Abstract
The activation of translation contributes to malignant transformation and is an emerging target for cancer therapies. RNA G-quadruplex structures are general inhibitors of cap-dependent mRNA translation and were recently shown to be targeted for oncoprotein translational activation. In contrast however, the G-quadruplex within the 5'UTR of the human vascular endothelial growth factor A (VEGF) has been shown to be essential for IRES-mediated translation. Since VEGF has a pivotal role in tumor angiogenesis and is a major target of anti-tumoral therapies, we investigated the structure/function relationship of the VEGF G-quadruplex and defined whether it could have a therapeutic potential. We found that the G-quadruplex within the VEGF IRES is dispensable for cap-independent function and activation in stress conditions. However, stabilization of the VEGF G-quadruplex by increasing the G-stretches length or by replacing it with the one of NRAS results in strong inhibition of IRES-mediated translation of VEGF. We also demonstrate that G-quadruplex ligands stabilize the VEGF G-quadruplex and inhibit cap-independent translation in vitro. Importantly, the amount of human VEGF mRNA associated with polysomes decreases in the presence of a highly selective stabilizing G-quadruplex ligand, resulting in reduced VEGF protein expression. Together, our results uncover the existence of functionally silent G-quadruplex structures that are susceptible to conversion into efficient repressors of cap-independent mRNA translation. These findings have implications for the in vivo applications of G-quadruplex-targeting compounds and for anti-angiogenic therapies.
Keywords: cap-independent, G-quadruplex, IRES, ligands, mRNA translation, VEGF
Abbreviations
- UTR
untranslated region
- G
guanine
Introduction
G-quadruplexes have received significant recent attention by virtue of increasing evidence favoring their multiple roles in gene expression. These non-canonical structures are formed by guanine-rich (G-rich) DNA or RNA sequences organized into stacks of tetrads (G-quartets), in which 4 guanines are assembled in a planar arrangement by Hoogsteen hydrogen bonding. An increasing number of reports support the view that RNA G-quadruplexes are important biological regulators playing a role in telomere maintenance, pre-mRNA processing (including splicing and polyadenylation), RNA turnover, mRNA targeting and translation.1,2 In particular, the majority of G-quadruplex structures located at the mRNA 5’ end function as repressors of mRNA translation.1,3-5 The general view is that RNA G-quadruplexes inhibit mRNA translation probably by compromising 5′ cap recognition and/or ribosome scanning in a way that is dependent not only on the thermodynamic stability6,7 but also on the position within the 5′ UTR.6,8 Although the majority of the naturally occurring G-quadruplexes displaying translationally regulatory properties are at least 3-G-quartet quadruplex structures,5 artificially engineered 2-G quartet quadruplexes displaying moderate stability have been shown to behave as temperature-sensitive RNA elements, or RNA thermometers.9 The suppressive effect on cap-dependent translation of stable G-quadruplexes can be modulated by small-molecule ligands able to selectively bind and either stabilize or destabilize these 4-stranded conformations.7,10-13 Importantly, RNA G-quadruplexes have been found associated to cancer relevant genes (including NRAS4 and TRF211) and recently shown to be targeted for oncoprotein translational activation.14 These findings have important implications for the development of RNA-directed drug design to modulate G-quadruplex function in mRNA translation of cancer relevant genes.
The vascular endothelial growth factor A (VEGF-A, hereafter called VEGF) is a potent angiogenic factor that has a pivotal role in tumor angiogenesis and the development of metastases. It thereby represents the main target for anti-angiogenic therapy. Translation initiation of VEGF mRNA is controlled by a number of sequence/structural cis-elements,15 including 2 internal ribosomal entry sites (IRES-A and IRES-B)16,17 that enable VEGF expression in response to various cellular stresses.18-21 IRESs comprise a variety of RNA sequences and structures, located at viral and cellular 5’ UTRs and recruit the 40S ribosomal subunit to the start codon independently of interactions between the translational machinery and cap. IRESs regulate the synthesis of the stress response, growth, and apoptotic factors under situations that compromise cap-dependent translation22 and their activity is regulated by IRES trans-acting factors (ITAFs).23 Although the mechanisms used by IRESs to recruit the ribosome are hugely diverse, the overall trend for cellular IRESs is the use of protein complexes that act as chaperones to remodel the IRES RNAs into structures able to recruit the ribosome.24 The growing body of evidence suggests that in the case of many cellular IRESs, the requirement for specific RNA secondary structures is not critical for function.24 In contrast however, the G-quadruplex embedded in the VEGF IRES-A has been shown to be a critical determinant for cap-independent translation initiation of VEGF mRNA. Indeed, a specific combination of G to U mutations in the G-rich sequence of the VEGF IRES-A eliminated intramolecular 2-G-quartet quadruplex formation, and resulted in a marked loss of IRES activity in translation.25 The question of whether and how G-quadruplexes associated to IRES regulate cap-independent mRNA translation is poorly investigated.
Here, we used different nucleotidic variants of the specific combination of mutations previously shown to invalidate the VEGF G-quadruplex structure, to analyze the structure/function relationship of the G-quadruplex motif in cap-independent mRNA translation. We show that the G-quadruplex at the VEGF 5’ UTR is not only dispensable for IRES function in cap-independent translation but also not required for VEGF IRES-A activation in stress conditions. However, stabilization of the VEGF IRES-A G-quadruplex structure by engineering G-stretch length or by ligand-targeting results in efficient inhibition of mRNA translation. Importantly, polysome analysis revealed that G-quadruplex targeting compounds reduces the translational efficiency of the endogenous VEGF mRNA from HeLa cells, resulting in lowered VEGF expression. Our data provide evidence that intrinsically stable or ligand-stabilized G-quadruplexes function as inhibitors of cap-independent translation.
Results
The G-quadruplex at IRES-A is dispensable for IRES function and its activation during ER-stress
The recently identified G-quadruplex-forming sequence GGAGGAGGGGGAGGAGG located at the VEGF IRES-A25 is highly conserved across various species though not rodents (Fig. S1) suggesting a potential biological function. To investigate the structure/function relationship of the G-quadruplex within the IRES-A of VEGF 5’ UTR, we used a dual-luciferase reporter, in which the VEGF 5’ UTR has been cloned in between the Renilla (RLuc) and the firefly (FLuc) luciferase genes. Using such a bicistronic reporters, the RLuc activity reflects cap-dependent translation and it is proportional to the amount of RNA present in the cell, whereas the FLuc activity measures the IRES-dependent translation. Therefore, the FLuc/RLuc ratio represents the IRES activity normalized to the amount of RNA present in the cell. We created variants of this bicistronic reporter based on data showing that the quadruple mutant G774,777,781,783U (nucleotide numbering refers to the VEGF 5’ UTR) eliminated any potential intramolecular G-quadruplex formation and completely abolished IRES function in mRNA translation.25 Our variants harbored substitutions of the Gs at these positions to Us (as in25, G>U), As (G>A) or Cs (G>C) (Fig. 1A). Concordant with previous data,25 we found that the G>U mutation induced a marked reduction in IRES activity compared to the wild-type sequence. Conversely, the G>A and G>C mutations had no significant effect on IRES function in translation (Fig. 1B). Since IRESs ensure translation activation of selected mRNAs during stress, we reasoned that the G-quadruplex at IRES-A might play a function in stress conditions, as recently proposed for the G-quadruplex at the p53 polyadenylation signal.26 This possibility was tested by measuring IRES-A activity of the WT and quadruple mutants in HeLa cells during endoplasmic reticulum (ER) stress. Indeed, VEGF mRNA is efficiently translated through an IRES-dependent mechanism during hypoxia18-20 or ischemia.21 Both stress conditions interfere with the ER function resulting in the activation of the unfolded protein response (UPR) through a pathway involving the activation of sensors such as PERK, a transmembrane protein that couples ER stress to translation inhibition by phosphorylating eIF2α, and the endoribonuclease IRE-1 that activates the transcription factor XBP1 by site-specific cleavage of its mRNA.27,28 This program allows the rapid downregulation of protein synthesis and upregulation of genes that promote cell survival.29 To induce ER stress we used DTT, a reducing agent that blocks disulfide bond formation necessary for the folding of many ER proteins. As shown in Figure 1C, the DTT induced both eIF2α phosphorylation and cytoplasmic cleavage of the XBP1 mRNA. In such ER stress response-activating conditions, we observed a 2-fold increase in VEGF IRES-A activity, indicating an upregulated initiation of translation at IRES-A (Fig. 1D). This activation was not modulated by any of the mutations within the G-quadruplex sequence (Fig. 1D). Similar results were obtained with bicistronic vectors containing only the VEGF IRES-A (Fig. S2), indicating that our conclusion on the dispensable role of the G-quadruplex in VEGF IRES function did not depend on the specific RNA context in which the G-quadruplex resided. Thus, while none of the G-quadruplex mutants interfered with IRES activation in stress conditions, the G>U mutant only impaired IRES function in cap-independent translation. To discriminate between a structure- and a sequence-dependent effect of the G-quadruplex mutations in VEGF mRNA translation, we assessed the ability of the WT and different quadruple mutants to adopt a G-quadruplex conformation. For this, we used the avian myeloblastosis virus (AMV)-mediated reverse transcription assay which exploits the ability of a G-quadruplex preferentially stabilized in the presence of K+ compared to Na+30, to pause reverse transcription in vitro. As shown in Figure 1E, when NaCl (or LiCl, Fig. S3) was substituted for KCl in the reverse transcription buffer, the strong pauses observed with K+ disappeared. Dideoxy sequencing of RNA indicated that the reverse transcriptase paused at G790, suggesting the occurrence of a G-quadruplex in the G-rich (+774 to +790) region (Fig. S3). This result is in agreement with previous data obtained using RNase T1, dimethylsulfate footprinting and CD spectroscopy showing the formation of a G-quadruplex in the presence of K+ at this position within the VEGF 5’ UTR.25 However, the K+-dependent AMV reverse transcriptase pausing was lost with the quadruple mutants, regardless of substituted nucleotide (Fig. 1E), suggesting that the presence of G at positions 774, 777, 781 and 783 of the VEGF 5’ UTR is required to enable the formation of a G-quadruplex.
Figure 1.
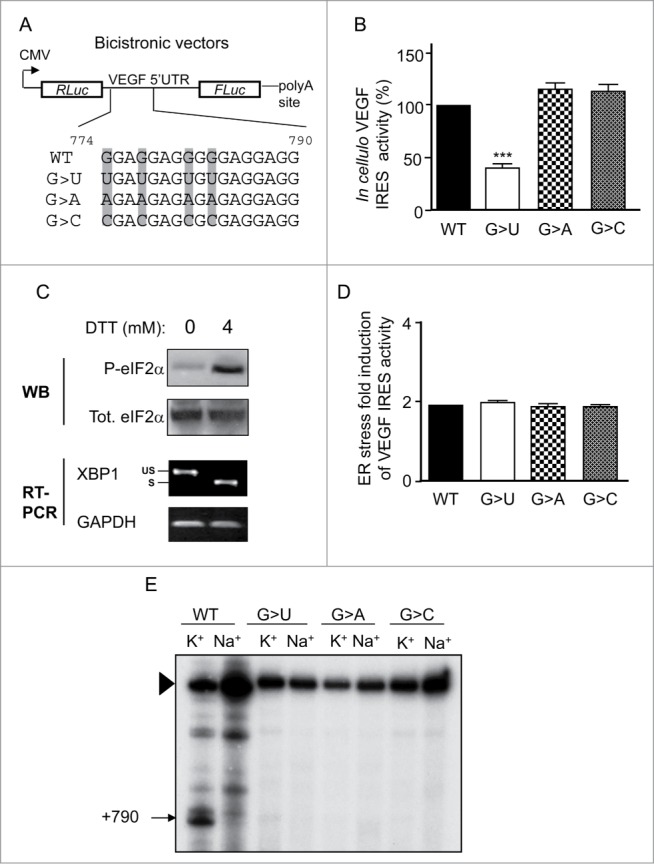
Structure/function relationship of the IRES-A-associated G-quadruplex in the full length VEGF 5’ UTR. (A) Illustration of the bicistronic vectors in which the VEGF 5’ UTR (nts 1-1038) has been cloned in between the Renilla (RLuc) and the firefly (FLuc) luciferase open reading frames. Individual clones carrying the G-quadruplex forming sequence wild-type (WT) or mutated, in which the Gs at positions 774, 777, 781, and 783 (in gray) of the full length VEGF 5’ UTR (with the first nucleotide being the transcription initiation site) are changed to Us (G>U), to As (G>A), or to Cs (G>C), are depicted. CMV, cytomegalovirus promoter. (B) VEGF cap-independent activity measured by the ratio of Firefly/Renilla luciferase activities (FLuc/RLuc) was determined using HeLa cells transfected with the WT, G>U, G>A and G>C reporter plasmids depicted in A. Statistical analysis of the mutant reporter plasmids was calculated relatively to the WT reporter plasmid (Anova test with Dunnett's multiple comparison test. ***, P <0.001). (C) Western blot analysis of total and phosphorylated eIF2α (Eukaryotic Initiation Factor 2 α) in HeLa cells treated or not with DTT (upper panel). RT-PCR analysis of XBP1 (X-box binding protein 1) mRNA splicing after DTT-induced ER stress (lower pannel). US, unspliced XBP1; S, spliced XBP1. (D) Contribution of the G-quadruplex to VEGF cap-independent activation by ER stress measured by transfection of the indicated plasmids in HeLa cells treated or not with the ER stress inducer DTT, followed by measurement of the FLuc/RLuc ratio. (E) Cation-dependent pausing of reverse transcription at VEGF RNA WT or mutated (G>U, G>A or G>C). Strong pauses of reverse transcriptase are indicated by arrowheads, with their positions within the VEGF 5’ UTR. Full-length extension products are indicated by a triangle.
Thus, although replacing the Gs at these positions by U, C or A abolished G-quadruplex formation, only the G>U mutant perturbed IRES activity, suggesting that this perturbation was not G-quadruplex structure-dependent. More importantly, this set of results shows that the G-quadruplex structuration at VEGF 5’ UTR is not required for IRES-driven translation initiation or stress-mediated IRES activation.
Sequence-dependent effect of the G>U mutation in IRES-mediated translation
Sequence analysis of the G-quadruplex forming sequence containing the G>U replacements revealed that the G777U substitution introduced an AUG followed by a 56 codon open reading frame (ORF). Upstream ORFs have been found to interfere with the translation of the principal downstream ORF in a codon number-dependent manner31,32 and can be translated through a cap-independent mechanism.33 With this in mind, we created 2 additional variants of the bicistronic plasmid containing the G to U quadruple mutant within the VEGF 5’ UTR (Fig. 2A). In the first variant, the G at 777 was substituted for A to modify the putative AUG start codon to a non-initiating AAG (G>U AUG/AAG). The second variant plasmid contained an A791U substitution that resulted in the formation of a stop codon in-frame with the AUG created by the G777U mutation (G>U STOP). As shown in Figure 2B, both replacements within the quadruple mutant almost completely restored the IRES activity, suggesting that the G to U mutation in the context of the G774,777,781,783U quadruple mutant affects VEGF IRES activity by introducing an inhibitory upstream ORF.
Figure 2.
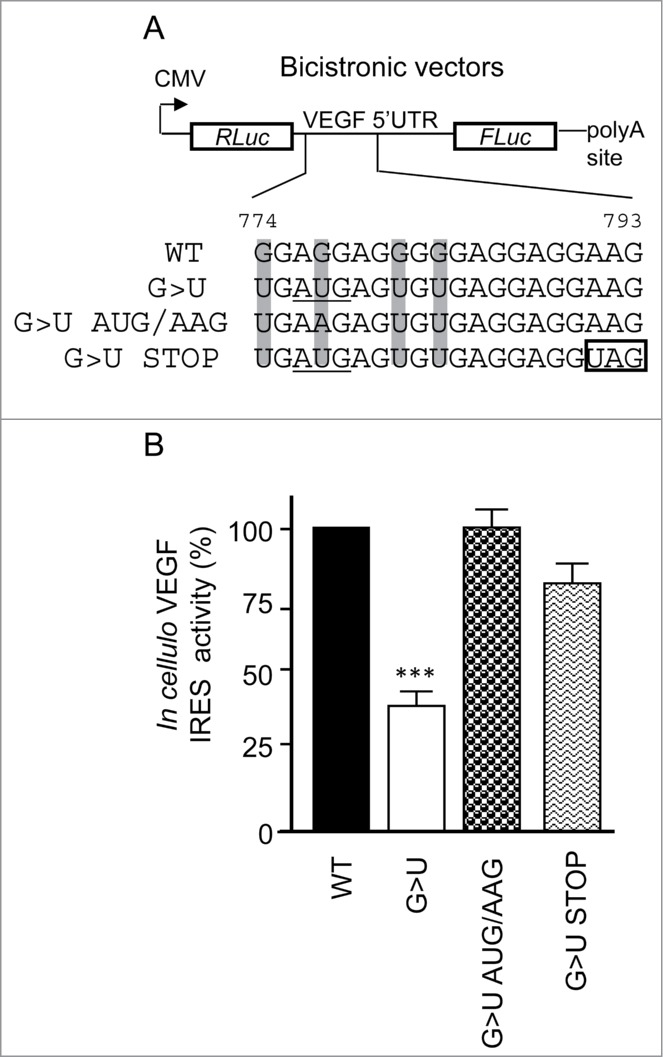
Characterization of the G>U mutation in IRES-mediated translation. (A) Illustration of the bicistronic vectors containing the full length VEGF 5’ UTR with the G-quadruplex WT, G>U (G774,777,781,783U; in gray) and 2 additional variants of the G>U construct: the G>U AUG/AAG, containing the G777A mutation to abolish formation of an AUG initiation codon (underlined), and the G>U STOP, containing a A791U mutation to insert a stop codon (boxed), resulting in a smaller open reading frame. (B) IRES activity of the aforementioned plasmids measured after transfections in HeLa cells. Statistical analysis of the mutant reporter plasmids were calculated relatively to the WT reporter plasmid (Anova test with Dunnett's multiple comparison test. ***, P <0.001).
Engineered three-G-quartet IRES-A G-quadruplex inhibits cap-independent translation
We then reasoned that although disruption of the G-quadruplex at the VEGF IRES-A was not necessary for IRES function and activation, its stabilization could interfere with cap-independent mRNA translation. Increasing the number of G-quartets has been shown to increase the stability of the G-quadruplex structure.9 Based on these data, we analyzed IRES activation of the IRES-A-containing bicistronic reporter in which the natural G-quadruplex sequence composed of 2 G-tetrads was either modified by inserting additional Gs (G4 3p) or replaced by the NRAS G-quadruplex sequence (NRAS),4 resulting in a 3-G-quartet RNA G-quadruplex (Fig. 3A). As shown in Figure 3B, both the artificial G4 3p motif and the naturally occurring NRAS G-quadruplex sequence resulted in inhibition of cap-independent translation. Mutating the G-stretches within the NRAS sequence (NRAS mut; Fig 3B) reverted its strong repression of the IRES-A activity. Cation-dependent reverse transcription showed that both the NRAS and G4 3p sequences but not the NRAS mut were able to efficiently pause the AMV reverse transcriptase (Fig. 3C), confirming that the natural 2-quartet G-quadruplex can be replaced by a sequence devoid of G-stretches without affecting IRES activity. This set of results shows that a 3-G-quartet RNA G-quadruplex represses IRES-mediated translation, suggesting that stable G-quadruplexes are inhibitory not only of cap-dependent5 but also cap-independent translation.
Figure 3.
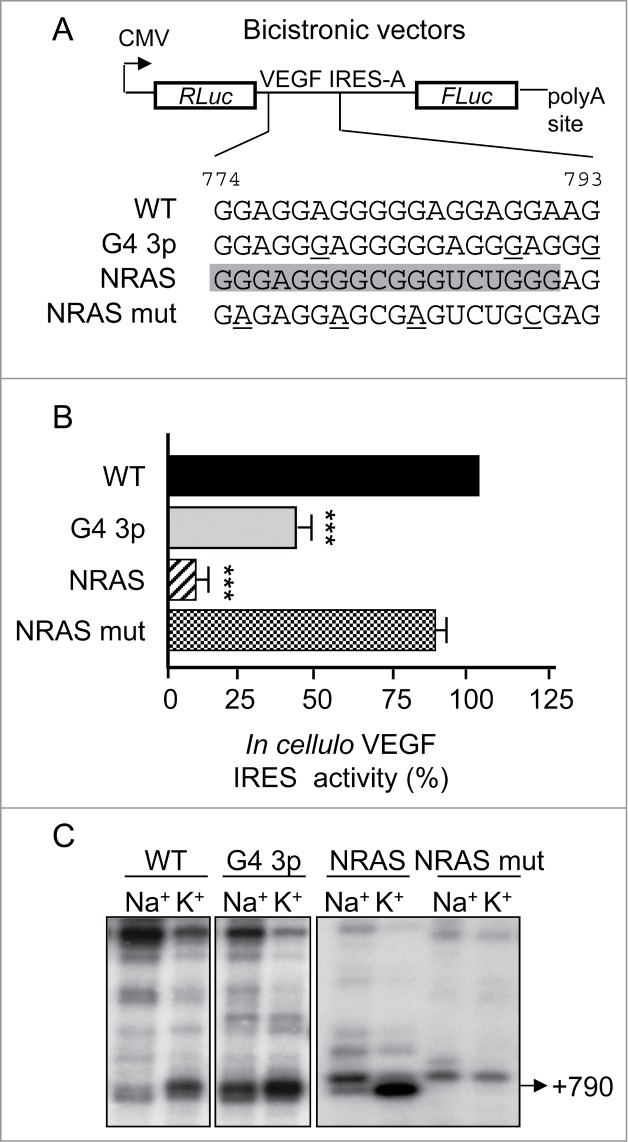
Engineered three-G-quartets IRES-A G-quadruplex inhibits cap-independent translation. (A) Illustration of the bicistronic vectors containing only the IRES-A (nts 746-1038) with the G-quadruplex sequence either wild-type (WT) or mutated to increase the G-stretches length (G4 3p, mutations underlined) or replaced by the NRAS G-quadruplex sequence (in gray) wild-type (NRAS) or mutated (NRAS mut; mutations underlined). (B) IRES activity of the aforementioned plasmids measured after transfections in HeLa cells. Statistical analysis of the mutant reporter plasmids were calculated relatively to the WT reporter plasmid (Anova test with Dunnett's multiple comparison test. ***, P <0.001). (C) Cation-dependent pausing of reverse transcription at VEGF RNA WT, G4 3p, NRAS and NRAS mut. The strong pause at position +790 is shown.
Stabilization of the IRES-A G-quadruplex inhibits cap-independent translation in vitro
To determine whether the IRES-A G-quadruplex is tuneable, we used small molecules able to selectively bind and stabilize G-quadruplex structures. Cation-dependent reverse transcription was performed in the presence of 3 highly selective G-quadruplex ligands (Fig. S4A): 360A, Phen-DC(3) and Phen-DC(6). 360A is a 2,6-pyridine-dicarboxamide derivative displaying the ability to bind stable RNA G-quadruplexes with high affinity and specificity and to further enhance their suppressive effect on translation.7,11 The Phen-DC(3) and Phen-DC(6) are 2 bisquinolinium compounds recently shown to stabilize RNA G-quadruplexes and modulate gene expression of TRF2 reporters in vitro.11 As shown in Figure 4A, all the compounds increased AMV-mediated reverse transcriptional pausing in conditions (i.e. Na+) less favorable to quadruplex formation. The most marked effect was observed with the 360A (Fig. 4A) but only with the wild-type sequence (Fig. S4B). To investigate the consequence of VEGF G-quadruplex stabilization on mRNA translation, we quantified cap-independent translation driven by the IRES-A containing the G-quadruplex sequence WT or mutated (G>A), adding the ligands 360A, Phen-DC(3) or Phen-DC(6) and using in vitro translation of in vitro transcribed bicistronic reporter RNAs (depicted in Fig. 4B). As shown in Figure 4C, the 3 G-quadruplex ligands had a more pronounced concentration-dependent inhibitory effect on the IRES activity in the WT than in the G-quadruplex mutated RNA construct. While additional experiments are needed to explore the possibility that the G-quadruplex ligands stabilize additional structures in the VEGF IRES sequence, our results indicate that the stabilization of the VEGF IRES-A G-quadruplex with specific ligands represses cap-independent translation in vitro.
Figure 4.

Stabilization of the G-quadruplex at VEGF IRES-A inhibits cap-independent translation in vitro. (A) Cation-dependent pausing of reverse transcription at VEGF RNA WT in the presence of increasing concentrations of the G-quadruplex ligands 360A (16 nM, 80 nM and 400 nM), Phen-DC(3) (1,6 nM, 8 nM and 40 nM), and Phen-DC(6) (1,6 nM, 8 nM and 40 nM). (B) Illustration of the in vitro transcribed, capped and polyadenylated (50 nts poly-A tail) bicistronic mRNAs containing only the IRES-A with the G-quadruplex sequence either wild-type (WT) or mutated (G>A). (C) VEGF IRES activity was determined using in vitro translation in RRL of in vitro transcribed bicistronic reporter RNAs depicted in B with increasing amounts of the ligands 360A, Phen-DC(3) and Phen-DC(6), as indicated. Statistical analysis of the G>A bicistronic mRNAs were calculated relatively to the WT bicistronic mRNAs for each G4-ligand concentration (2 tailed, Student's t-test *, P <0.05; **, P<0.01; ***, P<0.001).
G-quadruplex ligands inhibit VEGF mRNA translation in living cells
We next wished to investigate whether G-quadruplex-stabilizing ligands could impact the efficiency of endogenous VEGF mRNA translation in living cells. To this end, cytoplasmic extracts from HeLa cells treated with the Phen-DC(6) ligand were subjected to polysome analysis. This consisted in the separation of polysomes by density-gradient ultracentrifugation, followed by detection of RNA position in the gradient by the absorbance of UV light (254 nm). The resulting profile provided a snapshot of the global efficiency of translation. We found that the abundance of mRNAs in the polysomal fractions was slightly reduced after treatment with Phen-DC(6), suggesting that the average number of ribosomes per translated transcript was affected by the G-quadruplex ligand (Fig. 5A). In addition, the presence of Phen-DC(6) induced a modest but reproducible decrease in the 80S peak (corresponding to translating monosomes and inactive 80S not bound to mRNA) accompanied by a similar increase in the 60S peak, further indicating that the G-quadruplex ligand impacts global mRNA translation. This result is in agreement with the predicted over-representation of G-quadruplexes in translation regulatory regions of human mRNAs34 and with previous findings showing that stabilization of G-quadruplex by specific ligands results in reduced mRNA translation of reporter genes.10-12 To determine whether the G-quadruplex ligand could affect the efficiency of VEGF mRNA translation, we harvested mRNAs from each gradient fraction and analyzed by RT-qPCR the polysomal distribution of VEGF mRNA, as well as TRF2 and HPRT, that served as a positive11 and negative control, respectively, for the effect of the ligand on translation. G-quadruplex interactive agents have been shown to stabilize a G-quadruplex structure in the proximal promoter of the VEGF and downregulate VEGF mRNA levels in cells,35-38 suggesting a potential effect of Phen-DC(6) on VEGF mRNA transcription. To exclude the possibility that the observed effect of the ligand on VEGF mRNA polysomal distribution was due to a decrease in VEGF mRNA levels, we analyzed the ratio of polysomal to non-polysomal VEGF mRNA. As shown in Figure 5B, this ratio decreased for both the TRF2 and VEGF mRNAs, indicating that Phen-DC(6) reduces the association of ribosomes with either mRNA. Together, these data suggest that the G-quadruplex ligand Phen-DC(6) reduces the efficiency of VEGF mRNA translation. To determine whether Phen-DC(6) could impact VEGF IRES-mediated translation in living cells, we treated HeLa cells, transfected with in vitro transcribed bicistronic reporter RNAs (depicted inFig. 4B), with the bisquinolinium derivative and quantified cap-independent translation driven by the VEGF IRES-A containing the WT or mutated (G>A) G-quadruplex sequence. In agreement with the in vitro translation results, the G-quadruplex ligand had a more pronounced concentration-dependent inhibitory effect in cellulo on the VEGF IRES activity in the WT than in the G-quadruplex-mutated RNA constructs (Fig. 5C). Importantly, inhibition of VEGF IRES activity and the association of VEGF mRNA with actively translating ribosomes correlated with a reduction in VEGF protein expression, as revealed ELISA analysis (Fig. 5D).
Figure 5.
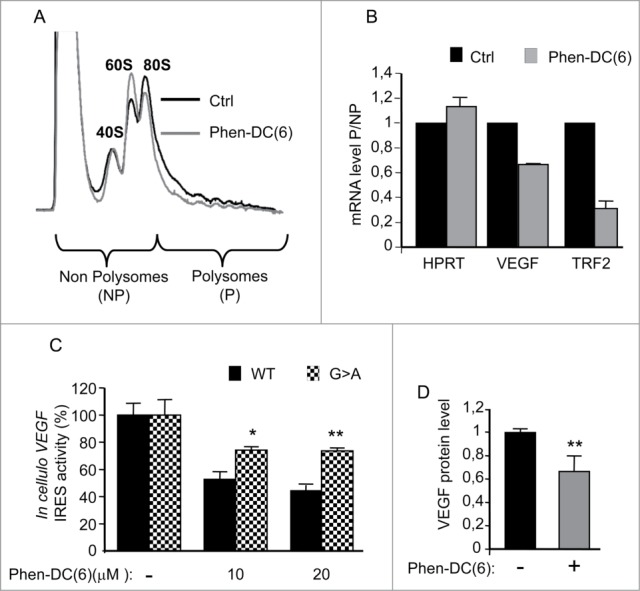
G-quadruplex ligands inhibit VEGF mRNA translation in living cells. (A) Representative polysome distribution profile obtained after centrifugation of cytoplasmic lysates over sucrose gradients and measurement of the UV-absorbance (254 nm). Lysates were prepared from either DMSO (Ctrl)- or Phen-DC(6)- treated HeLa cells (20μM). Fractions were collected and divided in 2 groups: non-polysome (NP, contain the ribonucleoproteins, the 40S, 60S and 80S ribosomal subunits) and polysome (P, contain mRNAs engaged in translation). (B) Quantitative RT-PCR was performed on each NP and P fractions using specific primers for TRF2 (Telomeric Repeat-binding Factor 2), VEGF and HPRT (Hypoxanthine Phosphoribosyltransferase 1) mRNAs. The mRNA levels in P out of NP in Phen-DC(6)- treated HeLa cells were plotted relatively to DMSO (Ctrl)- treated HeLa cells +/- SEM of two independent experiments. (C) In cellulo VEGF IRES activity was determined by transfecting HeLa cells with in vitro transcribed, capped and polyadenylated bicistronic mRNAs containing the IRES-A WT or mutated (G>A) (depicted in Fig. 4B) followed by treatment with Phen-DC(6). Statistical analysis of the G>A bicistronic mRNAs were calculated relatively to the WT bicistronic mRNAs for each Phen-DC(6) concentration (2 tailed, Student's t-test *, P <0.05; **, P<0.01). (D) Quantification of VEGF protein expression after treatment of HeLa cells with Phen-DC(6) (20μM) by using VEGF ELISA. Statistical analysis of the Phen-DC(6)-treated condition was calculated relatively to the DMSO treated condition (2 tailed, Student's t-test **, P<0.01).
Discussion
A growing body of evidence supports the link between deregulation of translational control and disruption of normal cell behavior in human cancers. RNA cis-regulatory elements located in the 5’-UTRs and translation initiation protein factors are considered to be active players in translational regulation. Among the cis-regulatory elements, G-quadruplex structures have been identified in a large number of genes, including cancer-associated genes, where they act as general repressors of cap-dependent translation. Recently, a link between RNA G-quadruplex disruption and cancer development mediated by the translation initiation factor eIF4A has been established.14 This G-quadruplex-dependent derepressive mechanism reinforces the view that small molecules stabilizing RNA G-quadruplexes can be potent antitumor chemotherapeutic agents.5 In contrast to the widely accepted repressive function of G-quadruplexes, the RNA G-quadruplex within the VEGF IRES has been shown to play a positive role in IRES-mediated translation initiation in HeLa cells. In this study, the quadruple mutant G774,777,781,783U, which lacks a sufficient number of G-stretches to adopt an intramolecular G-quadruplex conformation, was inactive in IRES-mediated translation, suggesting that the 2-quartet G-quadruplex at the VEGF is essential for cap-independent initiation. Our report provides a set of evidence to the contrary. We showed that mutating Gs at 774,777,781,783 positions to As or Cs (Fig. 1 and Fig. S2) or replacing the G-quadruplex forming element with the mutated NRAS sequence disrupted G-quadruplex formation (Figs. 1,3) but did not affect IRES activity (Fig. 1; Fig. S2). However, as previously shown, the mutation G774,777,781,783U resulted in significant decrease of IRES-directed translation in vitro and in cellulo (Figs. 1,2; Fig. S2). To explain this controversy, we propose that the G>U mutant affected VEGF IRES-A activity in a G-quadruplex-independent manner by introducing an inhibitory upstream open reading frame. Other possible explanations are that the G>U mutant affects the interaction with a protein regulator or introduce splicing sites. However, our data showing that mutations outside the G-quadruplex sequence revert the IRES activity (G>U STOP) as well as our in vitro studies using a heterologous cell-free system do not support these possibilities. In addition, in cellulo IRES activity was measured by using in vitro transcribed bicistronic reporters, thus excluding that splicing might be involved in the effect observed with the G>U mutant.
Extending the role of G-quadruplexes to cap-independent translation, our results show that intrinsically stable or ligand-stabilized G-quadruplexes function as inhibitors of IRES-directed translation. In view of the dynamic nature of IRESs and of the regulation of RNA interactions within them being a mechanism for regulating their activity (e.g. as shown for Apaf-139 or Bag-140), our results are consistent with a mechanism whereby stable G-quadruplex structures prevent the conformational changes necessary to recruit the ribosome. Previous data has indicated that the NRAS G-quadruplex efficiently blocks mRNA translation when it is positioned close to the 5’ end, within the first 50 nts of the NRAS 5’ UTR,4 yet loses its inhibitory activity when relocated further away.8 In addition, its artificial insertion in the VEGF IRES leads to a greater repression (c.a. 9 fold decrease, Fig. 3B) compared to that observed from its natural location close to the cap within NRAS 5’ UTR (c.a. 3.5 fold decrease).4 Thus, the extent of translational repression by G-quadruplexes might depend on whether the stable structure impairs the assembly and/or the scanning process of the 43S ribosomal subunit, or blocks the remodeling of the IRES RNA into structures able to recruit the ribosome. The existence of different position-dependent G-quadruplex mechanisms provide an explanation for the discrepancy between our data and previous works showing a lower degree of inhibition for the NRAS G-quadruplex4 and the ability of moderate G-quadruplexes to modulate translational efficiency.9
On the basis of our findings we propose that, in addition to intrinsically stable G-quadruplexes acting as translational repressors, there exist translationally silent structures that may be targeted to turn off mRNA translation (Fig. 6). The observation that G-quadruplex binders globally affect mRNA translation and have the potential to convert functionally silent quadruplex structures into efficient repressors of mRNA translation, has implications for the in vitro and in vivo applications of G-quadruplex-targeting compounds and should be taken into account when evaluating the cellular responses to these agents. In addition, our results discount the current application of G-quadruplex-interactive ligands to target G-quadruplexes formed in the promoter regions of many oncogenes, because of the presence of more ubiquitous and biologically relevant RNA G-quadruplexes in nature. Besides the action of small-molecule ligands, G-quadruplex modulation may occur through the binding and stabilizing activity of protein factors, such as nucleolin41 or Topo I,42 or nucleic acids including small RNAs that inhibit translation through the formation of intermolecular G-quadruplexes.13 Considering the critical role of VEGF expression in tumor angiogenesis, the G-quadruplex at VEGF IRES-A may represent a potential therapeutic target to downregulate VEGF expression in tumors. Our data together with others showing the G-quadruplex ligand-mediated downregulation of transcription of VEGF,36-38 HIF43 and the VEGFR-2 receptor,38 certainly corroborate the application of ligands in a cellular context to target G-quadruplexes acting on the VEGF axis and mediating tumor angiogenesis.38,43
Figure 6.
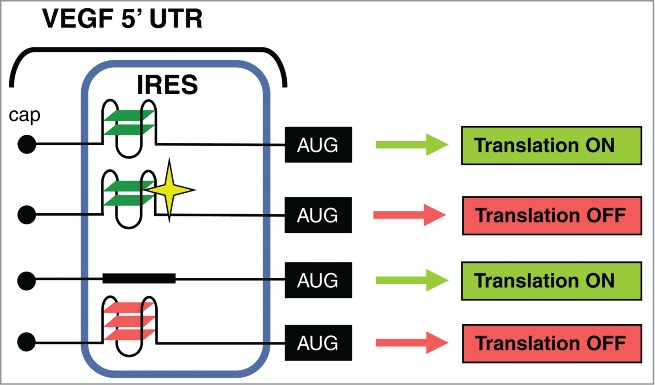
Model for the role of the IRES-A G-quadruplex in VEGF expression. The translationally silent G-quadruplex at the VEGF IRES-A can be converted into an efficient repressor of cap-independent translation by increasing the stability of the structure either by increasing the number of G-quartets or by adding a G-quadruplex ligand (depicted as a star).
Materials and Methods
Cell lines, culture and transfection
Human cervical carcinoma (HeLa) cells were grown in DMEM 1g/L glucose (Invitrogen, Cergy Pontoise, France) containing antibiotics (penicillin and streptomycin), 1% L-glutamine, and 10% fetal calf serum (FCS), at 37°C in a humidified 5% CO2 atmosphere. For transient transfection experiments, cells were seeded onto 20 4-well dishes (5 x 105 cells/well for bicistronic vectors or 10 x 105 cells/well for bicistronic mRNAs) 24 h prior to transfection. HeLa cells were transfected with (i) 250 ng of bicistronic vectors using the JetPEI transfection reagent (Polyplus Transfection) according to the manufacturer's instructions (ii) or 250 ng of bicistronic mRNAs using lipofectamine2000 reagent (Invitrogen) according to the manufacturer's instructions. To test cellular responses to drugs, cells were treated with (i) DTT (Sigma-aldrich) for 4h or (iii) Phen-DC(6) 10 or 20 µM for 24 h.
Plasmid constructions
To generate the different bicistronic constructs containing the VEGF IRES-A we used pCRVL and pCR31L plasmids44 derived from pRVL1 and pRVL2, respectively.16,45 Briefly, the 2 luciferase genes, Renilla luciferase (RLuc) and firefly luciferase (FLuc), are controlled by the cytomegalovirus promoter (CMV) and separated by either full 5’ UTR of the VEGF mRNA (nts 1 to 1038 for pCRVL) or by only VEGF IRES A sequence (nts 746 to 1038 for pCR31L). Human VEGF 5’ UTR was first PCR amplified from genomic DNA using the primers F-VEGF 5’ UTR-BglII (5’-AAAAGATCTCGCGGAGGCTTGGGGCAGCCG-3’) and R-VEGFA-NCOI (5’-TTTCCATGGTTTCGGAGGCCCGACCG-3’), then digested at the BglII, and NcoI sites and cloned into the BamHI and NcoI digested pCREL (bicistronic vector with EMCV IRES inserted between the 2 luciferase genes46) to create the plasmid pCRVL. VEGF IRES-A was PCR amplified from the pCRVL using the primers F-IRESA-BamHI (5’-AAAGGATCCTAGCTCGGGCCGGGAGGAGCCG-3’) and R-VEGFA-NCOI. PCR products were digested by BamHI and NcoI and ligated into the pCREL digested by BamHI and NcoI, leading to the pCR31L vector. The mutations of the G-quadruplex site in the VEGF 5’ UTR or IRES-A were performed using site directed mutagenesis (QuickChange, Stratagene). Finally, automated DNA Sequencing Analysis checked the nucleotide sequence of the all selected clones.
In vitro and in cellulo IRES activity analysis
Luciferase firefly bicistronic reporter mRNAs were produced by in vitro transcription using the mMESSAGE mMACHINE T7 kit (Ambion) as per the manufacturer's instructions. The templates for the in vitro transcription were PCR fragments generated from VEGF bicistronic vectors and using the following primers: 5'-GGATCCTAATACGACTCACTATAGGGCAAT TACAGCTCTTAACGTAATCTAGATGA-3' and 5'-(T)50-TATTACAATTTGGACTTTCCG CCC-3'. For the in vitro IRES activity analysis, 200 ng of in vitro transcribed luciferase firefly bicistronic reporter mRNAs (WT and G>A) were pre-incubated 30 min at room temperature with increasing amount of G-quadruplex ligands: 360A, Phen-DC(3) and Phen-DC(6). RRL (Flexi Rabbit Reticulocyte Lysate kit) were added to a final volume of 10 µl and the lysates were incubated 90 min at 30°C. 5µl of the reaction were used for the luciferase assay. For the in cellulo IRES activity analysis, the HeLa cells transfected with luciferase firefly bicistronic reporter mRNAs were harvested in 100µl of Passive Lysis Buffer (Promega). 10µl of this extract were analyzed with the luciferase assay.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Camille Mas and Corinne Hieblot for technical help. We thank Florian Hamon and Corine Guetta for the synthesis of Phen-DC(3) and Phen-DC(6) ligands. The authors also thank Dennis Gomez and Laurent Lacroix for G-quadruplex ligands and helpful discussions.
Funding
This work was supported by INSERM, Fondation de France, Ligue Nationale contre le Cancer and ARC (Association pour la Recherche sur le Cancer) (to S.M). A.D. was supported by INCa (Institut National Du Cancer), A.C by FRM (Fondation pour la Recherche Medicale) and A.L. by La Ligue Nationale Contre le Cancer.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1.Ji X, Sun H, Zhou H, Xiang J, Tang Y, Zhao C. Research progress of RNA quadruplex. Nucleic Acid Ther 2011; 21:185–200; PMID:21749296; http://dx.doi.org/ 10.1089/nat.2010.0272 [DOI] [PubMed] [Google Scholar]
- 2.Millevoi S, Moine H, Vagner S. G-quadruplexes in RNA biology. Wiley Interdiscip Rev RNA 2012; 3:495–507; PMID:22488917; http://dx.doi.org/ 10.1002/wrna.1113 [DOI] [PubMed] [Google Scholar]
- 3.Beaudoin JD, Perreault JP. 5'-UTR G-quadruplex structures acting as translational repressors. Nucleic Acids Res 2010; 38:7022–36; PMID:20571090; http://dx.doi.org/ 10.1093/nar/gkq557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumari S, Bugaut A, Huppert JL, Balasubramanian S. An RNA G-quadruplex in the 5' UTR of the NRAS proto-oncogene modulates translation. Nat Chem Biol 2007; 3:218–21; PMID:17322877; http://dx.doi.org/ 10.1038/nchembio864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bugaut A, Balasubramanian S. 5'-UTR RNA G-quadruplexes: translation regulation and targeting. Nucleic Acids Res 2012; 40:4727–41; PMID:22351747; http://dx.doi.org/ 10.1093/nar/gks068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halder K, Wieland M, Hartig JS. Predictable suppression of gene expression by 5'-UTR-based RNA quadruplexes. Nucleic Acids Res 2009; 37:6811–7; PMID:19740765; http://dx.doi.org/ 10.1093/nar/gkp696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halder K, Largy E, Benzler M, Teulade-Fichou MP, Hartig JS. Efficient suppression of gene expression by targeting 5'-UTR-based RNA quadruplexes with bisquinolinium compounds. Chembiochem 2011; 12:1663–8; PMID:21681881; http://dx.doi.org/ 10.1002/cbic.201100228 [DOI] [PubMed] [Google Scholar]
- 8.Kumari S, Bugaut A, Balasubramanian S. Position and stability are determining factors for translation repression by an RNA G-quadruplex-forming sequence within the 5' UTR of the NRAS proto-oncogene. Biochemistry 2008; 47:12664–9; PMID:18991403; http://dx.doi.org/ 10.1021/bi8010797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wieland M, Hartig JS. RNA quadruplex-based modulation of gene expression. Chem Biol 2007; 14:757–63; PMID:17656312; http://dx.doi.org/ 10.1016/j.chembiol.2007.06.005 [DOI] [PubMed] [Google Scholar]
- 10.Bugaut A, Rodriguez R, Kumari S, Hsu ST, Balasubramanian S. Small molecule-mediated inhibition of translation by targeting a native RNA G-quadruplex. Org Biomol Chem 2010; 8:2771–6; PMID:20436976; http://dx.doi.org/ 10.1039/c002418j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez D, Guedin A, Mergny JL, Salles B, Riou JF, Teulade-Fichou MP, Calsou P. A G-quadruplex structure within the 5'-UTR of TRF2 mRNA represses translation in human cells. Nucleic Acids Res 2010; 38:7187–98; PMID:20571083; http://dx.doi.org/ 10.1093/nar/gkq563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ofer N, Weisman-Shomer P, Shklover J, Fry M. The quadruplex r(CGG)n destabilizing cationic porphyrin TMPyP4 cooperates with hnRNPs to increase the translation efficiency of fragile X premutation mRNA. Nucleic Acids Res 2009; 37:2712–22; PMID:19273535; http://dx.doi.org/ 10.1093/nar/gkp130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito K, Go S, Komiyama M, Xu Y. Inhibition of translation by small RNA-stabilized mRNA structures in human cells. J Am Chem Soc 2011; 133:19153–9; PMID:22007660; http://dx.doi.org/ 10.1021/ja206353c [DOI] [PubMed] [Google Scholar]
- 14.Wolfe AL, Singh K, Zhong Y, Drewe P, Rajasekhar VK, Sanghvi VR, Mavrakis KJ, Jiang M, Roderick JE, Van der Meulen J., et al. RNA G-quadruplexes cause eIF4A-dependent oncogene translation in cancer. Nature 2014; 513:65–70; PMID:25079319; http://dx.doi.org/ 10.1038/nature13485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arcondeguy T, Lacazette E, Millevoi S, Prats H, Touriol C. VEGF-A mRNA processing, stability and translation: a paradigm for intricate regulation of gene expression at the post-transcriptional level. Nucleic Acids Res 2013; 41:7997–8010; PMID:23851566; http://dx.doi.org/ 10.1093/nar/gkt539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huez I, Creancier L, Audigier S, Gensac MC, Prats AC, Prats H. Two independent internal ribosome entry sites are involved in translation initiation of vascular endothelial growth factor mRNA. Mol Cell Biol 1998; 18:6178–90; PMID:9774635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller DL, Dibbens JA, Damert A, Risau W, Vadas MA, Goodall GJ. The vascular endothelial growth factor mRNA contains an internal ribosome entry site. FEBS Lett 1998; 434:417–20; PMID:9742966; http://dx.doi.org/ 10.1016/S0014-5793(98)01025-4 [DOI] [PubMed] [Google Scholar]
- 18.Akiri G, Nahari D, Finkelstein Y, Le SY, Elroy-Stein O, Levi BZ. Regulation of vascular endothelial growth factor (VEGF) expression is mediated by internal initiation of translation and alternative initiation of transcription. Oncogene 1998; 17:227–36; PMID:9674707; http://dx.doi.org/ 10.1038/sj.onc.1202019 [DOI] [PubMed] [Google Scholar]
- 19.Stein I, Itin A, Einat P, Skaliter R, Grossman Z, Keshet E. Translation of vascular endothelial growth factor mRNA by internal ribosome entry: implications for translation under hypoxia. Mol Cell Biol 1998; 18:3112–9; PMID:9584152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lang KJ, Kappel A, Goodall GJ. Hypoxia-inducible factor-1alpha mRNA contains an internal ribosome entry site that allows efficient translation during normoxia and hypoxia. Mol Biol Cell 2002; 13:1792–801; PMID:12006670; http://dx.doi.org/ 10.1091/mbc.02-02-0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bornes S, Prado-Lourenco L, Bastide A, Zanibellato C, Iacovoni JS, Lacazette E, Prats AC, Touriol C, Prats H. Translational induction of VEGF internal ribosome entry site elements during the early response to ischemic stress. Circ Res 2007; 100:305–8; PMID:17255526; http://dx.doi.org/ 10.1161/01.RES.0000258873.08041.c9 [DOI] [PubMed] [Google Scholar]
- 22.Spriggs KA, Bushell M, Willis AE. Translational regulation of gene expression during conditions of cell stress. Mol Cell 2010; 40:228–37; PMID:20965418; http://dx.doi.org/ 10.1016/j.molcel.2010.09.028 [DOI] [PubMed] [Google Scholar]
- 23.Komar AA, Hatzoglou M. Cellular IRES-mediated translation: the war of ITAFs in pathophysiological states. Cell Cycle 2011; 10:229–40; PMID:21220943; http://dx.doi.org/ 10.4161/cc.10.2.14472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plank TD, Kieft JS. The structures of nonprotein-coding RNAs that drive internal ribosome entry site function. Wiley Interdiscip Rev RNA 2012; 3:195–212; PMID:22215521; http://dx.doi.org/ 10.1002/wrna.1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris MJ, Negishi Y, Pazsint C, Schonhoft JD, Basu S. An RNA G-quadruplex is essential for cap-independent translation initiation in human VEGF IRES. J Am Chem Soc 2010; 132:17831–9; PMID:21105704; http://dx.doi.org/ 10.1021/ja106287x [DOI] [PubMed] [Google Scholar]
- 26.Decorsiere A, Cayrel A, Vagner S, Millevoi S. Essential role for the interaction between hnRNP H/F and a G quadruplex in maintaining p53 pre-mRNA 3'-end processing and function during DNA damage. Genes Dev 2011; 25:220–5; PMID:21289067; http://dx.doi.org/ 10.1101/gad.607011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koumenis C, Naczki C, Koritzinsky M, Rastani S, Diehl A, Sonenberg N, Koromilas A, Wouters BG. Regulation of protein synthesis by hypoxia via activation of the endoplasmic reticulum kinase PERK and phosphorylation of the translation initiation factor eIF2alpha. Mol Cell Biol 2002; 22:7405–16; PMID:12370288; http://dx.doi.org/ 10.1128/MCB.22.21.7405-7416.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blais JD, Addison CL, Edge R, Falls T, Zhao H, Wary K, Koumenis C, Harding HP, Ron D, Holcik M., et al. Perk-dependent translational regulation promotes tumor cell adaptation and angiogenesis in response to hypoxic stress. Mol Cell Biol 2006; 26:9517–32; PMID:17030613; http://dx.doi.org/ 10.1128/MCB.01145-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell; 40:280–93; PMID:20965422; http://dx.doi.org/ 10.1016/j.molcel.2010.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sundquist WI, Klug A. Telomeric DNA dimerizes by formation of guanine tetrads between hairpin loops. Nature 1989; 342:825–9; PMID:2601741; http://dx.doi.org/ 10.1038/342825a0 [DOI] [PubMed] [Google Scholar]
- 31.Kochetov AV. Alternative translation start sites and hidden coding potential of eukaryotic mRNAs. Bioessays 2008; 30:683–91; PMID:18536038; http://dx.doi.org/ 10.1002/bies.20771 [DOI] [PubMed] [Google Scholar]
- 32.Sachs MS, Geballe AP. Downstream control of upstream open reading frames. Genes Dev 2006; 20:915–21; PMID:16618802; http://dx.doi.org/ 10.1101/gad.1427006 [DOI] [PubMed] [Google Scholar]
- 33.Bastide A, Karaa Z, Bornes S, Hieblot C, Lacazette E, Prats H, Touriol C. An upstream open reading frame within an IRES controls expression of a specific VEGF-A isoform. Nucleic Acids Res 2008; 36:2434–45; PMID:18304943; http://dx.doi.org/ 10.1093/nar/gkn093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huppert JL, Bugaut A, Kumari S, Balasubramanian S. G-quadruplexes: the beginning and end of UTRs. Nucleic Acids Res 2008; 36:6260–8; PMID:18832370; http://dx.doi.org/ 10.1093/nar/gkn511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu Y, Zan LP, Wang XD, Lu YJ, Ou TM, Lin J, Huang ZS, Gu LQ. Stabilization of VEGF G-quadruplex and inhibition of angiogenesis by quindoline derivatives. Biochim Biophys Acta 2014; 1840:2970–7; PMID:24931695; http://dx.doi.org/ 10.1016/j.bbagen.2014.06.002 [DOI] [PubMed] [Google Scholar]
- 36.Sun D, Liu WJ, Guo K, Rusche JJ, Ebbinghaus S, Gokhale V, Hurley LH. The proximal promoter region of the human vascular endothelial growth factor gene has a G-quadruplex structure that can be targeted by G-quadruplex-interactive agents. Mol Cancer Ther 2008; 7:880–9; PMID:18413801; http://dx.doi.org/ 10.1158/1535-7163.MCT-07-2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun D, Guo K, Rusche JJ, Hurley LH. Facilitation of a structural transition in the polypurine/polypyrimidine tract within the proximal promoter region of the human VEGF gene by the presence of potassium and G-quadruplex-interactive agents. Nucleic Acids Res 2005; 33:6070–80; PMID:16239639; http://dx.doi.org/ 10.1093/nar/gki917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salvati E, Zizza P, Rizzo A, Iachettini S, Cingolani C, D'Angelo C, Porru M, Randazzo A, Pagano B, Novellino E, et al.. Evidence for G-quadruplex in the promoter of vegfr-2 and its targeting to inhibit tumor angiogenesis. Nucleic Acids Res 2014; 42:2945–57; PMID:24335081; http://dx.doi.org/ 10.1093/nar/gkt1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitchell SA, Spriggs KA, Coldwell MJ, Jackson RJ, Willis AE. The Apaf-1 internal ribosome entry segment attains the correct structural conformation for function via interactions with PTB and unr. Mol Cell 2003; 11:757–71; PMID:12667457; http://dx.doi.org/ 10.1016/S1097-2765(03)00093-5 [DOI] [PubMed] [Google Scholar]
- 40.Pickering BM, Mitchell SA, Spriggs KA, Stoneley M, Willis AE. Bag-1 internal ribosome entry segment activity is promoted by structural changes mediated by poly(rC) binding protein 1 and recruitment of polypyrimidine tract binding protein 1. Mol Cell Biol 2004; 24:5595–605; PMID:15169918; http://dx.doi.org/ 10.1128/MCB.24.12.5595-5605.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gonzalez V, Hurley LH. The C-terminus of nucleolin promotes the formation of the c-MYC G-quadruplex and inhibits c-MYC promoter activity. Biochemistry 2010; 49:9706–14; PMID:20932061; http://dx.doi.org/ 10.1021/bi100509s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arimondo PB, Riou JF, Mergny JL, Tazi J, Sun JS, Garestier T, Helene C. Interaction of human DNA topoisomerase I with G-quartet structures. Nucleic Acids Res 2000; 28:4832–8; PMID:11121473; http://dx.doi.org/ 10.1093/nar/28.24.4832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Welsh SJ, Dale AG, Lombardo CM, Valentine H, de la Fuente M, Schatzlein A, Neidle S. Inhibition of the hypoxia-inducible factor pathway by a G-quadruplex binding small molecule. Sci Rep 2013; 3:2799; PMID:24165797; http://dx.doi.org/ 10.1038/srep02799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karaa ZS, Iacovoni JS, Bastide A, Lacazette E, Touriol C, Prats H. The VEGF IRESes are differentially susceptible to translation inhibition by miR-16. RNA 2009; 15:249–54; PMID:19144909; http://dx.doi.org/ 10.1261/rna.1301109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bornes S, Boulard M, Hieblot C, Zanibellato C, Iacovoni JS, Prats H, Touriol C. Control of the vascular endothelial growth factor internal ribosome entry site (IRES) activity and translation initiation by alternatively spliced coding sequences. J Biol Chem 2004; 279:18717–26; PMID:14764596; http://dx.doi.org/ 10.1074/jbc.M308410200 [DOI] [PubMed] [Google Scholar]
- 46.Creancier L, Mercier P, Prats AC, Morello D. c-myc Internal ribosome entry site activity is developmentally controlled and subjected to a strong translational repression in adult transgenic mice. Mol Cell Biol 2001; 21:1833–40; PMID:11238920; http://dx.doi.org/ 10.1128/MCB.21.5.1833-1840.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


