Abstract
Recently, we reported that extracellular ubiquitin functions as another agonist of CXC chemokine receptor (CXCR)4. Whereas the cognate CXCR4 ligand, stromal cell-derived factor (SDF)-1α, is also a CXCR7 agonist, ubiquitin does not bind to CXCR7. Because both ligands are present in the extracellular environment, co-activation of CXCR4 appears to be physiologically relevant. CXCR4 mediated effects of ubiquitin, however, are not well understood and consequences of co-activation of CXCR4 with both ligands are unknown. Utilizing proximity ligation assays and flow cytometry, we detected CXCR4, but not CXCR7, on the cell surface of THP-1 cells, which suggests that confounding effects of CXCR7 are unlikely. Time course and magnitude of reduction of cell surface CXCR4 expression were comparable after stimulation of THP-1 cells with both ligands. SDF-1α was more efficacious than ubiquitin to mobilize Ca2+. Co-stimulation of THP-1 cells with both ligands resulted in synergistic effects on Ca2+ fluxes at suboptimal ligand concentrations. Homologous desensitization of Ca2+ fluxes was detectable with both ligands. SDF-1α pre-stimulation desensitized ubiquitin induced Ca2+ fluxes, but not vice versa. Effects of SDF-1α and ubiquitin on cAMP levels, Akt and ERK1/2 phosphorylation and chemotactic responses were additive. The chemotactic activities of ubiquitin and SDF-1α were sensitive to AMD3100, pertussis toxin, U73122, LY94002 and U0126. These data suggest that CXCR4 activation with SDF-1α and ubiquitin results in partially synergistic effects on cellular signaling events and in differential effects on receptor desensitization. The ligand ratio that is present in the extracellular environment may contribute to the regulation of CXCR4 mediated functions.
Keywords: Calcium, Cyclic AMP, CXCL12, Chemotaxis, CXCR7
1. Introduction
CXC chemokine receptor (CXCR)4 and its cognate ligand, stromal cell-derived factor (SDF)-1α (CXCL12), play important roles during development and in numerous disease processes, such as cancer metastasis, HIV, tissue repair, autoimmune and inflammatory diseases. Recently, we and others have reported that extracellular ubiquitin functions as an immune modulator and as another CXCR4 agonist [1–4]. Unlike SDF-1α, however, ubiquitin does not bind to CXCR7 and both ligands appear to activate CXCR4 through different mechanisms and separate binding sites on the receptor [5–7]. Both CXCR4 ligands are constitutively expressed in the systemic circulation and increased concentrations of SDF-1α and ubiquitin have been reported during infectious and sterile inflammation [1]. Whereas the consequences of CXCR4 activation with SDF-1α have been well characterized, ubiquitin mediated effects on CXCR4 are less well understood. Furthermore, CXCR4 appears to exist as a homodimer or as a heterodimer with CXCR7 [8,9], which suggests that each dimeric receptor unit might be engaged by a single ligand or simultaneously by the same or alternative ligands. CXCR4 mediated signaling events upon simultaneous activation with SDF-1α and ubiquitin, however, are unknown. As such an activation pattern appears to be physiologically relevant, the purpose of the present study was to assess whether separate and simultaneous activation of CXCR4 with SDF-1α and ubiquitin results in distinct effects on cell signaling and function in the human monocytic cell line THP-1.
2. Methods
2.1. Cell lines
THP-1 (human monocytic leukemia cells) cells were as described [2,7]. A7r5 (rat aortic smooth muscle cells, ATCC) cells were cultured in high glucose Dulbecco’s Modified’s Eagle Medium, 10 mg/mL sodium pyruvate, 2 mM l-glutamine, 10% fetal bovine serum, 100 U/mL penicillin, 100 µg/mL streptomycin.
2.2. Proteins and reagents
SDF-1α was as described [5,7]. Ubiquitin was obtained from R&D Systems. Bovine serum albumin, forskolin, AMD3100, pertussis toxin and U73122 were purchased from Sigma, LY94002 and U0126 from Cell Signaling and Trichostatin A (TSA) from Selleckchem. The Duolink proximity ligation assay was purchased from Olink Bioscience.
2.3. Fluorescence-activated cell sorting (FACS) analyses
Cells were labeled with polyclonal rabbit anti-CXCR4 (Abcam) and polyclonal rabbit anti-RDC1/CXCR7 (LS-Biolab) in combination with anti-rabbit fluorescein isothiocyanate (FITC) conjugated goat IgG (Abcam). Rabbit IgG (R&D Systems) in combination with FITC-conjugated anti-rabbit goat IgG (Abcam) were used as a negative control. The fluorescence intensities of at least 3×104 cells were recorded and analyzed using the FlowJo software (Tree Star).
2.4. Ca2+ assay
Intracellular calcium (Fluo-4NW Calcium Assay (Molecular Probes)) was measured as described [2,5,6].
2.5. cAMP assay
Quantification of cAMP levels was performed in forskolin treated cells as described [5–7].
2.6. Western blotting
Western blotting was performed as described [2,5]. In brief, 3 × 105 THP-1 cells were lysed in Laemmli sample buffer and 30 µL of the cell lysates were used for SDS–PAGE. Mouse monoclonal anti-phospho-p44/42 MAPK ERK1/2 (Thr202/Tyr204) (Cell Signaling) and rabbit monoclonal anti-phospho-Akt (Ser473) (Cell Signaling) were used in combination with anti-mouse or anti-rabbit IgG horseradish peroxidase-linked whole antibody (GE Healthcare), respectively. Rabbit anti-GAPDH (Cell Signaling) was used in combination with anti-rabbit IgG HRP-linked whole antibody (GE Healthcare) as a protein loading control.
2.7. Chemotaxis assay
Cell migration was assessed using the ChemoTx 96-well cell migration system, as described [5,7]. The chemotactic index (CI) was calculated as the ratio of cells that transmigrated through the filter in presence versus the absence (= PBS/control) of the test solutions.
2.8. In situ proximity ligation assays (PLA)
THP-1 cells were fixed on microscopic slides using 4% paraformaldehyde after cytospinning (2000 rpm, 10 min). A7r5 cells were grown and fixed on eight well tissue culture slides (Nunc). After blocking in 3% BSA/PBS, slides were incubated with rabbit anti-CXCR4 (1:400) or rabbit anti-CXCR7 (1:400) at 37 °C for 2 h in a humidifying chamber. As an antibody control, slides were incubated with PBS instead of primary antibody. Slides were washed and incubated (1 h, 37 °C) with secondary anti-rabbit antibodies conjugated with plus and minus Duolink II PLA probes (1:5). After washing, slides were then incubated with ligation-ligase solution (30 min, 37 °C) followed by incubation with amplification-polymerase solution (2 h, 37 °C), as per manufacturer’s protocol. Slides were then mounted with minimal volume of Duolink II Mounting medium with 4′,6-diamidino-2-phenylindole (DAPI) for 15–30 min and PLA signals (Duolink In Situ Detection Reagents Green (λexcitation/emission 495/527 nm) or Red (λexcitation/emission 598/634 nm)) were identified as fluorescent spots under a fluorescence microscope using 40×/100× objectives.
2.9. Data analyses
Data are expressed as mean ± SE. Data were analyzed with analysis of variance with Dunnett’s post-test to control for multiple testing using the GraphPad-Prism 5 software. Densitometric quantifications of chemiluminescence signals from Western blotting experiments were performed with the ImageLab Version 4.0.1 software (Biorad).
3. Results
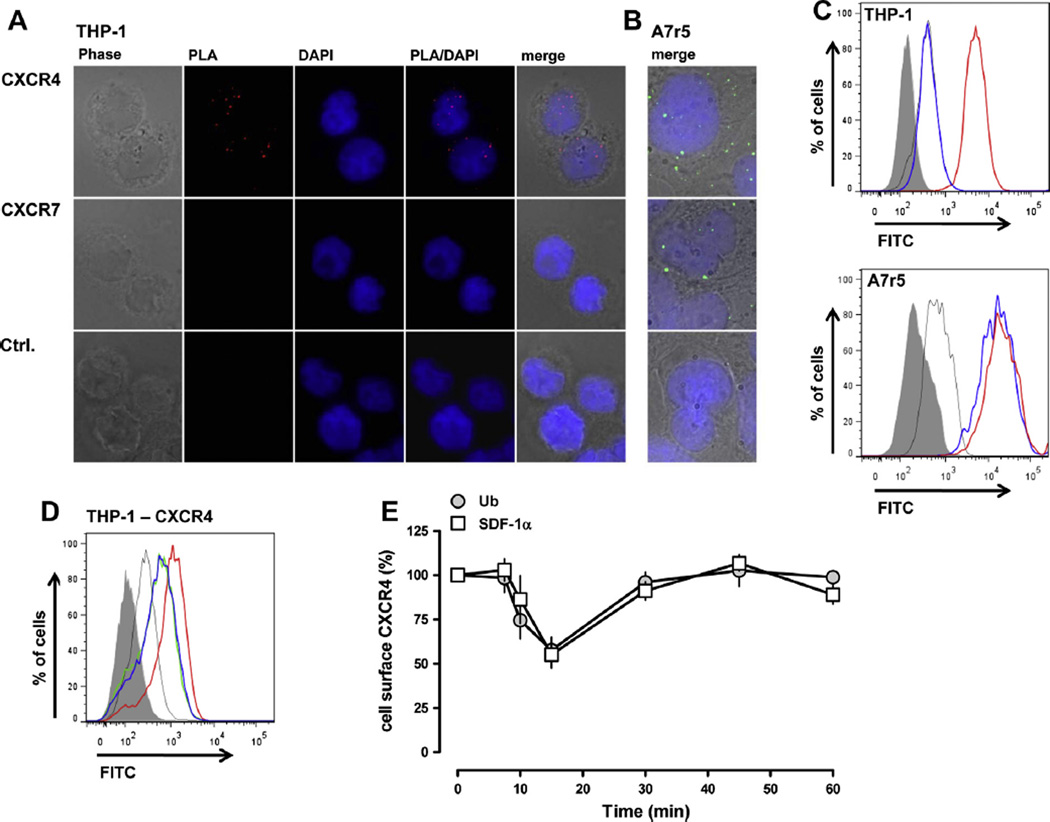
We first evaluated CXCR4/7 cell surface expression in THP-1 and A7r5 cells. A7r5 cells were used as a positive control because both receptors are known to be expressed on vascular smooth muscle cells [10]. A fluorescence signal for CXCR4 was detectable on THP-1 cells in in situ PLA, whereas CXCR7 could not be visualized (Fig. 1A). In contrast, PLA fluorescence signals for both receptors were detectable in A7r5 cells (Fig. 1B). Similarly, CXCR4, but not CXCR7, was detectable on the cell surface of THP-1 cells by FACS analyses, whereas A7r5 cells stained positive for both receptors (Fig. 1C).
Fig. 1.
(A) In situ proximity ligation assays (PLA) to detect CXCR4 and CXCR7 on THP-1 cells. The Duolink In Situ Detection Reagent Red was used to visualize CXCR4 (top row) and CXCR7 (center row). Bottom row: control, no primary antibody. From left to right: phase contrast, PLA – red, DAPI, merged PLA/DAPI and merged phase contrast/PLA/DAPI (merge). (B) In situ proximity ligation assays (PLA) to detect CXCR4 and CXCR7 on A7r5 cells. The Duolink In Situ Detection Reagent Green was used to visualize CXCR4 (top row) and CXCR7 (center row). Bottom row: control, no primary antibody. Merged phase contrast/PLA/DAPI images (merge) are shown. (C) Detection of CXCR4 and CXCR7 on THP-1 (top) and A7r5 cells (bottom) by FACS analyses. Thick red lines: cells labeled with rabbit anti-CXCR4/FITC-conjugated anti-rabbit goat IgG. Thick blue lines: cells labeled with rabbit anti-CXCR7/FITC-conjugated anti-rabbit goat IgG. Thin gray lines: control – cells labeled with rabbit anti-IgG/FITC-conjugated anti-rabbit goat IgG. Gray: unstained cells. (D) CXCR4 expression on THP-1 cells 15 min after treatment with 100 nM of the CXCR4 ligands or control (PBS) at 37 °C. Red thick line: cells labeled with rabbit anti-CXCR4/FITC-conjugated anti-rabbit goat IgG, control. Green thick line: cells labeled with rabbit anti-CXCR4/FITC-conjugated anti-rabbit goat IgG, SDF-1α treatment. Blue thick line: cells labeled with rabbit anti-CXCR4/FITC-conjugated anti-rabbit goat IgG, ubiquitin treatment. Thin gray line: cells stained with rabbit anti-IgG/FITC-conjugated anti-rabbit goat IgG, PBS treatment. Gray: unstained cells. (E) Quantification of CXCR4 expression on THP-1 cells after ligand treatment by FACS analyses, as in D. Cell surface CXCR4 expression is expressed as % of untreated control (n = 4–6). Open squares: 100 nM SDF-1α treatment. Gray circles: 100 nM ubiquitin treatment. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Next, we quantified CXCR4 cell surface expression in THP-1 cells after incubation with SDF-1α or ubiquitin. Fig. 1D shows typical FACS analyses for CXCR4 at the time point of maximal effects of the ligands and Fig. 1E shows the quantification of the changes in CXCR4 staining within 60 min of incubation with the CXCR4 agonists. SDF-1α and ubiquitin reduced the FACS signal for cell surface CXCR4 time dependently to 55 ± 8% and 57 ± 7% of control (t = 0 min-100%), respectively. Maximal effects were detectable after 15 min of incubation. The CXCR4 signal recovered to baseline levels within 60 min of incubation with both ligands.
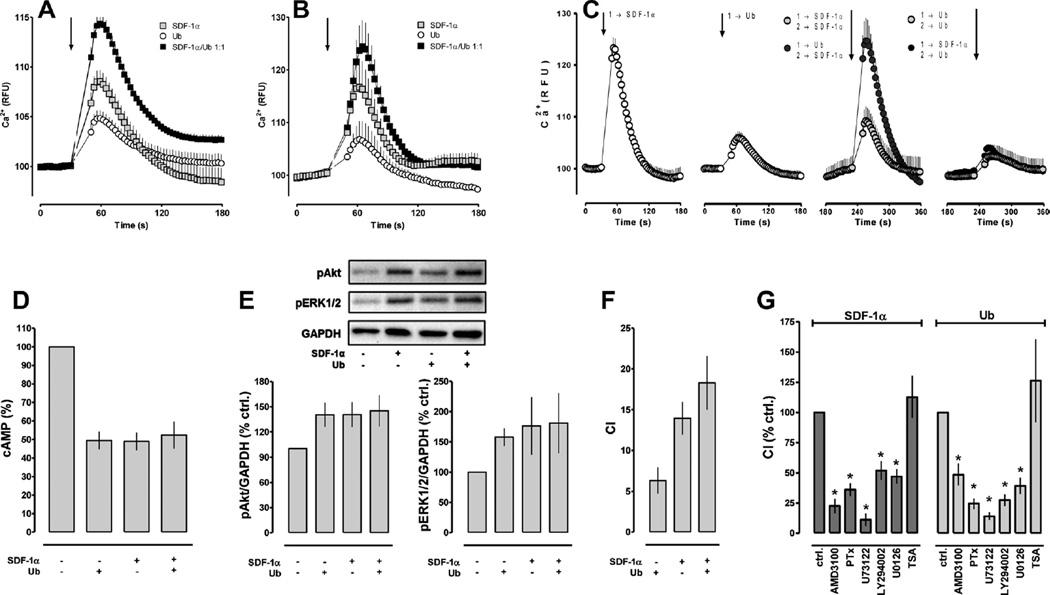
We then analyzed Ca2+ fluxes (Fig. 2A–C), cAMP levels (Fig. 2D) and protein kinase phosphorylation (Fig. 2E) in THP-1 cells as read outs for CXCR4 mediated cell signaling. SDF-1α, ubiquitin and the combination of both were tested in parallel in all experiments to control day to day variations of the overall magnitude of the cellular responses. When compared with the Ca2+ fluxes after stimulation with each CXCR4 agonist alone, co-stimulation with SDF-1α and ubiquitin at an equimolar concentration and a ligand ratio of 1:1(mol/mol) resulted in enhanced cellular Ca2+ mobilization at ligand concentrations in the lower nmolar range (10–20 nM; area under curve (AUC): ubiquitin-290; SDF-1α-502; SDF-1α/ubiquitin 1:1 (mol/mol)-1066; Fig. 2A). This effect could not be detected with confidence at a 10–20-fold higher ligand concentration (AUC at 200 nM: ubiquitin-524; SDF-1α-900; SDF-1α/ubiquitin 1:1 (mol/mol)-1139; Fig. 2B). When THP-1 cells were stimulated repetitively with either SDF-1α or ubiquitin, reduced Ca2+ fluxes upon subsequent stimulation were detectable at ligand concentrations of 10 nM (Fig. 2C), but not at ligand concentrations of 100 pM and 1 nM (not shown). Pre-treatment of THP-1 cells with 10 nM SDF-1α resulted in reduced Ca2+ fluxes upon subsequent stimulation with the same concentration of ubiquitin. Ubiquitin pre-treatment, however, did not reduce Ca2+ mobilization in response to SDF-1α, when compared to the Ca2+ fluxes upon initial stimulation with SDF-1α.
Fig. 2.
(A and B) Intracellular Ca2+ fluxes in THP-1 cells after stimulation with equimolar concentrations of SDF-1α (gray squares), ubiquitin (open squares) and SDF-1α plus ubiquitin 1:1 (mol/mol) (black squares). (A) Ligand concentration 10–20 nM; n = 7 (4 experiments with 10 nM and 3 experiments with 20 nM ligand concentration) with 5–10 replicates per experiment and condition. (B) Ligand concentration 200 nM; n = 3 with 5–10 replicates per experiment and condition. The arrow indicates the time point when SDF-1α/ubiquitin were added. RFU: relative fluorescence units. (C) Intracellular Ca2+ fluxes in THP-1 cells after repetitive stimulation with 10 nM SDF-1α or ubiquitin (n = 3–4 with 5–10 replicates per experiment and condition). From left to right: initial stimulation with SDF-1α (1→SDF-1α); initial stimulation with ubiquitin (1→Ub); subsequent stimulation with SDF-1α after initial stimulation with SDF-1α (gray circles; 1→SDF-1α 2→SDF-1α) or ubiquitin (black circles; 1→Ub 2→SDF-1α); subsequent stimulation with ubiquitin after initial stimulation with ubiquitin (gray circles; 1→Ub 2→Ub) or SDF-1α (black circles; 1→SDF-1α 2→Ub). The arrows indicate the time points when SDF-1α/ubiquitin were added. (D) Reduction of cAMP levels in forskolin stimulated THP-1 cells by 200 pM SDF-1α, 200 pM ubiquitin (Ub) or 100 pM SDF-1α plus 100 pM ubiquitin; n = 3. Data are expressed as % of untreated cells (=100%). (E) Top: Western blot analyses of Akt and ERK1/2 phosphorylation after stimulation (15 min, 37 °C) of THP-1 cells with 200 nM ubiquitin (Ub), SDF-1α or 100 nM of both ligands. Bottom: Quantification of the chemiluminescence signals after cell stimulation, as shown in the top panel; n = 3. Data are expressed as % of untreated cells (=100%). (F) Migration of THP-1 cells towards 10 nM of SDF-1α, 10 nM of ubiquitin (Ub) or 10 nM of both ligands. CI: chemotactic index. (G) Pharmacological inhibition of SDF-1α (left) and ubiquitin (right) induced chemotaxis in THP-1 cells. AMD3100: chemotaxis in the presence of 10 µM AMD3100. Cells were pre-incubated with all other inhibitors (pertussis toxin (PTx)-100 ng/mL, 2 h; U73122-5 µM, 30 min; LY294002-50 µM, 1 h; U0126-10 µM, 30 min; trichostatin A (TSA)-20 µM, 18 h) at 37 °C, washed and then used for filter migration assays. TSA was used as a negative control. Data are expressed as % control (no inhibitor = 100%). * p < 0.05 versus control.
The effects of co-incubation of forskolin stimulated THP-1 cells with SDF-1α and ubiquitin on cAMP levels are shown in Fig. 2D. When tested at equimolar concentrations, stimulation of cells with SDF-1α alone, ubiquitin alone or co-stimulation with both ligands at a ratio of 1:1(mol/mol) reduced cAMP levels to 49.5 ± 4.7%, 48.9 ± 4.8% and 52 ± 7.3%, respectively, when compared with cells in the absence of the CXCR4 ligands (=100%). Similarly, ubiquitin, SDF-1α and an equimolar concentration of both ligands at a 1:1(mol/mol) ratio resulted in comparable increases in Akt and ERK1/2 phosphorylation, as assessed by Western blotting (Fig. 2E).
We then utilized chemotactic movements of THP-1cells as a read out for CXCR4 mediated effects on cell function (Fig. 2F). At ligand concentrations of 10 nM, we observed chemotactic indices of 6 ± 1.6 for ubiquitin and of 14 ± 2 for SDF-1α. The chemotactic index was 18 ± 3 when cells migrated towards a mixture of 10 nM of each CXCR4 agonist. The chemotactic activities of SDF-1α and ubiquitin were sensitive to AMD3100, pertussis toxin, U73122, LY294002 and U0126, but not to trichostatin A (Fig. 2G).
4. Discussion
Our findings from PLAs and FACS analyses are consistent with the absence of cell surface CXCR7 in THP-1 cells which have not been differentiated into a macrophage-like phenotype [11]. This suggests that monocytic THP-1 cells are a suitable cell system to study the interaction of SDF-1α and ubiquitin on CXCR4, in which possible confounding effects of CXCR7, such as scavenging of SDF-1α or CXCR4-CXCR7 heterodimer formation, are unlikely.
The degree of CXCR4 cell surface reduction after stimulation of THP-1 cells with SDF-1α in the present study is comparable with previous observations [12]. We have shown recently that uptake of extracellular ubiquitin into THP-1 cells can be prevented with AMD3100 and SDF-1α [2]. Thus, our observation that magnitude and time course of reduction of cell surface CXCR4 in SDF-1α and ubiquitin stimulated THP-1 cells were indistinguishable suggests that binding of both proteins to CXCR4 induces a uniform pattern of ligand induced receptor internalization.
The findings that the effects of both ligands on cAMP levels and protein kinase phosphorylations were comparable, whereas the efficacy of SDF-1α to promote intracellular Ca2+ mobilization in THP-1 cells was higher than of ubiquitin, is consistent with previous observations in various cell lines [2,5,7]. While we could not detect synergy between SDF-1α and ubiquitin to reduce cellular cAMP levels or to induce phosphorylation of Akt and ERK1/2, we detected synergy to increase intracellular Ca2+ at ligand concentrations close to the equilibrium dissociation (Kd) constant of SDF-1α for CXCR4 binding (10–20 nM) [2,7]. The lack of synergistic effects on Ca2+ fluxes at ligand concentrations of 200 nM could be explained by a saturated dose–response relationship at a SDF-1α concentration >5 times the Kd. We have previously reported that the Kd of ubiquitin for CXCR4 (~100 nM) is several fold higher than the Kd of SDF-1α and that SDF-1α competes FITC-labeled ubiquitin off the receptor with a Ki that was 50% lower than the Ki for native ubiquitin [2]. Thus, an alternative explanation for the lack of synergy at high ligand concentrations could be that ubiquitin binding to CXCR4 in the presence of high concentrations of SDF-1α was reduced below the functionally relevant threshold.
Furthermore, homologous desensitization of Ca2+ fluxes was detectable for both ligands, but only SDF-1α pre-treatment resulted in heterologous desensitization. Signaling of G protein coupled receptors is thought to be regulated by internalization, downregulation and desensitization [13]. Because changes in CXCR4 cell surface expression upon SDF-1α and ubiquitin stimulation of THP-1 cells were comparable, differences in receptor internalization and down-regulation upon ligand activation of CXCR4 are unlikely to account for these observations. Thus, our findings could point towards differential regulation of G protein coupled receptor kinases, β-arrestin recruitment and ubiquitylation, which may desensitize the receptor but may also result in enhanced signaling independent of G proteins [14]. Our previous data, which suggested that both ligands interact with CXCR4 through distinct mechanisms and separate ligand binding sites on the receptor, could then be interpreted to provide a structural basis for agonist selective regulation of CXCR4 signaling [5,6]. Further studies are required to test this hypothesis.
Chemokine receptors and their ligands are known to be promiscuous, being able to bind multiple receptors/ligands [15]. Various chemokine receptor pairs have been described to synergistically enhance common signaling pathways upon co-activation with their ligands, which is thought to amplify the inflammatory response. To the best of our knowledge, synergy of two different ligands on the same receptor has not been reported previously and would add another level of complexity in the regulation of receptor signaling. It should be noted, however, that other mechanisms, such as interaction of the ligands with other receptors, receptor heterodimer formation or transactivation, could also account for such effects.
Our finding that chemotactic activities of SDF-1α and ubiquitin were sensitive to receptor blockade by the CXCR4 antagonist AMD3100, to pertussis toxin and inhibitors of phospholipase C, phosphatidylinositol 3 kinase and mitogen-activated protein kinase kinase 1/2, suggest that activation of CXCR4 with both ligands induces very similar pattern of cellular signaling events, through which cell function is regulated. The observation that co-stimulation of THP-1 cells, however, resulted in additive effects on chemotaxis could question the functional relevance of synergistic effects of both ligands on Ca2+ fluxes. On the other hand, the relationship between Ca2+ fluxes and chemotactic activity may not be linear and synergistic effects on other functional read outs of CXCR4 activation are possible.
In conclusion, the present study affirms and extends the observation that SDF-1α and ubiquitin activate the same signaling cascades downstream of CXCR4. In addition, we provide initial evidence that both ligands show partial synergy to activate cell signaling and differential effects on receptor desensitization. Whereas the molecular mechanisms underlying these events remain to be determined, our data suggest that the actual ligand ratio that is present in the extracellular environment contributes to the fine tuning of CXCR4 mediated functions.
Acknowledgements
This research was made possible, in part, by a grant that was awarded and administered by the U.S. Army Medical Research & Materiel Command (USAMRMC) and the Telemedicine & Advanced Technology Research Center (TATRC), at Fort Detrick, MD, under Contract Number W81XWH1020122. The views, opinions and/or findings contained in this research are those of the author(s) and do not necessarily reflect the views of the Department of Defense and should not be construed as an official DoD/Army position, policy or decision unless so designated by other documentation. No official endorsement should be made. This research was also supported, in part, by a grant from the American Heart Association (13GRNT17230072).
References
- 1.Majetschak M. Extracellular ubiquitin: immune modulator and endogenous opponent of damage-associated molecular pattern molecules. J Leukoc Biol. 2011;89:205–219. doi: 10.1189/jlb.0510316. [DOI] [PubMed] [Google Scholar]
- 2.Saini V, Marchese A, Majetschak M. CXC chemokine receptor 4 is a cell surface receptor for extracellular ubiquitin. J Biol Chem. 2010;285:15566–15576. doi: 10.1074/jbc.M110.103408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan L, Cai Q, Xu Y. The ubiquitin-CXCR4 axis plays an important role in acute lung-infection-enhanced lung tumor metastasis. Clin Cancer Res. 2013 Aug 6; doi: 10.1158/1078-0432.CCR-13-0011. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan C, Chen W, Wu Y, Chen S. Extracellular ubiquitin via CXC chemokine receptor 4 enhances platelet activity by ubiquitination of cycloxygenase-1. Heart. 2012;98(Suppl. 2):E10. [Google Scholar]
- 5.Saini V, Staren DM, Ziarek JJ, Nashaat ZN, Campbell EM, Volkman BF, et al. The CXC chemokine receptor 4 ligands ubiquitin and stromal cell-derived factor-1α function through distinct receptor interactions. J Biol Chem. 2011;286:33466–33477. doi: 10.1074/jbc.M111.233742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saini V, Marchese A, Tang WJ, Majetschak M. Structural determinants of ubiquitin-CXC chemokine receptor 4 interaction. J Biol Chem. 2011;286:44145–44152. doi: 10.1074/jbc.M111.298505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tripathi A, Saini V, Marchese A, Volkman BF, Tang WJ, Majetschak M. Modulation of the CXC chemokine receptor 4 agonist activity of ubiquitin through C-terminal protein modification. Biochemistry. 2013;52:4184–4192. doi: 10.1021/bi400254f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu B, Chien EY, Mol CD, Fenalti G, Liu W, Katritch V, et al. Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science. 2010;330:1066–1071. doi: 10.1126/science.1194396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levoye A, Balabanian K, Baleux F, Bachelerie F, Lagane B. CXCR7 heterodimerizes with CXCR4 and regulates CXCL12-mediated G protein signaling. Blood. 2009;113:6085–6093. doi: 10.1182/blood-2008-12-196618. [DOI] [PubMed] [Google Scholar]
- 10.Rajagopal S, Kim J, Ahn S, Craig S, Lam CM, Gerard NP, et al. Beta-arrestin-but not G protein-mediated signaling by the “decoy” receptor CXCR7. Proc Natl Acad Sci USA. 2010;107:628–632. doi: 10.1073/pnas.0912852107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma W, Liu Y, Ellison N, Shen J. Induction of C-X-C chemokine receptor type 7 (CXCR7) switches stromal cell-derived factor-1 (SDF-1) signaling and phagocytic activity in macrophages linked to atherosclerosis. J Biol Chem. 2013;288:15481–15494. doi: 10.1074/jbc.M112.445510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orsini MJ, Parent JL, Mundell SJ, Marchese A, Benovic JL. Trafficking of the HIV coreceptor CXCR4: role of arrestins and identification of residues in the C-terminal tail that mediate receptor internalization. J Biol Chem. 1999;274:31076–31086. doi: 10.1074/jbc.274.43.31076. [DOI] [PubMed] [Google Scholar]
- 13.Moore CA, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol. 2007;69:451–482. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- 14.Marchese A, Paing MM, Temple BR, Trejo J. G protein-coupled receptor sorting to endosomes and lysosomes. Annu Rev Pharmacol Toxicol. 2008;48:601–629. doi: 10.1146/annurev.pharmtox.48.113006.094646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy PM, Baggiolini M, Charo IF, Hebert CA, Horuk R, Matsushima K, et al. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52:145–176. [PubMed] [Google Scholar]