Abstract
Nurturing the development of cardiovascular physician-scientist investigators is critical for sustained progress in cardiovascular science and improving human health. The transition from an inexperienced trainee to an independent physician-scientist is a multifaceted process requiring a sustained commitment from the trainee, mentors, and institution. A cornerstone of this training process is a career development (K) award from the National Institutes of Health (NIH). These awards generally require 75% of the awardee’s professional effort devoted to research aims and diverse career development activities carried out in a mentored environment over a 5-year period. We report on recent success rates for obtaining NIH K awards, provide strategies for preparing a successful application and navigating the early career period for aspiring cardiovascular investigators, and offer cardiovascular division leadership perspectives regarding K awards in the current era. Our objective is to offer practical advice that will equip trainees considering an investigator path for success.
Keywords: awards and prizes, biomedical research, early-career investigators, fellowships and scholarships, grants, mentors
Introduction
The creativity, passion, and intellectual capital of cardiovascular physician-scientist investigators fuel advances in cardiovascular science that improve human health. Accordingly, these investigators are an indispensable resource. Unfortunately, ever-present financial pressures and myriad other forces now threaten the development of talented individuals for this mission and purpose, which may hinder continued innovation and scientific progress (1-3). Shortsightedness in this regard could have numerous adverse long-term consequences.
The 2014 National Institutes of Health (NIH) Physician-Scientist Workforce Working Group documented the aging and the declining size of the physician-scientist workforce (4). This decline is in stark contrast to increases in annual graduation rates of life science PhDs from <2,000 per year in 1993 to >8,000 per year in 2007 (5,6). If achieving independence is defined as obtaining a funded research project grant (R01), the average time for MD/PhD physician-scientists to achieve independence is 13 years from graduation and the average time for MD physician-scientists is 17 years from graduation (4). Thus, it takes a considerable time to develop physician-scientists who can pursue independent research. With numerous challenges and obstacles present and looming, a well-trained, diverse, replenishing, and adequately-sized cardiovascular physician-scientist workforce may now be in jeopardy.
Despite real challenges, this is also a time of unprecedented opportunity for elucidating pathophysiology and identifying novel preventive and therapeutic strategies that improve cardiovascular and overall health (2,7). To develop, harness, and effectively utilize the technological resources available to us, we need people—cardiovascular physician-scientists—to drive this enterprise. Accordingly, a team of authors including a National Heart Lung, and Blood Institute (NHLBI) program officer, a past chairman of the NHLBI K-grant study section, NIH K-grant study section reviewers, a cardiovascular division chief, and a fellowship program director, American College of Cardiology (ACC) leadership, K-award mentors, and current K-award recipients wrote this paper to inform, equip, and counsel the aspiring early-career cardiovascular physician-scientist. In several places throughout the paper, assertions are made without firm, quantitative data. As such, these assertions should be regarded as the consensus opinion of a broad authorship with extensive, multifaceted experience relevant to the subjects covered. Our objectives are to provide: 1) updated data for cardiovascular K-award applicants to the NHLBI regarding success rates for initial and resubmission applications; 2) guidance for preparation of each component of a competitive K-award application; and 3) practical counsel for what is important during this early career period (before and after K-award funding) to maximize one’s chance of achieving a sustained, independent research program.
NIH Career Development Awards (K Awards)
A K award is a mentored career development award (CDA) that is often the first step taken after fellowship training for those in pursuit of an independent research career (8-10). The NIH Mentored Clinical Scientist Research Career Development Award (K08) and NIH Mentored Patient-Oriented Research Career Development Award (K23) are the most common early-career awards for clinician-scientists at the instructor or assistant professor level (Table 1). Although most aspiring physician-scientists apply for a K08 or K23 award, some receive preparation as KL2 or K12 scholars funded by a Clinical and Translational Science Award (CTSA) or an Institutional K12 Award (Table 1). These CDAs typically provide up to 5 years (2 to 3 years for a K12 or KL2) of salary support ($75,000 per year for CDAs funded by the NHLBI) for the investigator, as well as research support ($25,000 to $50,000/year) for project expenses. The key advantage of these awards is the provision/requirement of 75% protected time for research and other career development activities. The remaining 25% is devoted to clinical, administrative, or teaching activities. For those in procedural specialties (including surgical specialties, electrophysiology, and interventional cardiology), it is often possible to request a reduction in research effort to no less than 50% (11). This exception is specific for each Institute within the NIH and needs to be discussed well in advance with a designated program officer on a case-by-case basis. In the final 2 years of a K award, effort may be reduced to no less than 50% and replaced with effort as a Principal Investigator (PI) on a qualifying research grant supported by the NIH (such as an R01 or a subproject of a multiproject NIH grant) or other Federal agencies, (12), provided the candidate remains in a mentored environment.
Table 1. NIH Career Development Awards (K Awards).
| KL2 or K12 |
|
|
| |
| K23 |
|
|
| |
| K08 |
|
NIH = National Institutes of Health.
Distinct from typical research awards, CDAs are designed as much (or more) to promote the development of the investigator as to accomplish specific scientific aims. The career development plan should be customized to the applicant, taking into account his or her background, experience, and future goals. The objective of the award is to provide a protected period of intensive mentored research that yields independent investigators with sustained and productive scientific careers.
NIH K awards–applicants and success rates
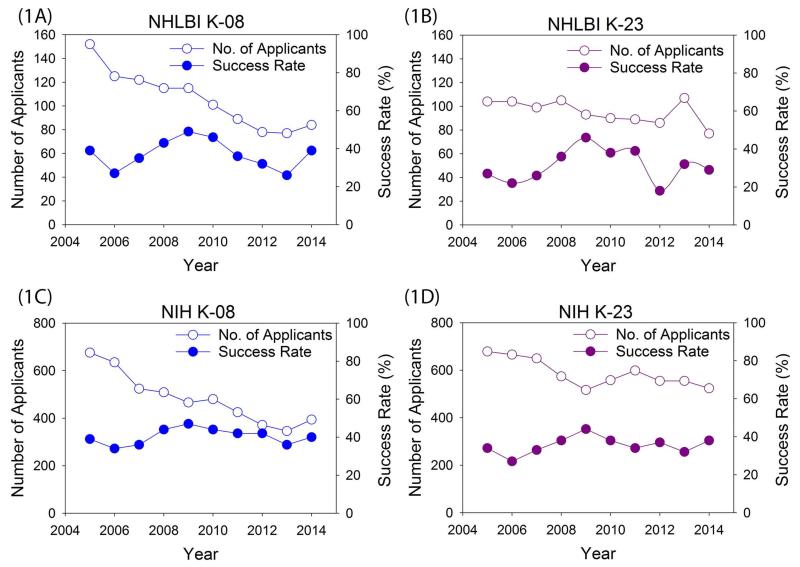
Over the last 10 years (2005 to 2014), there has been a substantial decline in the number of applicants for K awards to the NIH overall and to the NHLBI, particularly for K08 awards (Figure 1). Consistent with the 2014 NIH Physician-Scientist Workforce Working Group Report, these data indicate that the pool of early-career cardiovascular physician-scientists is shrinking (4). K-grant success rates have varied between 20% and 40% over this period, with a notable increase during 2009 to 2010 due to the American Recovery and Reinvestment Act. Despite the budgetary constraints of the last few years (2011 to 2014), the NHLBI has made an effort to maintain steady levels of support for research training and career development; therefore, success rates remained comparable to the years before the Recovery Act.
Figure 1. Temporal Trends for K Grants Between 2005 and 2014.
The NIH defines the success rate as the number of applications funded divided by the number of applications reviewed within a fiscal year; however, original submissions (A0) and resubmissions (A1) on the same project submitted within the same fiscal year are counted as 1 application. Accordingly, the success rate is on the basis of applicants per fiscal year (17). (A) NHLBI K-08. (B) NHLBI K-23. (C) Total NIH K-08. (D) Total NIH K-23. NIH = National Institutes of Health; NHLBI = National Heart, Lung, and Blood Institute.
The decline in K08 physician-scientists has been more marked than for K23 physician-scientists. Although we lack empiric data that identify the causes for these trends, we speculate that the following (and other) factors are likely contributing. K08 applicants tend to pursue more basic science research. Due to decreased funding at the NIH, increased competition from PhD-only scientists for NIH R01 dollars, and a paucity of funding sources for basic science research outside of the NIH, prospective K08 applicants may see a bleak, high-risk future and decide not to pursue a physician-scientist pathway. In contrast, prospective K23 applicants may see similar trends, but may also see more potential non-NIH funding options, including foundations and device and pharmaceutical companies, and less competition from PhD-only scientists for more clinically-focused research.
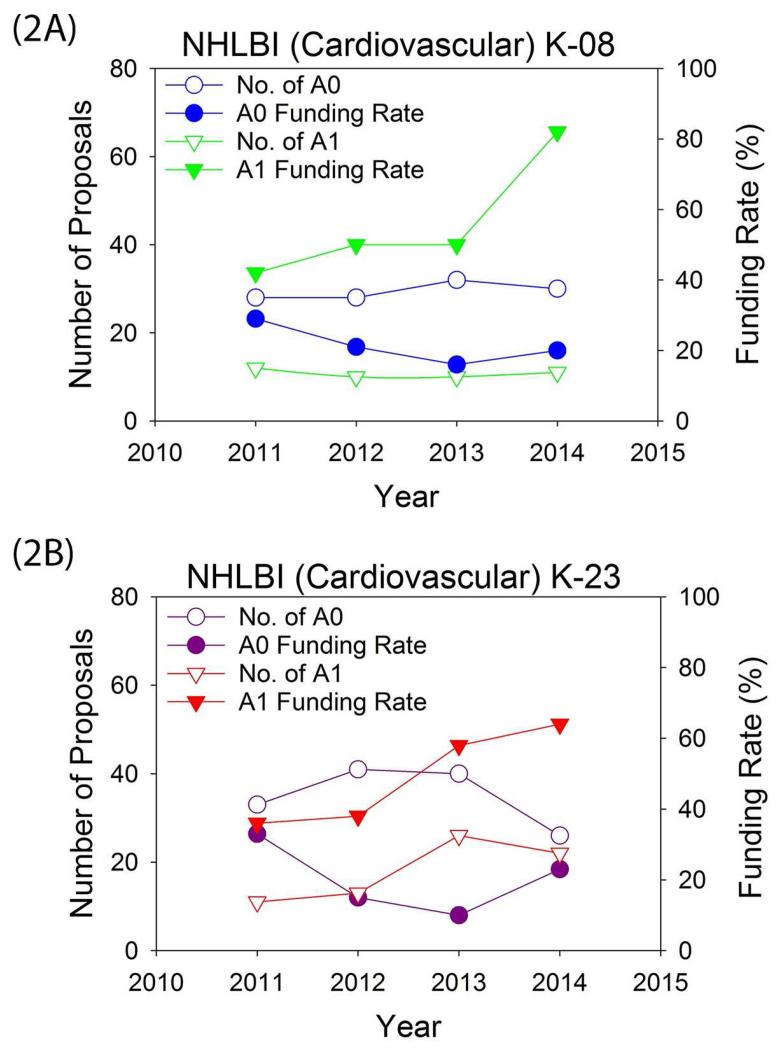
The data shown in Figure 2 for K-grant applications assigned to the Division of Cardiovascular Sciences at the NHLBI since 2011 provide the most current and likely the best estimate of success for aspiring cardiovascular physician-scientists. From 2011 to 2015, a total of 139 K08 and K23 applicants assigned to the Division of Cardiovascular Sciences made resubmissions. For this cohort, resubmitted proposals have a much higher funding rate than original submissions, highlighting that persistence pays off when applying for a K award (Figure 2). Those applications that were scored both initially (A0) and on resubmission (A1) had a median improvement of 13 points. For the entire A1 population, 84% received a better priority score on the resubmission than on the initial application. Among all of the resubmitted (A1) applications, 63% received scores in the high-impact range (priority score 10 to 30; potentially fundable) (Table 2). Even among 38 applicants whose initial proposal was not discussed (because reviewer feedback prior to the study section meeting indicated that it would not be competitive for funding), 37% of their resubmitted applications were judged to have a high impact. Despite these encouraging data on the success rate of resubmissions, less than half of applicants whose initial proposal was not funded resubmitted a K proposal (Table 2).
Figure 2. Original Submission Versus Resubmission Trends from the NHLBI Cardiovascular Sciences Division.
Funding rates are calculated per proposal. Data provided by the NHLBI Cardiovascular Sciences Division for 2011 to 2014. This period was chosen to reflect the current funding climate. (A) K-08. (B) K-23. A0 = original submission; A1 = resubmission. Abbreviations as in Figure 1.
Table 2. NHLBI (Cardiovascular) 2011-2014 Overall Rates for Original Proposals and Resubmissions—K08 and K23.
| Original (A0) proposals that were not discussed (triaged) | 35% |
| 2011-2014 resubmission rate for A0 proposals not funded | 46% |
| Resubmission (A1) applications that received a fundable priority score(≤30)* | 63% |
| A1 applications (initially not discussed/triaged) that received a fundable priority score (≤30) |
37% |
| Overall funding rate for 2011-2014, including original proposals and resubmissions | 31% |
In 2014-2015, a priority score ≤30 was funded.
NHLBI = National Heart, Lung, and Blood Institute.
Profile of a Successful K Award Application
The foundation for a successful K-award application begins with careful preparation long before the actual application is constructed and submitted (see Practical Steps–Before and After the Award). However, successful applicants have various levels of prior research training and experience. Some may have formal and more extensive research training (e.g., PhD or MSc), whereas others may not. Publication records may differ in length and impact. Ultimately, successful K award applicants are able to provide a compelling case for their likelihood to develop into an independent investigator, but there is no specific or precise mold into which one must fit.
The 5 scored components of K award proposals consist of: 1) the candidate; 2) the career development plan/career goals and objectives; 3) the research plan; 4) mentor(s), co-mentor(s), consultant(s), collaborator(s); and 5) the environment and institutional commitment to the candidate. These 5 components must cohesively and synergistically fit together in a unified and mutually reinforcing manner. We recommend that prospective applicants read several successful K award proposals (not necessarily in their area of proposed research) to provide ideas and guidance on how to integrate these 5 components into a compelling proposal. Many academic institutions formally or informally make sample applications available to prospective applicants. There is an art to crafting a competitive application that effectively sells the candidate and proposed plan.
Candidate
The applicant has to demonstrate commitment to and passion for research, as well as strong potential for achieving independence. Dedication of significant time during fellowship toward research, productivity during that time, and other evidence of initiative, drive, and persistence tangibly demonstrate this commitment and passion. The publication record in the candidate’s biosketch is particularly important. K-grant review panels look carefully, not just at the number of papers, but, more importantly, at their quality, author order, and genre (e.g., original research, review, or editorial) in relation to the candidate’s past experience and long-term goals. First-author research papers in well-regarded journals carry the most weight. One or more coauthorships with the mentor indicate that a working relationship has been established. One needs to communicate thoughtful long-term career goals and identify how the CDA will provide the necessary skills and foundation to achieve those goals. A research proposal in a unique niche or one that is likely to have a high impact with numerous avenues for potential future funding, will be viewed favorably, as it indicates a greater likelihood of long-term sustainability for the research program. The primary mentor’s letter and other reference letters must convey and corroborate the personal characteristics, qualities, and potential of the candidate. These individuals ought to know the candidate well and be able to give specific evidence of the candidate’s potential for a productive, independent scientific career.
Career Development Plan/Career Goals & Objectives
While there is no one-size-fits-all approach to career development plans, the applicant should clearly identify knowledge, skill, experiential, or other gaps to be addressed during this phase of training that will help facilitate development into an independent investigator. The career development plan must provide: 1) personalized steps toward meeting career goals; 2) harmonious integration with the research plan; and 3) a pathway toward independence that builds on prior experience and training. A program that involves foundational didactic courses (that may even lead to a degree) is appropriate for those with little or no formal research training; for those with a PhD, MPH, or MSc, a few selective courses to address specific knowledge gaps will be more appropriate. Informal or creative learning experiences, such as a visiting rotation, workshop, or other mechanisms to learn a new technique, method, or approach may also be incorporated into the plan. Formal and informal learning must directly support the scientific aims by topic and by timing (e.g., an applicant ought to take an epigenetics course during year 1 to support conducting research on a related specific aim in year 2). A clear and specific plan/schedule for mentor-mentee and advisory committee meetings (and content to be covered) should be outlined, along with reasons particular mentors were chosen. This plan should be corroborated in the letters from the primary mentor and mentor committee. Concrete milestones for progress should be included (e.g., number of abstracts or publications per year, recruitment goals, etc.), as well as specific plans for transitioning to an R01 application. Tables and timelines should be used to succinctly convey key components of the career development plan.
Research Plan
The proposed research must demonstrate scientific merit, appropriateness for career stage, and opportunities for obtaining new research skills. Although a simplistic scientific proposal will not be competitive, an overly ambitious proposal is commonly submitted and will be poorly received. There needs to be an appropriate balance between innovation and feasibility (in terms of budget, time, and scope). The aims ought to serve as a training vehicle for the applicant, so that important skills are acquired that can readily be translated to future work. The aims should also be connected, but not dependent on one another in such a way that if Aim 1 fails, then Aims 2 and 3 are no longer viable. Preliminary data (particularly when generated by the applicant) is certainly helpful, but, in many circumstances, not required for each Aim. If a relatively new technique is proposed, the applicant should demonstrate either personal experience with the technique (e.g., published paper), a mentor with expertise, or a clear plan to obtain relevant training. Analytic plans need to be sound and power calculations included where appropriate. The proposal must demonstrate an awareness of potential problems and indicate alternative strategies, integrate well with the career development plan and the expertise of the mentor, and build a foundation for the next step of experiments that are likely to be proposed in an R01.
Mentor(s), Co-Mentor(s), Consultant(s), Collaborator(s)
Mentoring is a crucial component of a successful K application. Although the analogy is not perfect, to some extent the mentor is viewed as the collateral on the NIH’s investment in an unproven and unpolished young investigator. The mentor will play a prominent and influential role in determining whether this investment yields a favorable return in the form of a productive, independent investigator. Accordingly, the mentor needs to be a recognized leader in his or her field of research, actively publishing, with a steady history of support from major peer-reviewed grants (e.g., NIH R01, VA MERIT Award, and the like). Applicants should explain how both the amount of resources available and the quality of science are equivalent to that typical of R01-funded investigators when their mentors are supported mostly by private foundations or industry. The qualities, track record of prior successful mentorship (i.e., trainee table), and commitment of the mentor are more important than an exact overlap between the mentor’s research and the proposed aims. The selection of the mentor needs to fit with the applicant’s stated goals, identified knowledge and skill gaps, and proposed research. The mentor letter must precisely describe the planned interaction with the mentee, the skills and knowledge that will be imparted, benchmarks for progression, criteria for evaluation, and plan for the transition to independence. This description should align with what is stated in the career development plan of the applicant. If there are questions regarding how and whether a specific portion of the mentor’s research portfolio will be passed on to the applicant, those should be addressed. The fingerprints and oversight of the mentor need to be evident in the crafting of the applicant’s proposal to demonstrate the engagement and commitment of the mentor; lack of such involvement is evident to experienced reviewers and raises a cautionary flag. While not required, a mentoring committee is commonly used to provide expertise in multiple areas that cannot be covered by just 1 person. A committee can also add expertise that does not exist in the home institution and can provide a balance of scientific and clinical perspectives. The primary mentor can coordinate and organize the individuals on the committee for the benefit of the candidate’s scientific and career development.
Environment and Institutional Commitment to the Candidate
There must be a clear commitment of the institution to the development of the applicant’s scientific career. This is demonstrated by the provision of sufficient resources in terms of time, space, equipment, technician or research coordinator, and start-up funds. The institutional provisions should not be conditional upon the applicant receiving the K award, as reviewers will interpret this as a lack of institutional commitment. While it may not be as important for applicants pursuing clinical research, appointment to a tenure-track position also demonstrates that the applicant is viewed as a promising contributor to the research mission of the institution. The provision of at least 75% protected time during the period of the award for research and career development activities needs to be clearly stated; if the applicant currently does not have that much protected time, it needs to be clear what and how certain clinical activities will be relieved. Someone who holds the authority to commit the institution needs to write a letter that expresses institutional commitment (e.g., department chair, medical school dean). Although sometimes discounted, the strengths and richness of the environment for the individual applicant and their specific research program needs to be clearly and specifically outlined, including intellectual resources, core facilities or unique equipment, collaborative relationships, statistical expertise, mock study sections for grant preparation, career development seminars, and so on.
Other Areas
There are a few nonscored areas that require careful planning. Training in the responsible conduct of research is a federal mandate, so a clear plan that demonstrates compliance is necessary. If animals are used, the Vertebrate Animal section needs to describe the proposed research (e.g., colony size and timing must be appropriate for the proposed research). For clinical studies, the Protection of Human Subjects section needs to be clearly and carefully completed. The budget needs to be realistic and appropriate for the proposed research. If the proposed aims cannot be carried out with the project support available for a K award, there must be a clear description of resources that will be used for the funding gap (e.g., mentor’s funds, start-up funds from the institution); such support should also be corroborated in the mentor’s letter and the institutional commitment letter. Resource sharing, biohazards, and select agents also need to be addressed, even when these elements are not part of the project. The current versions of the SF424 and Institute-specific instructions should be followed carefully. All applications receive an initial administrative review and those that are noncompliant are withdrawn from consideration, thereby delaying an eventual submission by 1 or more review cycles.
Writing an outstanding K award application requires time; it may take 6 to 12 months for all the pieces to come together into a competitive application (Table 3). Common pitfalls to avoid in the preparation of a K award application are outlined in Table 4.
Table 3. Checklist—Major Components of Preparing a K-Award Application.
| Identify a primary mentor |
| Identify a mentorship team |
| Develop and refine hypotheses and specific aims prior to writing the research plan |
| Research plan |
| Depending on prior training, identify an appropriate didactic curriculum |
| Identify informal learning experiences and other proposed career development activities |
| Career development plan |
| NIH Biosketch—exploit Section A |
| Protection of Human Subjects and/or Animal Use, Resource Sharing, Biohazards, Select Agents |
| Responsible Conduct of Research |
| Reference letters—suggest points that referees may highlight in their letters |
| Budget—allow time to receive quotes for services or equipment |
| Allow time for experienced colleagues or a mock study section to read and critique the grant as a whole |
| Letters from mentor, mentoring committee, collaborators, consultants, and institution (to demonstrate institutional commitment) |
NIH = National Institutes of Health.
Table 4. Common Pitfalls Responsible for Unsuccessful NIH K Award Applications.
| General |
|
|
|
|
|
| Candidate |
|
|
|
|
|
| Career Development Plan/Career Goals and Objectives |
|
|
|
|
|
| Research Plan |
|
|
|
|
|
| Mentor(s), Co-Mentor(s), Consultant(s), Collaborator(s) |
|
|
|
|
|
| Environment and Institutional Commitment to the Candidate |
|
|
|
CTSA = Clinical and Translational Science Award; NIH = National Institutes of Health; PI = principal investigator.
Practical Steps—Before and During the Award
Key steps during fellowship years
Mentors
The importance of mentorship cannot be overstated, especially during the early stages of development on an investigator path. Creativity, asking the “right”/fundable questions, project development, scientific writing, contextualizing, and messaging results, initiating collaborations, and other valuable skills are passed on, in part, via personal knowledge, in which the mentor transmits a craft or trade, more than facts to know or a formula to follow (13). In other words, these skills are imparted via an interactive and personal exchange, not by following a set of rules or guidelines. The mentor-mentee relationship is a reciprocal, collaborative alliance, rather than a 1-way relationship (14,15). In view of the increasing complexity and multidisciplinary nature of research and the breadth of knowledge and skills to be transmitted to the mentee, additional mentors are often needed. It is helpful to identify a cadre of additional mentors who have navigated different segments of the investigator path successfully, including those who are midcareer/senior, as well as more junior (perhaps on CDAs). Meetings specifically designed to convey wisdom and guidance from senior to junior investigators (e.g., How to Become a Cardiovascular Investigator, directed by Valentin Fuster, MD, PhD) are invaluable.
Projects and writing
Whereas initially one may get involved in research projects that are primarily those developed by a mentor, there needs to be some transition from functioning as a cog in the wheel of someone else’s research machine to developing and refining one’s own ideas. Learning from a seasoned mentor how to ask good questions, not just convenient questions, is an important skill. While overly ambitious initial project(s) are common, it is helpful to start small, learn the process, and then expand. Working to bring 1 or 2 projects to completion, rather than getting involved in multiple projects that fizzle, will establish traction and momentum. By turning a submitted abstract into a manuscript, one gains valuable experience with the process of communicating results and learning the art of scientific writing. Learning to write well is an incredibly important skill that is learned by practice, input from a constructively critical mentor(s), and via scientific writing workshops or courses.
Protected time
Carving out some protected time during fellowship is important to provide a sustained period (several months to a year or more) devoted to research. Basic science projects will require additional time (i.e., research time ≥2 years). If available at your institution, a NIH T32 (training grant) is an ideal mechanism to gain protected time. This time is often needed to lay an adequate foundation for a successful CDA application.
Introspection
Having spent so many years of training doing and saying the right thing to jump through the next hoop, one must now take an honest personal inventory (of strengths, weaknesses, passions, desires, and so on) and ask whether an investigator path is the right fit. For some, the desire and decision (to pursue or not pursue an investigator path) is clear and firm at an early point. For others to make an informed decision, clarity may require a 1 to 2 year substantive investment in research during fellowship (taking the steps above). Either way, one needs to honestly determine whether research is exhilarating and rewarding, or more of a chore and obligation. If there is no fire in the belly for an investigator path, it is important to recognize and acknowledge that early. Pragmatically, it is also important to consider some of the financial costs (at least initially) of choosing an investigator path that will typically include lower wages due to less clinical duties and volume (1). While this can be somewhat offset by programs such as the NIH loan repayment program, the near-term financial loss needs to be acknowledged.
Synergize your clinical focus and research focus
Given the limited time and numerous demands that early-career investigators face, synergizing one’s clinical niche and research focus to reinforce one another, rather than compete, can be particularly helpful. Numerous specialized clinical topics (e.g., cardio-oncology, genetics of cardiomyopathy, heart failure with preserved ejection fraction, valvular disease, vascular medicine, among others) permit a young investigator to develop a specialty clinic and a focused inpatient consult service. A prospective registry could be developed that enrolls all the patients seen, including detailed clinical and imaging data for careful phenotyping alongside a biobank of blood (and perhaps tissue) specimens, with prospective follow-up for clinical outcomes. Such a registry could provide preliminary data and the infrastructure for future intervention studies, and could also facilitate new intramural or extramural research collaborations, perhaps extending outside the immediate field of interest. Additional challenges are faced by those pursuing a procedure-based area of expertise (e.g., interventionalists, electrophysiologists), given the need to develop procedural competencies while finding sufficient time for research.
Focus, focus, focus
Narrow and deep
Those drawn to research are often curious about many topics, thus making it difficult to focus their investigative efforts. But if one’s research questions and effort are too diffuse, it will be difficult to gain traction in any area. It is vital to be narrow and deep before expanding and broadening.
First-author papers
At an early stage, one ought to focus on writing several first-author papers as the primary driver of the project and writing. This productivity lays the foundation for one’s independent research program and can lead to additional commitments from the institution, such as independent laboratory space and/or promotion. It is then often appropriate, in consultation with one’s mentor(s), to transition to senior-author papers, as evidence of one’s progression to independence and readiness for R01 funding. While there is a role for being a contributing/middle author on some papers, particularly at an early stage, avoid overcommitting on papers where you are not the first or last author. For a research track, middle-author papers count significantly less for advancement and establishing your expertise in a field.
Learn to say no
While it can be flattering to be asked by more senior colleagues to be on various committees (either institutional or in state or national organizations), it is important to be discerning and thoughtful about which invitations to accept. It is often important to say no to involvement on committees that will incur numerous meetings and tasks that will drain your time and distract you from development as an investigator. It may be strategic to say yes to select opportunities if they will purposefully advance your career development as a young investigator by enabling you to make important contacts that may include productive research collaborations and opportunities. Similarly, one should avoid too many educational commitments, particularly preparing time-consuming lectures for trainees or other audiences. A mentor can be helpful in providing protection from these requests, if needed.
Enter into mentoring slowly
Many young, energetic, early-stage investigators feel the desire to give back and pass on what they are learning by mentoring those more junior (medical students, residents, etc.). However, this responsibility can be time consuming and impede one’s ability to build a strong foundation when it is most critical. It is helpful to take a long view and realize that some selfishness with one’s time at an early stage is important and necessary to establish a viable independent research career. If this is done, one can mentor numerous trainees over a sustained career.
Compartmentalize clinical activity
It is essential to avoid clinical activities that bleed too much into time that is supposed to be protected for research. Large outpatient practices or a clinical leadership position can cause repetitive, unpredictable intrusions on research time and focus. It can be helpful to have defined blocks of clinical activity spaced out to allow for longer stretches of focused research activity. One should also try to avoid scheduling clinical activities during predictable grant application periods.
Resubmit your grant application
It can be devastating for an applicant who has put so much time, effort, and emotion into a grant application to have it receive an unfundable score (or not be scored at all). Although there may be some fatal flaws that cannot be overcome, usually the quickest way to get funded is to carefully review and responsively address the critiques of the reviewers and resubmit the grant. This applies for CDAs, as well as for many other applications. The success rate is much higher for resubmitted applications (Figure 2). One should allow time to get over feelings of disappointment and anger elicited by an unfavorable review and then work with mentors to improve the proposal to address the concerns of the reviewers. Contacting the NIH program officer or scientific review officer assigned to the application may provide strategic insights for making the revision more competitive.
Obtain additional funding
Research is expensive and the project support provided in a CDA is very limited. It is assumed that one’s mentor will help support the proposed work (emphasizing the importance of choosing a mentor[s] who is well-funded). However, even with mentor support, there is often a need for more money to support work that will provide a foundation for an independent R01 award. Recipients of CDAs are candidates for numerous potential awards prior to obtaining R01 funding (sometimes explicitly called “bridging awards”), many offered by foundations and some offered by industry (Table 5). It is vital to not overlook applying for awards that are related to one’s area of research, even though they are not specifically cardiovascular (e.g., funding agencies for research on diabetes, older adults, oncology, among others). To increase the chance of getting an idea/proposal funded by someone, the same idea (with appropriate adaptations pertinent to the particular award guidelines) may be submitted to multiple funding agencies. This is a more efficient strategy than submitting distinct grants to each potential funding agency. If multiple agencies fund a particular idea, the best award can generally be chosen.
Table 5. Examples of Additional Funding to Complement a K Award.
| American Heart Association (Grant-in-Aid, Mentored Clinical and Population Research Award, Fellow-to-Faculty Transition Award) |
| American College of Cardiology Foundation (ACCF/William F. Keating Endowment Career Development Award, ACCF/Merck Research Fellowships in Cardiovascular Disease and Cardiometabolic Disorders) |
| Doris Duke Charitable Foundation (Clinical Scientist Development Award) |
| Burroughs-Wellcome Fund (Career Awards for Medical Scientists) |
| NIH Investigator Research Supplement |
| Gilead Sciences Research Scholars Program in Cardiovascular Disease |
| Institutional pilot awards |
|
Related foundations (American Diabetes Association, Juvenile Diabetes Research Foundation. Career Development Award, Susan G. Komen Foundation, etc.) |
NIH = National Institutes of Health.
Develop collaborations, networks, and teams
Increasingly, there are research questions that cannot be answered by single-center studies and it is becoming more essential to collaborate with like-minded investigators across your institution and at other centers in networks and teams. One should be aware, however, that there can be challenges to carving out the individual contribution made in this setting (particularly at a junior stage) and some of these networks/teams can take years to grow. Leveraging existing registries (e.g., NCDR) to answer some questions may be more expeditious and efficient. The recently launched ACC Research Network seeks to connect investigators across the college and country to promote mentorship and productive research collaborations (16).
Preparing for an R01
The primary purpose of an NIH K award is to develop early-stage cardiovascular investigators into independent scientists, usually marked by an independent NIH R01 award. One should anticipate that the first R01 application may not get funded and require resubmission, so the time from initial submission to eventual funding could be up to 18 to 24 months. As such, an R01 application should be submitted approximately 2 to 3 years before the end of the CDA. To have a competitive and timely R01 application, a careful plan needs to be established to obtain the necessary preliminary data, papers that may be critical to the R01 application should be published, and the viability of collaborations that are important for the R01 proposal demonstrated.
From the Perspective of the Cardiovascular Division Leadership
Throughout the history of medicine, cardiovascular physician-scientists have played an important role in the advancement of medical knowledge and the development of new therapeutic approaches. Informed by their experience in caring for patients, and armed with rigorous training to ask and answer clinically important questions, physician-scientists are uniquely positioned at the crossroads between fundamental biomedical discovery and clinical practice. Accordingly, fostering the investigative careers of physicians in training and/or junior faculty is in the best interest of society, and thus is one of the most important missions of physicians in leadership positions. This statement, however, must be viewed with the knowledge that health care is under siege, now and for the indefinite future, and that fiscal stewardship of divisional resources is also an important role of leadership. Thus, from the divisional perspective, during a time of declining clinical margins, the challenge is to find a way to support research training and still maintain a balanced budget in order to preserve the other missions of the division and/or the department.
Although the value of physician-scientists to society and the world is, in our view, priceless, the cost of training physician-scientists is not an inexpensive undertaking from the divisional perspective. Training physician-scientists takes longer today than yesterday because the research questions are more complex; hence, the knowledge base required to be competitive takes longer to acquire. Furthermore, the increasing training requirements mandated by the Accreditation Council for Graduate Medical Education (ACGME) and American Board of Internal Medicine (ABIM) during the clinical years of training, and the complexity of clinical training resulting from the incredible advances in cardiovascular medicine leave little time for research training, let alone scholarship, during the clinical years of training. Whereas training physician-scientists could typically be accomplished within 3 years previously, given the complexity of training required to be competitive, it now requires 5 (or more) years of substantial protected time. Although the amount of time required for proper research training has never been clearly established, our experience has been that 75% to 80% protected research time for cognitive subspecialists and a minimum of 50% protected time for interventional/invasive subspecialists allows for proper training and support of physician-scientists. This requires a substantial financial commitment on the part of the sponsoring institution because of the need to cost-share that portion of the salary not supported by clinical revenue during training. Given the pressure on health care reimbursement, and the cutbacks that have occurred in cardiology over the past 5 years, the margins that used to support the training of physician scientists simply no longer exist in most divisional budgets. Supporting a cardiovascular physician-scientist until the time of their first R01 (or equivalent) grant, excluding start-up support, can (conservatively) cost between $375,000 to $750,000 per trainee. When multiplied by the number of physician-scientists in training at one time, the annual amount of support required can approach 1 million dollars per year, depending on the number of young research faculty. Viewed within this very pragmatic context, the need to encourage trainees and junior faculty to obtain CDAs is obvious. Beyond these pragmatic economic concerns, the most important reason to encourage trainees and junior faculty to obtain CDAs is to ensure that they obtain rigorous research training and are allowed the requisite time to develop into independent scientists with sustainable research programs. In the end, for those in positions of leadership and mentorship, the sense of satisfaction that one gets from watching trainees become independent investigators is simply an unparalleled professional experience.
Conclusion
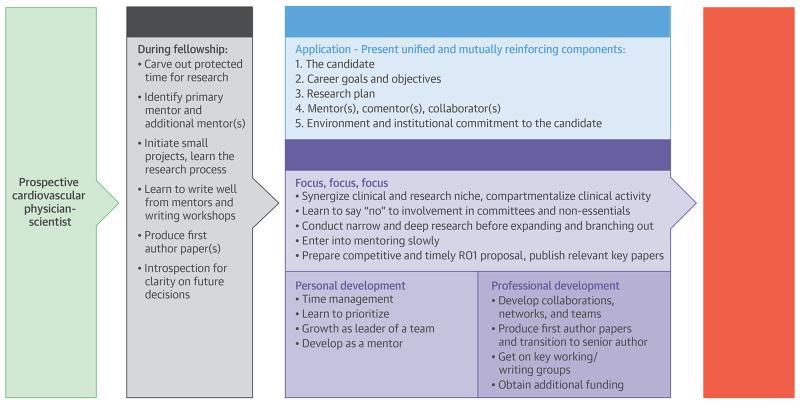
Tremendous opportunities now exist for advancing knowledge and improving cardiovascular health. In the face of myriad threats, the nurturing of talented, capable, and sufficiently equipped cardiovascular physician-scientists remains an indispensable priority. Shortcuts in this training mission will lead to an inability to make sustained advances in the years ahead. Individuals who desire to develop into independent investigators must recognize the need to be all in, with a clear-eyed awareness of the rigor, focus, and time commitment required to be prepared and trained for this role. That path often includes a K award early in the process. We have described strategies for success for obtaining a K award, but also for other aspects of development at this early stage (Central Illustration). The transition between a K award and an independent R01 has its own strategies and challenges, which we have alluded to, but not covered in detail. As emphasized, this is not a recipe or manual to simply read and follow; one needs to be connected with a mentor(s) to truly learn how to operationalize and execute these strategies to flourish.
Central Illustration. Strategies for Success as an Early-Stage Cardiovascular Physician-Scientist.
An NIH K award, combined with solid mentorship and multiple other aspects of personal and professional development, are instrumental to the training of an independent investigator. NIH = National Institutes of Health.
Acknowledgments
The authors thank Kristin West and Amalea Hijar from the American College of Cardiology for their assistance in the preparation of the manuscript.
Funding: Dr. Lindman is supported by NIH K23 HL116660, a Doris Duke Clinical Scientist Development Award (#2014106), and an American Heart Association (AHA) Clinical Research Program Award (#13CRP17080096). Dr. Tong is supported by NIH K08 HL114877 and a shared (Co-PI) AHA Grant-in-Aid 14GRNT20490025. Dr. Madhur is supported by NIH K08HL121671 and a Gilead Cardiovascular Scholars Award. Dr. Barac is supported by Georgetown-Howard Universities Center for Clinical Translational Science (GHUCCTS) KL2 Award, 5KL2TR000102-04. Dr. Abdalla is supported by NIH HL117323-02S2. Dr. Brittain is supported by an AHA Fellow-to-Faculty Award (#13TF16070002) and an Actelion Entelligence Young Investigator Award. Dr. Desai is supported by grant number K12HS023000 from the Agency for Healthcare Research and Quality. Dr. Mann is supported by NIH R01 HL111094 and NIH U10 HL110309.
Abbreviations
- CDA
career development award
- CTSA
Clinical and Translational Science Award
- NIH
National Institutes of Health
- NHLBI
National Heart, Lung and Blood Institute
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: Dr. Lindman has received research support from and served on the scientific advisory board for Roche Diagnostics. Dr. Jackson has consulted for McKesson and the American College of Cardiology and served as an expert witness for Motley Rice, LLC. Dr. Barac has received research support and honoraria for lectures from Genentech, Inc. and consultancy fees from Cell Therapeutics, Inc. Dr. Desai reports that he is a recipient of a research grant through Yale University from Johnson and Johnson to develop methods of clinical trial data sharing and from the Centers of Medicare and Medicaid Services (CMS) to develop and maintain performance measures that are used for public reporting. Dr. Freeman has served as a consultant for Gilead and as a speaker for Medtronic. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Disclaimer: The opinions expressed in this article are the authors’ own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, the Agency for Healthcare Research and Quality, or the United States Government.
References
- 1.Tong CW, Ahmad T, Brittain EL, et al. Challenges facing early career academic cardiologists. J Am Coll Cardiol. 2014;63:2199–208. doi: 10.1016/j.jacc.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins FS. Exceptional opportunities in medical science: a view from the National Institutes of Health. JAMA. 2015;313:131–2. doi: 10.1001/jama.2014.16736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrington RA, Barac A, Brush JE, Jr., et al. COCATS 4 Task Force 15: Training in Cardiovascular Research and Scholarly Activity. J Am Coll Cardiol. 2015;65:1899–906. doi: 10.1016/j.jacc.2015.03.032. [DOI] [PubMed] [Google Scholar]
- 4.The Physician-Scientist Workforce Working Group . National Institute of Health Physician-Scientist Workforce Working Group Report. Advisory Committee to the Director: Physician-Scientist Workforce, National Institutes of Health; [Accessed August 25, 2015]. 2014. Available at: http://acd.od.nih.gov/reports/PSW_Report_ACD_06042014.pdf. [Google Scholar]
- 5.Cyranoski D, Gilbert N, Ledford H, et al. Education: The PhD factory. Nature. 2011;472:276–9. doi: 10.1038/472276a. [DOI] [PubMed] [Google Scholar]
- 6.Alberts B, Kirschner MW, Tilghman S, et al. Rescuing US biomedical research from its systemic flaws. Proc Natl Acad Sci U S A. 2014;111:5773–7. doi: 10.1073/pnas.1404402111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lauer MS, Kiley JP, Mockrin SC, et al. National Heart, Lung, and Blood Institute (NHLBI) strategic visioning: setting an agenda together for the NHLBI of 2025. J Am Coll Cardiol. 2015;65:1130–3. doi: 10.1016/j.jacc.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Scott JD, Carlson DE. K08 and K99 cardiovascular training: comparisons and trends among current awardees. Circ Res. 2012;110:910–4. doi: 10.1161/RES.0b013e3182533291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Houser SR. How to obtain a National Heart, Lung, and Blood Institute-sponsored K08 and K99/R00 grant in the current funding climate. Circ Res. 2012;110:907–9. doi: 10.1161/RES.0b013e3182539d49. [DOI] [PubMed] [Google Scholar]
- 10.Sumandea CA, Balke CW. Funding opportunities for investigators in the early stages of career development. Circulation. 2009;119:1320–7. doi: 10.1161/CIRCULATIONAHA.107.752691. [DOI] [PubMed] [Google Scholar]
- 11.NOT-HL-06-118: Reduction in Percent Effort for NHLBI Mentored Career Development K Awards. National Heart, Lung and Blood Institute; [Accessed August 25, 2015]. 2006. Available at: http://grants.nih.gov/grants/guide/notice-files/NOT-HL-06-118.html. [Google Scholar]
- 12.NOT-OD-08-0605: Revision of NIH Policy Concerning Concurrent Support from Mentored Career Development (K) Award and a Research Grant. National Institutes of Health; [Accessed August 25, 2015]. 2009. Available at: http://grants.nih.gov/grants/guide/notice-files/NOT-OD-08-065.html. [Google Scholar]
- 13.Polanyi M. Personal Knowledge: Towards a Post-Critical Philosophy. University of Chicago Press; Chicago, IL: 1974. [Google Scholar]
- 14.Zerzan JT, Hess R, Schur E, et al. Making the most of mentors: a guide for mentees. Acad Med. 2009;84:140–4. doi: 10.1097/ACM.0b013e3181906e8f. [DOI] [PubMed] [Google Scholar]
- 15.Straus SE, Sackett DL. Mentorship in Academic Medicine. John Wiley & Sons, Inc.; Hoboken, NJ: 2014. [Google Scholar]
- 16.ACC Research Network [Accessed August 25, 2015];American College of Cardiology. Available at: http://www.acc.org/researchnetwork.
- 17.Office of Extramural Research (OER) Office of Planning, Analysis and Communications (OPAC) Division of Statistical Analysis & Reporting (OSAP) Table #204: NIH Career Development (K) Grants: Competing Applications, Awards, Success Rates, and Total Funding, by NIH Institutes/Centers and Activity Code Made with Direct Budget Authority Funds, Fiscal Years 2005-2014. National Institutes of Health; [Accessed August 25, 2015]. 2015. Available at: http://www.report.nih.gov/success_rates/index.aspx. [Google Scholar]
- 18.PA-14-049: Mentored Patient-Oriented Research Career Development Award (Parent K23) National Institutes of Health; [Accessed August 25, 2015]. 2015. Available at: http://grants.nih.gov/grants/guide/pa-files/PA-14-049.html. [Google Scholar]
- 19.PA-14-046: Mentored Clinical Scientist Research Career Development Award (Parent K08) National Institutes of Health; [Accessed August 25, 2015]. Available at: http://grants.nih.gov/grants/guide/pa-files/PA-14-046.html. [Google Scholar]