31829bd9e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song Z, Meyerson BA, Linderoth B. Spinal 5-HT receptors that contribute to the pain-relieving effects of spinal cord stimulation in a rat model of neuropathy. Pain. 2011;152:1666–1673. doi: 10.1016/j.pain.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Staud R, Bovee CE, Robinson ME, Price DD. Cutaneous C-fiber pain abnormalities of fibromyalgia patients are specifically related to temporal summation. Pain. 2008;139:315–323. doi: 10.1016/j.pain.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yakhnitsa V, Linderoth B, Meyerson BA. Spinal cord stimulation attenuates dorsal horn neuronal hyperexcitability in a rat model of mononeuropathy. Pain. 1999;79:223–233. doi: 10.1016/s0304-3959(98)00169-9. [DOI] [PubMed] [Google Scholar]
- 22.Yang F, Carteret AF, Wacnik PW, Chung CY, Xing L, Dong X, Meyer RA, Raja SN, Guan Y. Bipolar spinal cord stimulation attenuates mechanical hypersensitivity at an intensity that activates a small portion of A-fiber afferents in spinal nerve-injured rats. Neuroscience. 2011;199:470–480. doi: 10.1016/j.neuroscience.2011.09.049. [DOI] [PubMed] [Google Scholar]
- 23.Yang F, Xu Q, Cheong YK, Shechter R, Sdrulla A, He SQ, Tiwari V, Dong X, Wacnik PW, Meyer R, Raja SN, Guan Y. Comparison of intensity-dependent inhibition of spinal wide-dynamic range neurons by dorsal column and peripheral nerve stimulation in a rat model of neuropathic pain. Eur J Pain. 2014;18:978–988. doi: 10.1002/j.1532-2149.2013.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang F, Zhang C, Xu Q, Tiwari V, He SQ, Wang Y, Dong X, Vera-Portocarrero LP, Wacnik PW, Raja SN, Guan Y. Electrical Stimulation of Dorsal Root Entry Zone Attenuates Wide-Dynamic-Range Neuronal Activity in Rats. Neuromodulation. 2014 doi: 10.1111/ner.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]


