Abstract
AEOL 10150 is a catalytic metalloporphyrin superoxide dismutase mimic being developed as a medical countermeasure for radiation-induced lung injury (RILI). The efficacy of AEOL 10150 against RILI through a reduction of oxidative stress, hypoxia and pro-apoptotic signals has been previously reported. The goal of this study was to determine the most effective dose of AEOL 10150 (daily subcutaneous injections, day 1–28) in improving 180-day survival in CBA/J mice after whole-thorax lung irradiation (WTLI) to a dose of 14.6 Gy. Functional and histopathological assessments were performed as secondary end points. Estimated 180-day survival improved from 10% in WTLI alone to 40% with WTLI-AEOL 10150 at 25 mg/kg (P = 0.065) and to 30% at 40 mg/kg (P = 0.023). No significant improvement was seen at doses of 5 and 10 mg/kg or at doses between 25 and 40 mg/kg. AEOL 10150 treatment at 25 mg/kg lowered the respiratory function parameter of enhanced pause (Penh) significantly, especially at week 16 and 18 (P = 0.044 and P = 0.025, respectively) compared to vehicle and other doses. Pulmonary edema/congestion were also significantly reduced at the time of necropsy among mice treated with 25 and 40 mg/kg AEOL 10150 compared to WTLI alone (P < 0.02). In conclusion, treatment with AEOL 10150 at a dose of 25 mg/kg/day for a total of 28 days starting 24 h after WTLI in CBA/J mice was found to be the optimal dose with improvement in survival and lung function. Future studies will be required to determine the optimal duration and therapeutic window for drug delivery at this dose.
INTRODUCTION
A nuclear catastrophe, either deliberate (i.e., terrorist or state-sponsored attack) or accidental (i.e., nuclear melt-down), would result in mass casualty scenarios with serious consequences to human populations ranging from severe illness to death. After exposure to lethal doses of ionizing radiation, gastrointestinal (GI) and hematopoietic (H) acute radiation syndromes (ARS) are known to be major causes of death (1, 2). However, humans that survive H-ARS and GI-ARS sequelae may develop morbidity months or years later from pulmonary injury leading to organ failure and mortality or diminished quality of life (1, 3). Radiation-induced lung injury manifests in two phases: radiation pneumonitis, an inflammatory phase that occurs approximately 2–6 months after irradiation, followed by radiation fibrosis typically several months to 1 year after irradiation (4).
Due to the awareness of such risks from radiation exposure, one of the critical areas of ongoing research is aimed at discovering mitigators and/or treatments for radiation-induced injuries. Exposure to radiation induces direct reactive oxygen or nitrogen species (ROS/RNS) production, and continuous generation of free radicals by inflammatory mediators, mitochondrial leakage and oxidant generating enzymes such as nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (5–8). This continued production of ROS/RNS overpowers the endogenous antioxidant response leading to a chronic oxidative stress state that drives oxidative/nitroxidative modification of key intracellular signaling pathways (9, 10) and contributes to etiopathogenesis of radiation-induced cellular and tissue effects (11). The importance of the radioprotective effects of superoxide dismutase (SOD) has been studied since the 1970s (12, 13). This led to the development of SOD mimetic drugs as radioprotectants (9, 14, 15). Manganese-based SOD mimetics have been extensively researched and there are four known types: porphyrin, cyclic polyamine, salen and pyridoxyl ethyldiamine (PLED) (16). With specific reference to radiation-induced lung injury, metalloporphyrin-based SOD mimetics and salen-type MnSOD such as M40403 and EUK-207 have been in development as potential mitigators. We have previously described the use of the SOD mimetic AEOL 10113, which has been shown to reduce the severity of radiation-induced lung injury as demonstrated by histopathological changes, measurements of collagen deposition and plasma level of the profibrogenic cytokine transforming growth factor-beta (TGF-β) (9). Recently, EUK-207, which has both superoxide dismutase and catalase activities, was reported to demonstrate mitigation of radiation-induced pneumonitis and pulmonary fibrosis in rats when treatment was started 7 days after 13 Gy whole-thorax lung irradiation (WTLI) and stopped before pneumonitis (17). However, there was an insignificant change in morbidity or mortality associated with pneumonitis (17). The potential of manganese SOD gene overexpression to prevent the acute and late effects of radiation-induced lung damage has also been evaluated in murine studies by Epperly et al. (18). We have previously observed a lower incidence of lung damage as measured by breathing frequency, lung weight, macrophage count, percentage of activated TGF-β1 and collagen deposition in transgenic mice overexpressing extracellular-superoxide dismutase after WTLI compared to wild-type mice, further supporting the development of SOD mimetics as an approach to mitigate radiation-induced lung injury (19).
AEOL 10150 is a small molecular weight catalytic metalloporphyrin that has been under active investigation as a radiomitigator due to its superior antioxidant properties (5, 20, 21). In our previous studies, we have demonstrated the potential of AEOL 10150 to mitigate radiation-induced lung damage when administered before or after radiation exposure in a rat model of hemithoracic lung irradiation (20, 21). We have previously shown that AEOL 10150 administration results in significant reduction in DNA oxidation and CA9 expression (a hypoxia-inducible factor target-regulated gene) in irradiated lung tissue (21). Other studies have shown that in response to cerebral ischemia and reperfusion injury, AEOL 10150 administration blunted the increase in inflammatory genes including interleukin-6 (I-6), heat shock protein (HSP) and macrophage inflammatory protein-2 (MIP-2) (22). In response to tobacco smoke, AEO 10150 treatment results in reduced inflammatory markers in the bronchoalveolar lavage (BAL) fluid including MIP-2 and intercellular adhesion molecule-1 (ICAM-1) production (23). When murine-derived bone marrow macrophages were pretreated with AEOL 10150 followed by lipopolysaccharide stimulation, pro-inflammatory cytokine release (TNF-α, IL-1β) and ROS generation were suppressed (24).
While the above mentioned studies are encouraging and justify further development of AEOL 10150 as a mitigator of radiation-induced lung injury, its advancement into clinical trials or for regulatory approval requires appropriate dose selection that has the highest efficacy while minimizing dose-limiting toxicities. Therefore, the objective of this study was to determine the optimal dose(s) of AEOL 10150 for mitigating radiation pneumonitis from WTLI in the pneumonitisprone CBA/J mouse strain, the pathology of which is similar to humans (25–28).
METHODS AND MATERIALS
Animals
Female 180 CBA/J mice (Jackson Laboratory, Bar Harbor, ME) aged 7–10 weeks, were used with prior approval from the University of Maryland Baltimore’s Institutional Animal Care and Use Committee (IACUC). Mice were housed in microisolator cages (5 per cage) and were kept under standard laboratory conditions of temperature, pressure and humidity with a 12:12 h light–dark schedule. Mice were provided hyperchlorinated water ad libitum and fed certified commercial laboratory animal chow throughout the study (2018SX Teklad Global 18% Protein Extruded rodent diet; Harlan® Laboratories Inc., Indianapolis, IN). Animal health was monitored daily after irradiation and body weight acquired biweekly. Moribund animals were euthanized by pentobarbital overdose method (>100 mg/kg sodium pentobarbital, intraperitoneal injection) followed by thoracotomy after cessation of respiration for >1 min. All surviving animals were euthanized on day 182 after WTLI.
Animal Irradiation
CBA/J mice (≥20 g) were irradiated at 9–11 weeks of age. Prior to irradiation/sham irradiation mice were anesthetized with a mixture of ketamine (80–100 mg/kg) and xylazine (10–15 mg/kg) in saline. The anesthesia solution was administered intraperitoneally. Whole-thorax lung irradiation was performed on all mice using XRAD320 orthovoltage X-ray irradiator (Precision X-Ray, North Branford, CT). Animals received a total of 14.6 Gy in a single fraction delivered to the whole thorax through adjustable apertures (13 mm) at a dose rate of 1.25 Gy min−1 (filter = 2.00 mm Al, HVL ~1 mm Cu) with lead shielding of the head and abdomen. After irradiation, animals were kept on heating blankets until fully awake and returned to animal housing facility.
Mitigating Agent
AEOL 10150, a potent ROS scavenger, was supplied by Aeolus Pharmaceuticals Inc. (Mission Viejo, CA) as a solid and stored at room temperature. Different doses of AEOL 10150 (5, 10, 25 and 40 mg/kg) solutions were prepared by dissolving the appropriate amount of AEOL 10150 in vehicle (0.9% sodium chloride, injection, pH 5.0, sterile) and filtering with a 0.22 μm filtration system (Millipore, Billerica, MA) before treatment.
Administration of AEOL 10150
After irradiation, animals were randomized to groups (n = 20/irradiation or 10/sham irradiation) to receive no treatment, vehicle or 5, 10, 25 or 40 mg/kg/day AEOL 10150. AEOL 10150-vehicle were delivered by subcutaneous (s.c.) injection once per day for 28 days starting 24 h after irradiation.
Assessment of Respiratory Function
Respiratory function was evaluated using an unrestrained, noninvasive whole-body plethysmograph (WBP; Buxco Research Systems, Wilmington, NC). Respiratory function assessment was performed before irradiation to acclimate the mice to the method and obtain baseline measurements, and every two weeks for 6 months after WTLI. Mice were allowed to acclimate in a barometric chamber for 5 min, and recording of the breathing pattern was performed for 10 min.
Histology
After euthanasia, a bilateral thoracotomy was performed; lungs were excised, rinsed in PBS, blotted and wet lung weights recorded. The right lung was snap frozen in liquid nitrogen and stored at −80°C, while the left lung was inflated with 10% neutral buffered formalin (Fisher Scientific, Pittsburg, PA), placed in 10% formalin fixative for at least 48 h and paraffin embedded for histopathological damage analysis. Tissue sections (5 μm) were sectioned with a semi-automated microtome (cat. no. HM 355S; Thermo Fisher Scientific Inc., Waltham, MA), mounted on slides, and stained with Masson’s trichrome to assess morphological damage. Briefly, slides were deparaffinized and rehydrated in citrosolv and ethanol, respectively. After washing with deionized water, the slides were placed in preheated Bouin’s solution (50–60°C) for 30 min in an incubator. The slides were then washed with tap water and stained with Weigert’s iron hematoxylin working solution for 5 min. The slides were rinsed with running tap water for 5 min and then washed in deionized water for 1 min. The slides were stained with Biebrich scarlet-acid fuchsin solution for 6 min and then rinsed in deionized water. The slides were then incubated in phosphomolybdic/phosphotungstic acid solution for 5 min and immediately transferred to aniline blue solution for 6 min. After rinsing with deionized water, the slides were washed in 5% acetic acid (two washes with three dips in each) and then washed in distilled water. The slides were finally dehydrated through an alcohol series, cleared in citrosolv, and mounted with a cover slip. Brightfield acquisition of lung tissue images was obtained using the tile function of the ZEN 2011 Zeiss software and a Zeiss Imager.M2 AXIO microscope (Carl Zeiss MicroImaging Inc., Thornwood, NY). Images were taken using a 10× Zeiss Plan-Apochromat objective. The entire region of interest was scanned with numerous focus points. The multiple images were then processed using the fuse function in ZEN 2011.
Statistical Analysis
Data were analyzed using descriptive statistics (means, medians, standard deviations and standard errors). One-way ANOVA was used to test the significance of differences among groups. Survival analysis was performed using Kaplan-Meier analysis with log-rank test for comparison among the groups. A P value of ≤0.05 was considered statistically significant. Statistical analysis was performed using IBM SPSS Statistics V2.0 (IBM Corp., Armonk, New York)
RESULTS
AEOL 10150 Improves Survival when Administered 24 h after WTLI for 28 Days
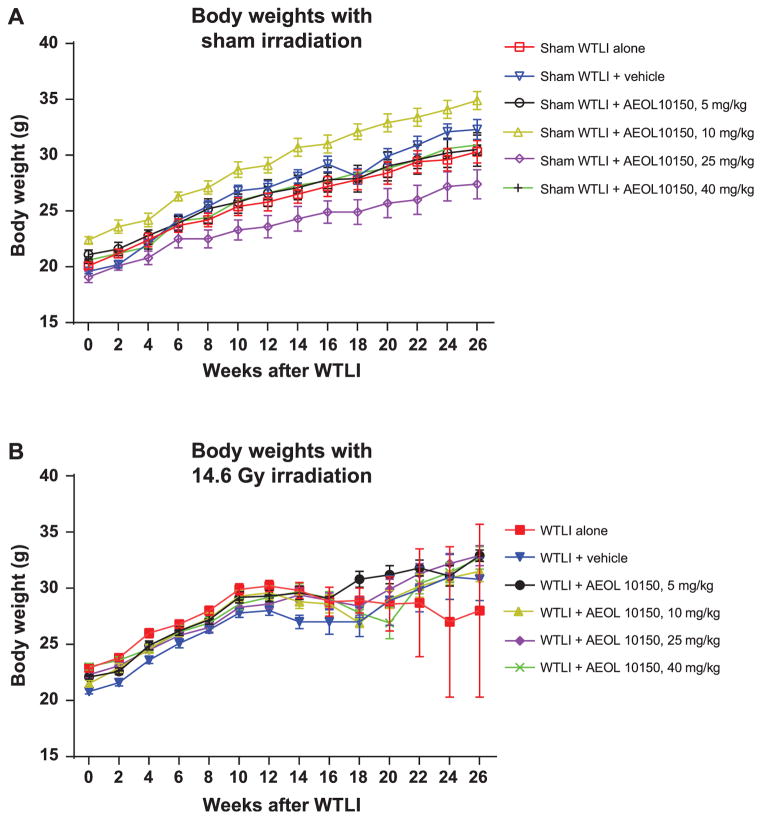
Ninety out of a total of 117 animals in the irradiated groups became morbid and were euthanized at 14–20 weeks after WTLI. The weight loss pattern is noted in Fig. 1A–B. Survival between the WTLI-alone vs. WTLI-vehicle groups was not statistically significant (P = 0.56), as shown in Fig. 2 and Table 1. However, in a comparison among the WTLI-vehicle cohorts, there was improvement in 180-day estimated survival noted from 21% (WTLI-vehicle) to 40% (WTLI-AEOL 10150, 25 mg/kg) and 30% (WTLI-AEOL 10150, 40 mg/kg), although this did not reach statistical significance (P = 0.173 and 0.235, respectively). Table 2 shows the dramatic increase in percentage mortality during the pneumonitis phase (week 14–20 postirradiation) among the WTLI mice from week 14 through week 20, represented by the steep portion of the dose-response curve. In addition, as shown in Table 2, low percentage mortality was observed in the irradiated cohorts treated with high doses of AEOL 10150 (25 and 40 mg/kg) during the pneumonitis phase.
FIG. 1.
Panel A: Longitudinal changes in body weight among sham-irradiated control CBA/J female mice demonstrate a healthy increase in body weight over the six-month follow-up period. Error bars represent ± SEM. Panel B: Longitudinal changes in body weight among the WTLI animals exposed to 14.6 Gy doses. During the period from week 12 to week 20 after exposure, mice in all WTLI groups (nontreated and treated) exhibited weight loss. After week 18, the surviving mice in AEOL 10150- and WTLI-vehicle groups started gaining body weight and continued to gain weight until they were euthanized after 180 days. Error bars represent ± SEM. The error bar for the WTLI-alone group at week 22, 24 and 26 is high because only two mice survived in this period with considerable difference between their body weights (35.7 g and 20.3 g at week 26).
FIG. 2.
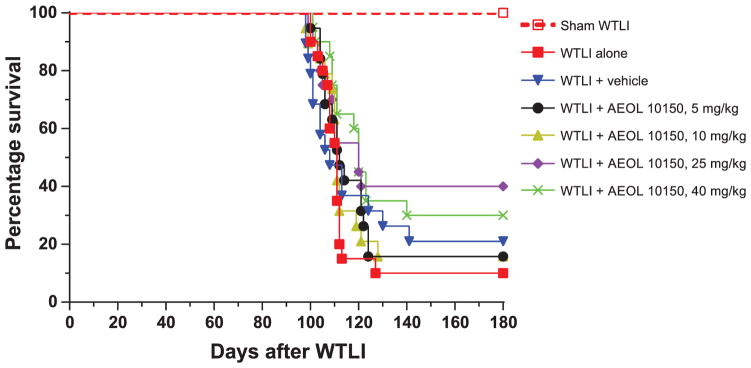
Kaplan-Meier survival curves for 180-day survival of sham (0 Gy) and WTLI (14.6 Gy) female CBA/J mice (320 kVp X rays; 1.25 Gy min−1, HVL ~1 mm Cu). AEOL 10150 and vehicle treatment were performed daily for 28 days starting 24 h after irradiation. See Tables 1 and 2.
TABLE 1.
180-Day Survival among All Treatment Groups of WTLI (14.6 Gy) and Sham-WTLI (0 Gy) CBA/J Female Mice
| Treatment group | Number of decedents/total | Percentage lethality | Mean survival time (days) | Median survival time (days) (25–75th percentile) |
|---|---|---|---|---|
| Sham WTLI (all groups)a | 1/60 | 1.7% | 180 ± 0.4 | 180 (180–180) |
| WTLI alone | 18/20 | 90% | 116 ± 5.0 | 111 (107–112) |
| WTLI-vehicle | 15/19 | 79% | 124 ± 7.3 | 108 (101–141) |
| WTLI-AEOL 10150, 5 mg/kg | 16/19 | 84% | 123 ± 6.1 | 112 (106–124) |
| WTLI-AEOL 10150, 10 mg/kg | 16/19 | 84% | 122 ± 6.2 | 111 (109–121) |
| WTLI-AEOL 10150, 25 mg/kg | 12/20 | 60% | 138 ± 8.0 | 120 (106–180) |
| WTLI-AEOL 10150, 40 mg/kg | 14/20 | 70% | 135 ± 7.0 | 120 (110–180) |
Sham WTLI (no treatment, treatment with vehicle or treatment with AEOL 10150).
TABLE 2.
Representation of Mortality during the Pneumonitis Phase
| Treatment group | Mortality rate (%)
|
|||
|---|---|---|---|---|
| Week 14 | Week 16 | Week 18 | Week 20 | |
| WTLI alone | 0 | 70 | 85 | 90 |
| WTLI-vehicle | 11 | 53 | 68 | 79 |
| WTLI-AEOL 10150, 5 mg/kg | 0 | 47 | 84 | 84 |
| WTLI-AEOL 10150, 10 mg/kg | 5 | 58 | 79 | 84 |
| WTLI-AEOL 10150, 25 mg/kg | 0 | 45 | 60 | 60 |
| WTLI-AEOL 10150, 40 mg/kg | 0 | 30 | 65 | 65 |
AEOL 10150 Improves Pulmonary Function during Pneumonitis Phase (14–20 Weeks after WTLI)
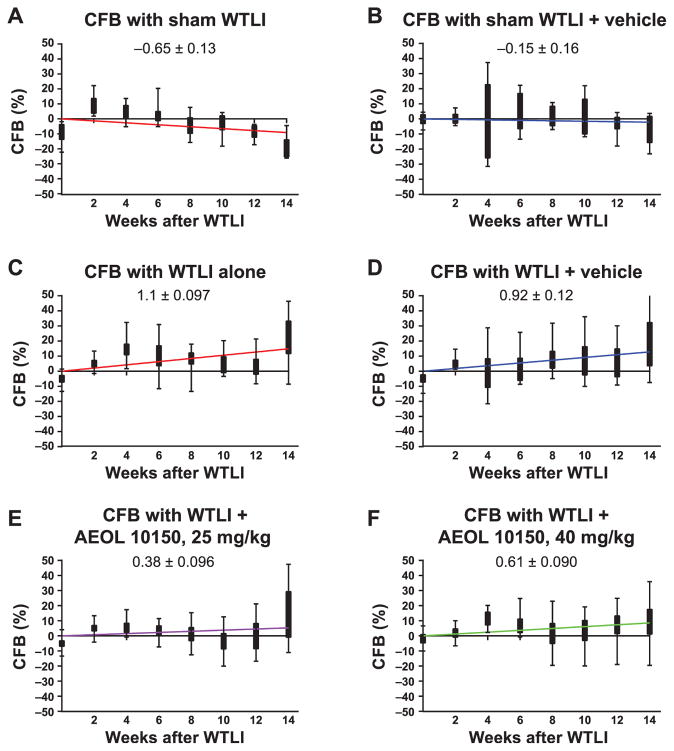
Respiratory frequency of all irradiated mice increased significantly from week 12 compared to the sham-irradiated group (Fig. 3). Respiratory frequency of mice in the WTLI-alone group remained slightly, insignificantly elevated for the duration of study compared to the WTLI-vehicle or AEOL 10150 treatment groups. A clear separation in respiratory frequency among the WTLI-AEOL 10150 doses is not evident at 14–18 weeks, but the frequency in the WTLI-AEOL 10150, 25 mg/kg group is slightly reduced compared to other treatment groups. When change in respiratory frequency from baseline (CFB) was evaluated, the sham-WTLI groups showed a consistent breathing pattern with a slope of <0.1 (Fig. 4). The WTLI cohort showed a markedly positive slope that trended towards normalization with increasing doses of AEOL 10150 up to 25 mg/kg followed by a rise at 40 mg/kg. This maintenance of pulmonary function is also apparent when the respiratory function data is presented in the form of Penh (Fig. 5). After week 14 postirradiation, all WTLI groups had a higher Penh than the sham-WTLI group. During the pneumonitis phase, 14–20 weeks, AEOL 10150 treatment at 25 mg/kg lowered the Penh significantly, especially at week 16 and 18, compared to treatment with vehicle or other doses.
FIG. 3.
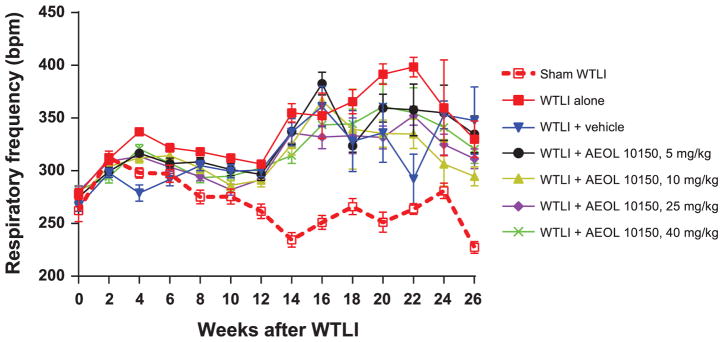
Transformed respiratory frequency (TRF) from WTLI (14.6 Gy) and representative sham-irrradiated (0 Gy) groups. Error bar represents the standard error in each study group. Based on the TRF, the data can be reviewed by two time periods, weeks 0–16 and weeks 18–26. The graph shows that the TRF for the WTLI-alone group is higher than for the WTLI and treatment groups (vehicle, 5, 10, 25 and 40 mg/kg) except at week 16 and 26. However, statistical analysis of the TRF in all groups reveals that in the first period of weeks 0–16, there is no statistical significance in most time points between the WTLI and treatment (vehicle, 5, 10, 25 and 40 mg/kg) and nontreatment, except at week 4, 8 and 10. From weeks 18–26, there is no statistical significance between WTLI and treatment (vehicle, 5, 10, 25 and 40 mg/kg) and nontreatment, except at week 22 where a significant difference is observed between the WTLI-alone and WTLI-vehicle groups. The lack of statistical significance from weeks 18–26 is due to greater standard errors observed in TRF, which are attributed to the lower number of live mice and larger variation in frequency.
FIG. 4.
Change in respiratory frequency from baseline (CFB) using the average of TRF at day 0 and 14 (two weeks) as baseline. A linear regression was performed to get an estimation of the slope (labeled in each graph), which indicates the relative change in frequency over time. Since the animals died after week 14 only the data from the first 14 weeks in each study group were analyzed. All the sham groups have the slope <0.1 (panel A), which indicates a consistent breathing pattern in the nonirradiated mice, with a shift towards zero with addition of vehicle (panel B). Among the irradiated groups, the slope of the CFB is highest for the WTLI-alone group (panel C) and WTLI-vehicle groups (panel D). Use of AEOL 10150 shows a shift in the trend back towards zero as the drug dose is increased, as with WTLI-AEOL 10150, 25 mg/kg (panel E) and WTLI-AEOL 10150, 40 mg/kg (panel F), suggesting a trend towards maintenance of a baseline breathing pattern despite the radiation exposure.
FIG. 5.
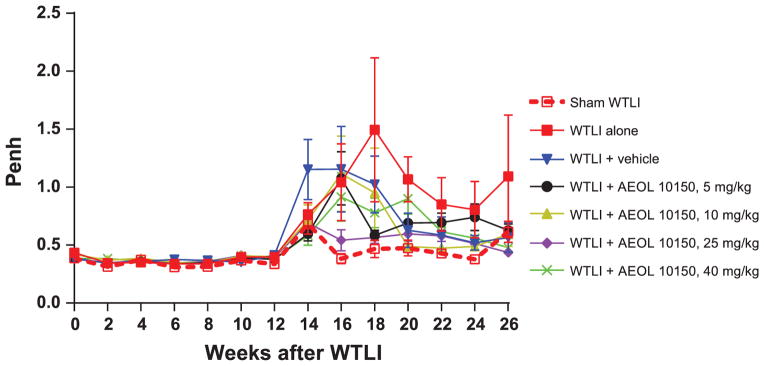
Penh in WTLI (14.6 Gy) and sham-irradiated (0 Gy) WTLI groups. Error bar represents the SEM in the group. On and after week 14 postirradiation, all WTLI groups have a higher Penh than the representative sham-irradiated groups. In the treatment groups from weeks 14–26, the WTLI-AEOL 10150, 10 mg/kg and WTLI-AEOL 10150, 25 mg/kg groups have lower Penh than the WTLI-AEOL 10150, 5 mg/kg and WTLI-AEOL 10150, 40 mg/kg groups. The following comparisons were statistically significant: WTLI vs. WTLI-AEOL 10150, 25 mg/kg, week 16 and 18, P = 0.044 and 0.025, respectively; WTLI-vehicle vs. WTLI-AEOL 10150, 25 mg/kg, week 16 and 18, P = 0.056 and 0.030, respectively; WTLI-AEOL 10150, 5 mg/kg vs. WTLI-AEOL 10150, 25 mg/kg, week 16, P = 0.028; WTLI-AEOL 10150, 10 mg/kg vs. WTLI-AEOL 10150, 25 mg/kg, week 16, P = 0.065; WTLI-AEOL 10150, 40 mg/kg vs. WTLI-AEOL 10150, 25 mg/kg, week 16, P = 0.006.
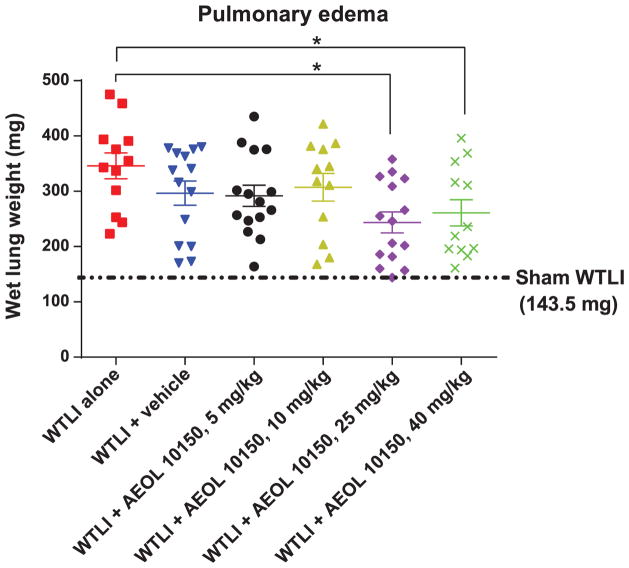
AEOL 10150 Ameliorates Radiation-Induced Pulmonary Edema
Figure 6 shows the wet lung weights of the mice from different treatment cohorts. Irradiated mice treated with AEOL 10150 at doses of 25 and 40 mg/kg showed significant reduction in pulmonary edema compared to irradiated mice with no treatment. The 5 and 10 mg/kg dose groups, as well as the vehicle-treated group, showed an insignificant reduction in pulmonary edema compared to the untreated group.
FIG. 6.
Wet lung weights (an indicator of edema and congestion associated with pneumonitis) among 14.6 Gy WTLI female CBA/J mice. Error bar represents the standard error in each study group. Analysis by pairwise comparison among groups was performed considering 0.05 as the level of significance. There was significant difference between the WTLI-alone group (mean = 346.0, SEM = 23.20) and both the WTLI-AEOL 10150, 25 mg/kg (mean = 244.0, SEM = 18.8, P = 0.002) group and WTLI-AEOL 10150, 40 mg/kg (mean = 261.0, SEM = 23.9, P = 0.046) group. The average wet lung weight of all sham-irradiated cohorts was 143.5 mg.
AEOL 10150 Improves Lung Structure Integrity after WTLI
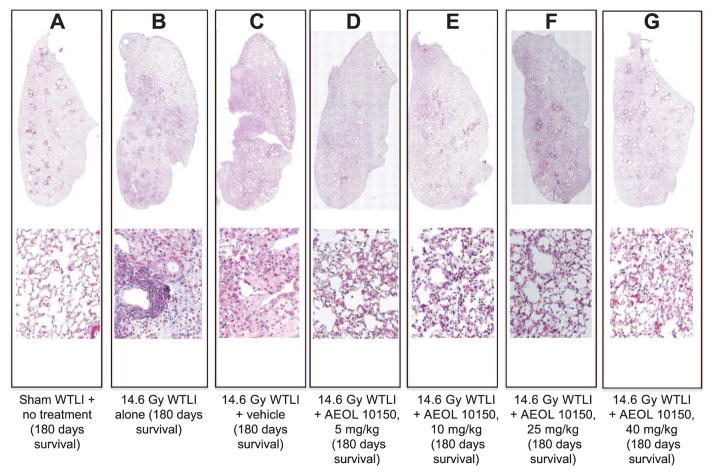
The lung structure integrity and degree of radiation fibrosis were evaluated using Masson’s trichrome staining. Histological sections of lung tissues from 180-day survivors exposed to radiation alone showed extensive injury to most of the parenchyma consistent with pneumonitis (alveolitis) (Fig. 7) including an abundance of lipid-laden alveolar macrophages, mononuclear cell infiltrates of the septa, interstitial and intra-alveolar edema and lymphocytic perivascular cuffing. A similar degree of inflammation and tissue damage was observed in the vehicle-treated groups. However, among survivors treated with AEOL 10150 at a dose of 5, 10, 25 and 40 mg/kg, a greater proportion of lung tissue displayed well-aerated alveoli and overall reduced tissue damage compared to the lungs from WTLI-alone or vehicle-treated groups. However, statistical comparisons could not be generated since only 2 animals survived in the WTLI-alone group and the study was not designed to collect histological samples in a serial manner.
FIG. 7.
Comparison of histopathological damage among representative surviving CBA/J mice (180 days after WTLI) from different treatment groups. The top panels represent the whole-lung tissue and the bottom panels represent a magnified portion of the whole lung. Masson’s trichrome staining of lung tissue shows apparent variation in histopathology between the sham-irradiated (0 Gy) and WTLI (14.6 Gy) groups [WTLI alone, WTLI-vehicle (saline) and WTLI-AEOL 10150]. Mice treated with AEOL 10150 show less lung tissue damage compared to vehicle or nontreated mice. Panel A: Lung tissue from a sham-irradiated mouse with no treatment appears normal, lung weight (LW) 150 mg. Panel B: Lung tissue from a WTLI-alone mouse displays increased alveolar wall thickness, congestion and lymphocytic inflammation, LW 223 mg. Panel C: Lung tissue from a WTLI-vehicle mouse shows increased alveolar wall thickness, congestion and inflammation, LW 173 mg. In the lung tissue from irradiated mice treated with AEOL 10150, relatively lower alveolar wall thickness, congestion and inflammation were seen with doses of: 5 mg/kg, LW 213 mg (panel D); 10 mg/kg, LW 180 mg (panel E); 25 mg/kg, LW 206 mg (panel F); and 40 mg/kg, LW 183 mg (panel G). Since CBA/J mice are prone to pneumonitis instead of fibrosis, even WTLI cohorts display relatively scant collagen staining (blue color). Instead, the changes are suggestive of representing an organized exudate.
DISCUSSION
Radiation-induced lung injury is a critical issue for survivors of accidental or deliberate total- or partial-body irradiation where much of the thorax is exposed (1, 29). Even in the setting of whole-thorax lung irradiation, percentage mortality in our current study increased from 0% at week 14 to 70% at week 16, 85% at week 18 and 90% at week 20. This study used pneumonitisprone CBA/J mice in which the pathology of radiation lung injury is comparable to the response in humans (25, 28). Additionally, the WTLI model represents a more realistic model for accidental/malintentional radiation exposure than hemi-thoracic radiation treatment used in the previous study. Since development of a chronic oxidative stress state from continued production of free radicals is thought to mediate radiation-induced cellular and tissue effects, including lung injury (9, 10), there is tremendous effort toward the development of ROS scavengers that can alleviate radiation-induced toxicities. AEOL 10150, a superoxide dismutase mimic, is one such compound that has been investigated as a radiomitigator with strong positive response in mitigating radiation-induced lung injury (20, 21, 30, 31). With accidental/malintentional radiation exposure of the population in mind, this current study was designed to define the best dose of AEOL 10150 to mitigate radiation pneumonitis when administered starting 24 h after exposure, consistent with the U.S. Government Concept of Operations (CONOPS). In our study, WTLI dose of 14.6 Gy in CBA/J female mice was used based on previously established dose-response curves (25, 28). AEOL 10150 was administered for a total duration of 28 days.
Survival differences observed at higher doses of AEOL 10150 (Fig. 2) were accompanied by improved functional and structural end points (Figs. 3–7). For instance, at week 4 the respiratory frequency is significantly higher in the WTLI-alone cohort when compared to the WTLI and treatment groups (vehicle, 5, 10 and 25 mg/kg). Similar effects were noted at week 8 among WTLI-alone group and WTLI and higher dose treatment groups (25 and 40 mg/kg) and at week 10 among WTLI-alone and WTLI and treatment groups (10 and 25 mg/kg). Significant differences in respiratory frequency were not seen in weeks 18–26, although at later time points the analysis was compromised by the lower number of surviving mice in the various cohorts (Fig. 3). While breathing rate has been used as a standard in radiobiological research to assess the efficacy of various therapeutic regimens against radiation pneumonitis (32), this does not often correlate with the survival outcomes. We employed a novel approach to analyze the respiratory frequency by measuring the CFB, as shown in Fig. 4. Interestingly, analysis of respiratory frequency in this manner allows a relatively simple representation of a complex problem and even gives an opportunity to see differential drug/dose effects within the first 14 weeks of radiation exposure, which were not discernible in Fig. 3. As shown, there is a trend towards normalization of the slope with increasing AEOL 10150 doses up to 25 mg/kg. Interestingly, the treatment cohort of 40 mg/kg had a higher slope of 0.61, similar to the 5 mg/kg cohort (slope of 0.79). These findings suggest that the optimal treatment dosage is likely between 25 to 40 mg/kg. This approach could not be used to analyze TRF beyond 14 weeks due to the increased number of deaths at later time points, which increases the confidence interval of the estimate. For these time points analysis of Penh, which is a marker of airway hyperactivity or airflow that typically predates a life-ending event, revealed that the WTLI-alone group had higher Penh than the WTLI-AEOL 10150 and vehicle groups on and after week 14 postirradiation (Fig. 5). Additionally, the wet lung weight measured at the time of euthanasia (planned or triggered) in the WTLI-alone group (mean = 346.0 mg, SEM = 23.2) was significantly higher compared to the WTLI-AEOL 10150, 25 mg/kg group (mean = 244.0 mg, SEM = 18.8, P = 0.002) and the WTLI-AEOL 10150, 40 mg/kg group (mean = 261.0 mg, SEM = 23.9, P = 0.018). Pneumonitis is characterized by accumulation of inflammatory cells in the lungs induced by secretion of pro-inflammatory cytokine and chemokines leading to vascular injuries manifesting as pulmonary edema (33, 34). Therefore, we used wet lung weight at the time of euthanasia as a surrogate marker for pulmonary edema. This was confirmed histopathologically, as the lung tissue in the WTLI-AEOL 10150 treatment groups had relatively lower alveolar walls thickness, congestion and inflammation than the vehicle and WTLI-along cohorts (Fig. 7). The improvements in Penh from week 14 onwards and the wet lung weight at euthanasia with AEOL 10150 treatment suggest the existence of a delayed effect in mitigation of lung injury even with short-course administration of 4 weeks.
While a dose response was seen in the various parameters between the doses of 5 and 10 mg/kg versus 25 mg/kg, the evaluation between 25 and 40 mg/kg was not entirely consistent. Kaplan-Meier survival analysis reveals that the survival difference reached statistical significance in the 40 mg/kg cohort (P = 0.023) and was borderline in the 25 mg/kg cohort (P = 0.065) compared to the WTLI-alone group, even though percentage lethality at the end of study was lower in the 25 mg/kg cohort. Interestingly, more delayed deaths were noted in the 40 mg/kg cohort. AEOL 10150 at a dose of 25 mg/kg lowered the Penh significantly at week 16 compared to all other doses, including 40 mg/kg. The reduction was also significant when compared to vehicle at weeks 16 and 18. In addition, when compared to the WTLI-alone group, the respiratory frequency in the WTLI-AEOL 10150, 25 mg/kg group was lower at weeks 4, 8 and 10, while the WTLI-AEOL 10150, 40 mg/kg group had lower frequency at only week 8. A similar trend was observed in the relative change in frequency from baseline (Fig. 4) that showed a decreasing slope as the AEOL 10150 dose increased from 5 mg/kg to 25 mg/kg followed by a reversal in trend at 40 mg/kg.
Survival in both WTLI and 25 and 40 mg/kg cohorts was not statistically superior to the WTLI-vehicle cohorts, although the mean survival time and percentage lethality was numerically better in the drug-treated cohorts. While the study was powered to determine statistical significance of each dosing regimen versus WTLI-vehicle, it was not powered for an inter-dose comparison, which would have required a much larger number of animals, thus further study is warranted. In this study, fluid administration alone resulted in a 10% improvement in survival. This has been repeatedly observed in our studies, regardless of the route of fluid administration (e.g., s.c. or oral gavage). Daily administration of 0.1 ml/day is approximately 3% of the daily fluid intake in female mice based on water consumption analysis conducted in our laboratory (unpublished data). Therefore, we hypothesize daily administration of fluids for 28 days contributed to the observed improvement in survival versus WTLI alone.
Additional studies are ongoing in nonhuman primates (NHP) using a whole-thorax lung irradiation model in rhesus macaques (31). In a pilot study, Garofalo et al. demonstrated improvement in survival (0 vs. 29%) of rhesus macaques with the use of AEOL 10150 at 5 mg/kg administered 24 h after 11.5 Gy WTLI and then continued as daily dose for a total of 4 weeks (31). In this comprehensive study, NHP were managed with supportive care including intravenous fluid support, transfusions, antibiotics and dexamethasone administration based on predefined criteria over a 180-day study duration. In addition to the delay in the requirement of dexamethasone support, there was about 33% reduction in mean dexamethasone support required per day alive in the study. Lower serum TGF-β1 levels and quantitative pneumonitis/fibrosis/effusions as identified by computed tomography scans (CT scans) were noted in the drug-treated cohorts (31).
Overall, with at least comparable outcomes between 25 and 40 mg/kg, and in the setting of delayed deaths in the 40 mg/kg cohort, we hypothesize that 25 mg/kg to be the best dose for further evaluation.
In our previous study with Fisher-344 rats irradiated with 28 Gy to the right hemithorax, we demonstrated that AEOL 10150 treatment, starting one day after irradiation and continuing for 10 weeks, resulted in a significant reduction in the degree of radiation-induced lung injury, as demonstrated by decreased structural damage, collagen deposition, macrophage accumulation and oxidative stress (21). This effect was seen for higher doses of AEOL 10150 (10 and 30 mg/kg/day) but not for a lower dose of 1 mg/kg/day. In a parallel study with fractionated radiation doses targeting the right hemithorax (40 Gy in 5 fractions), we demonstrated that AEOL 10150 treatment at 5 mg/kg 15 min before the first fraction and a second dose 8 h later, for each of the five days of radiation exposure, resulted in reduced HIF1α and CAIX (hypoxia markers), VEGF and CD-31 (angiogenesis), ED-1 (inflammation), 8-OHdG and 3-nitrotyrosine (oxidative stress) and TGF-β1, Smad3 and p-Smad2/3 (fibrosis) (20). This effect was not seen with a lower dose of 2.5 mg/kg. In another study, when AEOL 10150 was administered 2 h after 15 Gy WTLI in C57BL/6J mice, we observed a reduction in apoptotic nuclei, and caspase-3 activity with reduced PTEN, TGF-β1 and NOX4 expression, suggesting reduced radiation-induced pro-apoptotic signals (5). Additionally, we have demonstrated that treatment with AEOL 10150 restores basal level expression of 31 of 44 hypoxia-associated genes in mouse lung 6 weeks after radiation exposure when treatment was delivered for only 28 days (30).
Finally, it is unclear, however, if continued administration of AEOL 10150 after 4 weeks could possibly result in more effective mitigation of delayed radiation-induced effects. As noted above, in the previously reported study administration of AEOL 10150 for only a short duration, starting from the day prior to irradiation until one week postirradiation did not generate any differential response compared to irradiated controls, but a positive response was seen when the drug was continued for 10 weeks (21). Based on the findings of these two studies, AEOL 10150 at a dose of 25 mg/kg is the recommended dose for further evaluation in future rodent studies.
In conclusion, through this study we demonstrate a dose response with increasing doses of the AEOL 10150, which is a superoxide dismutase mimic. We noted that treatment with AEOL 10150 significantly improves survival and lung function when given at a dose of 25 mg/kg/day for a total of 28 days starting 24 h after WTLI in pneumonitisprone CBA/J mice. The higher dose of 40 mg/kg showed some effect in mitigation of lung toxicity but was associated with late deaths. A positive response seen in the WTLI-vehicle cohort suggests the importance of supportive care in management of radiation toxicity, which is being tested in current and previously reported NHP studies. Current studies are focused on the mechanism of action of AEOL 10150 and future studies will be performed to determine the optimal duration and therapeutic window for AEOL 10150 delivery at a dose of 25 mg/kg/day as an agent to mitigate radiation-induced lung injury from accidental or malintentional radiation exposure. There is also an opportunity to use this drug in clinical settings for radiation therapy in thoracic malignancies.
Acknowledgments
This project has been funded in whole or in part with federal funds from the Biomedical Advanced Research and Development Authority, Office of the Assistant Secretary for Preparedness and Response, Office of the Secretary, Department of Health and Human Services, under contract no. HHSO100201100007C and Aeolus Pharmaceuticals, Inc. (Mission Viejo, CA). Dr. Isabel Jackson is a consultant to Aeolus Pharmaceuticals, Inc.
References
- 1.Baranov AE, Selidovkin GD, Butturini A, Gale RP. Hematopoietic recovery after 10-Gy acute total body radiation. Blood. 1994;83:596–9. [PubMed] [Google Scholar]
- 2.Waselenko JK, MacVittie TJ, Blakely WF, Pesik N, Wiley AL, Dickerson WE, et al. Medical management of the acute radiation syndrome: recommendations of the Strategic National Stockpile Radiation Working Group. Ann Int Med. 2004;140:1037–51. doi: 10.7326/0003-4819-140-12-200406150-00015. [DOI] [PubMed] [Google Scholar]
- 3.Uozaki H, Fukayama M, Nakagawa K, Ishikawa T, Misawa S, Doi M. The pathology of multi-organ involvement: two autopsy cases from the Tokai-mura criticality accident. Br J Radiol. 2005;27:13–16. [Google Scholar]
- 4.Gross NJ. Pulmonary effects of radiation therapy. Ann Int Med. 1977;86:81–92. doi: 10.7326/0003-4819-86-1-81. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Zhang X, Rabbani ZN, Jackson IL, Vujaskovic Z. Oxidative stress mediates radiation lung injury by inducing apoptosis. Int J Radiat Oncol Biol Phys. 2012;83:740–8. doi: 10.1016/j.ijrobp.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rabbani ZN, Spasojevic I, Zhang X, Moeller BJ, Haberle S, Vasquez-Vivar J, et al. Antiangiogenic action of redox-modulating Mn(III) mesotetrakis(N-ethylpyridinium-2-yl)porphyrin, MnTE-2-PyP(5+), via suppression of oxidative stress in a mouse model of breast tumor. Free Radic Biol Med. 2009;47:992–1004. doi: 10.1016/j.freeradbiomed.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Batinic-Haberle I, Spasojevic I, Tse HM, Tovmasyan A, Rajic Z, St Clair DK, et al. Design of Mn porphyrins for treating oxidative stress injuries and their redox-based regulation of cellular transcriptional activities. Amino Acids. 2012;42:95–113. doi: 10.1007/s00726-010-0603-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson IL, Chen L, Batinic-Haberle I, Vujaskovic Z. Superoxide dismutase mimetic reduces hypoxia-induced O2*–, TGF-beta, and VEGF production by macrophages. Free Radic Res. 2007;41:8–14. doi: 10.1080/10715760600913150. [DOI] [PubMed] [Google Scholar]
- 9.Vujaskovic Z, Batinic-Haberle I, Rabbani ZN, Feng QF, Kang SK, Spasojevic I, et al. A small molecular weight catalytic metalloporphyrin antioxidant with superoxide dismutase (SOD) mimetic properties protects lungs from radiation-induced injury. Free Radic Biol Med. 2002;33:857–63. doi: 10.1016/s0891-5849(02)00980-2. [DOI] [PubMed] [Google Scholar]
- 10.Rabbani ZN, Anscher MS, Folz RJ, Archer E, Huang H, Chen L, et al. Overexpression of extracellular superoxide dismutase reduces acute radiation induced lung toxicity. BMC Cancer. 2005;5:59. doi: 10.1186/1471-2407-5-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rabbani ZN, Mi J, Zhang Y, Delong M, Jackson IL, Fleckenstein K, et al. Hypoxia inducible factor 1alpha signaling in fractionated radiation-induced lung injury: role of oxidative stress and tissue hypoxia. Radiat Res. 2010;173:165–74. doi: 10.1667/RR1816.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petkau A, Chelack WS, Pleskach SD, Meeker BE, Brady CM. Radioprotection of mice by superoxide dismutase. Biochem Biophys Res Commun. 1975;65:886–93. doi: 10.1016/s0006-291x(75)80468-2. [DOI] [PubMed] [Google Scholar]
- 13.Van Hemmen JJ, Meuling WJ. Inactivation of biologically active DNA by gamma-ray-induced superoxide radicals and their dismutation products singlet molecular oxygen and hydrogen peroxide. Acta Biochimica Biophys. 1975;402:133–41. doi: 10.1016/0005-2787(75)90031-3. [DOI] [PubMed] [Google Scholar]
- 14.Lefaix JL, Delanian S, Leplat JJ, Tricaud Y, Martin M, Nimrod A, et al. Successful treatment of radiation-induced fibrosis using Cu/Zn-SOD and Mn-SOD: an experimental study. Int J Radiat Oncol Biol Phys. 1996;35:305–12. doi: 10.1016/0360-3016(96)00061-2. [DOI] [PubMed] [Google Scholar]
- 15.Sorenson JR. Bis(3,5-diisopropylsalicylato)copper(II), a potent radioprotectant with superoxide dismutase mimetic activity. J Med Chem. 1984;27:1747–9. doi: 10.1021/jm00378a040. [DOI] [PubMed] [Google Scholar]
- 16.Karlsson JOG, Ignarro LJ, Lundstrom I, Jynge P, Almen T. Calmangafodipir [Ca4Mn(DPDP)(5)], mangafodipir (MnDPDP) and MnPLED with special reference to their SOD mimetic and therapeutic properties. Drug Discov Today. 2015;20:411–21. doi: 10.1016/j.drudis.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Gao F, Fish BL, Szabo A, Doctrow SR, Kma L, Molthen RC, et al. Short-term treatment with a SOD/catalase mimetic, EUK-207, mitigates pneumonitis and fibrosis after single-dose total-body or whole-thoracic irradiation. Radiat Res. 2012;178:468–80. doi: 10.1667/RR2953.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Epperly M, Bray J, Kraeger S, Zwacka R, Engelhardt J, Travis E, et al. Prevention of late effects of irradiation lung damage by manganese superoxide dismutase gene therapy. Gene Ther. 1998;5:196–208. doi: 10.1038/sj.gt.3300580. [DOI] [PubMed] [Google Scholar]
- 19.Kang SK, Rabbani ZN, Folz RJ, Golson ML, Huang H, Yu D, et al. Overexpression of extracellular superoxide dismutase protects mice from radiation-induced lung injury. Int J Radiat Oncol Biol Phys. 2003;57:1056–66. doi: 10.1016/s0360-3016(03)01369-5. [DOI] [PubMed] [Google Scholar]
- 20.Rabbani ZN, Salahuddin FK, Yarmolenko P, Batinic-Haberle I, Thrasher BA, Gauter-Fleckenstein B, et al. Low molecular weight catalytic metalloporphyrin antioxidant AEOL 10150 protects lungs from fractionated radiation. Free Radic Res. 2007;41:1273–82. doi: 10.1080/10715760701689550. [DOI] [PubMed] [Google Scholar]
- 21.Rabbani ZN, Batinic-Haberle I, Anscher MS, Huang J, Day BJ, Alexander E, et al. Long-term administration of a small molecular weight catalytic metalloporphyrin antioxidant, AEOL 10150, protects lungs from radiation-induced injury. Int J Radiat Oncol Biol Phys. 2007;67:573–80. doi: 10.1016/j.ijrobp.2006.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bowler RP, Sheng H, Enghild JJ, Pearlstein RD, Warner DS, Crapo JD. A catalytic antioxidant (AEOL 10150) attenuates expression of inflammatory genes in stroke. Free Radic Biol Med. 2002;33:1141–52. doi: 10.1016/s0891-5849(02)01008-0. [DOI] [PubMed] [Google Scholar]
- 23.Smith KR, Uyeminami DL, Kodavanti UP, Crapo JD, Chang LY, Pinkerton KE. Inhibition of tobacco smoke-induced lung inflammation by a catalytic antioxidant. Free Radic Biol Med. 2002;33:1106–14. doi: 10.1016/s0891-5849(02)01003-1. [DOI] [PubMed] [Google Scholar]
- 24.Tse HM, Milton MJ, Piganelli JD. Mechanistic analysis of the immunomodulatory effects of a catalytic antioxidant on antigen-presenting cells: implication for their use in targeting oxidation-reduction reactions in innate immunity. Free Radic Biol Med. 2004;36:233–47. doi: 10.1016/j.freeradbiomed.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 25.Jackson IL, Vujaskovic Z, Down JD. Revisiting strain-related differences in radiation sensitivity of the mouse lung: recognizing and avoiding the confounding effects of pleural effusions. Radiat Res. 2010;173:10–20. doi: 10.1667/RR1911.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson IL, Vujaskovic Z, Down JD. A further comparison of pathologies after thoracic irradiation among different mouse strains: finding the best preclinical model for evaluating therapies directed against radiation-induced lung damage. Radiat Res. 2011;175:510–18. doi: 10.1667/RR2421.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson IL, Xu P, Hadley C, Katz BP, McGurk R, Down JD, et al. A preclinical rodent model of radiation-induced lung injury for medical countermeasure screening in accordance with the FDA animal rule. Health Phys. 2012;103:463–73. doi: 10.1097/HP.0b013e31826386ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jackson IL, Xu PT, Nguyen G, Down JD, Johnson CS, Katz BP, et al. Characterization of the dose response relationship for lung injury following acute radiation exposure in three well-established murine strains: developing an interspecies bridge to link animal models with human lung. Health Phys. 2014;106:48–55. doi: 10.1097/HP.0b013e3182a32ccf. [DOI] [PubMed] [Google Scholar]
- 29.Van Dyk J, Keane TJ, Kan S, Rider WD, Fryer CJ. Radiation pneumonitis following large single dose irradiation: a re-evaluation based on absolute dose to lung. Int J Radiat Oncol Biol Phys. 1981;7:461–7. doi: 10.1016/0360-3016(81)90131-0. [DOI] [PubMed] [Google Scholar]
- 30.Jackson IL, Zhang X, Hadley C, Rabbani ZN, Zhang Y, Marks S, et al. Temporal expression of hypoxia-regulated genes is associated with early changes in redox status in irradiated lung. Free Radic Biol Med. 2012;53:337–46. doi: 10.1016/j.freeradbiomed.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garofalo MC, Ward AA, Farese AM, Bennett A, Taylor-Howell C, Cui W, et al. A pilot study in rhesus macaques to assess the treatment efficacy of a small molecular weight catalytic metalloporphyrin antioxidant (AEOL 10150) in mitigating radiation-induced lung damage. Health Phys. 2014;106:73–83. doi: 10.1097/HP.0b013e3182a4d967. [DOI] [PubMed] [Google Scholar]
- 32.Travis EL, Down JD, Holmes SJ, Hobson B. Radiation pneumonitis and fibrosis in mouse lung assayed by respiratory frequency and histology. Radiat Res. 1980;84:133–43. [PubMed] [Google Scholar]
- 33.Fleckenstein K, Zgonjanin L, Chen L, Rabbani Z, Jackson IL, Thrasher B, et al. Temporal onset of hypoxia and oxidative stress after pulmonary irradiation. Int J Radiat Oncol Biol Phys. 2007;68:196–204. doi: 10.1016/j.ijrobp.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szabo S, Ghosh SN, Fish BL, Bodiga S, Tomic R, Kumar G, et al. Cellular inflammatory infiltrate in pneumonitis induced by a single moderate dose of thoracic x radiation in rats. Radiat Res. 2010;173:545–56. doi: 10.1667/RR1753.1. [DOI] [PMC free article] [PubMed] [Google Scholar]