Abstract
Objective and Background
We examined sleep-related problems in adolescents and young adults after a mild traumatic brain injury (MTBI) or orthopedic injury. We extended the analysis of data from a study of early emotional and neuropsychological sequelae in these populations (McCauley et al. 2014. J Neurotrauma. 31:914).
Methods
We gave the Pittsburgh Sleep Quality Index (PSQI) to 77 participants with MTBI, 71 with orthopedic injury, and 43 non-injured controls. The age range was 12–30 years. We tested sleep quality within 96 hours of injury and at 1- and 3-month follow-up. Participants also completed measures of pain and fatigue, drug and alcohol use, and post-traumatic stress symptoms.
Results
Older participants (mean age = 25 years) in the MTBI group exhibited a sharp increase in sleep-related symptoms between the baseline assessment and 1 month, and still had difficulties at 3 months. Younger participants with MTBI (mean age = 15 years) and older participants with an orthopedic injury had modest increases in sleep difficulties between baseline and 1 month. The participants with MTBI also had more clinically significant sleep difficulties at all 3 assessments. At 3 months, PSQI scores in younger participants with MTBI and all participants with orthopedic injury did not differ significantly from the non-injured controls’. The controls had no significant change in their sleep symptoms during the 3 months.
Conclusions
Sleep difficulties in young adults may persist for ≤3 months after MTBI and exceed those after orthopedic injury. Clinicians should seek and treat sleep-related problems after MTBI.
Keywords: mild traumatic brain injury, sleep, Pittsburgh Quality Sleep Index, adolescents, young adults
Of the > 1 million traumatic brain injuries (TBIs) that occur each year in the United States, mild traumatic brain injuries (MTBIs) account for at least 79% (Langlois et al, 2006). Using incidence data, the US Centers for Disease Control and Prevention estimated that the total lifetime cost of MTBI for 1995, the most recent year studied, was $16.7 billion (National Center for Injury Prevention and Control, 2003). However, Thurman (2001) suggested that most estimates of the total cost of MTBI to society grossly underrepresent the actual figure because they fail to account for such hidden costs as lost productivity and the financial toll on caregivers and family members. It is important to isolate potential contributors to cognitive deficits and functional impairments that may continue past the acute phase of recovery and become chronic problems for individuals who have sustained an MTBI.
Among the numerous cognitive and psychological concerns that have been reported to arise after MTBI, some of the most frequent complaints are sleep deficits (Ayalon et al, 2007; Beetar et al, 1996; Orff et al, 2009; Ouellet et al, 2006; Parcell et al, 2006). In a large study comparing patients admitted to the emergency room after an MTBI with patients admitted for other reasons, Kraus et al (2009) found that 3 months post-injury, those with an MTBI exhibited poorer overall sleep quality, longer sleep latency (the time it takes to fall asleep), and more difficulties with daytime function than than the patients without head injuries.
Vanderploeg et al (2009) reported a risk of continued sleep problems in US Army veterans years after they had sustained an MTBI, even when the authors controlled for associated medical and emotional conditions, including symptoms of post-traumatic stress disorder (PTSD). Rao et al (2011) reported disturbances in polysomnograms and electroencephalographic sleep power spectra in a small sample of individuals with an MTBI. The authors suggested that sleep difficulties after MTBI alter sleep microarchitecture, anxiety, or awareness of sleep problems. Sleep problems after TBI have been linked to symptoms of anxiety and depression, slow information processing, and general difficulties with rehabilitation (Fogelberg et al, 2012; Mathias and Alvaro, 2012; Ponsford et al, 2012).
Sleep problems after TBI are not limited to adults (Blinman et al, 2009; Tham et al, 2012). Tham et al (2012) found that children who had sustained a mild to severe TBI were at greater risk of having sleep difficulties up to 24 months post-injury than were controls with orthopedic injuries, and that worse sleep difficulties were related to poorer functional outcomes at 24 months.
Despite findings of sleep problems in both children and adults after MTBI, few if any studies have systematically examined age differences in these sleep problems. Documented age differences could help determine developmental risk and protective factors and might lead to improvements in detection and treatment for particularly vulnerable populations.
Many of the studies investigating sleep problems after TBI have had limitations such as the lack of an adequate control group (eg, no controls at all, only orthopedic controls, or only typically developing controls), small samples or samples restricted by age and injury severity, and limited or no follow-up. Our study addressed these limitations by measuring sleep quality over time (ie, baseline assessment and 1 and 3 months after injury) in individuals with MTBI, individuals with orthopedic injuries only, and non-injured controls. We studied participants from a broad age range (12 to 30 years) to define sleep issues for adolescents and young adults after MTBI. Compared with other investigations, our approach allowed us to explore how MTBI can affect individuals across the age range, and to suggest whether age effects should be studied more closely. These questions appear particularly timely as our 2014 study (McCauley et al, 2014), upon which this report builds using some of the same participants, suggested a differential impact of age on acute symptoms after MTBI.
In the present study, we assessed sleep variables via the Pittsburgh Sleep Quality Index (PSQI) (Buysse et al, 1989). The PSQI has demonstrated satisfactory reliability and validity when used with a head-injured population (Buysse et al, 1989; Fichtenberg et al, 2012; Mahmood et al, 2004). We hypothesized that individuals with an MTBI would exhibit greater sleep problems at 1-month follow-up than would orthopedic and non-injured controls, and that these problems would persist at 3-month follow-up. Based on our 2014 paper (McCauley et al, 2014), we anticipated that our older participants would have worse sleep problems. Thus, our major aim in this study was to determine whether young people had sleep difficulties in the post-acute phase after MTBI and to explore how age might interact with any observed sleep-related problems.
METHODS
The data that we present in this report were collected during a larger study that our group conducted on the neurocognitive outcomes of MTBI. Some of our participants with MTBI and orthopedic injury (OI) took part in that study, and the procedures were similar. Two articles from the larger study have already been published: Hanten et al (2013) and McCauley et al (2014). These articles add details about our current study’s participants and procedures.
Participants
We studied 191 right-handed individuals between 12 and 30 years of age: 77 who had sustained an MTBI, 71 controls who had sustained an OI, and 43 non-injured controls.
Between December 2008 and January 2013, we recruited the participants with MTBI and OI from consecutive admissions to 3 Level I trauma centers within the Texas Medical Center, in Houston, Texas: Memorial Hermann Hospital-Texas Medical Center, Ben Taub General Hospital, and Texas Children’s Hospital. All of these participants were admitted to the emergency department, treated, and released < 24 hours after their injury. During the admission, the staff excluded post-traumatic amnesia in all of the participants with MTBI using the Galveston Orientation and Amnesia Test (Levin et al, 1979). None of the participants with OI had a history of post-traumatic amnesia, loss of consciousness, or intracranial injury.
We defined MTBI according to recommendations from the US Centers for Disease Control and Prevention (National Center for Injury Prevention and Control, 2003). That is, our participants with MTBI had to have suffered an injury to the head from blunt trauma or from acceleration or deceleration forces, and they had to have experienced at least 1 of the following: 1) observed or self-reported confusion, disorientation, memory dysfunction around the time of the injury, or loss of consciousness for no longer than 30 minutes after the injury; and 2) headache, seizures, dizziness, irritability, fatigue, or poor concentration soon after the injury.
As recommended by the World Health Organization task force on MTBI (Carroll et al, 2004), our participants with MTBI had to have a Glasgow Coma Scale (Teasdale and Jennett, 1974) score ≥13 (minor brain injury) when examined at an emergency center, had no abnormal findings on a computed tomography scan of the brain, and experienced any post-traumatic amnesia for < 24 hours and any loss of consciousness for < 30 minutes. Of note, 3 of our participants with MTBI lacked Glasgow Coma Scale scores; we included them in the study because the emergency room staff judged them to have sustained an MTBI.
Further, our participants with MTBI had to have an extracranial Abbreviated Injury Scale (Petrucelli et al, 1981) score ≤ 3 (“serious” on a scale of 1 [minor] to 6 [maximal] and, within the Scale, a modified Injury Severity Score of < 12 (on a scale of 1 [most minor] to 75 [most critical]). The Abbreviated Injury Scale defines the type, body region, and severity of a physical injury. We selected cut-off scores in keeping with the conventions of previous TBI investigations. For the participants with OI, the Abbreviated Injury Scale had to be ≤3 for any body region, and the modified Injury Severity Scale had to be ≤12, with no head region injury.
Our second control group comprised non-injured controls, also aged 12 to 30 years. Between December 2008 and January 2013, we recruited these volunteers from the Houston area through personal referrals and community advertising. These controls had no history of a head injury or orthopedic injury. All other inclusion and exclusion criteria were identical to those for the other study groups.
Exclusion criteria for all 3 study groups are detailed in Hanten et al (2013). Briefly, we studied only individuals who spoke English or Spanish. The injured individuals could not have a blood alcohol level > 200 mg/dL when they were admitted to the emergency room. We also excluded candidates if they had a previous hospitalization for head injury, a neurodevelopmental disorder, or a serious and persistent mental health condition such as schizophrenia.
Each of the control groups enabled us to make specific comparisons. We matched the participants with OI to those with MTBI for age at time of injury (within 1 year) and for composite socioeconomic status, which includes years of education (see Socioeconomic Composite Index below for an explanation of the education measure). We used the OI group to control for risk factors that predispose to injury, such as pre-existing behavioral problems, subtle learning disabilities, and family variables, as well as to equate for the experience of stress and discomfort of having a traumatic injury and being hospitalized (Bijur and Haslum, 1995; Hanten et al, 2013; McCauley et al, 2014; Stancin et al, 1998, 2001). Researchers also consider individuals with OI to be well-suited to serve as controls to individuals with TBI because of the traumatic nature of their injury (Hanten et al, 2013).
We matched the non-injured controls to both groups of injured participants for age (within 1 year), sex, race, and overall socioeconomic status. As in previous studies with this cohort (Hanten et al, 2013; McCauley et al, 2014), we used the non-injured controls to estimate the effect of time unrelated to injury (ie, sleep problems that might arise spontaneously in this population) and to compare the injured participants’ recovery to changes over time in age-matched and demographically similar peers.
Table 1 shows the participants’ demographic and injury characteristics.
TABLE 1.
Demographic Statistics and Summary Scores of the Study Groups
| Mild Traumatic Brain Injury (n = 77) | Orthopedic Injury (n = 71) | Non-Injured Controls (n = 43) | P | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | Mean | SD | Range | |||
| Age at injury (years) | 20.4 | 5.4 | 12.2–30.6 | 20.7 | 5.5 | 12.1–30.8 | 21.0 | 5.0 | 12.0–30.3 | 0.8300 | |
| Socioeconomic Composite Index1 | −0.18 | 0.72 | −1.59–1.99 | 0.00 | 0.75 | −1.31–2.34 | 0.17 | 0.85 | −1.20–2.43 | 0.0578 | |
| Alcohol Use Disorders Identification Test2 | 3.28 | 4.09 | 0–19 | 2.57 | 3.28 | 0–13 | 3.05 | 3.27 | 0–14 | 0.4949 | |
| Drug Abuse Screening Test3 | 1.01 | 1.59 | 0–8 | 0.86 | 1.48 | 0–7 | 0.86 | 1.23 | 0–5 | 0.7768 | |
| Pain | Baseline | 27.20 | 26.85 | 0–100 | 21.93 | 21.78 | 0–76 | 3.49 | 7.56 | 0–44 | < 0.0001 |
| 1 month | 11.55 | 18.21 | 0–73 | 11.63 | 21.20 | 0–82 | 3.92 | 12.29 | 0–75 | 0.0263 | |
| 3 months | 14.08 | 23.47 | 0–90 | 3.66 | 9.55 | 0–56 | 2.26 | 6.98 | 0–41 | < 0.0001 | |
| Fatigue | Baseline | 33.04 | 31.13 | 0–100 | 21.94 | 27.09 | 0–100 | 21.29 | 25.81 | 0–97 | 0.1257 |
| 1 month | 24.64 | 28.66 | 0–100 | 18.26 | 26.67 | 0–92 | 11.55 | 15.79 | 0–51 | 0.0872 | |
| 3 months | 27.77 | 28.58 | 0–100 | 15.86 | 21.35 | 0–95 | 7.36 | 12.65 | 0–67 | 0.0002 | |
| Post-traumatic stress disorder | Baseline | 33.89 | 14.40 | 17–82 | 23.50 | 8.12 | 17–57 | 23.79 | 6.46 | 17–41 | < 0.0001 |
| 1 month | 31.02 | 11.83 | 17–64 | 23.11 | 7.70 | 17–52 | 21.36 | 5.14 | 17–39 | < 0.0001 | |
| 3 months | 31.28 | 13.31 | 17–83 | 22.46 | 7.27 | 17–61 | 24.80 | 10.69 | 17–66 | < 0.0001 | |
| MTBI N (%) | OI N (%) | NIC N (%) | |||||||||
| Sex | Male | 52 (67.53%) | 53 (74.65%) | 25 (58.14%) | 0.1851 | ||||||
| Female | 25 (32.47%) | 18 (25.35%) | 18 (41.86%) | ||||||||
| Race | Black | 30 (38.96%) | 29 (40.85%) | 15 (34.88%) | 0.8173 | ||||||
| Non-Black | 47 (61.04%) | 42 (59.15%) | 28 (65.12%) | ||||||||
| Mechanism of injury | Motor vehicle | 38 (49.35%) | 10 (14.08%) | NA | < 0.0001 | ||||||
| Non-motor vehicle | 30 (38.96%) | 54 (76.06%) | NA | ||||||||
| Assault | 9 (11.69%) | 7 (9.86%) | NA | ||||||||
| Glasgow Coma Scale score4* | 13 | 3 (4.05%) | NA | NA | N/A | ||||||
| 14 | 12 (16.22%) | ||||||||||
| 15 | 59 (79.73%) | ||||||||||
Bold type indicates significance at P < 0.05.
Three of the 77 participants with MTBI had no Glasgow Coma Scale score.
MTBI = mild traumatic brain injury. OI = orthopedic injury. NIC = non-injured controls. SD = standard deviation. NA = not applicable.
The study protocols were approved by the institutional review board of Baylor College of Medicine. Before beginning the study, we obtained informed consent from the participants who were of legal age and from the parents of the minors. The adolescent participants also gave their assent before taking part.
Procedures
We recruited and conducted baseline testing for the participants with MTBI or OI within 96 hours of their injury. We repeated their tests 1 and 3 months post-injury. We tested the non-injured controls at the same intervals as the injured participants: at baseline, 1 month, and 3 months. We contacted the participants to remind them about their follow-up appointments.
We did the testing for all 3 groups at author H.S.L.’s laboratory at Baylor College of Medicine. We gave all the measures as paper-and-pencil tasks in a standardized manner, and we gave the participants as much time as they needed to complete the tasks.
Pittsburgh Sleep Quality Index
The PSQI (Buysse et al, 1989) is a brief, reliable, valid and widely used self-report measure of sleep quality that can be given repeatedly to adolescents and adults to track symptoms of sleep disturbance (Backhaus et al, 2002; Buysse et al, 1989; Geng et al, 2013; Megdal and Schernhammer, 2007; Morgan et al, 2003; Tan et al, 2012). The PSQI has also demonstrated validity for assessing sleep disturbances after traumatic head injury (Fictenberg et al, 2001) as well as with psychiatrically at-risk adolescents (Lunsford-Avery et al, 2013).
The measure includes 14 multiple-choice questions and 5 short-answer items. Most respondents can complete it in 5 to 10 minutes. In our study, all participants completed the PSQI at each of the 3 testing sessions. At the baseline assessment, they were asked to respond by describing their sleep patterns during the month before their injury.
The PSQI total score combines the responses to 7 subscales: duration of sleep, sleep disturbance, sleep latency, “day dysfunction due to sleepiness,” sleep efficiency, overall sleep quality, and “need meds to sleep” (Buysse et al, 1989). A total score > 5 indicates clinically significant sleep problems. Our study focused on the total sleep score, to provide an overview of sleep-related problems.
Visual Analog Scales for Pain and Fatigue
As explained in McCauley et al (2014), during each assessment we asked the participants to make a mark indicating their current level of pain on a 100-millimeter line on a piece of paper. The line had anchor points at 0 (no pain at all) to 100 (worst pain in your whole life). Participants also made a mark for their current level of fatigue on a similar line with anchor points at 0 (not tired at all) to 100 (very tired). We used the distance between the participants’ marks and the 0 anchor point as a measure of their relative pain and fatigue.
Drug Abuse Screening Test and Alcohol Use Disorders Identification Test
We gave these 2 brief measures to evaluate, respectively, a participant’s history of drug and alcohol abuse symptoms (Bohn et al, 1995; Saunders et al, 1993; Skinner, 1982; Yudko et al, 2007). Both tests have been used in other studies of MTBI (McCauley et al, 2014) and have shown adequate reliability and validity.
Socioeconomic Composite Index
As described by Yeates et al (1997, 2004), the Socioeconomic Composite Index is a measure of family socioeconomic status based on 3 variables: years of maternal education, annual family income (Moos and Moos, 1994), and the Duncan Occupational Status Index (Stevens and Featherman, 1981). In this study, we used years of maternal education for our participants aged 12–18; however, departing from the usual measure, we used the individual’s own years of education for our participants aged 19–30.
Values for the 3 variables are transformed into z scores, averaged together, and then standardized so that the mean = 0 and the standard deviation (SD) = 1. The Socioeconomic Composite Index has been used as a general measure of socioeconomic status in a population with TBI (Schmidt et al, 2010; Yeates et al, 1997, 2004).
Completeness of Collected Data
Of our 191 total participants, 137 completed all 3 visits, 30 missed 1 visit, and 24 missed 2 visits. The participants with missing data did not differ from those with complete data on the outcome measure of PSQI total score: At the baseline assessment, t(181) = −0.04, P = 0.9675; at 1 month, t(159) = −0.01, P = 0.9952; at 3 months, t(149) = −0.88, P = 0.3795. The 3 study groups did not differ in numbers of participants lost to follow-up or numbers of missing sessions: chi-square (2) = 0.55, P = 0.7588. Within the MTBI group, the mechanism of injury (motor vehicle accident, non-motor vehicle accident, or assault) did not show an effect on the missing data: chi-square (2) = 1.45, P = 0.4847. Based on these findings, we judged the missing data to be random, ie, participants were lost to follow-up for reasons such as time conflicts or not wishing to continue with the study, not because of the outcome measure, injury status, or mechanism.
Statistical Analysis
We compared the demographic variables using analysis of variance for continuous variables (age and the Socioeconomic Composite Index), and chi-square test for categorical variables (sex, race, and mechanism of injury). We used a generalized linear mixed model approach to analyze the participants’ pattern of PSQI total scores.
We examined the randomness of intercept, slope, and change of slope of the recovery curves. Only the variance of intercept was significant, and the individual growth curves confirmed the randomness of the intercept. Therefore, we set the intercept as random; however, we fixed the linear (slope) and quadratic (change of slope) terms of the interval. We also entered into the model the data for group, age, Socioeconomic Composite Index, sex, PTSD, pain, and fatigue, to check their effect on the intercept and growth (slope and change of slope). Because the effects of Socioeconomic Composite Index, sex, PTSD, pain, and fatigue were not significant, we dropped them from the model.
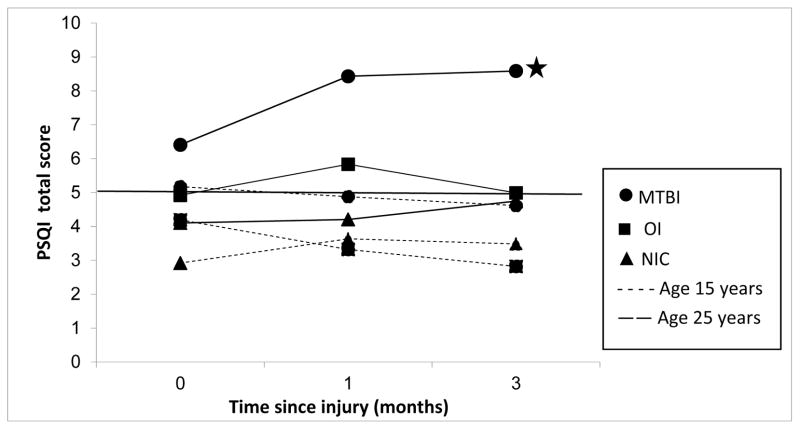
Age, a continuous variable, was centered at its grand mean of 20 years for all 3 study groups. We used fitted values for Figure 1 (see Table 2).
FIGURE 1.
Group differences in Pittsburgh Sleep Quality Index (PSQI) (Buysse et al, 1989) total scores for the 3 study groups: mild traumatic brain injury (MTBI), orthopedic injury (OI), and non-injured controls (NIC). PSQI scores differed by post-injury interval and age. The “younger” subgroup had a mean age of 15 years minus 1 standard deviation. The “older” subgroup had a mean age of 25 years plus 1 standard deviation. The curve marked by a star shows that the MTBI group mean score was significantly worse than the NIC group at baseline assessment, and significantly worse than both the NIC and OI groups at the 1- and 3-month follow-up assessments. The reference line at “5” represents the PSQI cutoff: A score > 5 indicates a sleep disturbance.
TABLE 2.
Parameter Estimates from the Growth Curve Model on the Pittsburgh Sleep Quality Index1 Total Score
| Estimate | t | P | |
|---|---|---|---|
| Intercept (NIC) | 3.5116 | 7.33 | < 0.0001 |
| Group (MTBI versus NIC) | 2.2792 | 3.72 | 0.0002 |
| Group (OI versus NIC) | 1.0461 | 1.69 | 0.0930 |
| Age at baseline | 0.1184 | 1.24 | 0.2148 |
| Age*group (MTBI versus NIC) | 0.0049 | 0.04 | 0.9669 |
| Age*group (OI versus NIC) | −0.0458 | −0.38 | 0.7013 |
| Interval (slope) | 0.5140 | 0.84 | 0.3991 |
| Interval*group (MTBI versus NIC) | 0.6476 | 0.83 | 0.4056 |
| Interval*group (OI versus NIC) | −0.3711 | −0.47 | 0.6401 |
| Interval*age | −0.0934 | −0.77 | 0.4441 |
| Interval*age*group (MTBI versus NIC) | 0.3959 | 2.65 | 0.0084 |
| Interval*age*group (OI versus NIC) | 0.3374 | 2.20 | 0.0288 |
| Interval 2(change of slope) | −0.1042 | −0.60 | 0.5493 |
| Interval 2*group (MTBI versus NIC) | −0.1929 | −0.88 | 0.3794 |
| Interval 2*group (OI versus NIC) | −0.0159 | −0.07 | 0.9432 |
| Interval 2*age | 0.0320 | 0.92 | 0.3597 |
| Interval 2*age* group (MTBI versus NIC) | −0.1024 | −2.46 | 0.0144 |
| Interval 2*age* group (OI versus NIC) | −0.0973 | −2.23 | 0.0263 |
Bold type indicates significance at P < 0.05. The superscript “2” denotes the quadratic function of the interval.
NIC = non-injured controls. MTBI = mild traumatic brain injury. OI = orthopedic injury.
To facilitate interpretation of the results, we also compared the groups on pain, fatigue, and PTSD (Table 1) at each assessment using a log-linear model, and on the dichotomized sleep disturbance variable (PSQI total score > 5) at each assessment using the chi-square test. We also prepared summary statistics for each of the 7 categories of sleep difficulties at each assessment. Because the non-injured control group had a low rate of sleep difficulties, we were unable to use a more advanced model to adjust for other demographic variables, as we could with the continuous variables; however, this level of analysis is not likely needed to provide a valid interpretation of the data.
RESULTS
Demographic Variables
As shown in Table 1, the 3 study groups were balanced for age, sex, race, and socioeconomic level. At all 3 assessments, the participants with MTBI had a significantly higher PTSD score than either the OI or the non-injured control group (P < 0.0001).
Groups differed significantly on the pain score at the baseline assessment (P < 0.0001), 1 month (P = 0.0125), and 3 months (P < 0.0001): The MTBI and OI groups evidenced similarly elevated pain levels at the baseline assessment and 1-month follow-up, while the OI and non-injured control groups had similarly low pain at 3 months. The 3 groups also showed a significant difference on fatigue scores at 1 month (P = 0.0468) and 3 months (P < 0.0001), with the MTBI group again scoring highest and the non-injured control group scoring lowest at each assessment. The 3 groups did not differ significantly on substance use as measured by their scores on the Drug Abuse Screening Test (Yudko et al, 2007) and Alcohol Use Disorders Identification Test (Saunders et al, 1993). We found no group-by-age interactions on pain, fatigue, PTSD, or either substance abuse test.
PSQI Results
Table 2 shows that there was a significant 3-way interaction of group by age and quadratic function of post-injury interval: F (2,292) = 3.34, P = 0.0369, indicating that participants’ sleep-related difficulties after MTBI depended on their age at injury. Figure 1 shows that the older participants with MTBI (25 years old = mean age plus 1 SD) had a sharp increase in their sleep symptoms from the baseline assessment to 1 month post-injury, and their symptoms remained troublesome 3 months post-injury. The younger participants with MTBI (15 years old = mean age minus 1 SD) had fewer sleep-related difficulties at the baseline assessment than did their older counterparts. Unlike the older group, which had a worsening of symptoms with each assessment, the younger subgroup reported a slight drop in symptoms at 1 month and again at 3 months.
At the baseline assessment, the older participants with OI had almost as low a level of sleep-related difficulties as the younger participants with MTBI. However, the older OI subgroup had an increase in sleep-related difficulties at 1 month, followed by a drop at 3 months. The younger OI subgroup had a low level of sleep-related symptoms at the baseline assessment and even fewer symptoms at 1 and 3 months, bringing them very close to the level of the younger non-injured controls at the same assessments. At 3 months, both the younger MTBI subgroup and the older OI subgroup had improved their symptoms to levels similar to those of the older non-injured controls.
Using Bonferroni correction and setting a significance level at 0.0005, we performed post hoc analyses of the PSQI results (pairwise group comparisons, shown in Table 3) and found that the younger participants in all 3 groups did not differ significantly from 1 another at any testing session. However, for the older participants, the MTBI subgroup was significantly worse than the non-injured controls at the baseline assessment (P = 0.0036), and significantly worse than both the OI subgroup and non-injured controls at 1 month and 3 months (P < 0.0001).
TABLE 3.
Post Hoc Comparisons on Pittsburgh Sleep Quality Index1 Total Score for Younger and Older Participants (alpha = 0.0005)
| Age Group | Time of Evaluation | Comparison | Estimate | Standard Error | t | P |
|---|---|---|---|---|---|---|
| Younger: Mean minus 1 standard deviation = 15 years old | Baseline | MTBI versus OI | 0.9796 | 0.7833 | 1.25 | 0.2121 |
| MTBI versus NIC | 2.2547 | 0.9109 | 2.48 | 0.0139 | ||
| OI versus NIC | 1.2750 | 0.9368 | 1.36 | 0.1745 | ||
| 1 month | MTBI versus OI | 1.5548 | 0.7132 | 2.18 | 0.0301 | |
| MTBI versus NIC | 1.24 | 0.8769 | 1.42 | 0.1577 | ||
| OI versus NIC | −0.3127 | 0.8973 | −.35 | 0.7277 | ||
| 3 months | MTBI versus OI | 1.7967 | 0.7503 | 2.39 | 0.0173 | |
| MTBI versus NIC | 1.1312 | 0.9123 | 1.24 | 0.2160 | ||
| OI versus NIC | −0.6655 | 0.9427 | −0.71 | 0.4808 | ||
| Older: Mean plus 1 standard deviation = 25 years old | Baseline | MTBI versus OI | 1.4865 | 0.6998 | 2.12 | 0.0345 |
| MTBI versus NIC | 2.3036 | 0.7844 | 2.94 | 0.0036 | ||
| OI versus NIC | 0.8171 | 0.7774 | 1.05 | 0.2941 | ||
| 1 month | MTBI versus OI | 2.5948 | 0.6561 | 3.95 | < 0.0001 | |
| MTBI versus NIC | 4.2256 | 0.7690 | 5.50 | < 0.0001 | ||
| OI versus NIC | 1.6308 | 0.7692 | 2.12 | 0.0349 | ||
| 3 months | MTBI versus OI | 3.5958 | 0.6857 | 5.24 | < 0.0001 | |
| MTBI versus NIC | 3.8400 | 0.7937 | 4.84 | < 0.0001 | ||
| OI versus NIC | 0.2441 | 0.7842 | 0.31 | 0.7558 |
Bold type indicates significance at P < 0.05.
MTBI = mild traumatic brain injury. OI = orthopedic injury. NIC = non-injured controls.
From a diagnostic point of view, PSQI scores > 5 are considered to signal clinically meaningful sleep disturbance. Thus, we also analyzed the group difference on sleep disturbance (PSQI total score > 5) at each assessment. The MTBI group had a higher rate of sleep disturbance than the other groups at the baseline assessment (P = 0.0115), 1 month (P = 0.0177), and 3 months (P < 0.0001) (Table 4). These results are consistent with those using the PSQI total score.
TABLE 4.
Descriptive Statistics for Sleeping Categories and Sleep Disturbance on the Pittsburgh Sleep Quality Index (PSQI)1
| Assessment (Time Post-Injury) | PSQI Subscale | Mild Traumatic Brain Injury (n = 77) | Orthopedic Injury (n = 71) | Non-Injured Controls (n = 43) | P* | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | SD | Mean | Median | SD | Mean | Median | SD | |||
| Baseline | Quality | 1.18 | 1 | 0.86 | 0.91 | 1 | 0.66 | 0.70 | 1 | 0.51 | 0.0083 |
| Latency | 1.71 | 2 | 0.77 | 1.48 | 1 | 0.62 | 1.68 | 2 | 0.75 | 0.3139 | |
| Duration | 0.58 | 0 | 0.83 | 0.60 | 0 | 0.90 | 0.23 | 0 | 0.48 | 0.0465 | |
| Efficiency | 0.69 | 0 | 1.14 | 0.64 | 0 | 1.00 | 0.30 | 0 | 0.64 | 0.1850 | |
| Disturbance | 1.33 | 1 | 0.61 | 0.94 | 1 | 0.38 | 0.88 | 1 | 0.45 | < 0.0001 | |
| Medication | 0.39 | 0 | 0.90 | 0.13 | 0 | 0.52 | 0.28 | 0 | 0.63 | 0.0949 | |
| Day dysfunction | 0.51 | 0 | 0.75 | 0.28 | 0 | 0.51 | 0.28 | 0 | 0.45 | 0.1381 | |
| PSQI total | 6.04 | 5 | 3.61 | 4.53 | 4 | 2.77 | 3.65 | 3 | 2.48 | 0.0005 | |
| 1 month | Quality | 1.33 | 1 | 0.79 | 0.89 | 1 | 0.69 | 0.90 | 1 | 0.64 | 0.0011 |
| Latency | 1.88 | 2 | 0.70 | 1.56 | 1 | 0.73 | 1.42 | 1 | 0.61 | 0.0197 | |
| Duration | 0.92 | 0 | 1.14 | 0.49 | 0 | 0.87 | 0.31 | 0 | 0.66 | 0.0076 | |
| Efficiency | 0.86 | 0 | 1.24 | 0.80 | 0 | 1.11 | 0.58 | 0 | 0.92 | 0.5686 | |
| Disturbance | 1.23 | 1 | 0.64 | 1 | 1 | 0.61 | 0.85 | 1 | 0.37 | 0.0038 | |
| Medication | 0.46 | 0 | 0.91 | 0.32 | 0 | 0.81 | 0.31 | 0 | 0.66 | 0.6416 | |
| Day Dysfunction | 0.57 | 0 | 0.72 | 0.40 | 0 | 0.64 | 0.33 | 0 | 0.53 | 0.1670 | |
| PSQI total | 6.77 | 6 | 4.00 | 4.77 | 4 | 3.32 | 3.95 | 3 | 2.81 | 0.0006 | |
| 3 months | Quality | 1.20 | 1 | 0.75 | 0.80 | 1 | 0.62 | 0.86 | 1 | 0.77 | 0.0048 |
| Latency | 1.78 | 2 | 0.70 | 1.27 | 1 | 0.56 | 1.76 | 2 | 0.83 | 0.0013 | |
| Duration | 1.08 | 1 | 1.15 | 0.44 | 0 | 0.76 | 0.57 | 0 | 1.04 | 0.0019 | |
| Efficiency | 0.92 | 0 | 1.16 | 0.47 | 0 | 0.84 | 0.57 | 0 | 0.98 | 0.0991 | |
| Disturbance | 1.15 | 1 | 0.48 | 0.86 | 1 | 0.44 | 0.83 | 1 | 0.51 | 0.0011 | |
| Medication | 0.43 | 0 | 0.93 | 0.21 | 0 | 0.68 | 0.11 | 0 | 0.40 | 0.1166 | |
| Day Dysfunction | 0.58 | 0 | 0.81 | 0.25 | 0 | 0.51 | 0.26 | 0 | 0.44 | 0.0263 | |
| PSQI total | 6.72 | 6 | 4.00 | 3.86 | 3 | 2.52 | 4.06 | 4 | 3.48 | < 0.0001 | |
| Sleep disturbance (ie, total PSQI score > 5) | MTBI N (%) | OI N (%) | NIC N (%) | P | |||||||
| Baseline | Yes | 33 (45.8%) | 19 (27.9%) | 9 (20.9%) | 0.0115 | ||||||
| No | 39 (54.2%) | 49 (72.1%) | 34 (79.1%) | ||||||||
| 1 month | Yes | 33 (54.1%) | 21 (34.4%) | 11 (28.2%) | 0.0177 | ||||||
| No | 28 (45.9%) | 40 (65.6%) | 28 (71.8%) | ||||||||
| 3 months | Yes | 32 (53.3%) | 10 (17.9%) | 6 (17.1%) | < 0.0001 | ||||||
| No | 28 (46.7%) | 46 (82.1%) | 29 (82.9%) | ||||||||
Bold type indicates significance at P < 0.05.
P values for PSQI subscales are from Wilcoxon rank sum (nonparametric) test.
DISCUSSION
Our study examined the trajectory of sleep-related symptoms after an MTBI or OI, compared to non-injured controls. We found an interaction between age and injury group: The older (mean age = 25 years) participants with MTBI experienced a significantly greater increase than the other groups in sleep symptoms from the baseline assessment to 1 month post-injury. This group also had persistent sleep-related problems at 3 months and more clinically meaningful elevations on the PSQI at all 3 assessments. This difference became highly significant at the 3-month follow-up. Although the older OI subgroup also had increases in sleep-related difficulties at 1 month, by 3 months their symptoms had subsided to near those at the baseline assessment. Thus, not only did the older MTBI subgroup have higher levels of sleep difficulties at the baseline assessment, but they also had increases in sleep-related problems throughout the study and these difficulties were more pronounced when compared to the younger MTBI subgroup and the OI and non-injured control groups.
We are not aware of other studies that have shown age-related differences in sleep difficulties after an MTBI. However, previous investigations have reported sleep-related difficulties in children after a TBI, and MTBI specifically was a significant risk factor for these symptoms at 24 months post-injury (Tham et al, 2012). Our study did not reveal persistent sleep difficulties in our younger participants, as did Tham and colleagues (2012), perhaps because their methods differed from ours. We used the PSQI to study sleep difficulties in 12- to 30-year-olds with MTBI for 3 months; they studied children of all ages with TBIs ranging from mild to severe for 24 months, using a single-item parent report of sleep problems. Thus, design and methods may account for the differences in findings between the 2 studies. Although the PSQI has been used in other investigations, it is also possible that a self-report instrument may not be sensitive to sleep difficulties in younger people, especially adolescents.
Our findings showed clear evidence of persistent sleep-related difficulties in our older participants with MTBI, those between 20 and 30 years of age. We must be cautious in interpreting how age affects sleep symptoms after MTBI in individuals of other ages. Although our older OI subgroup also had an initial rise in sleep difficulties 1 month post-injury, their symptoms returned to near their baseline assessment levels at 3-month follow-up and were not significantly different from those of the non-injured controls at 3 months.
This pattern suggests that factors related to the experience of being injured might not fully account for the persistent sleep difficulties in the MTBI group. Context-related factors such as stress and anxiety may contribute. For example, young people who sustain a mild head injury may feel especially stressed and anxious about their long-term cognitive, psychiatric, and physical function and their psychosocial and occupational future.
McCauley and colleagues’ (2014) study, using some of the same participants as this analysis, indicated higher levels of perceived stress and psychiatric symptoms in the MTBI group than in the OI and non-injured control groups. McCauley et al also found evidence for an age-by-group interaction such that at the baseline assessment, the participants in the MTBI group experienced higher levels of self-reported cognitive, emotional, and somatic symptoms with increasing age.
Our findings complement those of McCauley et al and suggest that individuals with a mean age = 25 years have more difficulties than younger people throughout the process of recovering from a mild head injury. The consistency between our findings and McCauley et al’s is somewhat perplexing given that both investigations were restricted to 12- to 30-year-olds. This consistency suggests that:
Adults experience more significant mild injuries, possibly as a result of different mechanisms of injury such as more high-speed motor-vehicle accidents.
Adults are more vulnerable to even mild head injuries.
Adults experience other significant factors (eg, pain, fatigue, stress, depression, PTSD symptoms) that either predispose them to or complicate their recovery from mild TBI.
Adults are more likely to have a history of unreported previous closed head injuries.
Pre-injury sleep difficulties predispose some individuals to sustaining an MTBI.
The constraints of our study make it difficult for us to rule out these potential confounds. We screened the participants via a structured historical interview for any previous head injuries. We intended this process to minimize the likelihood of occult head trauma; however, it is possible that the participants sustained very mild head injuries that did not result in any hospitalization, loss of consciousness, or other noticeable effects, but that still may have contributed to our findings. Therefore, although we made every attempt to screen out previous head trauma, our results may have been influenced by a subset of individuals with a history of undisclosed mild head injury.
To control thoroughly for this possibility, future investigations of differences in sleep-related variables should compare a group with a documented history of a single MTBI and a group with a history of multiple mild injuries. Future studies should also seek out age-by-mechanism of injury interactions and age-by-perceived level of stress interactions that might alter the interpretation of our current findings and those of McCauley et al (2014). It would also be valuable to follow a large cohort of individuals with elevated sleep-related problems to determine if over time they have experienced increases in rates of MTBI or other serious injuries.
One interesting possibility about the age differences that we and McCauley et al observed is that adolescents may be less vulnerable to the impact of mild TBI because they have protective factors that the adults lack. The protection could be psychosocial—supportive family and peers—or neurodevelopmental, conferred by the stage of brain development. Studies using neuroimaging techniques would help to explore this hypothesis.
Although it is likely that stress and sleep difficulties have a reciprocal relationship (Lallukka et al, 2012; Petersen et al, 2013), within our study population it is impossible to disentangle whether sleep problems caused or exacerbated participants’ stress, or whether the sleep problems themselves could have been directly caused by stress. Importantly, our MTBI group had significantly elevated PTSD symptoms at all 3 assessments, and this group had more pain and fatigue than the OI group at both 1 and 3 months.
Although it is conceivable that these variables accounted for some of the differences in sleep outcomes in the MTBI group, our analyses did not indicate an age-by-PTSD, -pain, or -fatigue interaction. This suggests that, at least in adults, sleep-related difficulties are important and unique contributors to outcomes after mild head injury. Unfortunately, limitations on our sample size and variability prevented us from using more sophisticated statistical models that would let us be more firm in our causal inferences. Finally, as noted, it is also difficult to determine whether pre-injury sleep problems increase an individual’s vulnerability to sustaining an MTBI, which may then exacerbate the individual’s sleep-related issues.
We should note several important additional limitations of the study. First, the data reflect only self-report measures of sleep disturbance. Future research may gain more information by incorporating partner and parent reports. Similarly, our data represent only subjective sleep problems. We might have obtained different results if we had been able to use objective measures of sleep quality such as polysomnograms.
Second, although we conducted baseline assessments ≤96 hours after our injured participants’ trauma, it is possible that they under-reported their sleep difficulties because of the injury itself, ie, the “good old days” bias (Iverson, 2010). Although given our study’s time frame such bias appears unlikely, this type of under-reporting could exaggerate group differences.
Third, as alluded to above, although we compared the 3 groups on a number of variables that might influence sleep-related problems, the non-injured control group’s relatively low and stable PSQI scores prevented us from conducting a detailed analysis of additional covariants (eg, PTSD symptoms, pain, fatigue, and substance use) that may have influenced the trajectory of sleep-related difficulties. As discussed, however, our analyses indicated no group-by-age interactions in these variables, thus lessening the possibility that these variables altered the patterns of sleep problems.
Likewise, we did not evaluate other potentially exacerbating variables such as depressed mood. Reporting on the same data, McCauley and colleagues (2014) suggested that at the baseline assessment, pain and fatigue scores did not affect group differences in other important variables of interest, eg, emotional factors, post-concussive symptoms, and neuropsychological function.
Thus, these variables may not have differentially affected sleep quality among the groups. Further, the results did not indicate any pain-by-age or fatigue-by-age interactions. Still, we evaluated pain and fatigue symptoms with only a single measure because of testing time constraints, and a more robust assessment of these factors may prove informative in future studies.
As a fourth limitation, although a previous study (Lunsford-Avery et al, 2013) used the PSQI with adolescents, it may not be the most sensitive measure of sleep-related difficulties with this age group. It is possible that our use of the PSQI in this study underestimated the extent of sleep problems in the younger participants. However, given the fact that young adults were included along with adolescents in our subgroup of younger participants, we think it unlikely that the PSQI would grossly underestimate sleep problems in this entire age cohort.
As a final limitation, because we followed participants only through the post-acute phase of their recovery, we cannot draw any conclusions about chronic sleep problems after MTBI.
Compared to earlier work, however, the strengths of our study are our relatively large and homogeneous sample of participants with MTBI, our inclusion of 2 control groups, our use of a well-validated and reliable measure of sleep problems, and our following our participants for 3 months. We were able to underscore the persistent nature of sleep problems after MTBI and to suggest that these difficulties are not the result of other injury-related factors. Further, our suggestion of an interaction between age and sleep problems should be a focus for future research.
Though limited in scope, this study is an important first step toward establishing the extent of sleep problems in young people with MTBI and suggests that patients, especially young adults, deserve assessment and intervention. Basic sleep hygiene training is effective in improving mood and cognitive skills in patients in the post-acute phase of TBI (Wiseman-Hakes et al, 2013). Our findings suggest that such simple recommendations may also help individuals with milder injuries.
Clinicians treating individuals who have suffered a mild head injury should evaluate their patients’ sleep quality for at least the 3- to 6-month recovery period to determine if they need further assessments and treatment. Patients with serious and prolonged sleep difficulties after an MTBI have been helped not only by sleep hygiene and sleep extension but also by cognitive behavioral therapy and relaxation training, all of which have been shown to be effective for a variety of psychiatric and neurologic populations (Dewald-Kaufmann et al, 2014; Martínez et al, 2014; Ruff et al, 2012).
Future research should explore whether sleep difficulties account for variability in outcome after MTBI and whether they account for a significant amount of the variance in post-concussive symptoms or cognitive function in general. Studies that follow patients with MTBI past the post-acute phase of recovery are needed to determine whether sleep problems continue and become a chronic deficit for these individuals. Given the connection between sleep difficulties and cognitive impairments, our findings advance the understanding of the variability in outcome after MTBI by documenting that many adult survivors suffer from sleep problems as a persistent deficit. Finally, our findings may help practitioners both recognize sleep difficulties as important sequelae of MTBI, especially in adults, and identify patients who may require monitoring and support services.
Acknowledgments
Supported in part by National Institute of Neurological Disorders and Stroke Grant 5 P01 NS056202 (H.S.L.).
Glossary
- MTBI
mild traumatic brain injury
- OI
orthopedic injury
- PSQI
Pittsburgh Sleep Quality Index
- PTSD
post-traumatic stress disorder
- SD
standard deviation
- TBI
traumatic brain injury
Footnotes
The authors declare no conflicts of interest.
References
- Ayalon L, Borodkin K, Dishon L, et al. Circadian rhythm sleep disorders following mild traumatic brain injury. Neurology. 2007;68:1136–1140. doi: 10.1212/01.wnl.0000258672.52836.30. [DOI] [PubMed] [Google Scholar]
- Backhaus J, Junghanns K, Broocks A, et al. Test-retest reliability and validity of the Pittsburghh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002;53:737–740. doi: 10.1016/s0022-3999(02)00330-6. [DOI] [PubMed] [Google Scholar]
- Beetar JT, Guilmette TJ, Sparadeo FR. Sleep and pain complaints in symptomatic traumatic brain injury and neurologic populations. Arch Phys Med. 1996;77:1298–1302. doi: 10.1016/s0003-9993(96)90196-3. [DOI] [PubMed] [Google Scholar]
- Benitez A, Gunstad J. Poor sleep quality diminishes cognitive functioning independent of depression and anxiety in healthy young adults. Clin Neuropsychol. 2012;26:214–223. doi: 10.1080/13854046.2012.658439. [DOI] [PubMed] [Google Scholar]
- Bijur P, Haslum M. Cognitive, behavioral, and motoric sequelae of mild head injury in a national birth cohort. In: Broman SH, Michel ME, editors. Traumatic Head Injury in Children. New York, New York: Oxford; 1995. pp. 147–164. [Google Scholar]
- Blinman TA, Houseknecht E, Snyder C. Postconcussive symptoms in hospitalized pediatric patients after mild traumatic brain injury. J Pediatr Surg. 2009;44:1223–8. doi: 10.1016/j.jpedsurg.2009.02.027. [DOI] [PubMed] [Google Scholar]
- Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): validation of a screening instrument for use in medical settings. J Stud Alcohol. 1995;56:423–432. doi: 10.15288/jsa.1995.56.423. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, III, Monk TH. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Carroll LJ, Cassidy JD, Holm L, et al. Methodological issues and research recommendations for mild traumatic brain injury: the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004;43:113–125. doi: 10.1080/16501960410023877. [DOI] [PubMed] [Google Scholar]
- Dean PJ, Sterr A. Long-term effects of mild traumatic brain injury on cognitive performance. Front Hum Neurosci. 2013;7:1–11. doi: 10.3389/fnhum.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewald-Kaufmann JF, Oort FJ, Meijer AM. The effects of sleep extension and sleep hygiene advice on sleep and depressive symptoms in adolescents: a randomized controlled trial. J Child Psychol Psychiatry. 2014;55:273–283. doi: 10.1111/jcpp.12157. [DOI] [PubMed] [Google Scholar]
- Fichtenberg NL, Zafonte RD, Putnam S, et al. Insomnia in a post-acute brain injury sample. Brain Inj. 2012;16:197–206. doi: 10.1080/02699050110103940. [DOI] [PubMed] [Google Scholar]
- Fictenberg NL, Putnam SH, Mann NR, et al. Insomnia screening in postacute traumatic brain injury: utility and validity of the Pittsburgh Sleep Quality Index. Am J Phys Med Rehabil. 2001;80:339–345. doi: 10.1097/00002060-200105000-00003. [DOI] [PubMed] [Google Scholar]
- Fogelberg DJ, Hoffman JM, Dikmen S, et al. Association of sleep and co-occurring psychological conditions at 1 year after traumatic brain injury. Arch Phys Med. 2012;93:1313–1318. doi: 10.1016/j.apmr.2012.04.031. [DOI] [PubMed] [Google Scholar]
- Geng F, Fan F, Mo L, et al. Sleep problems among adolescent survivors following the 2008 Wenchuan earthquake in China: a cohort study. J Clin Psychiatry. 2013;74:67–74. doi: 10.4088/JCP.12m07872. [DOI] [PubMed] [Google Scholar]
- Hanten G, Li X, Ibarra A, et al. Updating memory after mild traumatic brain injury and orthopedic injuries. J Neurotrauma. 2013;30:618–624. doi: 10.1089/neu.2012.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus J, Hsu P, Schaffer K, et al. Preinjury factors and 3-month outcomes following emergency department diagnosis of mild traumatic brain injury. J Head Trauma Rehabil. 2009;24:344–354. doi: 10.1097/HTR.0b013e3181ae35fd. [DOI] [PubMed] [Google Scholar]
- Lallukka T, Ferrie JE, Kivimäki M, et al. Economic difficulties and subsequent sleep problems: evidence from British and Finnish occupational cohorts. Sleep Med. 2012;13:680–685. doi: 10.1016/j.sleep.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Thomas KE. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations, and Deaths. Atlanta, Georgia: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2006. [Accessed November 7, 2014]. Available at http://www.cdc.gov/ncipc/pub-res/TBI_in_US_04/TBI_ED.htm. [Google Scholar]
- Levin HS, O’Donnell VM, Grossman RG. The Galveston Orientation and Amnesia Test: a practical scale to assess cognition after head injury. J Nerv Ment Dis. 1979;167:675–684. doi: 10.1097/00005053-197911000-00004. [DOI] [PubMed] [Google Scholar]
- Lunsford-Avery JR, Orr JM, Gupta T, et al. Sleep dysfunction and thalamic abnormalities in adolescents at ultra high-risk for psychosis. Schizophr Res. 2013;151:148–153. doi: 10.1016/j.schres.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood O, Rapport LJ, Hanks RA, et al. Neuropsychological performance and sleep disturbance following traumatic brain injury. J Head Trauma Rehabil. 2004;19:378–390. doi: 10.1097/00001199-200409000-00003. [DOI] [PubMed] [Google Scholar]
- Martínez MP, Miró E, Sánchez AI, et al. Cognitive-behavioral therapy for insomnia and sleep hygiene in fibromyalgia: a randomized controlled trial. J Behav Med. 2014;37:683–697. doi: 10.1007/s10865-013-9520-y. [DOI] [PubMed] [Google Scholar]
- Mathias JL, Alvaro PK. Prevalence of sleep disturbances, disorders, and problems following traumatic brain injury: a meta-analysis. Sleep Med. 2012;13:898–905. doi: 10.1016/j.sleep.2012.04.006. [DOI] [PubMed] [Google Scholar]
- McCauley SR, Wilde EA, Barnes A, et al. Patterns of early emotional and neuropsychological sequelae after mild traumatic brain injury. J Neurotrauma. 2014;31:914–925. doi: 10.1089/neu.2012.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megdal SP, Scheernhammer ES. Correlates for poor sleepers in a Los Angeles high school. Sleep Med. 2007;9:60–63. doi: 10.1016/j.sleep.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Moos RH, Moos BS. LISRES-A: Life Stressors and Social Resources Inventory: Adult Form. Professional Manual. Odessa, Florida: Psychological Assessment Resources; 1994. [Google Scholar]
- Morgan K, Dixon S, Mathers N, et al. Psychological treatment for insomnia in the management of long-term hypnotic drug use: a pragmatic randomised controlled trial. Br J Gen Pract. 2003;53:923–928. [PMC free article] [PubMed] [Google Scholar]
- National Center for Injury Prevention and Control. Report to Congress on Mild Traumatic Brain Injury in the United States: Steps to Prevent a Serious Public Health Problem. Atlanta, Georgia: Centers for Disease Control and Prevention; 2003. [Accessed November 07, 2014]. Available online at http://www.cdc.gov/ncipc/pub-res/mtbi/mtbireport.pdf. [Google Scholar]
- Ouellet MC, Beaulieu-Bonneau S, Morin CM. Insomnia in patients with traumatic brain injury: frequency, characteristics, and risk factors. J Head Trauma Rehabil. 2006;21:199–212. doi: 10.1097/00001199-200605000-00001. [DOI] [PubMed] [Google Scholar]
- Orff HJ, Ayalon L, Drummond SP. Traumatic brain injury and sleep disturbance: a review of current research. J Head Trauma Rehabil. 2009;24:155–165. doi: 10.1097/HTR.0b013e3181a0b281. [DOI] [PubMed] [Google Scholar]
- Parcell DL, Ponsford JL, Rajaratnam SM, et al. Self-reported changes to nighttime sleep after traumatic brain injury. Arch Phys Med. 2006;87:278–285. doi: 10.1016/j.apmr.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Petersen H, Kecklund G, D’Onofrio P, et al. Stress vulnerability and the effects of moderate daily stress on sleep polysomnography and subjective sleepiness. J Sleep Res. 2013;22:50–57. doi: 10.1111/j.1365-2869.2012.01034.x. [DOI] [PubMed] [Google Scholar]
- Petrucelli E, States J, Hames L. The abbreviated injury scale: evolution, usage and future adaptability. Accid Anal Prev. 1981;13:29–35. [Google Scholar]
- Ponsford JL, Ziino C, Parcell DL, et al. Fatigue and sleep disturbance following traumatic brain injury--their nature, causes, and potential treatments. J Head Trauma Rehabil. 2012;27:224–233. doi: 10.1097/HTR.0b013e31824ee1a8. [DOI] [PubMed] [Google Scholar]
- Rao V, Bergey A, Hill H, et al. Sleep disturbance after mild traumatic brain injury: indicator of injury? J Neuropsychiatry Clin Neurosci. 2011;23:201–205. doi: 10.1176/jnp.23.2.jnp201. [DOI] [PubMed] [Google Scholar]
- Ruff RL, Riechers RG, II, Wang XF, et al. For veterans with mild traumatic brain injury, improved posttraumatic stress disorder severity and sleep correlated with symptomatic improvement. J Rehabil Res Dev. 2012;49:1305–1320. doi: 10.1682/jrrd.2011.12.0251. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption—II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Schmidt AT, Hanten GR, Li X, et al. Emotion recognition following pediatric traumatic brain injury: longitudinal analysis of emotional prosody and facial expression. Neuropsychologia. 2010;48:2869–2877. doi: 10.1016/j.neuropsychologia.2010.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner H. The drug abuse screening test. Addict Behav. 1982;7:363–371. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- Stancin T, Kaugars AS, Thompson GH, et al. Child and family functioning 6 and 12 months after serious pediatric fracture. J Trauma. 2001;51:51–76. doi: 10.1097/00005373-200107000-00011. [DOI] [PubMed] [Google Scholar]
- Stancin T, Taylor HG, Thompson GH, et al. Acute psychosocial impact of pediatric orthopedic trauma with and without accompanying brain injuries. J Trauma. 1998;45:1031–1038. doi: 10.1097/00005373-199812000-00010. [DOI] [PubMed] [Google Scholar]
- Stevens G, Featherman DL. A revised socioeconomic index of occupational status. Soc Sci Res. 1981;10:364–395. [Google Scholar]
- Tan E, Healey D, Gray AR, et al. Sleep hygiene intervention for youth aged 10 to 18 years with problematic sleep: a before-after pilot study. BMC Pediatr. 2012;12:189. doi: 10.1186/1471-2431-12-189. Available online at http://www.biomedcentral.com/1471-2431/12/189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness: a practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Tham SW, Palermo TM, Vavilala MS, et al. The longitudinal course, risk factors, and impact of sleep disturbances in children with traumatic brain injury. J Neurotrauma. 2012;29:154–161. doi: 10.1089/neu.2011.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman D. The epidemiology and economics of head trauma. In: Miller L, Hayes R, editors. Head Trauma: Basic, Preclinical and Clinical Directions. New York, New York: Wiley & Sons; 2001. [Google Scholar]
- Vanderploeg RD, Belanger HG, Curtiss G. Mild traumatic brain injury and posttraumatic stress disorder and their associations with health symptoms. Arch Phys Med. 2009;90:1084–1093. doi: 10.1016/j.apmr.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Wiseman-Hakes C, Murray B, Moineddin R, et al. Evaluating the impact of treatment for sleep/wake disorders on recovery of cognition and communication in adults with chronic TBI. Brain Inj. 2013;27:1364–1376. doi: 10.3109/02699052.2013.823663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates KO, Swift E, Taylor HG, et al. Short- and long-term social outcomes following pediatric traumatic brain injury. J Int Neuropsychol Soc. 2004;10:412–426. doi: 10.1017/S1355617704103093. [DOI] [PubMed] [Google Scholar]
- Yeates KO, Taylor HG, Drotar DD, et al. Preinjury family environment as a determinant of recovery from traumatic brain injuries in school-age children. J Int Neuropsychol Soc. 1997;3:617–630. [PubMed] [Google Scholar]
- Yudko E, Lozhkina O, Fouts A. A comprehensive review of the psychometric properties of the Drug Abuse Screening Test. J Subst Abuse Treat. 2007;32:189–198. doi: 10.1016/j.jsat.2006.08.002. [DOI] [PubMed] [Google Scholar]