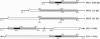Abstract
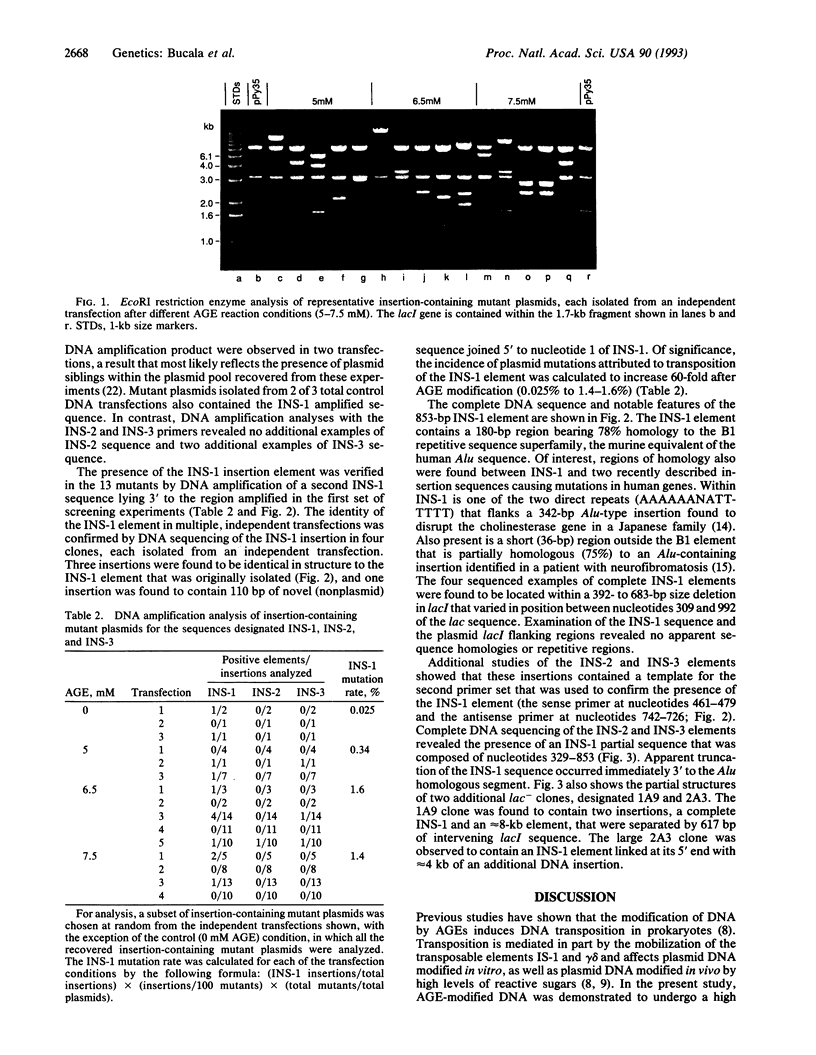
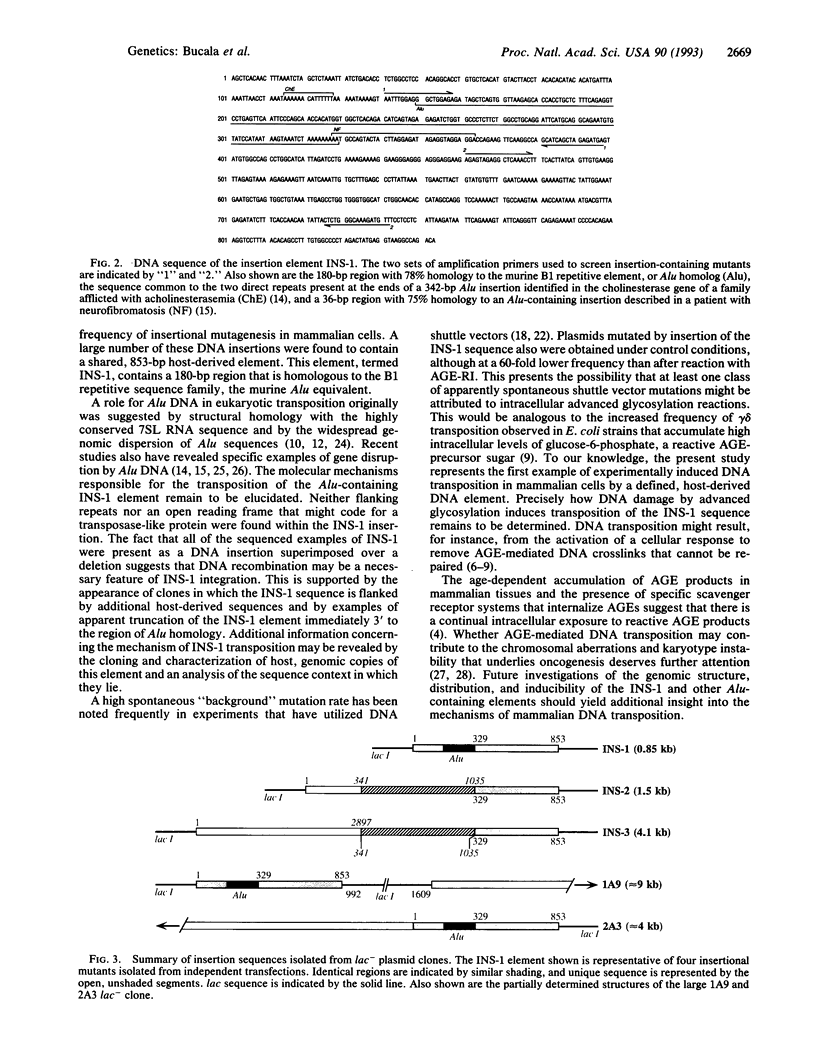
Advanced glycosylation endproducts react with DNA and cause mutations and DNA transposition in bacteria. To investigate the mutagenic effect of advanced glycosylation in mammalian cells, plasmid DNA containing the lacI mutagenesis marker was modified by advanced glycosylation endproducts in vitro, transfected into murine lymphoid cells, recovered, and analyzed for mutations, plasmid size changes, and the presence of shared insertion sequences. An 853-bp host-derived DNA sequence, designated INS-1, was identified as an insertion element common to plasmids recovered from multiple independent transfections. Modification of DNA by advanced glycosylation increased by 60-fold the apparent frequency of INS-1 transposition: from 0.025% to 1.5%. The INS-1 element contains a 180-bp region that is homologous to the Alu repetitive sequence family. INS-1 was also observed to be present within larger insertional mutations and, in two cases, an apparently truncated version of INS-1 that lacks the Alu region was identified. These results demonstrate the experimental induction of DNA transposition involving mammalian chromosomal elements and suggest that advanced glycosylation may play a role in the formation of Alu-containing insertions that have been found to disrupt human genes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bucala R., Cerami A. Advanced glycosylation: chemistry, biology, and implications for diabetes and aging. Adv Pharmacol. 1992;23:1–34. doi: 10.1016/s1054-3589(08)60961-8. [DOI] [PubMed] [Google Scholar]
- Bucala R., Model P., Cerami A. Modification of DNA by reducing sugars: a possible mechanism for nucleic acid aging and age-related dysfunction in gene expression. Proc Natl Acad Sci U S A. 1984 Jan;81(1):105–109. doi: 10.1073/pnas.81.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucala R., Model P., Russel M., Cerami A. Modification of DNA by glucose 6-phosphate induces DNA rearrangements in an Escherichia coli plasmid. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8439–8442. doi: 10.1073/pnas.82.24.8439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucala R., Tracey K. J., Cerami A. Advanced glycosylation products quench nitric oxide and mediate defective endothelium-dependent vasodilatation in experimental diabetes. J Clin Invest. 1991 Feb;87(2):432–438. doi: 10.1172/JCI115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns J. The origin of human cancers. Nature. 1981 Jan 29;289(5796):353–357. doi: 10.1038/289353a0. [DOI] [PubMed] [Google Scholar]
- Calabretta B., Robberson D. L., Barrera-Saldaña H. A., Lambrou T. P., Saunders G. F. Genome instability in a region of human DNA enriched in Alu repeat sequences. Nature. 1982 Mar 18;296(5854):219–225. doi: 10.1038/296219a0. [DOI] [PubMed] [Google Scholar]
- Calos M. P., Lebkowski J. S., Botchan M. R. High mutation frequency in DNA transfected into mammalian cells. Proc Natl Acad Sci U S A. 1983 May;80(10):3015–3019. doi: 10.1073/pnas.80.10.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombroski B. A., Mathias S. L., Nanthakumar E., Scott A. F., Kazazian H. H., Jr Isolation of an active human transposable element. Science. 1991 Dec 20;254(5039):1805–1808. doi: 10.1126/science.1662412. [DOI] [PubMed] [Google Scholar]
- Fanning T. G., Singer M. F. LINE-1: a mammalian transposable element. Biochim Biophys Acta. 1987 Dec 8;910(3):203–212. doi: 10.1016/0167-4781(87)90112-6. [DOI] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Jagadeeswaran P., Forget B. G., Weissman S. M. Short interspersed repetitive DNA elements in eucaryotes: transposable DNA elements generated by reverse transcription of RNA pol III transcripts? Cell. 1981 Oct;26(2 Pt 2):141–142. doi: 10.1016/0092-8674(81)90296-8. [DOI] [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Lebkowski J. S., DuBridge R. B., Antell E. A., Greisen K. S., Calos M. P. Transfected DNA is mutated in monkey, mouse, and human cells. Mol Cell Biol. 1984 Oct;4(10):1951–1960. doi: 10.1128/mcb.4.10.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A. T., Cerami A. Elevated glucose 6-phosphate levels are associated with plasmid mutations in vivo. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8311–8314. doi: 10.1073/pnas.84.23.8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A. T., Cerami A. Induction of gamma delta transposition in response to elevated glucose-6-phosphate levels. Mutat Res. 1991 Jul;249(1):125–133. doi: 10.1016/0027-5107(91)90139-f. [DOI] [PubMed] [Google Scholar]
- Lee A. T., Cerami A. The formation of reactive intermediate(s) of glucose 6-phosphate and lysine capable of rapidly reacting with DNA. Mutat Res. 1987 Aug;179(2):151–158. doi: 10.1016/0027-5107(87)90305-8. [DOI] [PubMed] [Google Scholar]
- Lehrman M. A., Goldstein J. L., Russell D. W., Brown M. S. Duplication of seven exons in LDL receptor gene caused by Alu-Alu recombination in a subject with familial hypercholesterolemia. Cell. 1987 Mar 13;48(5):827–835. doi: 10.1016/0092-8674(87)90079-1. [DOI] [PubMed] [Google Scholar]
- Mathias S. L., Scott A. F., Kazazian H. H., Jr, Boeke J. D., Gabriel A. Reverse transcriptase encoded by a human transposable element. Science. 1991 Dec 20;254(5039):1808–1810. doi: 10.1126/science.1722352. [DOI] [PubMed] [Google Scholar]
- Mitchell G. A., Labuda D., Fontaine G., Saudubray J. M., Bonnefont J. P., Lyonnet S., Brody L. C., Steel G., Obie C., Valle D. Splice-mediated insertion of an Alu sequence inactivates ornithine delta-aminotransferase: a role for Alu elements in human mutation. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):815–819. doi: 10.1073/pnas.88.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muratani K., Hada T., Yamamoto Y., Kaneko T., Shigeto Y., Ohue T., Furuyama J., Higashino K. Inactivation of the cholinesterase gene by Alu insertion: possible mechanism for human gene transposition. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11315–11319. doi: 10.1073/pnas.88.24.11315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njoroge F. G., Monnier V. M. The chemistry of the Maillard reaction under physiological conditions: a review. Prog Clin Biol Res. 1989;304:85–107. [PubMed] [Google Scholar]
- Nowell P. C. The clonal evolution of tumor cell populations. Science. 1976 Oct 1;194(4260):23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- Ullu E., Tschudi C. Alu sequences are processed 7SL RNA genes. Nature. 1984 Nov 8;312(5990):171–172. doi: 10.1038/312171a0. [DOI] [PubMed] [Google Scholar]
- Vlassara H., Brownlee M., Cerami A. High-affinity-receptor-mediated uptake and degradation of glucose-modified proteins: a potential mechanism for the removal of senescent macromolecules. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5588–5592. doi: 10.1073/pnas.82.17.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassara H., Brownlee M., Manogue K. R., Dinarello C. A., Pasagian A. Cachectin/TNF and IL-1 induced by glucose-modified proteins: role in normal tissue remodeling. Science. 1988 Jun 10;240(4858):1546–1548. doi: 10.1126/science.3259727. [DOI] [PubMed] [Google Scholar]
- Wallace M. R., Andersen L. B., Saulino A. M., Gregory P. E., Glover T. W., Collins F. S. A de novo Alu insertion results in neurofibromatosis type 1. Nature. 1991 Oct 31;353(6347):864–866. doi: 10.1038/353864a0. [DOI] [PubMed] [Google Scholar]