Abstract
Osteoclasts are the exclusive cells of bone resorption. Abnormally activating osteoclasts can lead to low bone mineral density, which will cause osteopenia, osteoporosis, and other bone disorders. To date, the mechanism of how osteoclast precursors differentiate into mature osteoclasts remains elusive. MicroRNAs (miRNAs) are novel regulatory factors that play an important role in numerous cellular processes, including cell differentiation and apoptosis, by post-transcriptional regulation of genes. Recently, a number of studies have revealed that miRNAs participate in bone homeostasis, including osteoclastic bone resorption, which sheds light on the mechanisms underlying osteoclast differentiation. In this review, we highlight the miRNAs involved in regulating osteoclast differentiation and bone resorption, and their roles in osteoporosis.
Keywords: MicroRNA, osteoclast, osteoporosis
Abbreviations
- GM-CSF
Granulocyte macrophage colony-stimulating factor
- MiRNA
microRNA
- 3’-UTR
3’ untranslated region
- BMMs
bone marrow macrophages
- PDCD4
programmed cell death 4
- FasL
Fas ligand
- PIO
particle-induced osteolysis
- Calcr
calcitonin receptor
- RDX
radixin
- M-RIP
myosin phosphatase-Rho interacting protein
- ITGA5
integrin α5
- Fzd3
frizzled 3
- ALP
alkaline phosphatase
- TRAP
tartrate-resistant acid phosphatase
- CXCL11
chemokine (C-X-C motif) ligand 11
- CXCR3
chemokine (C-X-C motif) receptor 3
- SLC39A1
solute carrier family (zinc transporter) member 1
- TRAF6
TNF receptor-associated factor 6
- OVX
ovariectomy
- MAFB
V-maf musculoaponeurotic fibrosarcoma oncogene homolog B
- CBL
Casitas B-lineage lymphoma proto-oncogene
- PAG1
phosphoprotein associated with glycosphingolipid microdomains
- TOB2
transducer of ERBB2
- sICAM1
soluble intracellular adhesion molecule
Introduction
Osteoporosis is caused by an imbalance between osteoblastic bone formation and osteoclastic bone resorption.1 Osteoclasts, the sole cell type capable of bone resorption in the human body, originate from haematopoietic progenitors which can also differentiate into monocytes and macrophages.2 The process of osteoclast differentiation is regulated by a variety of cytokines, growth factors and hormones, including M-CSF, RANKL, sex hormones, and parathyroid hormone (PTH). Of these signaling molecules, RANKL binds the membrane receptor RANK on osteoclast precursors, activates downstream signaling pathways, such as the MAPK, PI3K and NF-κB pathways, and regulates the expression of various transcription factors, including c-Fos, PU.1, Fra-1 and NFATc1.3-5 These pathways and transcription factors modulate osteoclast differentiation, maturation and survival. Investigation of the molecular mechanisms that mediate osteoclastic differentiation as well as the regulation of their pathological function will contribute to a better understanding of the pathogenesis of osteoporosis and the development of new treatment strategies for this disease.
MicroRNAs (MiRNAs), a class of noncoding RNAs 19–25 nucleotides in length, control gene expression at the post-transcriptional level.6 Since 1993, when the first miRNA, named lin-4, was discovered in Caenorhabditis elegans,7 numerous miRNAs have been identified in different organisms. The latest miRBase release (v20, June 2013) contained 24,521 miRNA loci from 206 species that can produce 30,424 mature miRNA products.8 A number of miRNAs regulate various pathophysiological events, including organ development, haematopoietic function, organogenesis, apoptosis, proliferation and tumorigenesis. MiRNAs are also involved in bone cell differentiation and function, bone development and diseases. MiR-223, which is almost exclusively expressed in mouse bone marrow, is regulated by the enhancer modulators PU.1 and CAAT.9,10 In addition, miR-125b,11 miR-26a,12 miR-133 and miR-135 13 have all been implicated in the differentiation of osteoblasts. The expression pattern of miRNAs throughout the differentiation of osteoclast precursors into mature osteoclasts has also been explored, and the expression of 44 miRNAs increased by more than 2-fold.14 Moreover, the involvement of Dicer (part of the Rnase III family, cleaves double-stranded RNA or pre-microRNA into short double-stranded RNA fragments) in regulating osteoclastic bone resorption indicates that miRNAs are novel regulatory factors of osteoclastogenesis.15 Therefore, miRNAs may represent new therapeutic targets for the pharmacological control of bone diseases. Recently, some researchers reviewed the importance of miRNAs in post-transcriptional regulation of skeletal development.16-18 The functions of miR-21, miR-155 and miR-223 in osteoclast differentiation have been summarized in previous reviews.19,20 In this paper, we reviewed the role of all relevant miRNAs in osteoclasts and related bone diseases, such as osteoporosis (Table 1).
Table 1.
Summary of miRNAs, their targets, expression, and effects on osteoclast differentiation
| miRNAs | species | Target gene(s) | Endogenous miRNA expression | miRNA function | Related disease(s) | References |
|---|---|---|---|---|---|---|
| miR-21 | mouse | FasL PDCD4 |
Upregulated in RANKL-induced osteoclastogenesis | Enhances osteoclast differentiation Inhibits osteoclast apoptosis |
Postmenopausal osteoporosis; osteoporosis | 23,25 |
| miR-29a | rat | Not mentioned | Reduced expression in glucocorticoid-induced bone loss | Inhibits GC-induced osteoclast differentiation | Glucocorticoid-induced bone loss | 33 |
| miR-29b | human | c-Fos, MMP2 | Downregulated in RANKL induced osteoclastogenesis | Inhibits osteoclast differentiation | Multiple myeloma-related bone disease | 34 |
| miR-29 | mouse | CDC42 SRGAP2 NFIA, CD93 CALCR |
Increased during osteoclast differentiation | miR-29 family member that sustains migration and commitment of precursors to osteoclastogenesis | Possibly related to increased osteoclast formation with aging | 35 |
| miR-223 | mouse | Not mentioned | Downregulated in osteoclast differentiation | Overexpression completely blocks osteoclast formation | Might be related to bone metabolic disorders | 14,52 |
| human | NFIA | Downregulated in osteoclast differentiation | Inhibits osteoclast differentiation | Bone destruction in rheumatoid arthritis | 53 | |
| miR-378 | mouse | Not mentioned | Upregulated in osteoclast differentiation | Not mentioned | Osteolytic bone metastasis | 14,64 |
| miR-146a |
mouse | Not mentioned | Downregulated upon RANKL treatment upregulated upon TNF-α/RANKL treatment | Suppresses osteoclastogenesis | Not mentioned | 14 |
| human | TRAF6 | Not mentioned | Inhibits PBMC differentiation into osteoclast | Joint destruction in arthritis | 49 | |
| miR-133a | human |
CXCL11 CXCR3 SLC39A1 |
Up-regulated in low BMD postmenopausal women | Not mentioned | Postmenopausal osteoporosis | 46 |
| miR-503 | human | RANK | Markedly reduced in postmenopausal osteoporosis women | Inhibits RANKL-induced osteoclast differentiation | Postmenopausal osteoporosis | 59 |
| miR-31 | mouse | RhoA | Highly upregulated during osteoclast development upon RANKL stimulation | promote ring-shaped mature osteoclasts formation, attributed to cytoskeleton organization | Not mentioned | 38 |
| miR-124 | mouse | NFATc1 RhoA Rac1 |
Decreased rapidly upon stimulation of BMMs with RANKL | Negatively regulates osteoclastogenesis, reduces the proliferation and motility of osteoclast precursors | Not mentioned | 42 |
| miR-125a | human | TRAF6 | Dramatically downregulated during osteoclastogenesis | Inhibit osteoclastogenesis | May be involved in metabolic disease | 67,68 |
| miR-148a | Human mouse |
MAFB | Upregulated during osteoclast differentiation | Promote osteoclastogenesis | Osteoporosis, contributes to low BMD in lupus patients | 67 |
| miR-155 | mouse | SOCS1 MITF SHIP |
RANKL treatment suppress miR-155 levels in BMMs from Dicer-deficient mice | Suppress osteoclast differentiation | Osteoclast-mediated diseases | 15,76,77 |
| miR-422a | human |
CBL CD226 IGF1 PAG1 TOB2 |
Upregulated with marginal significance in the low BMD postmenopausal women | Not mentioned | Postmenopausal osteoporosis | 80 |
*Italics: potential targets
MiR-21
Mature miR-21, which is encoded by a single gene, is conserved in mammals. The mouse miR-21 includes miR-21a, miR-21b and miR-21c, of which the genes are mapped to chromosome 11, chromosome 3 and chromosome 8 respectively. As to human miR-21, of which the gene is located in chromosome 17 between the stop codon and poly A signal of the 3’-UTR (3’ untranslated region) of transmembrane protein 49 (TMEM49).21,22 MiR-21 is involved in the RANKL-induced differentiation of osteoclasts derived from mouse bone-marrow macrophages (BMMs). Transcription factors for osteoclastogenesis, such as c-Fos and PU.1, trigger miR-21 transcription via the AP-1 and PU.1 binding sites in the miR-21 promoter.22 Sugatani et al. reported that miR-21 regulates osteoclast formation through a positive feedback loop that involves c-Fos/miR-21/PDCD4 (programmed cell death 4). In this process, the osteoclastogenesis transcription factor c-Fos upregulates the expression of miR-21. miR-21 then downregulates PDCD4 protein expression, which in turn represses the PDCD4-induced inhibition of c-Fos and thereby promotes RANKL-induced osteoclastogenesis.23 Sugatani and colleagues also discovered that miR-21 plays a critical role in estrogen-controlled osteoclastogenesis. Notably, estrogen inhibits osteoclastogenesis and induces osteoclastic apoptosis.24 Estrogen downregulates miR-21 biogenesis so that protein levels of FasL (Fas ligand), a target of miR-21, are elevated post-transcriptionally and thus induce osteoclastic apoptosis.25 Wear particles induce aggressive osteoclastic bone resorption after joint replacement, which causes aseptic loosening.26 Zhou et al. found that miR-21 was markedly upregulated in particle-induced osteolysis (PIO) model animals and knockout miR-21 in particle-stimulated tissue could ameliorate the symptoms of osteolysis.27 In short, the upregulation of miR-21 plays an important role in the pathological processes underlying osteoporosis and osteolysis during aseptic implant loosening in joint replacement patients.
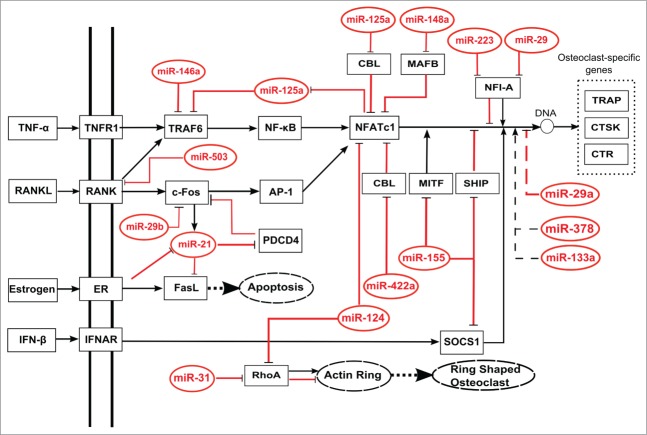
Figure 1.
Schematic regulatory networks of miRNAs regulating osteoclast differentiation. MiRNAs can directly (solid lines) or indirectly (dotted lines) repress transcriptional factors, and affect osteoclast differentiation, apoptosis, or phenotype. Bold dotted lines indicate the promotion of cellular processes, dotted boxes indicate osteoclast specific genes, and dotted ovals indicate cellular processes or phenotypes.
MiR-29
The miR-29 family includes miR-29a, miR-29b and miR-29c. MiR-29b is represented by 2 members, miR-29b-1 and miR-29b-2. MiR-29a was first discovered in cervical cancer cells (HeLa).28,29 Based on seed sequence target prediction, miR-29 family members may regulate >6,000 gene expressions.30 An earlier study by Mott et al. has suggested that NF-κB activation suppresses miR-29b-1 and miR-29a promoter function, and the expression of mature miR-29b is mediated via the recognition of NF-κB binding sites.31 As NF-κB is an important regulator of osteoclast differentiation, researchers have started to focus on whether miR-29a is active in osteoclasts. Several studies confirmed that miR-29 is a key mediator of osteoclast differentiation. Gong et al. reported that miR-29a was down expressed in SBC-5 small-cell lung cancer cells in bone metastases. However, the downregulation of miR-29a had no correlation with osteolytic lesions.32 Wang et al. discovered that osteoporosis caused by glucocorticoids was associated with reduced expression of miR-29a, and a gain of miR-29a function reduced glucocorticoid-induced osteoclast differentiation in vitro. Pre-miR-29a precursor treatment in rats significantly alleviated glucocorticoid-induced bone loss, while knockout miR-29a accelerated bone resorption by osteoclasts, cortical bone porosity and bone fragility. Moreover, they demonstrated that miR-29a might be involved in the Wnt and Dkk-1 signaling pathways to promote osteoblast differentiation and mineral deposition in bone.33 Consistent with this finding, Rossi et al. showed that miR-29b expression decreased progressively in the differentiation of CD14+ haematopoietic stem cells to osteoclasts stimulated by RANKL and M-CSF in vitro. Furthermore, the overexpression of miR-29b has pronounced negative effects on the TRAP expression, collagen degradation and lacunae generation, which are all characteristics of osteoclast activity.34 However, others have reported contradictory results. Franceschetti et al. demonstrated that miR-29 family members are positive regulators of osteoclast formation by targeting mRNAs encoding NFI-A and the calcitonin receptor (Calcr), both of which are important for cytoskeletal organization, commitment, and osteoclast resorption and survival. The expression of miR-29 family increases during osteoclast differentiation, while the inhibition of miR-29a, miR-29b and miR-29c suppresses the formation of TRAP-positive multinucleated osteoclasts. MiR-29a knockdown generates smaller osteoclasts from both primary bone marrow cells and the monocytic cell line RAW264.7. MiR-29 knockdown also impaired commitment and migration of osteoclast precursors. However, miR-29 knockdown does not influence cell viability, actin ring formation, or apoptosis in mature osteoclasts. NFI-A is a negative regulator of the M-CSF receptor in osteoclasts and downregulation of the NFI-A mRNA by miR-29 may play a role in promoting osteoclastogenesis.35 In short, the miR-29 family plays a critical role in osteoclast differentiation, although there are conflicting reports as to their mechanisms of action.
MiR-31
MiR-31 is encoded by a single locus and is expressed in a wide variety of tissues and cells in humans and mice. In addition, miR-31 is the only member of a broadly conserved miRNA ‘seed family’ that is present in vertebrates and Drosophila.36 Corresponding target predications using bioinformatic analyses showed that miR-31 targeted >200 different mRNAs in animal cells. Genes encoding proteins that are active in cell motility, including cell polarity, cytoskeletal dynamics and cell adhesion, were significantly enriched in these predicted targets. In in vitro assays, miR-31 directly inhibited at least 6 mRNAs that are important for the progression of tumors, including RhoA, RDX (radixin), MMP16 (matrix metallopeptidase 16), M-RIP (myosin phosphatase-Rho interacting protein), ITGA5 (integrin α5) and Fzd3 (frizzled 3).37 Mizoguchi et al. found that RANKL treatment enhanced miR-31 expression by up to 18-fold in bone marrow cells in mice, and miR-31 inhibition with specific antagomirs inhibited RANKL-induced bone resorption and osteoclast formation. However, cell fusion was not affected by miR-31. Therefore, they suggested that cytoskeleton disorganization caused impaired bone resorption and the formation of osteoclasts resulting from the inhibition of miR-31. The same research group also demonstrated that RhoA, a molecular switch that transduces extracellular signals to actin and the microtubule cytoskeleton, might be a target of miR-31 in osteoclasts.38 Therefore, miR-31 inhibits osteoclastic bone resorption by the repression of osteoclast formation.
MiR-124
Many overexpression and loss-of-function studies in vertebrates suggest that miR-124, a highly abundant brain-enriched miRNA, is a progenitor self-renewal inhibitor and enhancer of neuronal differentiation.39 MiR-124 has also been shown to regulate the myogenic and adipogenic differentiation of bone marrow-derived mesenchymal stem cells.40,41 Lee et al. demonstrated that osteoclastogenesis from mouse BMMs is regulated by miR-124 via the suppression of NFATc1 expression. The inhibition of osteoclastogenesis by miR-124 was prevented by overexpression of a constitutively active form of NFATc1. The motility and proliferation of osteoclast precursors were also found to be affected by miR-124. These changes coincided with reduced levels of RhoA and Rac1 expression, both of which are central actors in motility.42 In summary, miR-124 inhibits osteoclast formation through the suppression of differentiation and the migration of osteoclast precursors.
MiR-133a
MiR-133a was initially considered to be a muscle-specific miRNA involved in the regulation of muscle-cell differentiation and the pathogenesis of myogenic disease and heart disease.43 A recent study confirmed that miR-133a plays a key role in skeletal system regulation. Mesenchymal cells have been shown to express miR-133a in a lineage-related pattern. This miRNA was shown to directly target the Runx2 gene 3’-UTR when overexpressed in MC3T3, an osteoblast cell line, and suppress ALP (alkaline phosphatase, a marker of osteoblast formation) production and osteoblast differentiation.44 Ji et al. determined that miR-133a is downregulated in primary human osteosarcoma tissues and osteosarcoma cell lines and these changes significantly correlated with tumor progression.45 In addition, miR-133a has also been shown to be involved in osteoclast regulation. Wang et al. compared miR-133a expression in PBMCs (peripheral blood monocytes) between postmenopausal female Caucasians with normal or low bone mineral density (BMD) and found that miR-133a expression in the low BMD group was significantly higher than in the high BMD group. Bioinformatics target gene analysis demonstrated that miR-133a negatively regulates 3 potential osteoclast-related target genes, CXCL11 [chemokine (C-X-C motif) ligand 11], CXCR3 [chemokine (C-X-C motif) receptor 3] and SLC39A1 [solute carrier family (zinc transporter), member 1], although it appears that all the 3 genes do not have significant roles in osteoclast differentiation.46 Therefore, miR-133a is an important regulator of circulating monocytes in postmenopausal women and may serve as a biomarker for postmenopausal osteoporosis.
MiR-146a
MiR-146a was initially found in a human acute monocytic leukemia cell line called THP-1. In THP-1, the elevation of miR-146a expression by LPS (lipopolysaccharides) stimulation depends on NF-κB, which controls Toll-like receptor and cytokine signaling via a negative feedback loop and suppression of the protein levels of TRAF6 (TNF receptor-associated factor 6) and IL-1 receptor-associated kinase 1.47,48 Both TRAF6 and NF-κB are important molecules in osteoclastogenesis. RANKL treatment causes the time-dependent downregulation of miR-146a expression, while miR-146a is highly expressed during osteoclast differentiation in TNF-α/RANKL-treated cells relative to RANKL-treated cells.14 Nakasa et al. transfected PBMCs from healthy volunteers with double-stranded miR-146a, cultured them in the presence of M-CSF and either TNF-α or RANKL, and found that miR-146a inhibits the differentiation of PBMCs into osteoclasts in a dose-dependent manner in both osteoclastogenesis culture stimulation systems. At the same time, c-Jun, NF-ATc1, PU.1, and TRAP expression levels were also downregulated. The intravenous injection of miR-146a prevented bone erosion in mice with collagen-induced arthritis, although this treatment did not completely ameliorate the associated inflammation49 These investigations suggest that miR-146a expression induced by TNF-α/RANKL treatment may serve as a negative regulator of osteoclastogenesis.
MiR-223
MiR-223 is almost exclusively expressed in bone marrow and is specifically expressed in CD11b+ myeloid cell lineages.9 Although a preliminary study could not detect an increase in myeloid output from the bone marrow upon miR-223 expression, subsequent studies have suggested a profound role for miR-223 in myelopoiesis.50 Moreover, IKK-α, an important regulator of the NF-κB pathway, has been confirmed as a regulated target of miR-223.51 In vitro studies showed that the differentiation of human monocytes into macrophages with GM-CSF was accompanied by a reduction in the expression of miR-223, which led to a substantial increase in IKK-α protein expression levels and eventually inhibited the NF-κB signaling pathway.51 These results indicated that miR-223 influences the differentiation of monocytes/macrophages. As osteoclasts differentiate from monocytes/macrophages, osteoclast differentiation would be likely to be a target of miR-223 regulation. Further investigations by Sugatani et al. using RAW264.7, a mouse osteoclast precursor cell line, demonstrated that miR-223 plays a critical role in osteoclast differentiation. Overexpression of miR-223 completely inhibited TRAP+ multinucleated cell formation. However, these researchers were unable to identify the miR-223 target mRNA during RAW264.7 osteoclast precursor differentiation.52 In a subsequent study, Kagiya et al. used microarrays to screen for the expression of mature miRNAs in RAW264.7 cells treated with a combination of TNF-α and RANKL and found that miR-223 was downregulated during osteoclast differentiation using qRT-PCR.14 When miR-223 was overexpressed in PBMCs, the number of TRAP+ multinucleated osteoclasts decreased. NFI-A is confirmed as a validated miR-223 target gene, and thus the expression of this gene was not detected when miR-223 was overexpressed. Although no significant difference was observed in the expression of NFI-A mRNA between the ds-miR-223 and non-specific dsRNA groups, Western blotting demonstrated that NFI-A protein was still downregulated by miR-223, which suggested a mechanism by which miR-223 suppresses osteoclastogenesis.53 In summary, miR-223 is downregulated during osteoclast differentiation, and therefore it is possible to inhibit osteoclast differentiation and maturation and ultimately reduce bone erosion through the upregulation of miR-223 expression.
MiR-503
MiR-503 was first identified in human retinoblastoma tissues using the miRNA microarray technique.54 These methods demonstrated that miR-503 expression was upregulated in human parathyroid and adrenocortical carcinomas.54-56 MiR-503 influences the proliferation, migration, adhesion, and network formation of endothelial cells.57 MiR-503 is transcribed as a polycistronic message when monocytes differentiate into macrophages and targets an overlapping set of cell cycle regulators to induce G1 arrest.58 Chen et al. reported that, when compared with healthy postmenopausal women, miR-503 expression was significantly reduced in the circulating progenitors of osteoclast-CD14+ PBMCs from postmenopausal women with osteoporosis. When miR-503 was overexpressed in human PBMCs, RANKL-induced osteoclast differentiation was significantly inhibited, while the silencing of miR-503 promoted osteoclastogenesis. In the same study, RANK was shown to be a target of miR-503. In the OVX (ovariectomy) mouse, a miR-503 antagonist was able to increase the expression of the RANK protein, which led to enhanced bone resorption and a reduction in BMD. Therefore, mimicking overexpression of miR-503 by using agomir inhibited bone resorption and prevented osteoporosis in OVX mice.59 Taken together, these findings suggest that miR-503 may play an important role in the pathogenesis of postmenopausal osteoporosis.
MiR-378
MiR-378 is generally considered to be a tumor suppressor and is downregulated in gastric, colon and throat cancer tissues.60,61 In a test of bone marrow from patients with myelodysplastic syndrome, Dostalova et al. determined that miR-378 expression was downregulated in patients with a deletion of the long arm of chromosome 5, which indicated that miR-378 may play a role in the regulation of bone marrow cells.62 Furthermore, in a study by Kahai et al., miR-378 was shown to affect osteoblast differentiation by regulating the expression of nephronectin.63 MiR-378 expression increased by 59-fold in TNF-α/RANKL-treated RAW264.7 cells.14 EII et al. reported that miR-378 was associated with bone metastases in cancer. MiR-378 expression was elevated during osteoclast differentiation in the serum of mice with bone metastases. Moreover, miR-378 regulated the expression of sICAM1 in serum, while sICAM1 was secreted by bone metastatic cancer cells and activated the NF-κB signaling pathway.64 Therefore, the upregulation of miR-378 appears to play a regulatory role in the erosion of tumor bone metastases by affecting the NF-κB signaling pathway.
MiR-125a
In human cells, miR-125 consists of 3 homologs, miR-125a, miR-125b-1, and miR-125-2. A number of genes, including matrix-metalloproteases, transcription factors, members of Bcl-2 family and others are targets of miR-125, and changes in these genes could lead to enhanced metastasis, proliferation, and invasiveness. Moreover, the ectopic expression of miR-125a was shown to inhibit the proliferation and metastasis of hepatocellular carcinoma by targeting MMP11 and VEGF.65,66 In addition, miR-125a is downregulated during osteoclast differentiation stimulated by M-CSF and RANKL.67 Guo et al. discovered that CD14+ PBMCs were prevented from differentiating into osteoclasts by miR-125a overexpression, which then suppressed the levels of TRAP and NFATc1 mRNA. They also observed that transfecting antagomirs of miR-125a into PBMCs promoted osteoclast formation. TRAF6 is a target gene of miR-125a, and NFATc1, a downstream target of TRAF6, binds to the promoter of miR-125a to modulate the expression of miR-125a.68 Thus, miR-125a forms a negative feedback loop with TRAF6 and NFATc1 to modulate osteoclast differentiation and maturation.
MiR-148a
MiR-148a is expressed aberrantly in normal tissue, and the expression of miR-148a is significantly altered in some neoplastic and non-neoplastic diseases. To elaborate, miR-148a was found to be significantly upregulated in plasma of patients with multiple myeloma, and high levels of miR-148a have been related to shorter relapse-free survival durations.69 Cheng et al. found that miR-148a was dramatically upregulated during M-CSF- and RANKL-induced osteoclast differentiation from CD14+ PBMCs. They also showed that patients with lupus experienced significantly elevated levels of miR-148a in CD14+ PBMCs, a process that resulted in enhanced osteoclastogenesis and lower BMD. Overexpression of miR-148a promotes the formation of osteoclasts, whereas the inhibition of miR-148a attenuates their formation. OVX mice that were intravenously injected with antagomirs of miR-148a decreased bone resorption and increased bone mass. These regulatory functions of miR-148a were achieved by binding the 3'-UTR of MAFB (V-maf musculoaponeurotic fibrosarcoma oncogene homolog B) mRNA and inhibiting MAFB protein expression.67 Furthermore, MAFB negatively regulates RANKL-induced osteoclastogenesis by attenuating DNA binding of 3 key regulators, NFATc1, c-Fos, and Mitf.70 Therefore, the miR-148a-induced modulation of MAFB regulates NFATc1, c-Fos, and other regulatory factors that ultimately modulate osteoclast differentiation.
MiR-155
MiR-155, which is highly specifically expressed by haematopoietic cells, plays a regulatory role in a variety of cancers, including leukemia, lymphoma, liver cancer, lung cancer, and breast cancer.6,71 MiR-155 also regulates various inflammatory cytokines in rheumatoid arthritis (RA). The expression of miR-155 is elevated in the PBMCs, synovial tissue, synovial fluid, and fibroblasts of patients with RA and thus plays an important role in bone destruction.72-74 In a model of K/BxN serum-transfer arthritis that requires only innate effector mechanisms, a significant reduction of localized bone destruction was observed in miR-155-/- mice, and this observation was linked to reduced osteoclast generation.75 Compared to WT (wild type) mice, osteoclast-specific Dicer deficient mice had increased bone mass and trabecular thickness, decreased osteoclast formation, and reduced expressions of TRAP and NFATc1. MiR-155 levels in BMMs from Dicer-deficient mice were inhibited by RANKL treatment. In contrast, miR-155 levels in WT cells were not significantly changed by RANKL treatment. When bone marrow-derived macrophages from osteoclast-specific Dicer-deficient mice were stimulated with RANKL, TRAP+-cells grown in Dicer-deficient bone-marrow cell culture were typically smaller in size than WT cells. Determining the number of multinucleated TRAP+-cells showed that Dicer-deficient osteoclasts inhibited the RANKL-induction of TRAP+-cell development. This study also showed that Dicer-deficient osteoclasts inhibited Trap mRNA expression and slightly suppressed the level of Nfatc1 mRNA. These researchers speculated that miR-155 was suppressed by Dicer deficiency upon RANKL treatment, which may then cause the upregulation of SHIP, an osteoclast inhibitor.15 Mann et al. found that miR-155 expression increased rapidly during the process of RAW264.7 differentiating into macrophages, and miR-155 inhibited the osteoclast differentiation of RAW264.7 cells by inhibiting MITF (microphthalmia-associated transcription factor), which is a transcription factor essential for osteoclast differentiation.76 Zhang et al. reported that IFN-β induced miR-155 and mediated IFN-β-induced suppression of osteoclast differentiation by interacting with MITF and SOCS1, positive regulators of osteoclastogenesis.77Taken together, these findings demonstrate that miR-155 regulates osteoclastogenesis via targeting several essential transcriptional factors.
MiR-422a
MiR-422a plays an important role in human diseases, such as colon cancer and multiple sclerosis and has been shown to inhibit pathways that stimulate tumor cell proliferation in osteosarcoma.78 In the field of skeletal research, one study has shown that miR-422a was down-regulated by peptide-15 (analog of the sequence766GTPGPQGIAGQRGVV780 in the collagen α 1 (I) chain), which is known to increase bone formation.79 In osteoclasts, Cao et al. discovered that miR-422a is significantly upregulated in a low BMD group of postmenopausal women relative to a high BMD group. Moreover, several target genes were predicted to be related to osteoclastogenesis, including CBL (Casitas B-lineage lymphoma proto-oncogene), CD226 (cluster of differentiation 226), IGF1 (insulin-like growth factor 1), PAG1 (phosphoprotein associated with glycosphingolipid microdomains), and TOB2 (transducer of ERBB2). This group also demonstrated an apparent negative correlation between each of these 5 genes and miR-422a, although none of the correlations reached statistical significance.80 These studies suggest that miR-422a is possibly involved in the regulation of postmenopausal osteoporosis and might be a potential biomarker of osteoporosis.
Others
During the differentiation and maturation of osteoclasts, there are other miRNA expression changes in addition to the ones discussed above. Kaiya et al. found 52 mature miRNAs with expression changes when RAW264.7 cells were treated with RANKL during osteoclast formation. In addition to the miRNAs already discussed, expression levels of miR-483, miR-680, miR-689, miR-714, and miR-721 were shown to be elevated in a time-dependent fashion, while miR-23b and miR-342-3p expression levels decreased. Notably, miR-26a, miR-199a-3p, and miR-1224 expression levels declined at 24 h after treatment and then increased 82 h after treatment with RANKL. EII et al. cultured the RAW264.7 cells with tumor-conditioned media, and detected increased expression levels of 42 miRNAs that were upregulated by more than 2-fold; they also found that 45 miRNAs were downregulated.64 However, the molecular mechanism and function of these miRNAs in osteoclasts remain unclear.
Conclusions
Bone homeostasis is maintained by a balance between osteoblastic bone formation and osteoclastic bone resorption. Metabolic bone disorders, such as osteopenia, osteoporosis, and RA-related bone destruction, occur when osteoclastic bone resorption exceeds osteoblastic bone formation. The treatment of osteoporosis is typically focused on inhibiting the excessive activation of osteoclasts. Therefore, understanding the mechanisms of osteoclast differentiation and maturation will assist in developing treatments for osteoporosis. The discovery of the OPG/RANK/RANKL pathway in osteoclast differentiation and maturation is a major landmark in osteoporosis research. However, more studies are necessary regarding the molecular mechanism underlying osteoclast differentiation. MiRNAs have been shown to be important regulators of many biological functions. The studies reviewed here show that miRNAs are deeply involved in the regulation of osteoclast differentiation and bone resorption (Fig. 1). The overexpression or inhibition of specific miRNAs effectively inhibits osteoclast differentiation and bone resorption. MiRNA-based therapeutics have shown some promise for the treatment of osteoporosis. However, further studies are required if miR-based therapeutics are to be used in the clinic, and many more questions are raised. For example, what other important miRNAs can regulate osteoclasts? What specific pathways are regulated by miRNAs in osteoclasts? What side effects would occur with the regulation of osteoclasts by miRNAs in clinic? How can miRNAs be effectively delivered in vivo? All of these questions will need to be answered in the future.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
P-F Tang, Q. Xiong and L-H Zhang are supported by the National Natural Science Foundation of China (31370947). W. Ge is supported by the National Natural Science Foundation of China (81373150).
References
- 1. Teitelbaum SL. Bone resorption by osteoclasts. Science 2000; 289:1504-8; PMID:10968780 [DOI] [PubMed] [Google Scholar]
- 2. Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature 2003; 423:337-42; PMID:12748652; http://dx.doi.org/ 10.1038/nature01658 [DOI] [PubMed] [Google Scholar]
- 3. Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation. Bone 2007; 40:251-64; PMID:17098490; http://dx.doi.org/ 10.1016/j.bone.2006.09.023 [DOI] [PubMed] [Google Scholar]
- 4. Leibbrandt A, Penninger JM. RANK/RANKL: regulators of immune responses and bone physiology. Ann N Y AcadSci 2008; 1143:123-50; PMID:19076348; http://dx.doi.org/ 10.1196/annals.1443.016 [DOI] [PubMed] [Google Scholar]
- 5. Negishi-Koga T, Takayanagi H. Ca2+-NFATc1 signaling is an essential axis of osteoclast differentiation. Immunol Revi 2009; 231:241-56; PMID:19754901; http://dx.doi.org/ 10.1111/j.1600-065X.2009.00821.x [DOI] [PubMed] [Google Scholar]
- 6. Landgraf P, Rusu M, Sheridan R, Sewer A, Iovino N, Aravin A, Pfeffer S, Rice A, Kamphorst AO, Landthaler M, et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell 2007; 129:1401-14; PMID:17604727; http://dx.doi.org/ 10.1016/j.cell.2007.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993; 75:843-54; PMID:8252621; http://dx.doi.org/ 10.1016/0092-8674(93)90529-Y [DOI] [PubMed] [Google Scholar]
- 8. Kozomara A, Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucl Acid Res 2014; 42:D68-73; PMID:24275495; http://dx.doi.org/ 10.1093/nar/gkt1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science 2004; 303:83-6; PMID:14657504; http://dx.doi.org/ 10.1126/science.1091903 [DOI] [PubMed] [Google Scholar]
- 10. Fukao T, Fukuda Y, Kiga K, Sharif J, Hino K, Enomoto Y, Kawamura A, Nakamura K, Takeuchi T, Tanabe M. An evolutionarily conserved mechanism for microRNA-223 expression revealed by microRNA gene profiling. Cell 2007; 129:617-31; PMID:17482553; http://dx.doi.org/ 10.1016/j.cell.2007.02.048 [DOI] [PubMed] [Google Scholar]
- 11. Mizuno Y, Yagi K, Tokuzawa Y, Kanesaki-Yatsuka Y, Suda T, Katagiri T, Fukuda T, Maruyama M, Okuda A, Amemiya T, et al. miR-125b inhibits osteoblastic differentiation by down-regulation of cell proliferation. Biochem Biophys Res Commun 2008; 368:267-72; PMID:18230348; http://dx.doi.org/ 10.1016/j.bbrc.2008.01.073 [DOI] [PubMed] [Google Scholar]
- 12. Luzi E, Marini F, Sala SC, Tognarini I, Galli G, Brandi ML. Osteogenic differentiation of human adipose tissue-derived stem cells is modulated by the miR-26a targeting of the SMAD1 transcription factor. J Bone Miner Res 2008; 23:287-95; PMID:18197755; http://dx.doi.org/ 10.1359/jbmr.071011 [DOI] [PubMed] [Google Scholar]
- 13. Li Z, Hassan MQ, Volinia S, van Wijnen AJ, Stein JL, Croce CM, Lian JB, Stein GS. A microRNA signature for a BMP2-induced osteoblast lineage commitment program. Proc Nat Acad Sci U S A 2008; 105:13906-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kagiya T, Nakamura S. Expression profiling of microRNAs in RAW264.7 cells treated with a combination of tumor necrosis factor alpha and RANKL during osteoclast differentiation. J Periodontal Res 2013; 48:373-85; PMID:23078176; http://dx.doi.org/ 10.1111/jre.12017 [DOI] [PubMed] [Google Scholar]
- 15. Mizoguchi F, Izu Y, Hayata T, Hemmi H, Nakashima K, Nakamura T, Kato S, Miyasaka N, Ezura Y, Noda M. Osteoclast-specific Dicer gene deficiency suppresses osteoclastic bone resorption. J Cell Biochem 2010; 109:866-75; PMID:20039311 [DOI] [PubMed] [Google Scholar]
- 16. van der Eerden BC. MicroRNAs in the skeleton: cell-restricted or potent intercellular communicators? Arch Biochem Biophys 2014; 561C:46-55; PMID:24832391; http://dx.doi.org/ 10.1016/j.abb.2014.04.016 [DOI] [PubMed] [Google Scholar]
- 17. Gamez B, Rodriguez-Carballo E, Ventura F. MicroRNAs and post-transcriptional regulation of skeletal development. J Mol Endocrinol 2014; 52:R179-97; PMID:24523514; http://dx.doi.org/ 10.1530/JME-13-0294 [DOI] [PubMed] [Google Scholar]
- 18. Lian JB, Stein GS, van Wijnen AJ, Stein JL, Hassan MQ, Gaur T, Zhang Y. MicroRNA control of bone formation and homeostasis. Nat Rev Endocrinol 2012; 8:212-27; PMID:22290358; http://dx.doi.org/ 10.1038/nrendo.2011.234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xia Z, Chen C, Chen P, Xie H, Luo X. MicroRNAs and their roles in osteoclast differentiation. Frontiers of medicine 2011; 5:414-9; PMID:22198753; http://dx.doi.org/ 10.1007/s11684-011-0168-0 [DOI] [PubMed] [Google Scholar]
- 20. van Wijnen AJ, van de Peppel J, van Leeuwen JP, Lian JB, Stein GS, Westendorf JJ, Oursler MJ, Im HJ, Taipaleenmaki H, Hesse E, et al. MicroRNA functions in osteogenesis and dysfunctions in osteoporosis. Curr Osteoporos Rep 2013; 11:72-82; PMID:23605904; http://dx.doi.org/ 10.1007/s11914-013-0143-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Krichevsky AM, Gabriely G. miR-21: a small multi-faceted RNA. J Cell Mol Med 2009; 13:39-53; PMID:19175699; http://dx.doi.org/ 10.1111/j.1582-4934.2008.00556.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fujita S, Ito T, Mizutani T, Minoguchi S, Yamamichi N, Sakurai K, Iba H. miR-21 Gene expression triggered by AP-1 is sustained through a double-negative feedback mechanism. J Mol Biol 2008; 378:492-504; PMID:18384814; http://dx.doi.org/ 10.1016/j.jmb.2008.03.015 [DOI] [PubMed] [Google Scholar]
- 23. Sugatani T, Vacher J, Hruska KA. A microRNA expression signature of osteoclastogenesis. Blood 2011; 117:3648-57; PMID:21273303; http://dx.doi.org/ 10.1182/blood-2010-10-311415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Garcia Palacios V, Robinson LJ, Borysenko CW, Lehmann T, Kalla SE, Blair HC. Negative regulation of RANKL-induced osteoclastic differentiation in RAW264.7 Cells by estrogen and phytoestrogens. J Bio Chem 2005; 280:13720-7; PMID:15644335; http://dx.doi.org/ 10.1074/jbc.M410995200 [DOI] [PubMed] [Google Scholar]
- 25. Sugatani T, Hruska KA. Down-regulation of miR-21 biogenesis by estrogen action contributes to osteoclastic apoptosis. J Cell Biochem 2013; 114:1217-22; PMID:23238785; http://dx.doi.org/ 10.1002/jcb.24471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Holt G, Murnaghan C, Reilly J, Meek R. The biology of aseptic osteolysis. Clin Orthopaed relat Res 2007; 460:240-52; PMID:17620815 [DOI] [PubMed] [Google Scholar]
- 27. Zhou Y, Liu Y, Cheng L. miR-21 expression is related to particle-induced osteolysis pathogenesis. J Orthop Res 2012; 30:1837-42; PMID:22508494; http://dx.doi.org/ 10.1002/jor.22128 [DOI] [PubMed] [Google Scholar]
- 28. Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science 2001; 294:853-8; PMID:11679670; http://dx.doi.org/ 10.1126/science.1064921 [DOI] [PubMed] [Google Scholar]
- 29. Wang Y, Zhang X, Li H, Yu J, Ren X. The role of miRNA-29 family in cancer. Eur Journal Cell Bio 2013; 92:123-8; PMID:23357522; http://dx.doi.org/ 10.1016/j.ejcb.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 30. Liston A, Papadopoulou AS, Danso-Abeam D, Dooley J. MicroRNA-29 in the adaptive immune system: setting the threshold. Cell Mol Life Sci 2012; 69:3533-41; PMID:22971773; http://dx.doi.org/ 10.1007/s00018-012-1124-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mott JL, Kurita S, Cazanave SC, Bronk SF, Werneburg NW, Fernandez-Zapico ME. Transcriptional suppression of mir-29b-1/mir-29a promoter by c-Myc, hedgehog, and NF-kappaB. J Cell Biochem 2010; 110:1155-64; PMID:20564213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gong M, Ma J, Guillemette R, Zhou M, Yang Y, Yang Y, Hock JM, Yu X. miR-335 inhibits small cell lung cancer bone metastases via IGF-IR and RANKL pathways. Mol Cancer Research 2014; 12:101-10; PMID:23966614; http://dx.doi.org/ 10.1158/1541-7786.MCR-13-0136 [DOI] [PubMed] [Google Scholar]
- 33. Wang FS, Chuang PC, Lin CL, Chen MW, Ke HJ, Chang YH, Chen YS, Wu SL, Ko JY. MicroRNA-29a protects against glucocorticoid-induced bone loss and fragility in rats by orchestrating bone acquisition and resorption. Arthritis Rheum 2013; 65:1530-40; PMID:23529662; http://dx.doi.org/ 10.1002/art.37948 [DOI] [PubMed] [Google Scholar]
- 34. Rossi M, Pitari MR, Amodio N, Di Martino MT, Conforti F, Leone E, Botta C, Paolino FM, Del Giudice T, Iuliano E, et al. miR-29b negatively regulates human osteoclastic cell differentiation and function: implications for the treatment of multiple myeloma-related bone disease. J Cell Phys 2013; 228:1506-15; PMID:23254643; http://dx.doi.org/ 10.1002/jcp.24306 [DOI] [PubMed] [Google Scholar]
- 35. Franceschetti T, Kessler CB, Lee SK, Delany AM. miR-29 promotes murine osteoclastogenesis by regulating osteoclast commitment and migration. J Biol Chem 2013; 288:33347-60; PMID:24085298; http://dx.doi.org/ 10.1074/jbc.M113.484568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Valastyan S, Weinberg RA. miR-31: a crucial overseer of tumor metastasis and other emerging roles. Cell Cycle 2010; 9:2124-9; PMID:20505365; http://dx.doi.org/ 10.4161/cc.9.11.11843 [DOI] [PubMed] [Google Scholar]
- 37. Valastyan S, Benaich N, Chang A, Reinhardt F, Weinberg RA. Concomitant suppression of three target genes can explain the impact of a microRNA on metastasis. Genes Dev 2009; 23:2592-7; PMID:19875476; http://dx.doi.org/ 10.1101/gad.1832709 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38. Mizoguchi F, Murakami Y, Saito T, Miyasaka N, Kohsaka H. miR-31 controls osteoclast formation and bone resorption by targeting RhoA. Arthritis Res Ther 2013; 15:R102; PMID:24004633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sonntag KC, Woo TU, Krichevsky AM. Converging miRNA functions in diverse brain disorders: a case for miR-124 and miR-126. Exp Neur 2012; 235:427-35; PMID:22178324; http://dx.doi.org/ 10.1016/j.expneurol.2011.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Laine SK, Alm JJ, Virtanen SP, Aro HT, Laitala-Leinonen TK. MicroRNAs miR-96, miR-124, and miR-199a regulate gene expression in human bone marrow-derived mesenchymal stem cells. J Cellular Biochem 2012; 113:2687-95; PMID:22441842; http://dx.doi.org/ 10.1002/jcb.24144 [DOI] [PubMed] [Google Scholar]
- 41. Cai B, Li J, Wang J, Luo X, Ai J, Liu Y, Wang N, Liang H, Zhang M, Chen N, et al. microRNA-124 regulates cardiomyocyte differentiation of bone marrow-derived mesenchymal stem cells via targeting STAT3 signaling. Stem cells 2012; 30:1746-55; PMID:22696253; http://dx.doi.org/ 10.1002/stem.1154 [DOI] [PubMed] [Google Scholar]
- 42. Lee Y, Kim HJ, Park CK, Kim YG, Lee HJ, Kim JY, Kim HH. MicroRNA-124 regulates osteoclast differentiation. Bone 2013; 56:383-9; PMID:23867221; http://dx.doi.org/ 10.1016/j.bone.2013.07.007 [DOI] [PubMed] [Google Scholar]
- 43. Rao PK, Kumar RM, Farkhondeh M, Baskerville S, Lodish HF. Myogenic factors that regulate expression of muscle-specific microRNAs. Proc Nat Acad Sci U S A 2006; 103:8721-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang Y, Xie RL, Croce CM, Stein JL, Lian JB, van Wijnen AJ, Stein GS. A program of microRNAs controls osteogenic lineage progression by targeting transcription factor Runx2. Proc Nat Acad Sci U S A 2011; 108:9863-8; PMID:21628588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ji F, Zhang H, Wang Y, Li M, Xu W, Kang Y, Wang Z, Wang Z, Cheng P, Tong D, et al. MicroRNA-133a, downregulated in osteosarcoma, suppresses proliferation and promotes apoptosis by targeting Bcl-xL and Mcl-1. Bone 2013; 56:220-6; PMID:23756231; http://dx.doi.org/ 10.1016/j.bone.2013.05.020 [DOI] [PubMed] [Google Scholar]
- 46. Wang Y, Li L, Moore BT, Peng XH, Fang X, Lappe JM, Recker RR, Xiao P. MiR-133a in human circulating monocytes: a potential biomarker associated with postmenopausal osteoporosis. PloS One 2012; 7:e34641; PMID:22506038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Nat Acad Sci U S A 2006; 103:12481-6; PMID:16885212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bhaumik D, Scott GK, Schokrpur S, Patil CK, Orjalo AV, Rodier F, Lithgow GJ, Campisi J. MicroRNAs miR-146a/b negatively modulate the senescence-associated inflammatory mediators IL-6 and IL-8. Aging 2009; 1:402-11; PMID:20148189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nakasa T, Shibuya H, Nagata Y, Niimoto T, Ochi M. The inhibitory effect of microRNA-146a expression on bone destruction in collagen-induced arthritis. Arthritis Rheum 2011; 63:1582-90; PMID:21425254; http://dx.doi.org/ 10.1002/art.30321 [DOI] [PubMed] [Google Scholar]
- 50. O'Connell RM, Zhao JL, Rao DS. MicroRNA function in myeloid biology. Blood 2011; 118:2960-9; PMID:21725054; http://dx.doi.org/ 10.1182/blood-2011-03-291971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li T, Morgan MJ, Choksi S, Zhang Y, Kim YS, Liu ZG. MicroRNAs modulate the noncanonical transcription factor NF-kappaB pathway by regulating expression of the kinase IKKalpha during macrophage differentiation. Nature immunology 2010; 11:799-805; PMID:20711193; http://dx.doi.org/ 10.1038/ni.1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sugatani T, Hruska KA. MicroRNA-223 is a key factor in osteoclast differentiation. J Cell Biochem 2007; 101:996-9; PMID:17471500; http://dx.doi.org/ 10.1002/jcb.21335 [DOI] [PubMed] [Google Scholar]
- 53. Shibuya H, Nakasa T, Adachi N, Nagata Y, Ishikawa M, Deie M, Suzuki O, Ochi M. Overexpression of microRNA-223 in rheumatoid arthritis synovium controls osteoclast differentiation. Mod Rheumatol 2013; 23:674-85; PMID:22903258; http://dx.doi.org/ 10.1007/s10165-012-0710-1 [DOI] [PubMed] [Google Scholar]
- 54. Zhao JJ, Yang J, Lin J, Yao N, Zhu Y, Zheng J, Xu J, Cheng JQ, Lin JY, Ma X. Identification of miRNAs associated with tumorigenesis of retinoblastoma by miRNA microarray analysis. Child's Nervous System 2009; 25:13-20; PMID:1881893319926710 [DOI] [PubMed] [Google Scholar]
- 55. Corbetta S, Vaira V, Guarnieri V, Scillitani A, Eller-Vainicher C, Ferrero S, Vicentini L, Chiodini I, Bisceglia M, Beck-Peccoz P, et al. Differential expression of microRNAs in human parathyroid carcinomas compared with normal parathyroid tissue. Endocr Relat Cancer 2010; 17:135-46; PMID:19926710; http://dx.doi.org/ 10.1677/ERC-09-0134 [DOI] [PubMed] [Google Scholar]
- 56. Tombol Z, Szabo PM, Molnar V, Wiener Z, Tolgyesi G, Horanyi J, Riesz P, Reismann P, Patocs A, Liko I, et al. Integrative molecular bioinformatics study of human adrenocortical tumors: microRNA, tissue-specific target prediction, and pathway analysis. Endocrine-related cancer 2009; 16:895-906; PMID:19546168; http://dx.doi.org/ 10.1677/ERC-09-0096 [DOI] [PubMed] [Google Scholar]
- 57. Caporali A, Meloni M, Vollenkle C, Bonci D, Sala-Newby GB, Addis R, Spinetti G, Losa S, Masson R, Baker AH, et al. Deregulation of microRNA-503 contributes to diabetes mellitus-induced impairment of endothelial function and reparative angiogenesis after limb ischemia. Circulation 2011; 123:282-91; PMID:21220732; http://dx.doi.org/ 10.1161/CIRCULATIONAHA.110.952325 [DOI] [PubMed] [Google Scholar]
- 58. Forrest AR, Kanamori-Katayama M, Tomaru Y, Lassmann T, Ninomiya N, Takahashi Y, de Hoon MJ, Kubosaki A, Kaiho A, Suzuki M, et al. Induction of microRNAs, mir-155, mir-222, mir-424 and mir-503, promotes monocytic differentiation through combinatorial regulation. Leukemia 2010; 24:460-6; PMID:19956200; http://dx.doi.org/ 10.1038/leu.2009.246 [DOI] [PubMed] [Google Scholar]
- 59. Chen C, Cheng P, Xie H, Zhou HD, Wu XP, Liao EY, Luo XH. MiR-503 regulates osteoclastogenesis via targeting RANK. J Bone Miner Res 2014; 29:338-47; PMID:23821519; http://dx.doi.org/ 10.1002/jbmr.2032 [DOI] [PubMed] [Google Scholar]
- 60. Guo J, Miao Y, Xiao B, Huan R, Jiang Z, Meng D, Wang Y. Differential expression of microRNA species in human gastric cancer versus non-tumorous tissues. JGastroenterol Hepatol 2009; 24:652-7; PMID:19175831; http://dx.doi.org/ 10.1111/j.1440-1746.2008.05666.x [DOI] [PubMed] [Google Scholar]
- 61. Wang YX, Zhang XY, Zhang BF, Yang CQ, Chen XM, Gao HJ. Initial study of microRNA expression profiles of colonic cancer without lymph node metastasis. J Dig Dis 2010; 11:50-4; PMID:20132431; http://dx.doi.org/ 10.1111/j.1751-2980.2009.00413.x [DOI] [PubMed] [Google Scholar]
- 62. Dostalova Merkerova M, Krejcik Z, Votavova H, Belickova M, Vasikova A, Cermak J. Distinctive microRNA expression profiles in CD34+ bone marrow cells from patients with myelodysplastic syndrome. Eur J Hum Genet 2011; 19:313-9; PMID:21150891; http://dx.doi.org/ 10.1038/ejhg.2010.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kahai S, Lee SC, Lee DY, Yang J, Li M, Wang CH, Jiang Z, Zhang Y, Peng C, Yang BB. MicroRNA miR-378 regulates nephronectin expression modulating osteoblast differentiation by targeting GalNT-7. PloS One 2009; 4:e7535; PMID:19844573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ell B, Mercatali L, Ibrahim T, Campbell N, Schwarzenbach H, Pantel K, Amadori D, Kang Y. Tumor-induced osteoclast miRNA changes as regulators and biomarkers of osteolytic bone metastasis. Cancer Cell 2013; 24:542-56; PMID:24135284; http://dx.doi.org/ 10.1016/j.ccr.2013.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bi Q, Tang S, Xia L, Du R, Fan R, Gao L, Jin J, Liang S, Chen Z, Xu G, et al. Ectopic expression of MiR-125a inhibits the proliferation and metastasis of hepatocellular carcinoma by targeting MMP11 and VEGF. PloS One 2012; 7:e40169; PMID:22768249; http://dx.doi.org/ 10.1371/journal.pone.0040169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sun YM, Lin KY, Chen YQ. Diverse functions of miR-125 family in different cell contexts. J Hematol Oncol 2013; 6:6; PMID:23321005; http://dx.doi.org/ 10.1186/1756-8722-6-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cheng P, Chen C, He HB, Hu R, Zhou HD, Xie H, Zhu W, Dai RC, Wu XP, Liao EY, et al. miR-148a regulates osteoclastogenesis by targeting V-maf musculoaponeurotic fibrosarcoma oncogene homolog B. J BoneMiner Res 2013; 28:1180-90; PMID:23225151; http://dx.doi.org/ 10.1002/jbmr.1845 [DOI] [PubMed] [Google Scholar]
- 68. Guo LJ, Liao L, Yang L, Li Y, Jiang TJ. MiR-125a TNF receptor-associated factor 6 to inhibit osteoclastogenesis. Exp Cell Res 2014; 321:142-52; PMID:24360988; http://dx.doi.org/ 10.1016/j.yexcr.2013.12.001 [DOI] [PubMed] [Google Scholar]
- 69. Huang JJ, Yu J, Li JY, Liu YT, Zhong RQ. Circulating microRNA expression is associated with genetic subtype and survival of multiple myeloma. Med Oncol 2012; 29:2402-8; PMID:22447484; http://dx.doi.org/ 10.1007/s12032-012-0210-3 [DOI] [PubMed] [Google Scholar]
- 70. Kim K, Kim JH, Lee J, Jin HM, Kook H, Kim KK, Lee SY, Kim N. MafB negatively regulates RANKL-mediated osteoclast differentiation. Blood 2007; 109:3253-9; PMID:17158225; http://dx.doi.org/ 10.1182/blood-2006-09-048249 [DOI] [PubMed] [Google Scholar]
- 71. Chen Z, Ma T, Huang C, Hu T, Li J. The pivotal role of microRNA-155 in the control of cancer. J Cell Physiol 2014; 229:545-50; PMID:24122356; http://dx.doi.org/ 10.1002/jcp.24492 [DOI] [PubMed] [Google Scholar]
- 72. Pauley KM, Satoh M, Chan AL, Bubb MR, Reeves WH, Chan EK. Upregulated miR-146a expression in peripheral blood mononuclear cells from rheumatoid arthritis patients. Arthritis Res Ther 2008; 10:R101; PMID:18759964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Stanczyk J, Pedrioli DM, Brentano F, Sanchez-Pernaute O, Kolling C, Gay RE, Detmar M, Gay S, Kyburz D. Altered expression of MicroRNA in synovial fibroblasts and synovial tissue in rheumatoid arthritis. Arthritis Rheum 2008; 58:1001-9; PMID:18383392; http://dx.doi.org/ 10.1002/art.23386 [DOI] [PubMed] [Google Scholar]
- 74. Pandis I, Ospelt C, Karagianni N, Denis MC, Reczko M, Camps C, Hatzigeorgiou AG, Ragoussis J, Gay S, Kollias G. Identification of microRNA-221/222 and microRNA-323-3p association with rheumatoid arthritis via predictions using the human tumour necrosis factor transgenic mouse model. Ann Rheum Dis 2012; 71:1716-23; PMID:22562984 [DOI] [PubMed] [Google Scholar]
- 75. Bluml S, Bonelli M, Niederreiter B, Puchner A, Mayr G, Hayer S, Koenders MI, van den Berg WB, Smolen J, Redlich K. Essential role of microRNA-155 in the pathogenesis of autoimmune arthritis in mice. Arthritis Rheum 2011; 63:1281-8; PMID:21321928; http://dx.doi.org/ 10.1002/art.30281 [DOI] [PubMed] [Google Scholar]
- 76. Mann M, Barad O, Agami R, Geiger B, Hornstein E. miRNA-based mechanism for the commitment of multipotent progenitors to a single cellular fate. Proc Nat Acad SciU S A 2010; 107:15804-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhang J, Zhao H, Chen J, Xia B, Jin Y, Wei W, Shen J, Huang Y. Interferon-beta-induced miR-155 inhibits osteoclast differentiation by targeting SOCS1 and MITF. FEBS Letters 2012; 586:3255-62; PMID:22771905; http://dx.doi.org/ 10.1016/j.febslet.2012.06.047 [DOI] [PubMed] [Google Scholar]
- 78. Gougelet A, Pissaloux D, Besse A, Perez J, Duc A, Dutour A, Blay JY, Alberti L. Micro-RNA profiles in osteosarcoma as a predictive tool for ifosfamide response. Int J Cancer 2011; 129:680-90; PMID:20949564; http://dx.doi.org/ 10.1002/ijc.25715 [DOI] [PubMed] [Google Scholar]
- 79. Palmieri A, Pezzetti F, Brunelli G, Martinelli M, Lo Muzio L, Scarano A, Degidi M, Piattelli A, Carinci F. Peptide-15 changes miRNA expression in osteoblast-like cells. Implant Dent 2008; 17:100-8; PMID:18332763; http://dx.doi.org/ 10.1097/ID.0b013e318166d182 [DOI] [PubMed] [Google Scholar]
- 80. Cao Z, Moore BT, Wang Y, Peng XH, Lappe JM, Recker RR, Xiao P. MiR-422a as a potential cellular microRNA biomarker for postmenopausal osteoporosis. PloS One 2014; 9:e97098; PMID:24820117; http://dx.doi.org/ 10.1371/journal.pone.0097098 [DOI] [PMC free article] [PubMed] [Google Scholar]