Abstract
BACKGROUND
Cardiac dysfunction influences candidate selection for kidney transplantation. There is a paucity of data regarding predictors of myocardial recovery following kidney transplantation as well as long-term outcomes.
OBJECTIVES
The purpose of this study was to identify the extent of reverse remodeling in our kidney transplant population as well as predictors of such changes and assess outcomes in these patients.
METHODS
We reviewed 232 patients who underwent kidney transplantation at the Cleveland Clinic from 2003 to 2013 and had baseline and post-transplant echocardiograms, excluding those with simultaneous heart transplantation.
RESULTS
Post-transplant median left ventricular ejection fraction (LVEF) improved in those with left ventricular (LV) dysfunction (increased from 41% to 50%; p < 0.0001; n = 66). There was significant improvement in other parameters including diastolic function, LV end-diastolic dimension, LV mass, and right ventricular systolic pressure. After adjusting for multiple clinical variables, increased hemoglobin following transplantation was associated with improved LVEF (odds ratio: 1.50; 95% confidence interval [CI]: 1.07 to 2.14; p = 0.016) and reduced mortality (hazard ratio [HR]: 0.65; 95% CI: 0.49 to 0.87; p = 0.004). Improved LVEF ≥10% predicted survival independent of pre-transplant LVEF (HR: 0.46; 95% CI: 0.21 to 0.93; p = 0.031).
CONCLUSIONS
Kidney transplantation is associated with improved cardiac structure and function. A rise in post-transplant hemoglobin was a significant factor associated with such changes, in addition to conferring a survival advantage.
Keywords: anemia, cardiorenal syndrome, heart failure, reverse remodeling
In patients with end-stage renal disease (ESRD), progressive cardiorenal compromise often results in the development of adverse cardiovascular outcomes – one of the leading causes of mortality in this population (1). While ESRD was previously thought to be the result of accelerated atherosclerosis and subsequent coronary artery disease, it is now believed that other pathophysiologic processes play a contributing role. Although patients with ESRD have a high prevalence of “conventional” cardiovascular risk factors including hypertension, diabetes, and hyperlipidemia, these do not account for all the cardiovascular risks (2). Other contributing factors include hemodynamic overload from volume and pressure, anemia, arteriovenous shunts, and arterial remodeling, as well as biochemical mediators and uremic toxins, such as alterations in calcium, phosphate, parathyroid hormone, urea, homocysteine, and endothelin (3).
Despite significant progress in the care of patients with heart failure (HF), individuals with ESRD and concomitant cardiac dysfunction are generally considered less suitable candidates for kidney transplantation due to increased risk of operative morbidity and mortality. Nevertheless, an emerging concept has challenged this notion that cardiac dysfunction is a forbidding comorbidity. In fact, reports have shown that kidney transplantation may actually improve cardiac function as measured by serial radionuclide ventriculography (4), a factor that should be taken into consideration when evaluating these patients. Herein, our objective is to describe the longitudinal experience of cardiac remodeling in our kidney transplant population, their respective mortality risk following transplantation, and their propensity for reverse remodeling, as well as elements that influence these changes.
METHODS
STUDY POPULATION
We retrospectively reviewed our single-center experience of adult patients with ESRD undergoing living-donor and cadaveric kidney transplantation at the Cleveland Clinic between January 2003 and December 2013 who had pre- and post-transplant transthoracic echocardiograms. All subjects were in chronic stable condition and carefully reviewed by an interdisciplinary committee that deemed them eligible for transplantation after weighing the risks and benefits. All transplant recipients received a protocol-driven standardized post-transplant medical regimen including tacrolimus, mycophenolate mofetil, and prednisone. We excluded those who did not have post-transplant follow-up documented in our electronic medical record or those with simultaneous heart-kidney transplantation. The Cleveland Clinic Institutional Review Board approved the project.
Demographic, clinical, echocardiographic, and laboratory data were obtained directly from the electronic medical record. Estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease equation (5). Reference values for echocardiographic parameters were defined based on the recent American Society of Echocardiography (ASE) guidelines (6) as follows: mild systolic dysfunction, left ventricular ejection fraction (LVEF) ≤51% for men and ≤53% for women, but >40%; moderate dysfunction, LVEF ≤40% for both sexes; abnormal LV mass indexed to body surface area (BSA), >115 g/m2 in men and >95 g/m2 in women; and severely abnormal LV mass/BSA, >148 g/m2 in men and >121 g/m2 in women. Hypertension was defined by a cutoff of ≥130/80 mm Hg (7), and alternatively as ≥140/90 mm Hg based on the more recent Eighth Joint National Committee (JNC-8) guidelines (8). Anemia was defined as hemoglobin <13 g/dl in men and <12 g/dl in women based on KDIGO (Kidney Disease – Improving Global Outcomes) guidelines for patients with chronic kidney disease (CKD) (9). These parameters were obtained from the most recent pre-transplant and 12-month post-transplant office visits. One exception was echocardiographic data, the timing of which could not be controlled for in this retrospective study. Echocardiograms were obtained during the pre-transplant evaluation, with the most recent one taken into account, and the time closest to 12 to 24 months post-transplantation (see Results section).
ASSESSMENT OF CARDIAC STRUCTURE AND FUNCTION
All echocardiograms were performed at our institution. For each echocardiography examination, interventricular septal (IVS) diameter, posterior wall (PW) diameter, and left ventricular end-diastolic dimension (LVDd) were measured according to ASE recommendations (6). LVEF was calculated by the biplane Simpson method. LV mass, calculated using the ASE equation, was defined as 0.8 × (1.04 × [IVS diameter + PW diameter + LVDd]3 − [LVDd3]) + 0.6 and was indexed to BSA. LV diastolic function was graded as either normal or stages I to IV based on mitral inflow profiles and tissue Doppler imaging. Right ventricular systolic pressure (RVSP) was estimated from the tricuspid regurgitation velocity using the modified Bernoulli equation.
STATISTICAL ANALYSIS
Continuous variables were reported as mean ± SD if normally distributed or as median and interquartile range (IQR) if non-normally distributed. Normality was assessed by the Shapiro-Wilk W test. Differences in clinical variables were assessed using the Student t test or analysis of variance. Linear regression analysis was used to analyze the association between clinical variables and reverse remodeling. Odds ratio (OR) for predicting clinical outcomes was calculated using logistic regression analysis and evaluated according to the likelihood ratio test. Kaplan-Meier survival analysis was used to compare unadjusted survival and the log-rank test assessed the differences between groups. The Cox proportional hazards model was used to test for independent predictors of mortality. All p values reported are from 2-sided tests and a p value < 0.05 was considered statistically significant. Statistical analyses were performed using JMP 11.2.0 (SAS Institute, Cary, North Carolina).
RESULTS
Of the 1,697 adult patients who underwent kidney transplantation, we identified a total of 232 subjects who fulfilled the inclusion and exclusion criteria (Figure 1). Per the baseline demographic data (Table 1), the predominant etiology of ESRD was diabetes (31%) and hypertension (23%). The majority of patients were dialysis-dependent (mostly intermittent hemodialysis) and the median duration of dialysis was 778 days (IQR: 239 to 1,741). Preemptive transplantation occurred in 17% of subjects. Simultaneous kidney-pancreas or kidney-liver transplantation accounted for 11% of the cohort. The pre-transplant echocardiogram was performed at a median duration of 257 days (IQR: 125 to 460) versus the post-transplant echocardiogram, which occurred at a median of 422 days (IQR: 241 to 735). Baseline echocardiogram revealed a reduced LVEF in 28% of patients (taking all 1,375 patients who underwent pre-transplant echocardiogram into account, 18% had LV dysfunction). An abnormal LV mass/BSA was identified in 65% of patients.
FIGURE 1. Patient Inclusion and Exclusion.
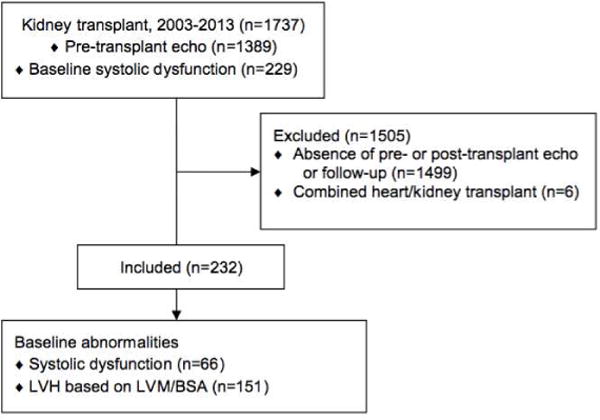
Out of a kidney transplant patient base of 1,697, a total of 232 patients were eventually included in this study. LVH = left ventricular hypertrophy; LVM/BSA = left ventricular mass indexed to body surface area.
TABLE 1.
Baseline Patient Characteristics
| Pre- and Post-Tx TTE (n = 232) | Pre-Tx TTE (n = 1,143) | No TTE (n = 322) | p Value | |
|---|---|---|---|---|
| Transplant age, yrs | 54 ± 12 | 50 ± 12 | 44 ± 13 | <0.001 |
| Male | 145 (63) | 718 (63) | 191 (59) | 0.475 |
| Caucasian race | 156 (67) | 856 (75) | 246 (76) | 0.129 |
| BMI, kg/m2 | 28 ± 6 | 27 ± 5 | 27 ± 6 | 0.081 |
| Renal condition* | ||||
| Diabetes | 72 (31) | 366 (32) | 47 (15) | <0.001 |
| Hypertension | 54 (23) | 225 (20) | 81 (25) | 0.034 |
| Glomerulonephritis | 36 (16) | 229 (20) | 83 (26) | 0.011 |
| Polycystic kidney disease | 14 (6) | 138 (12) | 41 (13) | 0.022 |
| Pre-emptive transplantation | 39 (17) | 250 (22) | 87 (27) | 0.015 |
| Hemodialysis (among dialysis patients) | 163 (84) | 673 (75) | 155 (66) | 0.011 |
| Arteriovenous access | 123 (75) | 507 (75) | 103 (66) | 0.044 |
| Median dialysis duration, days | 778 (239–1,741) | 893 (377–1,626) | 644 (298–1,108) | <0.001 |
| Combined transplantation (liver or pancreas) | 25 (11) | 164 (14) | 23 (7) | <0.001 |
| Comorbidities | ||||
| Diabetes | 107 (46) | 469 (41) | 73 (22) | <0.001 |
| Hypertension | 191 (82) | 853 (75) | 226 (70) | 0.031 |
| Hyperlipidemia | 132 (57) | 381 (33) | 72 (22) | <0.001 |
| Coronary artery disease | 61 (26) | 148 (13) | 15 (5) | <0.001 |
| Heart failure | 72 (31) | 132 (12) | 6 (2) | <0.001 |
| LVEF | 54 ± 10 | 57 ± 8 | <0.001 | |
| LV dysfunction† | 66 (28) | 178 (16) | <0.001 |
Values are mean ± SD, median (interquartile range), or n (%).
Some patients had combined diabetic and hypertensive nephropathy.
Reference values defined by American Society of Echocardiography guidelines (6).
BMI = body mass index; BSA = body surface area; LV = left ventricular; LVEF = left ventricular ejection fraction; TTE = transthoracic echocardiogram; Tx = transplant.
As expected, hypertension was a prevalent comorbidity, accounting for 82% of our cohort, and anemia was present in 71% of subjects.
REVERSE REMODELING FOLLOWING KIDNEY TRANSPLANTATION
The changes in echocardiographic, clinical, and laboratory variables in the post-transplant period are summarized in Table 2. During the study period, none of these patients underwent coronary revascularization or cardiac resynchronization therapy to explain improvements seen, and only 1 patient had an aortic valve replacement. LVEF improved by 9 (± 13) percentage points in those with any degree of systolic dysfunction (p < 0.0001; n = 66), and 15 (± 13) percentage points in those with moderate systolic dysfunction (p < 0.0001; n = 28). Regression in LV mass was noted following transplantation. LV mass/BSA improved by 20 g/m2 (p < 0.0001; n = 151) in those with baseline abnormalities. Statistically significant improvements in LVDd, wall diameter, diastolic function, and RVSP were similarly observed (Table 2). Those with a baseline systolic blood pressure (SBP) ≥130 mm Hg or diastolic blood pressure (DBP) ≥80 mm Hg demonstrated an 18 mm Hg (± 26) and 10 mm Hg (± 17) improvement, respectively (p < 0.0001). These results remained consistent when a cutoff of ≥140/90 mm Hg was used.
TABLE 2.
Post-transplant Outcomes
| Pre-transplant | Post-transplant | p Value | |
|---|---|---|---|
| LVEF | |||
|
| |||
| All subjects | 53 ± 10 | 56 ± 10 | 0.002 |
| Baseline LVSD (n = 66)* | 41 ± 10 | 50 ± 12 | <0.0001 |
| Baseline moderate LVSD* (n = 28) | 32 ± 7 | 47 ± 14 | <0.0001 |
|
| |||
| LVM/BSA, g/m2 | |||
|
| |||
| All subjects | 132 ± 46 | 125 ± 42 | 0.032 |
| Baseline LVH* (n = 151) | 154 ± 40 | 134 ± 45 | <0.0001 |
| Baseline severe LVH* (n = 90) | 176 ± 36 | 138 ± 42 | <0.0001 |
|
| |||
| LVDd, cm | |||
|
| |||
| All subjects | 4.8 ± 0.7 | 4.7 ± 0.7 | 0.005 |
| Baseline ≥5.6 (n = 33) | 6.0 ± 0.4 | 5.0 ± 0.8 | <0.0001 |
|
| |||
| IVS diameter, cm | |||
|
| |||
| All subjects | 1.4 ± 0.3 | 1.4 ± 0.3 | 0.984 |
| Baseline ≥1.2 (n = 180) | 1.5 ± 0.2 | 1.4 ± 0.3 | <0.001 |
|
| |||
| PW diameter, cm | |||
|
| |||
| All subjects | 1.2 ± 0.3 | 1.2 ± 0.3 | 0.974 |
| Baseline ≥1.2 (n = 140) | 1.4 ± 0.2 | 1.3 ± 0.3 | <0.0001 |
|
| |||
| RVSP, mm Hg | |||
|
| |||
| All subjects | 35 ± 11 | 34 ± 12 | 0.728 |
| Baseline ≥40 (n = 35) | 48 ± 8 | 38 ± 15 | <0.001 |
|
| |||
| Diastolic dysfunction | |||
|
| |||
| Stage 2 | 56 (24) | 50 (22) | |
| Stage 3 | 7 (3) | 6 (3) | |
|
| |||
| Blood pressure, mm Hg | |||
|
| |||
| All subjects SBP | 136 ± 25 | 132 ± 22 | 0.026 |
| All subjects DBP | 76 ± 13 | 74 ± 13 | 0.080 |
| Baseline SBP ≥130 (n = 130) | 152 ± 19 | 134 ± 22 | <0.0001 |
| Baseline SBP ≥140 (n = 95) | 159 ± 18 | 137 ± 23 | <0.0001 |
| Baseline DBP ≥80 (n = 86) | 88 ± 10 | 78 ± 14 | <0.0001 |
| Baseline DBP ≥90 (n = 28) | 99 ± 11 | 81 ± 13 | <0.0001 |
|
| |||
| Hemoglobin, g/dl | |||
|
| |||
| All subjects | 11.8 ± 1.5 | 12.3 ± 2.0 | <0.001 |
| Baseline anemia† (n = 165) | 11.1 ± 1.1 | 12.2 ± 2.1 | <0.0001 |
|
| |||
| Medications | |||
|
| |||
| Beta-blocker | 141 (61) | 148 (64) | 0.733 |
| ACE inhibitor or ARB | 67 (29) | 37 (16) | <0.001 |
| Aldosterone antagonist | 0 (0) | 3 (1) | 0.083 |
| Hydralazine | 24 (10) | 19 (8) | 0.354 |
| Nitrate | 21 (9) | 17 (7) | 0.347 |
|
| |||
| Mortality | |||
|
| |||
| 1 year | 15 (7) | ||
| 3 years | 25 (11) | ||
| 5 years | 33 (14) | ||
Values are mean ± SD or n (%).
Reference values defined by American Society of Echocardiography guidelines (6).
Defined as hemoglobin <13 g/dl in males and <12 g/dl in females (9).
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; DBP = diastolic blood pressure; IVS = interventricular septum; LVDd = left ventricular end-diastolic dimension; LVH = left ventricular hypertrophy; LVM/BSA = left ventricular mass indexed to body surface area; LVSD = left ventricular systolic dysfunction; PW = posterior wall; RVSP = right ventricular systolic pressure; SBP = systolic blood pressure; other abbreviations as in Table 1.
Anemia and changes in hemoglobin were closely associated with changes in cardiac structure and function. In subjects with pre-transplant anemia, post-transplant hemoglobin improved by 1.1 g/dl (p < 0.0001). Table 3 summarizes the correlation between clinical variables and reverse remodeling. Change in hemoglobin showed correlation with change in LVEF (in all 232 patients: coefficient = 1.22; 95% CI: 0.61 to 1.83; p < 0.001; in those with baseline LV dysfunction: coefficient = 2.17; 95% CI: 0.94 to 3.40; p < 0.001; Central Illustration). Additionally, multiple factors correlated with LV mass regression including post-transplant improvements in hemoglobin, SBP, DBP, and lower baseline body mass index (BMI). Change in hemoglobin was the only independent factor associated with improved LVEF ≥10 percentage points using logistic regression analysis. This held true when corrected for multiple demographic parameters including transplant age, sex, race, and BMI (OR: 1.68; 95% CI: 1.20 to 2.39; p = 0.002), as well as clinical factors, including dialysis duration, eGFR at 12 months, and changes in SBP and DBP (OR: 1.50; 95% CI: 1.07 to 2.14; p = 0.016).
TABLE 3.
Correlation between Clinical Variables and Reverse Remodeling*
| Δ LVEF | Δ LVM/BSA | |||
|---|---|---|---|---|
| Variable | Coefficient (95% CI) | p Value | (95% CI) | p Value |
| Transplant age | −0.03 (−0.16–0.08) | 0.545 | −0.20 (−0.75–0.34) | 0.463 |
| BMI | −0.22 (−0.48–0.04) | 0.095 | 1.34 (0.14–2.53) | 0.029 |
| Dialysis duration | 0.00 | 0.00 | ||
| eGFR at 12 | ||||
| months | 0.06 (0.01–0.11) | 0.017 | −0.13 (−0.36–0.10) | 0.260 |
| Δ SBP | −0.03 (−0.08–0.02) | 0.281 | 0.63 (0.43–0.83) | <0.0001 |
| Δ DBP | −0.07 (−0.16–0.02) | 0.115 | 0.76 (0.38–1.15) | <0.001 |
| Δ Hemoglobin | 1.22 (0.61–1.83) | <0.001 | −7.76 (−10.40 to −5.13) | <0.0001 |
CLINICAL OUTCOMES
Unadjusted mortality was higher in patients with abnormal baseline LVEF as assessed in all 1,375 patients who underwent pre-transplant echocardiogram (log-rank p < 0.001; Figure 2A). Nevertheless, despite pre-transplant systolic dysfunction, those who improved their LVEF ≥10 percentage points had similar mortality outcomes to those with normal pre-transplant LV function (log-rank p = 0.120; Figure 2B). Changes in hemoglobin predicted improved survival when adjusted for demographic and clinically relevant variables, independent of pre-transplant LVEF or post-transplant improvement in LVEF (hazard ratio: 0.65; 95% CI: 0.49 to 0.87; p = 0.004).
FIGURE 2. Impact of Impaired Pre-Transplant LVEF on Post-Transplant Clinical Outcomes.
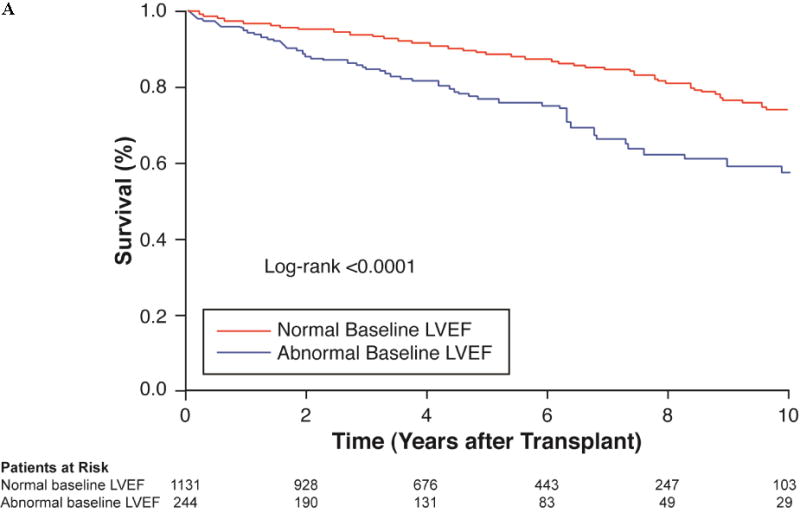
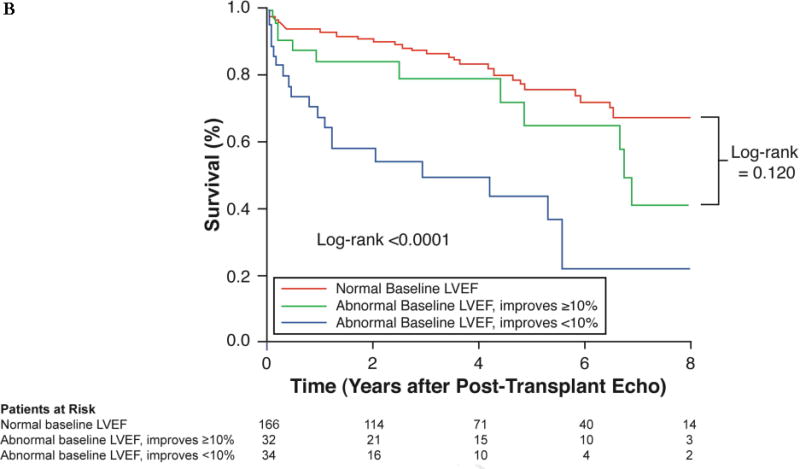
The Kaplan-Meier curves demonstrated (A) a significant difference in survival based on baseline LVEF, but (B) no significant difference in survival in the subgroup of patients who underwent reverse remodeling. In the latter analysis, censor time was calculated from the date of post-transplant echo and not date of transplant, to reduce possible confounding. LVEF = left ventricular ejection fraction.
DISCUSSION
We observed an overall favorable impact of kidney transplantation on cardiac structure and function, with corresponding improvement in long-term survival seen with reverse remodeling in kidney transplant recipients with baseline cardiac dysfunction. Specifically, LV systolic dysfunction is not uncommonly observed in patients undergoing kidney transplantation; amounting to 1 out of 6 patients in this contemporary series with echocardiographic evaluation. Although the presence of baseline LV systolic dysfunction was associated with poorer overall long-term outcomes following kidney transplantation, improvement of LVEF ≥10 percentage points following kidney transplantation in patients with underlying LV systolic dysfunction was associated with better long-term outcomes. Another key finding is that improvement and preservation of hemoglobin was identified as a major contributor to both reverse remodeling and prognosis. Taken together, our current findings imply that metabolic factors associated with ESRD likely contribute to cardiac dysfunction and that structural and functional parameters of cardiac dysfunction may reverse with metabolic improvement following kidney transplantation. Patients with ESRD and underlying LV systolic dysfunction showed remarkable and consistent improvements in LVEF following kidney transplantation, with a mean increase of 15% percentage points in those with baseline LVEF ≤40%. Ventricular dilation, as assessed by LVDd, improved and LV hypertrophy, as assessed by LV mass, substantially regressed post-transplantation. Moreover, positive changes were seen in diastolic function and RVSP in the post-transplant period. A few prior studies had demonstrated positive changes in LV systolic function following kidney transplantation, albeit in very small cohorts (10–13). Specifically, our findings are consistent with findings from Wali and colleagues more than a decade ago, identifying 103 patients with LV systolic dysfunction by radionuclide ventriculography gated-blood pool scan, who significantly improved their LVEF in the post-transplant period (4). Similarly, studies have shown improvements in LV mass post-transplantation (14), as well as a correlation between LV mass regression and SBP (15). Being the largest and most contemporary study to address this question to date, we demonstrate here a similar correlation; in addition, we have identified correlations between LV mass regression and DBP and lower baseline BMI.
An understanding of cardiorenal interactions is paramount in appreciating the pathophysiological processes that explain such reverse remodeling (16). Hemodynamic abnormalities in ESRD result in increased afterload, a phenomenon that had been considered the primary driver for cardiac dysfunction. A multitude of factors contribute, including interdialytic volume overload, elevated blood pressure, and decreased vessel compliance (17). Nevertheless, it is the nonhemodynamic derangements that occur in ESRD including anemia (18), secondary hyperparathyroidism (19), overactivity of the renin-angiotensin-aldosterone system (20,21), as well as the presence of uremic toxins (22), that likely contribute to a hostile inflammatory milieu for the myocardium. Ultimately, cardiac dysfunction ensues, further activating neurohormonal pathways, culminating in the vicious cycle of cardiorenal syndrome. Although there may be a point at which the uremic milieu induces irreversible cardiac damage, our study theoretically demonstrates continued overall benefit to the heart following kidney transplantation despite being preceded by dialysis for a median duration of more than 2 years.
There is a paucity of data on factors associated with reverse remodeling following kidney transplantation. In our study, hemoglobin was a significant predictor of improved LVEF and LV mass in the post-transplant period (Central Illustration). This improvement in anemia translated into superior outcomes. Despite correcting for important clinical variables (including baseline LVEF, post-transplant improvement in LVEF, and post-transplant eGFR), improved hemoglobin remained an independent factor associated with reduced mortality. Although it is not possible to determine causality in this observational study, the impact of anemia on cardiac structure and clinical outcomes has been extensively analyzed. Multiple studies have demonstrated anemia to be a predictor of mortality in the HF population (23–25). Additionally, an inverse correlation was noted between changes in hemoglobin and LV mass on cardiac magnetic resonance (CMR) (26).
The interconnected relationship between CKD, HF, and anemia has been referred to as the “cardiorenal anemia syndrome” (27). The predominant etiology of anemia in this setting is a combination of reduced erythropoietin production and function related to CKD and the inflammatory state of HF, medication-related inhibition of the pro-erythropoetic effects of angiotensin, and disturbances in iron metabolism (28). Iron deficiency in patients with HF is a highly prevalent but often overlooked condition. Although its presence may be suspected in the setting of anemia, it should be noted that in one study, 32% of nonanemic HF patients were iron deficient (29). Randomized placebo-controlled trials have assessed intravenous iron in the setting of heart failure. The recent CONFIRM-HF (Ferric CarboxymaltOse evaluatioN on perFormance in patients with IRon deficiency in coMbination with chronic Heart Failure) trial identified improvements in functional capacity, symptoms, quality of life, and reduced risk of HF hospitalization (30). Importantly, these benefits were irrespective of hemoglobin, highlighting the potential adverse nature of iron-deficiency independent of anemia. Other trials have similarly demonstrated improved symptoms, health status measures, N-terminal pro-B-type natriuretic peptide, peak VO2, and improvements in cardiac and renal function (31–33).
Patients with baseline systolic dysfunction had expected worse outcomes based on unadjusted analyses in our study. However, we highlighted a salient point; specifically, those who underwent reverse remodeling fared no worse than those with normal pre-transplant LVEF. Therefore, impaired cardiac function should not necessarily preclude a patient from undergoing kidney transplantation, especially in the absence of other criteria that indicate the presence of underlying advanced HF (e.g., severe functional impairment or hemodynamic compromise). Continued efforts should be made to identify those factors that mighty predict reverse remodeling.
STUDY LIMITATIONS
One of the limitations of the study is its observational nature, which inherently may have resulted in some bias. This study cohort only included those selected for kidney transplantation and those who survived over the treatment period to allow both pre- and post-transplant echocardiography; this may influence the prognostic conclusions that can be drawn. However, we did use the date of post-transplant echo for censoring to reduce possible confounding. Moreover, we could not control for the timing of echocardiograms. Given the high prevalence of cardiac structural abnormalities in ESRD, as well as variations in intravascular volume in dialysis, echocardiography may have some limitations in this population (34). CMR is considered the “gold standard” for assessing cardiac dimensions and mass, as it is independent of geometric assumptions (35). However, considering its availability and practicality, echocardiography remains an important clinical and research tool when assessing these parameters. The study did not assess for symptoms and functional status, or metabolic parameters including iron studies, parathyroid hormone, phosphorous, and calcium.
CONCLUSIONS
In kidney transplant patients, post-transplant improvement in anemia was an important factor associated with the significant reverse remodeling that was observed, as well as an independent factor associated with reduced mortality. Importantly, we demonstrated favorable survival in patients with pre-transplant LV dysfunction who underwent reverse remodeling. Additional studies should analyze the prognostic implications that these changes pose, and the findings from this study, as well as others, should be taken into consideration when determining criteria for kidney transplant candidacy.
CENTRAL ILLUSTRATION. Reverse Remodeling and Kidney Transplantation: Relationships between change in LVEF and change in hemoglobin.
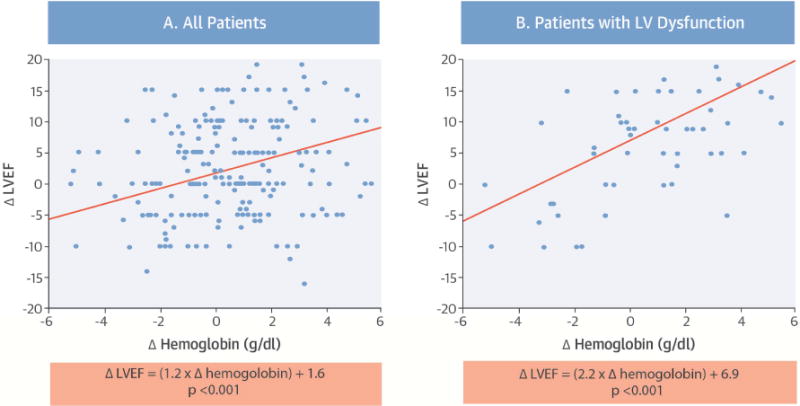
In patients who have undergone kidney transplantation, the need exists to gather data on predictors of myocardial recovery post-transplantation. In exploring the relationship between change in left ventricular ejection fraction (LVEF) and change in hemoglobin, there were significant correlations between change in those two parameters in all patients (A), Δ LVEF = (1.2 × Δ hemoglobin) + 1.6 (p < 0.001), and in patients with LV dysfunction (B), Δ LVEF = (2.2 × Δ hemoglobin) + 6.9 (p < 0.001).
TABLE 4.
Clinical Predictors of Mortality
| Hazard Ratio* (95%CI) | p Value | |
|---|---|---|
| Pre-transplant LVEF† | 0.73 (0.67–0.81) | <0.001 |
| Pre-transplant LVM/BSA | 1.20 (0.95–1.50) | 0.129 |
| Pre-transplant LVDd | 1.25 (0.96–1.60) | 0.090 |
| Multivariable Model (corrected for age, sex, race, dialysis duration, eGRF at 12 months)‡ | ||
| Pre-transplant LVEF | 0.61 (0.49–0.78) | <0.001 |
| Improved LVEF ≥ 10% | 0.46 (0.21–0.93) | 0.031 |
| Δ Hemoglobin | 0.65 (0.49–0.87) | 0.004 |
Hazard ratio per 1 standard deviation increment for continuous variables.
Pre-transplant LVEF was assessed in all 1,375 patients who had a pre-transplant echocardiogram.
Censor time was calculated from the date of post-transplant echo and not date of transplant, to reduce possible confounding.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE
In patients with mild to moderate ventricular dysfunction, cardiac structure and function can improve following kidney transplantation concurrent with improvement in anemia.
TRANSLATIONAL OUTLOOK
Further studies are needed to explore the role of iron metabolism in the myocardial reverse remodeling that follows kidney transplantation.
Acknowledgments
Source of Funding: This research was supported by grant from the National Institutes of Health (R01HL103931).
ABBREVIATIONS AND ACRONYMS
- BSA
body surface area
- DBP
diastolic blood pressure
- eGFR
estimated glomerular filtration rate
- ESRD
end-stage renal disease
- LV
left ventricular
- LVEF
left ventricular ejection fraction
- SBP
systolic blood pressure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: None
References
- 1.Collins AJ. Cardiovascular mortality in end-stage renal disease. Am J Med Sci. 2003;325:163–7. doi: 10.1097/00000441-200304000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Fleischmann EH, Bower JD, Salahudeen AK. Are conventional cardiovascular risk factors predictive of two-year mortality in hemodialysis patients? Clin Nephrol. 2001;56:221–30. [PubMed] [Google Scholar]
- 3.Meeus F, Kourilsky O, Guerin AP, Gaudry C, Marchais SJ, London GM. Pathophysiology of cardiovascular disease in hemodialysis patients. Kidney Int Suppl. 2000;76:S140–7. doi: 10.1046/j.1523-1755.2000.07618.x. [DOI] [PubMed] [Google Scholar]
- 4.Wali RK, Wang GS, Gottlieb SS, et al. Effect of kidney transplantation on left ventricular systolic dysfunction and congestive heart failure in patients with end-stage renal disease. J Am Coll Cardiol. 2005;45:1051–60. doi: 10.1016/j.jacc.2004.11.061. [DOI] [PubMed] [Google Scholar]
- 5.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. modification of diet in renal disease study group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 6.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 8.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 9.KDIGO anemia work group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl. 2012;2:279–335. [Google Scholar]
- 10.Burt RK, Gupta-Burt S, Suki WN, Barcenas CG, Ferguson JJ, Van Buren CT. Reversal of left ventricular dysfunction after renal transplantation. Ann Intern Med. 1989;111:635–40. doi: 10.7326/0003-4819-111-8-635. [DOI] [PubMed] [Google Scholar]
- 11.Parfrey PS, Harnett JD, Foley RN, et al. Impact of renal transplantation on uremic cardiomyopathy. Transplantation. 1995;60:908–14. [PubMed] [Google Scholar]
- 12.Ferreira SR, Moises VA, Tavares A, Pacheco-Silva A. Cardiovascular effects of successful renal transplantation: A 1-year sequential study of left ventricular morphology and function, and 24-hour blood pressure profile. Transplantation. 2002;74:1580–7. doi: 10.1097/00007890-200212150-00016. [DOI] [PubMed] [Google Scholar]
- 13.Casas-Aparicio G, Castillo-Martinez L, Orea-Tejeda A, Abasta-Jimenez M, Keirns-Davies C, Rebollar-Gonzalez V. The effect of successful kidney transplantation on ventricular dysfunction and pulmonary hypertension. Transplant Proc. 2010;42:3524–8. doi: 10.1016/j.transproceed.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 14.Rigatto C, Foley RN, Kent GM, Guttmann R, Parfrey PS. Long-term changes in left ventricular hypertrophy after renal transplantation. Transplantation. 2000;70:570–5. doi: 10.1097/00007890-200008270-00006. [DOI] [PubMed] [Google Scholar]
- 15.Montanaro D, Gropuzzo M, Tulissi P, et al. Effects of successful renal transplantation on left ventricular mass. Transplant Proc. 2005;37:2485–7. doi: 10.1016/j.transproceed.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 16.Bock JS, Gottlieb SS. Cardiorenal syndrome: New perspectives. Circulation. 2010;121:2592–600. doi: 10.1161/CIRCULATIONAHA.109.886473. [DOI] [PubMed] [Google Scholar]
- 17.Konings CJ, Dammers R, Rensma PL, et al. Arterial wall properties in patients with renal failure. Am J Kidney Dis. 2002;39:1206–12. doi: 10.1053/ajkd.2002.33392. [DOI] [PubMed] [Google Scholar]
- 18.Bonomini M, Sirolli V. Uremic toxicity and anemia. J Nephrol. 2003;16:21–8. [PubMed] [Google Scholar]
- 19.Horl WH. The clinical consequences of secondary hyperparathyroidism: Focus on clinical outcomes. Nephrol Dial Transplant. 2004;19(Suppl 5):V2–8. doi: 10.1093/ndt/gfh1049. [DOI] [PubMed] [Google Scholar]
- 20.Vlahakos DV, Hahalis G, Vassilakos P, Marathias KP, Geroulanos S. Relationship between left ventricular hypertrophy and plasma renin activity in chronic hemodialysis patients. J Am Soc Nephrol. 1997;8:1764–70. doi: 10.1681/ASN.V8111764. [DOI] [PubMed] [Google Scholar]
- 21.Sato A, Funder JW, Saruta T. Involvement of aldosterone in left ventricular hypertrophy of patients with end-stage renal failure treated with hemodialysis. Am J Hypertens. 1999;12:867–73. doi: 10.1016/s0895-7061(99)00066-7. [DOI] [PubMed] [Google Scholar]
- 22.Vanholder R, Glorieux G, Lameire N, European Uremic Toxin Work Group Uraemic toxins and cardiovascular disease. Nephrol Dial Transplant. 2003;18:463–6. doi: 10.1093/ndt/18.3.463. [DOI] [PubMed] [Google Scholar]
- 23.Al-Ahmad A, Rand WM, Manjunath G, et al. Reduced kidney function and anemia as risk factors for mortality in patients with left ventricular dysfunction. J Am Coll Cardiol. 2001;38:955–62. doi: 10.1016/s0735-1097(01)01470-x. [DOI] [PubMed] [Google Scholar]
- 24.Mozaffarian D, Nye R, Levy WC. Anemia predicts mortality in severe heart failure: The prospective randomized amlodipine survival evaluation (PRAISE) J Am Coll Cardiol. 2003;41:1933–9. doi: 10.1016/s0735-1097(03)00425-x. [DOI] [PubMed] [Google Scholar]
- 25.Tang WH, Tong W, Jain A, Francis GS, Harris CM, Young JB. Evaluation and long-term prognosis of new-onset, transient, and persistent anemia in ambulatory patients with chronic heart failure. J Am Coll Cardiol. 2008;51:569–76. doi: 10.1016/j.jacc.2007.07.094. [DOI] [PubMed] [Google Scholar]
- 26.Anand I, McMurray JJ, Whitmore J, et al. Anemia and its relationship to clinical outcome in heart failure. Circulation. 2004;110:149–54. doi: 10.1161/01.CIR.0000134279.79571.73. [DOI] [PubMed] [Google Scholar]
- 27.Silverberg DS, Wexler D, Iaina A, Schwartz D. The interaction between heart failure and other heart diseases, renal failure, and anemia. Semin Nephrol. 2006;26:296–30. doi: 10.1016/j.semnephrol.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Anand IS. Heart failure and anemia: Mechanisms and pathophysiology. Heart Fail Rev. 2008;13:379–86. doi: 10.1007/s10741-008-9088-8. [DOI] [PubMed] [Google Scholar]
- 29.Jankowska EA, Rozentryt P, Witkowska A, et al. Iron deficiency: An ominous sign in patients with systolic chronic heart failure. Eur Heart J. 2010;31:1872–80. doi: 10.1093/eurheartj/ehq158. [DOI] [PubMed] [Google Scholar]
- 30.Ponikowski P, van Veldhuisen DJ, Comin-Colet J, et al. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiencydagger. Eur Heart J. 2015;36:657–68. doi: 10.1093/eurheartj/ehu385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anker SD, Comin Colet J, Filippatos G, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361:2436–48. doi: 10.1056/NEJMoa0908355. [DOI] [PubMed] [Google Scholar]
- 32.Toblli JE, Lombrana A, Duarte P, Di Gennaro F. Intravenous iron reduces NT-pro-brain natriuretic peptide in anemic patients with chronic heart failure and renal insufficiency. J Am Coll Cardiol. 2007;50:1657–65. doi: 10.1016/j.jacc.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 33.Okonko DO, Grzeslo A, Witkowski T, et al. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency FERRIC-HF: A randomized, controlled, observer-blinded trial. J Am Coll Cardiol. 2008;51:103–12. doi: 10.1016/j.jacc.2007.09.036. [DOI] [PubMed] [Google Scholar]
- 34.Mark PB, Patel RK, Jardine AG. Are we overestimating left ventricular abnormalities in end-stage renal disease? Nephrol Dial Transplant. 2007;22:1815–9. doi: 10.1093/ndt/gfm224. [DOI] [PubMed] [Google Scholar]
- 35.Glassock RJ, Pecoits-Filho R, Barberato SH. Left ventricular mass in chronic kidney disease and ESRD. Clin J Am Soc Nephrol. 2009;4(Suppl 1):S79–91. doi: 10.2215/CJN.04860709. [DOI] [PubMed] [Google Scholar]


