Abstract
Objective
The relationship between subjective memory complaints (SM) and objective memory (OM) performance in aging has been variably characterized in a substantial literature, to date. In particular, cross-sectional studies often observe weak or no associations. We investigated whether subjective memory complaints and objectively measured cognition influence each other over time, and if so, which is the stronger pathway of change – objective to subjective, or subjective to objective – or whether they are both important.
Method
Using bivariate latent change score modeling in data from a population study (N=1980) over 5 annual assessment cycles, we tested 4 corresponding hypotheses: 1) no coupling between SM and OM over time; 2) SM as leading indicator of change in OM; 3) OM as leading indicator of change in SM; 4) dual coupling over time, with both SM and OM leading subsequent change in the other. We also extended objective cognition to two other domains, language and executive functions.
Results
The dual-coupling models best fit the data for all three objective cognitive domains. The SM – OM temporal dynamics differ qualitatively compared to other domains, potentially reflecting changes in insight and self-awareness specific to memory impairment.
Conclusions
Subjective memory and objective cognition reciprocally influence each other over time. The temporal dynamics between subjective and objective cognition in aging are nuanced, and must be carefully disentangled to shed light on the underlying processes.
Keywords: aging, latent change score modeling, Age-Related Memory Disorders (MeSH), epidemiology, meta-cognition, anosognosia
The role of subjective memory complaints in cognitive health in aging has been recurrently debated. As an initial proposed criterion for mild cognitive impairment (MCI) (Petersen et al., 1999), in conjunction with memory deficits on objective assessments, some studies have reported that the subjective complaint component did not contribute to the diagnostic or prognostic value of MCI (Jungwirth et al., 2004; Lautenschlager, Flicker, Vasikaran, Leedman, & Almeida, 2005; Lenehan, Klekociuk, & Summers, 2012; Mitchell, 2008; Purser, Fillenbaum, & Wallace, 2006). In contrast, other studies have reported prognostic value of subjective complaints in MCI for subsequent clinical progression to more severe impairment (Lam, Lui, Tam, & Chiu, 2005; Mitchell & Shiri-Feshki, 2009; Schofield et al., 1997). In the Alzheimer’s disease (AD) research community, the conceptual framework of ‘subjective cognitive decline’ has emerged, which characterizes subjective cognitive concerns in the absence of objectively measured cognitive deficits as a putative pre-MCI clinical state, and potentially the first indicator of subsequent cognitive decline for some older individuals (Jessen et al., 2014; Reisberg, Shulman, Torossian, Boksay, et al., 2010).
It is clear from cross-sectional studies of non-demented older adults that, although objective memory (OM) and subjective memory (SM) deficits are associated with each other across groups, they are often incongruent within individuals. In a review by Mitchell (2008), SM complaints were present in only 38% of individuals with MCI defined via objective cognitive assessment. In contrast, studies of preclinical AD, especially those seeking to characterize biomarkers, often identify individuals with SM complaints but without OM deficits, as described above (Amariglio et al., 2012; Hafkemeijer et al., 2013; Jessen et al., 2014; Rowe et al., 2010; Saykin et al., 2006). The question of which is the starting point for cognitive decline (or whether either may be) is best addressed in longitudinal studies.
From a theoretical standpoint, Hermann (Herrmann, 1982) in an early work noted that individual differences in subjective ratings of memory symptom severity may obscure cross-sectional relationships between subjective and objectively measured memory. Therefore, longitudinal change in both subjective and objective memory should yield higher associations, as each individual serves as his/her own ‘baseline’ and reference point for change (Hertzog & Pearman, 2014). Other investigators have argued that non-cognitive factors, such as personality traits, depressive symptoms and socially determined self-perceived efficacy (Bandura, 1989; Perrig-Chiello, Perrig, & Stahelin, 2000) are more strongly predictive of subjective memory evaluation in aging than is objectively measured performance, and this explains the weak relationship.
A number of longitudinal studies have investigated baseline SM predicting longitudinal change in OM (or more broadly measured cognitive decline), with mixed findings (Dik et al., 2001; Dufouil, Fuhrer, & Alpérovitch, 2005; Hohman, Beason-Held, Lamar, & Resnick, 2011; Mol, van Boxtel, Willems, & Jolles, 2006; Schofield et al., 1997; Wang et al., 2004). Far fewer studies have included any measures of longitudinal change in SM. A small study of 100 healthy older participants found no change over 2.5 years on self-rated cognitive errors (Cognitive Failures Questionnaire) regardless of whether there was decline on objective memory testing, concluding a lack of correspondence between objective and subjective changes over time (Weaver Cargin, Collie, Masters, & Maruff, 2008). In contrast, Parisi et al. (Parisi et al., 2011) used parallel process latent growth curve modeling with data from n=1,301 healthy participants in the ACTIVE cognitive training intervention trial, observing that OM slope was associated with SM slope over five years. Zimprich et al. (2003) also reported an association between change in subjective cognitive complaints and change in OM over two measurement occasions over 4 years in n=442 participants from the German population-based Interdisciplinary Study on Life Development, using latent change score modeling. These two larger studies indicate significant associations between changes over time in SM to changes in OM; however, they did not specifically address questions of temporal sequence or direction of influence over time between the two constructs. Jorm et al. (2001) addressed these questions using structural equation modeling in a community sample across a cognitive spectrum, including dementia, of n=331 over 3 waves and 7.6 years follow-up. Measuring OM and SM at each wave, they reported a significant OM to SM path, as well as an SM to OM path, across wave 1 to 2, and wave 2 to 3. These results indicate cross-wave mutual influences of levels of OM and SM, respectively, on each other; of note, the model did not address questions of change, per se, in either SM or OM.
Building on results of these longitudinal studies, the aim of the present investigation was to model the temporal dynamics between SM and OM in a large population-based cohort of older adult over 5 annually spaced assessments. We used bivariate latent change score (LCS) models (Ferrer & McArdle, 2010; McArdle, 2009), to model change over time in OM and SM across waves, and to test four alternative hypotheses about possible temporal directions of mutual influence: 1) A “no-coupling” model represented the hypothesis of no relationship between changes in OM and SM. 2) An “OM single pathway” model represented objectively-measured memory as a leading indicator of subjective memory, in that it precedes changes in subjective memory ratings. 3) An “SM single pathway” model represented subjectively-reported memory as the leading indicator of objective memory, in that it precedes changes in objective memory performance. 4) A “dynamic coupling” hypothesis tested whether both kinds of memory outcomes show evidence of leading and lagging the other, such that levels of each variable influence subsequent changes in the other. The broader study goal was to better understand the interplay between subjective and objective cognition, and informing future refinement of assessment approaches for early detection of pathological cognitive aging.
Finally, we expanded the objective cognitive measurement to other domains beyond OM, including objective language (OL) and objective executive functions (OEF). The rationale for doing so included the clinical observation that older individuals often generically complain of ‘memory problems” but when probed describe language- or semantic-based symptoms, such as word-finding difficulties, or executive dysfunction, such as difficulty organizing one’s personal or financial affairs, working- or prospective memory failures, etc. (Burmester, Leathem, & Merrick, 2014).
Method
Study Site and Population
Our study cohort named the Monongahela-Youghiogheny Healthy Aging Team (MYHAT) is an age-stratified random population sample drawn from the publicly available voter registration lists for a small-town region of Pennsylvania (USA.) Community outreach, recruitment, and assessment protocols were approved by the University of Pittsburgh Institutional Review Board for protection of human subjects. All participants provided written informed consent. Recruitment criteria were (a) age 65 years or older, (b) living within the selected towns, (c) not already in long-term care institutions. Individuals were ineligible if they (d) were too ill to participate, (e) had severe vision or hearing impairments, (f) were decisionally incapacitated. We recruited 2036 individuals over a two-year period, and screened out 54 who at study entry exhibited moderate or severe cognitive impairment by scoring <21/30 on an age-education-corrected Mini-Mental State Exam (MMSE); (Folstein, Folstein, & McHugh, 1975; Mungas, Marshall, Weldon, Haan, & Reed, 1996). The remaining 1982 individuals underwent a detailed in-home assessment including, but not limited to, the elements below. (Ganguli et al., 2009)
Assessments
At baseline and at each annual data collection cycle, we assessed cognitive functioning with a comprehensive neuropsychological test battery tapping multiple cognitive domains including memory, attention/processing speed, visuospatial reasoning, language and executive functioning (Ganguli, Snitz, et al., 2010). Objective memory tests included Wechsler Memory Scale-Revised Logical Memory and Visual Reproduction (Wechsler, 1987), and the FULD Object Memory Evaluation (Fuld, 1981). Objective language tests included the Indiana University token test (Unverzagt et al., 1999), semantic fluency (animals), and the Boston Naming Test (Kaplan, Goodglass, & Weintraub, 2001). Objective executive functions tests included Trail Making Part B (connections / second), clock drawing (Freedman et al., 1994), and phonemic fluency (FAS). We created composite scores for these three domains by averaging test Z-scores referenced to the cohort at baseline (higher Z indicates better test performance). Subjective memory was assessed a self-report measure of subjective cognitive complaints (Ganguli, Dodge, Shen, & DeKosky, 2004; Snitz et al., 2012), 16 items of which related to memory complaints specifically (Supplemental Table 1). The sum of 16 items (each scored 0 vs. 1 for complaint absent / present) were Z-score transformed, referenced to the cohort at baseline for the present analysis, with higher scores representing greater number of complaints / memory symptoms endorsed.
Other assessments administered in annual cycles included the modified Center for Epidemiologic Studies Depression scale (mCESD) (Ganguli, Gilby, Seaberg, & Belle, 1995), the Clinical Dementia Rating (CDR) scale (Ganguli, Chang, et al., 2010; Morris, 1993), self-reported health history and medication count. Genotyping for APOE*4 allele was completed on a subset of n=1778 participants at baseline.
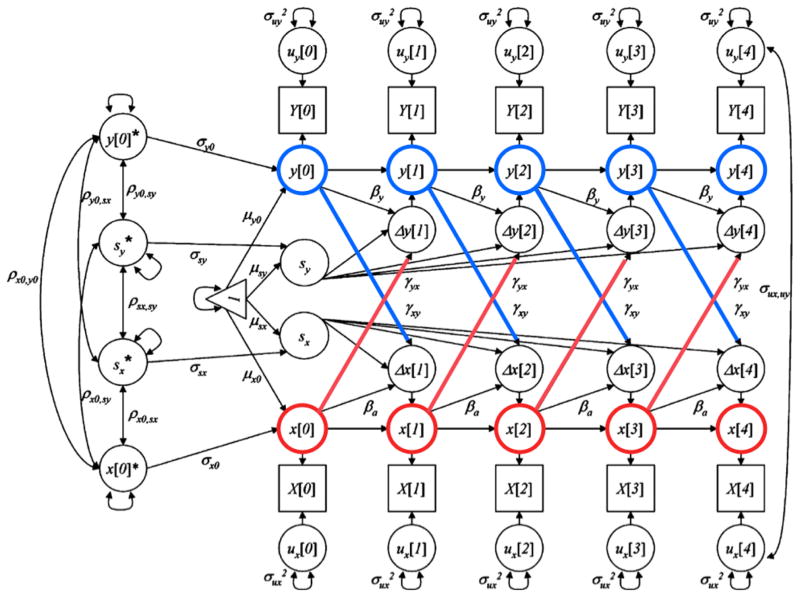
Data Analysis
Analysis of the changes in subjective memory and objective cognitive measures took place in two stages. First, univariate latent change score models were applied to each variable to describe changes across the follow-up period (for reviews, see (Ferrer & McArdle, 2010; McArdle, 2009). Next, we examined dynamic relationships between 1) SM and OM, 2) SM and OL, and 3) SM and OEF by fitting bivariate latent change score models. Figure 1 shows the path diagram for a prototypic model between an x variable (e.g., SM) and a y variable (e.g., OM). As a guide to these models, the latent difference scores (e.g., Δx[t]) are influenced by a constant component (i.e., sx), as well as changes that are related to prior level of performance (βx). In the dual coupling model, this latent difference score is also predicted by previous scores from the opposite variable through the use of a coupling parameter (e.g., γxy). The statistical significance of the coupling parameters indicates whether prior scores on one variable (e.g., y[0]) influences changes on the other (e.g., Δx[1]). Using these models, we examined changes in model fit (deviation in the -2LL, AIC, and BIC) across four sequential hypotheses that are tested for each objective domain: a) no relationship between outcomes; b) level of SM predicting subsequent change in objective cognition; c) level of objective cognition predicting subsequent change in SM; and d) dual coupling between the two outcomes (Small, Dixon, McArdle, & Grimm, 2012). The convergence criterion for the dual coupling model between SM and OEF was constrained to 0.05 to account for high correlation between the temporal changes of the two cognitive functions. The convergence criterion for all other models was 0.00005.
Figure 1.
Path diagram of dual latent change score model. X = Subjective memory; Y = Objective cognition [Objective memory (OM), Objective language (OL), & Objective executive functions (OEF), respectively]
For each outcome, scores were expressed as z-scores based upon means and SDs at baseline. Covariates included were age at baseline (treated continuously), depression score at baseline (treated continuously), gender, and education (categorized at < HS; HS; > HS). Our goal for covariate selection was to control for potential demographic confounders of objective-subjective memory associations. We also included depressive symptoms as an established correlate of subjective memory complaints (Reid & Maclullich, 2006). Analyses were completed with M-Plus (v.7.1;(Muthen & Muthen, 2010). All available data at each cycle were included in all models.
Results
Demographic and Clinical Characteristics
At baseline (study entry, cycle 1), the MYHAT cohort (N=1980) was representative of older adults in the targeted communities, with a mean (SD) age of 77.6 (7.4) years and a median educational level of high school graduate; 61.0% were women and 94.8% were of mixed European descent (Table 1). Regarding general health indicators, about 17% rated themselves as in only ‘fair’ or ‘poor’ health, and over half the cohort was taking at least 4 prescription medications. Of note, 71.3% were rated CDR=0 (no dementia), 27.6% CDR 0.5 (very mild / questionable dementia) and 1.2% were rated CDR=1 (mild dementia), so that by CDR a broad range of cognition was included (with moderate to severe impairment screened out at entry by the MMSE criterion of < 21, age- and education-corrected). Over the first 5 years of the study, the cohort experienced 41.2% attrition as would be expected in an aging population-based cohort. Inspection of summary demographic and clinical variables, including CDR, MMSE, and APOE*4 allele status, across the 5 cycles suggest broadly comparable cohort characteristics despite the loss to follow-up.
Table 1.
Cohort characteristics (mean / SD or n / %) by annual assessment cycle
| Cycle 1 | Cycle 2 | Cycle 3 | Cycle 4 | Cycle 5 | |
|---|---|---|---|---|---|
| N | 1980 | 1697 | 1467 | 1320 | 1161 |
| Age, mean (SD) | 77.65 (7.44) | 78.47 (7.34) | 79.16 (7.20) | 79.90 (7.15) | 80.51 (7.07) |
| % female | 61.05 | 62.05 | 62.99 | 63.26 | 63.05 |
| % ≥ HS education | 86.23 | 86.74 | 87.71 | 87.42 | 87.42 |
| % white | 94.70 | 94.87 | 94.72 | 95.15 | 95.09 |
| CDR | |||||
| % 0 | 71.29 | 73.72 | 72.14 | 71.89 | 73.56 |
| % 0.5 | 27.55 | 24.99 | 25.85 | 25.68 | 23.77 |
| % > = 1 | 1.16 | 1.30 | 2.0 | 2.42 | 2.67 |
| % APOE*4 allele | 20.92 | 21.23 | 21.36 | 21.03 | 21.17 |
| Subjective health | |||||
| % Poor/Fair | 17.44 | 15.26 | 14.91 | 14.24 | 16.62 |
| % Good | 45.70 | 47.26 | 48.93 | 50.53 | 46.94 |
| % Very Good/Excellent | 36.86 | 37.48 | 36.16 | 35.23 | 36.43 |
| Number Rx meds | |||||
| % 0 | 8.8 | 8.37 | 7.35 | 7.12 | 6.46 |
| % 1–3 | 35.76 | 34.20 | 35.20 | 33.18 | 33.07 |
| % >= 4 | 55.44 | 57.43 | 57.45 | 59.70 | 60.47 |
| MMSE, mean (SD) | 26.94 (2.43) | 27.04 (2.72) | 27.03 (2.68) | 27.0 (3.03) | 26.94 (3.17) |
| mCESD score (SD) | 0.94 (2.08) | 0.72 (1.92) | 0.67 (1.87) | 0.52 (1.49) | 0.48 (1.55) |
| Objective Memory | |||||
| WMS-R Logical Memory IR, mean (SD) | 19.67 (7.27) | 20.49 (7.38) | 21.36 (7.46) | 21.89 (7.67) | 22.52 (7.84) |
| WMS-R Visual Reproduction IR, mean (SD) | 27.56 (7.19) | 28.37 (7.02) | 28.08 (7.09) | 27.84 (7.27) | 28.10 (7.16) |
| Fuld OME, mean (SD) | 21.86 (4.30) | 22.92 (4.75) | 23.33 (4.85) | 23.36 (5.14) | 23.46 (5.20) |
| Objective Language | |||||
| Semantic fluency, mean (SD) | 15.57 (4.83) | 15.63 (4.89) | 15.60 (5.02) | 15.62 (4.99) | 15.55 (4.81) |
| IU Token Test, mean (SD) | 22.67 (1.80) | 22.72 (1.84) | 22.75 (1.88) | 22.79 (1.86) | 22.83 (1.88) |
| BNT, mean (SD) | 52.96 (6.04) | 53.15 (6.11) | 53.26 (6.33) | 53.19 (6.30) | 53.17 (6.66) |
| Objective Executive Functions | 22.67 (1.80) | 22.72 (1.84) | 22.75 (1.88) | 22.79 (1.86) | 22.83 (1.88) |
| Trail Making B, (connections / s) mean (SD) | 0.2372 (0.1011) | 0.2440 (0.1129) | 0.2567 (0.1114) | 0.2553 (0.1100) | 0.2539 (0.1127) |
| Phonemic fluency (FAS), mean (SD) | 11.97 (4.46) | 12.09 (4.62) | 12.31 (4.38) | 12.52 (4.54) | 12.83 (4.52) |
| Clock drawing | 13.50 (1.79) | 13.52 (1.87) | 13.56 (1.81) | 13.66 (1.87) | 13.69 (1.85) |
| Subjective memory symptoms, mean (SD) (range 0 – 16) | 2.08 (2.30) | 1.80 (2.27) | 1.89 (2.31 | 2.0 (2.41) | 2.0 (2.48) |
Note. CDR = Clinical Dementia Rating scale; MMSE = Mini Mental State Exam; WMS-R = Wechsler Memory Scale – Revised; OME = Object Memory Evaluation; IU = Indiana University; BNT = Boston Naming Test. APOE*4 = Apolipoprotein E gene, presence of at least one E*4 allele; mCESD = modified Center for Epidemiologic Studies Depression scale.
Univariate Latent Change Score Models
The estimated parameters for subjective memory, objective memory, objective language, and objective executive functions across the follow-up period are reported in Table 2. The results from these models indicate that statistical significant changes over time were observed, with declines in SM complaints and increases for OM and OL performance, independent of age at baseline, gender and years of education. Among the covariates, older age was associated with higher baseline SM complaint scores and increasing SM complaints over time. Older age was associated with lower scores in OM, OL and OEF at baseline, as well as with decline in OM and OL across time. Female gender was associated with lower baseline SM complaints and higher baseline OM and OEF scores, but had no association with changes over time. Finally, more years of education was associated with decline in SM complaints over time, and with higher OM, OL and OEF scores over time.
Table 2.
Univariate latent change score model results [estimates (se)] for subjective memory and objective memory, language & objective executive functions, with age, gender, education level, and baseline depression score as covariates
| Subjective Memory | Objective Memory | Objective Language | Objective Executive Functions | |
|---|---|---|---|---|
| Proportion, β | −.237 (.049)*** | −.084 (.044) | −.095 (.042)* | .082 (.085) |
| Level mean, μ0 | −1.150 (.248)*** | 3.345 (.185)*** | 2.883 (.183)*** | 3.080 (.179)*** |
| Slope mean, μs | −.955 (.139)*** | .886 (.188)*** | .694 (.154)*** | .035 (.034) |
| Level variance, σ02 | .654 (.028)*** | .409 (.015)*** | .394 (.015)*** | .350 (.015)*** |
| Slope variance, σs2 | .081 (.018)*** | .024 (.007)*** | .028 (.007)*** | .008 (.002)*** |
| Residual variance, σe2 | .269 (.006)*** | .092 (.015)*** | .109 (.002)*** | .147 (.003)*** |
| σ0,1 | .111 (.029)*** | .049 (.018)** | .046 (.017)** | −.020 (.031) |
| Covariates | ||||
| Age → Level | .015 (.003)*** | −.047 (.002)*** | −.042 (.002)*** | −.045 (.002)*** |
| Age → Slope | .014 (.002)*** | −.011 (.003)*** | −.010 (.002)*** | .000 (.004) |
| Gender → Level | −.177 (.044)*** | .151 (.032)*** | .007 (.032) | .083 (.031)** |
| Gender → Slope | −.011 (.019) | .006 (.012) | .005 (.011) | −.014 (.011) |
| Education_1 → Level | −.070 (.066) | .219 (.049)*** | .419 (.049)*** | .345 (.047)*** |
| Education_1 → Slope | −.097 (.029)** | .002 (.018) | .016 (.024) | −.036 (.032) |
| Education_2 → Level | −.078 (.068) | .401 (.050)*** | .565 (.050)*** | .464 (.049)*** |
| Education_2 → Slope | −.106 (.030)*** | .021 (.023) | .031 (.029) | −.047 (.041) |
| Depression → Level | .101 (.010)*** | −.040 (.008)*** | −.038 (.008)*** | −.035 (.007)*** |
| Depression → Slope | .008 (.006) | −.001 (.003) | −.003 (.003) | .002 (.004) |
| Fit Statistics | ||||
| χ2 (28) | 225.07*** | 174.89*** | 95.09*** | 89.529*** |
| CFI | .960 | .984 | .992 | .991 |
Note. CFI = comparative fit index.
p < .05
p < .01
p < .001
Bivariate Latent Change Score Models
Table 3 displays a summary of the model fit indices for each of the four models that were evaluated concerning the relationships between changes in subjective memory and objective memory. As a guide to the table, the no-coupling model is the reference model; change in chi-square (Δχ2) allows us to evaluate whether any improvement in model fit is statistically significant relative to the number of parameters that were added to the model. Interpretation of the bivariate LCS model results are aided by vector field plots in the Figure. These plots (Boker & McArdle, 1995; Small et al., 2012) were constructed to illustrate the dynamic relationships between the paired variables. For a given pair of subjective and objective cognitive scores, the arrow indicates the expected changes in both subjective and objective cognition at the next measurement occasion. The direction of the arrows indicates whether future changes will be negative, positive, or neutral and the relative size of the arrow relates to the relative size of predicted changes. Details of the results, as specifically reflected in the vector field plots, are presented separately by cognitive domain.
Table 3.
Bivariate latent change score summary results for subjective memory coupled with 1) objective memory, 2) objective language, and 3) objective executive functions
| Model | χ2 | df | CFI | Δχ2 |
|---|---|---|---|---|
| Objective Memory | ||||
| No Coupling | 426.32 | 76 | .976 | - |
| SM to OM | 402.91 | 75 | .978 | 23.41*** |
| OM to SM | 424.01 | 75 | .976 | 2.31 |
| Dual Coupling | 385.83 | 74 | .979 | 40.49*** |
| Objective Language | ||||
| No coupling | 374.21 | 76 | .978 | - |
| SM to OL | 364.77 | 75 | .979 | 9.44** |
| OL to SM | 323.47 | 75 | .982 | 50.74*** |
| Dual coupling | 296.11 | 74 | .984 | 78.10*** |
| Objective Executive Functions | ||||
| No coupling | 345.53 | 76 | .978 | - |
| SM to OEF | 334.24 | 75 | .978 | 11.29** |
| OEF to SM | 316.03 | 75 | .980 | 29.50*** |
| Dual coupling | 283.52 | 74 | .983 | 62.01*** |
Note. CFI = comparative fit index.
p < .05
p < .01
p < .001
Subjective memory and objective memory
As compared to the no-coupling model, adding a unidirectional path from subjective memory to changes in objective memory improved the fit of the model. However, the inclusion of a unidirectional path from objective memory to changes in subjective memory did not fit the data better than the no-coupling model. Finally, the model that included both paths (dual coupling model) provided the best fit to the data. The parameter estimate for the subjective memory to objective memory relationship was −0.55 (SE = 0.12, p < .001) and the path from objective memory to subjective memory was 0.65 (SE = 0.17, p < .001). The negative parameter estimate for SM to OM indicates that fewer subjective memory complaints were related to greater improvement in objective memory performance; or, alternatively, that more subjective complaints predicted decline in objective memory scores. For the opposite path, worse objective memory was associated with greater decline in subjective memory complaints over time. These relationships are illustrated in Figure 2.
Figure 2.
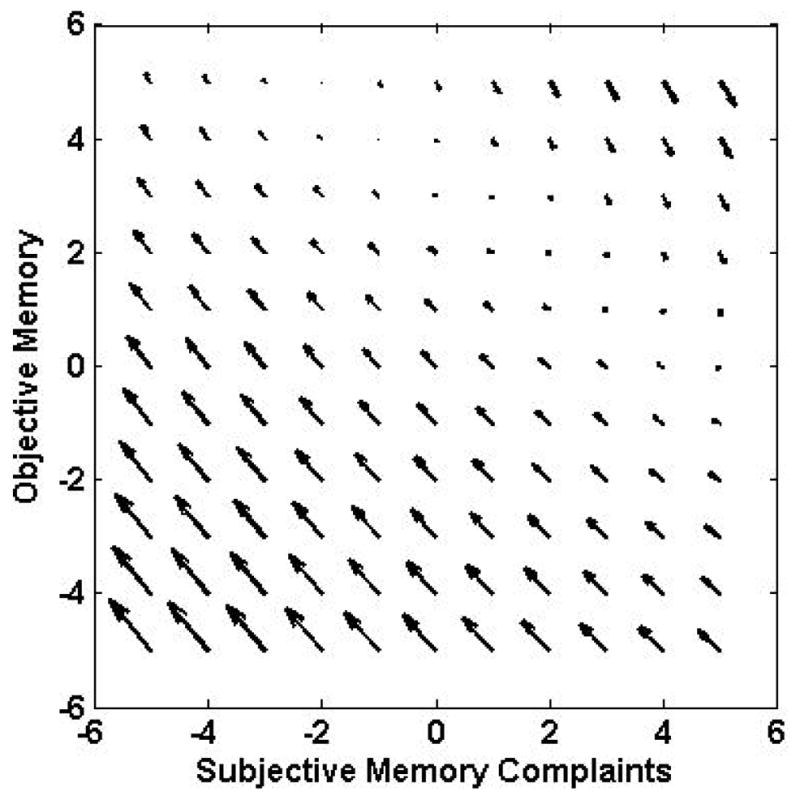
Vector field plots for subjective memory and objective memory. The plots illustrate the dynamic relationships between the paired variables. For a given pair of subjective and objective cognitive scores, the arrow indicates the expected changes in both subjective and objective cognition at the next measurement occasion. The direction of the arrows indicates whether future changes will be negative, positive, or neutral and the relative size of the arrow relates to the relative size of predicted changes.
Subjective memory and objective language
Relative to the no-coupling model, the dual coupling model provided the best fit to the data (see Table 3). The parameter estimate for the subjective memory to objective language pathway was 0.332 (SE 0.086, p < .001) and the path from OL to SM was −1.358 (SE 0.215, p < .001). The positive SM to OL estimate indicates fewer subjective memory complaints were associated with declines in objective language. The negative parameter estimate for OL to SM indicates worse objective language scores were associated with subsequent increase in subjective memory complaints, or alternatively, better OL scores associated with decline in SM complaints. Inspection of the vector field plot (Figure 3) suggests 1) that both these patterns are driving the negative OL to SM parameter estimate; and 2) that the OL to SM pathway is stronger than the SM to OL pathway, consistent with relative size of the estimates.
Figure 3.
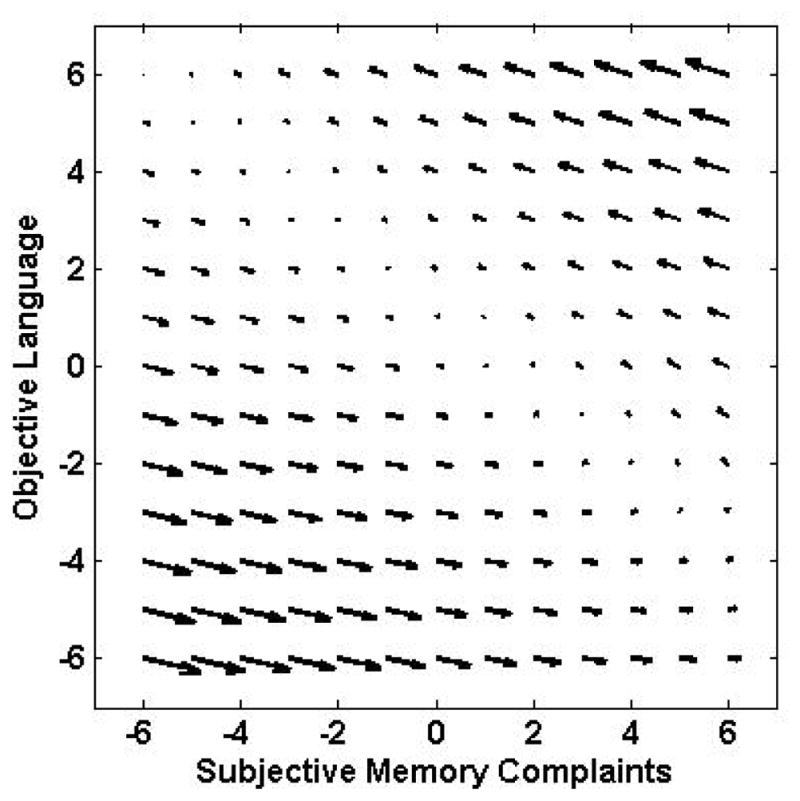
Vector field plots for subjective memory and objective language.
Subjective memory and objective executive functions
The model fit parameters for the SM and OEF models are shown in Table 3. Although the inclusion of the single pathways improved the fit of the model relative to the no-coupling model, the best fit was provided by the dual coupling model. Both of the parameter estimates were statistically significant (p < .001); the estimate for the subjective memory to objective executive functions pathway was 0.396 (SE 0.090) and the estimate for the OEF to SM pathway was −4.839 (SE 0.809). The positive parameter estimate indicated that fewer subjective memory complaints were associated with decreases in OEF performance. The negative estimates indicate that worse executive function scores are associated with subsequent increases in subjective memory complaints, or, that better executive functions scores are associated with decreases in subjective memory complaints. These relationships are illustrated in the Figure 4. As with OL-SM model, the vector field plot suggests that the objective to subjective pathway is the stronger one, consistent with the size of the estimates.
Figure 4.
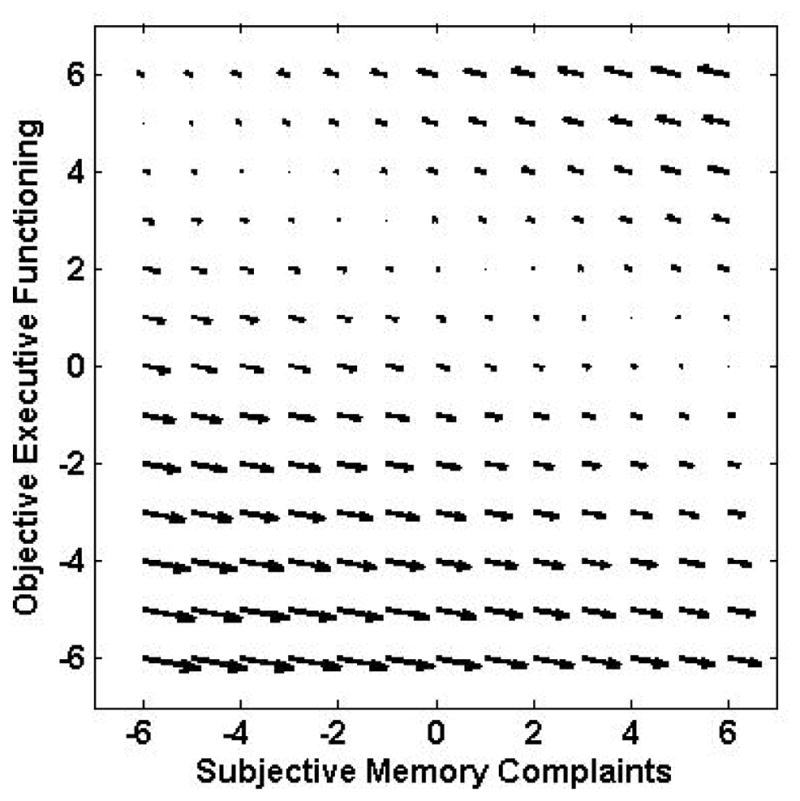
Vector field plots for subjective memory and objective executive functions.
Discussion
We sought to better understand the temporal dynamic relationships between subjectively-perceived and objectively-measured cognition over time. within a large, representative, population-based cohort of older adults. We applied bivariate latent change score modeling to data measured annually over five years and tested four competing models of change (within each of 3 domains). Results indicate that dual-pathway models best fit the data for all three cognitive domains. In all cases, the relationship between subjective memory ratings and objective cognitive performance represented a mutual influence of the two constructs on each other, with regard to sequential change over time. This study adds to a growing literature indicating that bidirectional influences are important in cognitive aging, including such paired constructs as cognition - activity level (Mousavi-Nasab, Kormi-Nouri, & Nilsson, 2014; Small et al., 2012; Wilson, Segawa, Boyle, & Bennett, 2012), cognition - physical functioning (Krall, Carlson, Fried, & Xue, 2014) and cognition - sense of well-being (Allerhand, Gale, & Deary, 2014).
The directions of effects in the present study were complex and partly unexpected. With regard to the memory domain, low subjective complaint scores were associated with the most improvement (i.e., practice) on memory test performance. This relationship reflects a correspondence between subjective and objective measures of cognitive health and suggests good insight in this range of lower subjective memory complaints. The other pathway, however, indicated lower objective memory scores were associated with subsequent decline in subjective complaints, revealing a subjective-objective discordance. This pathway suggests poor insight in this range of lower objective memory scores, possibly consistent with anosognosia. Interestingly, the pathway effects for both language and executive functions domains were opposite those for the memory domain. Lower objective language and executive function scores were associated with subsequent increases in subjective complaints, reflecting concordance in the outcomes. Among the three objective cognitive domains, memory uniquely reveals subjective-objective discordance at the poorer objective functioning range of the spectrum. This observation is consistent with the substantial literature on memory monitoring (meta-memory) deficits in clinical memory disorders, including amnestic MCI (Perrotin et al., 2011; Vogel et al., 2004) AD (Clare, 2004; Harwood, Sultzer, & Wheatley, 2000; Lopez, Becker, Somsak, Dew, & DeKosky, 1994; Mograbi, Brown, & Morris, 2009) and Korsakoff syndrome (Pannu & Kaszniak, 2005). Empirically, it is also consistent with studies showing objective episodic memory performance correlates with metamemory deficits (Shaked et al., 2014) and anosognosia (Orfei et al., 2010) in MCI and AD. Some theoretical accounts, as well, highlight the centrality of episodic memory to cognitive mechanisms underlying anosognosia for memory impairment. For instance, in the Morris and Hannisdottir (2007; 2004) Cognitive Awareness Model, a mnemonic sub-type of anosognosia in AD refers to failure to update a personal knowledge base of memory failures (or successes) over time, despite intact recognition of a memory failure at the time (i.e., intact error detection). This ‘forgetting I’ve forgotten’ mechanism, theorized to be one route to anosognosia in clinical dementia, may be reflected in the population level by a subtler form of deficit awareness in the present results.
Our results are consistent with other studies using sensitive modeling techniques and showing associations between objective and subjective memory changes (slopes) over time (Parisi et al., 2011; Zimprich et al., 2003), as well as across-occasion correlations (Jorm et al., 2001). All of these studies indicate significant general associations between subjective and objective memory across time in older populations.
Building on the previous literature, this study modeled specific temporal dynamics of change over time, allowing us to address the question of sequential influences of one variable on the other. According to present findings, the question of which is the earlier indicator of cognitive decline is not simply answered but is multifaceted and cognitive-domain dependent, as well as clinical state-dependent. At lower objective memory performance level, the expected concordance between subjective and objective memory is obscured, presumably by impaired insight, and shows the opposite relationship. In contrast, at higher levels of subjective memory complaints, objective memory subsequently declines for high objective memory performers (but tends to increase for lower objective memory performers). These results validate clinical observations that the reliability of patient self-reporting on day-to-day memory functioning usually varies with degree of objective memory impairment, and reflects self-awareness. To our knowledge this relationship has rarely been examined empirically at the population level. The observed subjective-objective memory discordances may help explain cross-study inconsistences in a large cross-sectional and longitudinal literature vis a vis subjective memory complaints as valid indicators or predictors of objective cognitive status (Crumley, Stetler, & Horhota, 2014; Reid & Maclullich, 2006). For instance, studies reporting subjective memory concordantly associated (i.e., in the predicted direction) with objective memory or predictive of clinical decline on follow-up tend to include participants screened for healthy, unimpaired cognitive status at baseline (Geerlings, Jonker, Bouter, Ader, & Schmand, 1999; Jessen et al., 2010; Reisberg, Shulman, Torossian, Leng, et al., 2010; St John & Montgomery, 2002; Wang et al., 2004). On the other hand, studies reporting low subjective-objective memory associations or concordance tend to include a broader spectrum of participants or patients that includes some degree of clinical memory impairment (Grut et al., 1993; Jorm et al., 1997; Jungwirth et al., 2004; Mitchell, 2008; O’Connor, Pollitt, Roth, Brook, & Reiss, 1990; Purser et al., 2006; Roberts, Clare, & Woods, 2009). Baseline cognitive status is likely a significant moderating variable in understanding the subjective-objective memory literature and important to formerly evaluate in future meta-analytic work.
Subjective memory complaints were also influenced by -- and in turn also influenced subsequent change in -- objective language and objective executive functions. This is consistent with other reports of subjective memory ratings showing associations with objectively assessed domains beyond episodic memory (Benito-León, Mitchell, Vega, & Bermejo-Pareja, 2010; Minett, Dean, Firbank, English, & O’Brien, 2005; Snitz, Morrow, Rodriguez, Huber, & Saxton, 2008). Thus, there is good evidence that subjective – objective cognitive associations are not specific to memory, even when the wording of subjective items are targeted toward memory. In the present study subjective memory items (Supplemental Table 1) were a culled from a larger scale based on their wording reflecting “memory” symptoms and face validity for episodic memory processes. Results nevertheless reinforce the observation that patients (or study participants) often think of extra-mnemonic processes in daily functioning, such word-finding or planning, sequencing, prospective memory, attentional control and working memory (i.e., “multi-tasking”), as very much belonging to the collective subjective appraisal of one’s “memory.”
In contrast to the objective memory domain, objective language and objective executive functions show similar and more concordant patterns of temporal dynamics with subjective memory complaints, respectively. Although the dual-pathway model best fit the data in these non-memory domains, as well, inspection of the vector field plots suggest that a dominant pattern is one of lower objective cognition leading subsequent increases in subjective complaints. Few studies have examined predictors of change in subjective memory appraisal over time. Present results offer evidence of validity for measures of longitudinal change (e.g., increase) in subjective memory complaints vis a vis their associations with lower cognitive performance scores, specifically in non-memory domains.
Strengths of the present study include a large population-based cohort from small-town communities, five annual cycles of measurements, and relatively comprehensive and detailed neuropsychological assessments. The sample size and longitudinal follow-up period allows application of sophisticated modeling approaches of change in subjective and objective cognition. The random population sample strengthens the external validity of findings, often a limitation in cognitive aging studies with more selected volunteer-, patient- or clinic-based samples.
Limitations of the study include the assumption in latent change score modeling that attrition is random. As well, re-test effects were observed for objective memory and language, masking absolute decline in these cognitive functions over time. Re-test effects are a challenging problem in cognitive aging research with little consensus regarding analytic approaches (Ghisletta & De Ribaupierre, 2005; Hoffman, Hofer, & Sliwinski, 2011; Salthouse, Schroeder, & Ferrer, 2004). However, individual differences in the degree of re-test effects (i.e., practice gains) have been shown to be predictive of significant health-related outcomes such as dementia risk (Darby, Maruff, Collie, & McStephen, 2002; Galvin et al., 2005) and terminal decline (Dodge, Wang, Chang, & Ganguli, 2011). Thus, although absolute decline in objective memory and language was not observed in univariate models adjusted for covariates, we believe the relative patterns of slope differences in these outcomes are meaningful with respect to the study questions. Finally, although the advantages of the representativeness of the cohort are noted above, the cohort was primarily of European descent, reflecting the elderly population of the southwest Pennsylvania study area.
In summary, we tested four competing models of mutual and sequential influence on change in subjective and objective cognition over time. The best fitting model was one in which subjective memory leads subsequent change in objective memory, and objective memory leads subsequent change in subjective memory. We found the same pattern of mutual influence over time for objective language and executive functions domains, as well. The temporal dynamic relationships between subjective and objective cognition are complex and can reveal discordant relationships, as we observed with subjective-objective memory dynamics, likely reflecting clinical changes in insight and self-awareness. With regard to the larger question of whether subjective memory complaints are a valid indicator of dysfunction, decline or disease, our study suggests that the answer is likely dependent on level of objective memory functioning, and that caution is warranted at the lower end of the spectrum.
Supplementary Material
Acknowledgments
This research has been supported by grants from the National Institute on Aging, US Department of Health and Human Services (# R01 AG023651, K07 AG044395, K23AG083479). We gratefully acknowledge the staff and study participants of the MYHAT Project, without whose time and effort this work would not be possible
Footnotes
The authors report no conflicts of interest regarding this study.
Contributor Information
Beth E. Snitz, University of Pittsburgh
Brent J. Small, University of South Florida
Tianxiu Wang, University of Pittsburgh.
Chung-Chou H. Chang, University of Pittsburgh
Tiffany F. Hughes, University of Pittsburgh
Mary Ganguli, University of Pittsburgh.
References
- Allerhand M, Gale CR, Deary IJ. The dynamic relationship between cognitive function and positive well-being in older people: A prospective study using the English Longitudinal Study of Aging. Psychology and Aging. 2014;29(2):306. doi: 10.1037/a0036551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amariglio RE, Becker JA, Carmasin J, Wadsworth LP, Lorius N, Sullivan C, Sperling RA. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia. 2012 doi: 10.1016/j.neuropsychologia.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandura A. Regulation of cognitive processes through perceived self-efficacy. Developmental Psychology. 1989;25(5):729. [Google Scholar]
- Benito-León J, Mitchell AJ, Vega S, Bermejo-Pareja F. A population-based study of cognitive function in older people with subjective memory complaints. Journal of Alzheimer’s Disease. 2010;22(1):159–170. doi: 10.3233/JAD-2010-100972. [DOI] [PubMed] [Google Scholar]
- Boker SM, McArdle J. Statistical vector field analysis applied to mixed cross-sectional and longitudinal data. Experimental Aging Research. 1995;21(1):77–93. doi: 10.1080/03610739508254269. [DOI] [PubMed] [Google Scholar]
- Burmester B, Leathem J, Merrick P. Assessing subjective memory complaints: a comparison of spontaneous reports and structured questionnaire methods. International psychogeriatrics/IPA. 2014:1–17. doi: 10.1017/S1041610214001161. [DOI] [PubMed] [Google Scholar]
- Clare L. Awareness in early-stage Alzheimer’s disease: A review of methods and evidence. British Journal of Clinical Psychology. 2004;43(2):177–196. doi: 10.1348/014466504323088042. [DOI] [PubMed] [Google Scholar]
- Crumley JJ, Stetler CA, Horhota M. Examining the relationship between subjective and objective memory performance in older adults: A meta-analysis. Psychology and Aging. 2014;29(2):250. doi: 10.1037/a0035908. [DOI] [PubMed] [Google Scholar]
- Darby D, Maruff P, Collie A, McStephen M. Mild cognitive impairment can be detected by multiple assessments in a single day. Neurology. 2002;59(7):1042–1046. doi: 10.1212/wnl.59.7.1042. [DOI] [PubMed] [Google Scholar]
- Dik MG, Jonker C, Comijs HC, Bouter LM, Twisk JW, van Kamp GJ, Deeg DJ. Memory complaints and APOE-epsilon4 accelerate cognitive decline in cognitively normal elderly. [Research Support, Non-U.S. Gov’t] Neurology. 2001;57(12):2217–2222. doi: 10.1212/wnl.57.12.2217. [DOI] [PubMed] [Google Scholar]
- Dodge HH, Wang CN, Chang CCH, Ganguli M. Terminal decline and practice effects in older adults without dementia The MoVIES project. Neurology. 2011;77(8):722–730. doi: 10.1212/WNL.0b013e31822b0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufouil C, Fuhrer R, Alpérovitch A. Subjective Cognitive Complaints and Cognitive Decline: Consequence or Predictor? The Epidemiology of Vascular Aging Study. Journal of the American Geriatrics Society. 2005;53(4):616–621. doi: 10.1111/j.1532-5415.2005.53209.x. [DOI] [PubMed] [Google Scholar]
- Ferrer E, McArdle JJ. Longitudinal modeling of developmental changes in psychological research. Current Directions in Psychological Science. 2010;19(3):149–154. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Freedman M, Leach L, Kaplan E, Winocur G, Shulman KI, Delis DC. Clock drawing: A neuropsychological analysis. Oxford University Press; 1994. [Google Scholar]
- Fuld PA. Fuld Object-Memory Evaluation. Woodale, IL: Stoelting Company; 1981. [Google Scholar]
- Galvin JE, Powlishta KK, Wilkins K, McKeel DW, Xiong C, Grant E, Morris JC. Predictors of preclinical Alzheimer disease and dementia: a clinicopathologic study. Archives of Neurology. 2005;62(5):758–765. doi: 10.1001/archneur.62.5.758. [DOI] [PubMed] [Google Scholar]
- Ganguli M, Chang CCH, Snitz BE, Saxton JA, Vanderbilt J, Lee CW. Prevalence of mild cognitive impairment by multiple classifications: the Monongahela-Youghiogheny Healthy Aging Team (MYHAT) project. The American Journal of Geriatric Psychiatry. 2010;18(8):674–683. doi: 10.1097/JGP.0b013e3181cdee4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguli M, Dodge HH, Shen C, DeKosky ST. Mild cognitive impairment, amnestic type: an epidemiologic study. Neurology. 2004;63(1):115–121. doi: 10.1212/01.wnl.0000132523.27540.81. [DOI] [PubMed] [Google Scholar]
- Ganguli M, Gilby J, Seaberg E, Belle S. Depressive symptoms and associated factors in a rural elderly population: the MoVIES Project. American Journal of Geriatric Psychiatry. 1995;3:144–160. doi: 10.1097/00019442-199500320-00006. [DOI] [PubMed] [Google Scholar]
- Ganguli M, Snitz B, Vander Bilt J, Chang CC, Ganguli M, Snitz B, Chang CCH. How much do depressive symptoms affect cognition at the population level? The Monongahela-Youghiogheny Healthy Aging Team (MYHAT) study. [Research Support, N.I.H., Extramural] International Journal of Geriatric Psychiatry. 2009;24(11):1277–1284. doi: 10.1002/gps.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguli M, Snitz BE, Lee CW, Vanderbilt J, Saxton JA, Chang CCH. Age and education effects and norms on a cognitive test battery from a population-based cohort: the Monongahela–Youghiogheny Healthy Aging Team. Aging and Mental Health. 2010;14(1):100–107. doi: 10.1080/13607860903071014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerlings MI, Jonker C, Bouter LM, Ader HJ, Schmand B. Association between memory complaints and incident Alzheimer’s disease in elderly people with normal baseline cognition.[see comment] American Journal of Psychiatry. 1999;156(4):531–537. doi: 10.1176/ajp.156.4.531. [DOI] [PubMed] [Google Scholar]
- Ghisletta P, De Ribaupierre A. A dynamic investigation of cognitive dedifferentiation with control for retest: evidence from the Swiss Interdisciplinary Longitudinal Study on the Oldest Old. Psychology and Aging. 2005;20(4):671. doi: 10.1037/0882-7974.20.4.671. [DOI] [PubMed] [Google Scholar]
- Grut M, Jorm AF, Fratiglioni L, Forsell Y, Viitanen M, Winblad B. Memory complaints of elderly people in a population survey: variation according to dementia stage and depression. Journal of the American Geriatrics Society. 1993 doi: 10.1111/j.1532-5415.1993.tb06478.x. [DOI] [PubMed] [Google Scholar]
- Hafkemeijer A, Altmann-Schneider I, Oleksik AM, van de Wiel L, Middelkoop HA, van Buchem MA, Rombouts SA. Increased functional connectivity and brain atrophy in elderly with subjective memory complaints. Brain connectivity. 2013;3(4):353–362. doi: 10.1089/brain.2013.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannesdottir K, Morris RG. Primary and secondary anosognosia for memory impairment in patients with Alzheimer’s disease. Cortex. 2007;43(7):1020–1030. doi: 10.1016/s0010-9452(08)70698-1. [DOI] [PubMed] [Google Scholar]
- Harwood DG, Sultzer DL, Wheatley MV. Impaired insight in Alzheimer disease: association with cognitive deficits, psychiatric symptoms, and behavioral disturbances. Cognitive and Behavioral Neurology. 2000;13(2):83. [PubMed] [Google Scholar]
- Herrmann DJ. Know thy memory: The use of questionnaires to assess and study memory. Psychological Bulletin. 1982;92(2):434. [Google Scholar]
- Hertzog C, Pearman A. Memory complaints in adulthood and old age. In: Perfect TJ, Lindsay DS, editors. The SAGE Handbook of Applied Memory. London: SAGE; 2014. [Google Scholar]
- Hoffman L, Hofer SM, Sliwinski MJ. On the confounds among retest gains and age-cohort differences in the estimation of within-person change in longitudinal studies: a simulation study. Psychology and Aging. 2011;26(4):778. doi: 10.1037/a0023910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohman TJ, Beason-Held LL, Lamar M, Resnick SM. Subjective cognitive complaints and longitudinal changes in memory and brain function. Neuropsychology. 2011;25(1):125. doi: 10.1037/a0020859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chételat G, van der Flier WM. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s & Dementia. 2014 doi: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen F, Wiese B, Bachmann C, Eifflaender-Gorfer S, Haller F, Kolsch H Dementia in Primary Care Patients Study G. Prediction of dementia by subjective memory impairment: effects of severity and temporal association with cognitive impairment. Archives of General Psychiatry. 2010;67(4):414–422. doi: 10.1001/archgenpsychiatry.2010.30. [DOI] [PubMed] [Google Scholar]
- Jorm A, Christensen H, Korten A, Henderson A, Jacomb P, Mackinnon A. Do cognitive complaints either predict future cognitive decline or reflect past cognitive decline? A longitudinal study of an elderly community sample. Psychological Medicine. 1997;27(01):91–98. doi: 10.1017/s0033291796003923. [DOI] [PubMed] [Google Scholar]
- Jorm A, Christensen H, Korten A, Jacomb P, Henderson A. Memory complaints as a precursor of memory impairment in older people: a longitudinal analysis over 7–8 years. Psychological Medicine. 2001;31(3):441–449. [PubMed] [Google Scholar]
- Jungwirth S, Fischer P, Weissgram S, Kirchmeyr W, Bauer P, Tragl KH. Subjective memory complaints and objective memory impairment in the Vienna-Transdanube aging community. Journal of the American Geriatrics Society. 2004;52(2):263–268. doi: 10.1111/j.1532-5415.2004.52066.x. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. 2. Philedelphia: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- Krall JR, Carlson MC, Fried LP, Xue Q-L. Examining the Dynamic, Bidirectional Associations Between Cognitive and Physical Functioning in Older Adults. American Journal of Epidemiology. 2014:kwu198. doi: 10.1093/aje/kwu198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam LCW, Lui VWC, Tam CWC, Chiu HFK. Subjective memory complaints in Chinese subjects with mild cognitive impairment and early Alzheimer’s disease. International Journal of Geriatric Psychiatry. 2005;20(9):876–882. doi: 10.1002/gps.1370. [DOI] [PubMed] [Google Scholar]
- Lautenschlager NT, Flicker L, Vasikaran S, Leedman P, Almeida OP. Subjective memory complaints with and without objective memory impairment: relationship with risk factors for dementia. The American Journal of Geriatric Psychiatry. 2005;13(8):731–734. doi: 10.1176/appi.ajgp.13.8.731. [DOI] [PubMed] [Google Scholar]
- Lenehan ME, Klekociuk SZ, Summers MJ. Absence of a relationship between subjective memory complaint and objective memory impairment in mild cognitive impairment (MCI): is it time to abandon subjective memory complaint as an MCI diagnostic criterion? International Psychogeriatrics. 2012;24(09):1505–1514. doi: 10.1017/S1041610212000695. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Becker JT, Somsak D, Dew MA, DeKosky ST. Awareness of cognitive deficits and anosognosia in probable Alzheimer’s disease. European Neurology. 1994;34(5):277–282. doi: 10.1159/000117056. [DOI] [PubMed] [Google Scholar]
- McArdle JJ. Latent variable modeling of differences and changes with longitudinal data. Annual Review of Psychology. 2009;60:577–605. doi: 10.1146/annurev.psych.60.110707.163612. [DOI] [PubMed] [Google Scholar]
- Minett TS, Dean JL, Firbank M, English P, O’Brien JT. Subjective memory complaints, white-matter lesions, depressive symptoms, and cognition in elderly patients. The American Journal of Geriatric Psychiatry. 2005;13(8):665–671. doi: 10.1176/appi.ajgp.13.8.665. [DOI] [PubMed] [Google Scholar]
- Mitchell AJ. Is it time to separate subjective cognitive complaints from the diagnosis of mild cognitive impairment? Age and Ageing. 2008;37(5):497–499. doi: 10.1093/ageing/afn147. [DOI] [PubMed] [Google Scholar]
- Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia – meta-analysis of 41 robust inception cohort studies. Acta Psychiatrica Scandinavica. 2009;119(4):252–265. doi: 10.1111/j.1600-0447.2008.01326.x. [DOI] [PubMed] [Google Scholar]
- Mograbi DC, Brown RG, Morris RG. Anosognosia in Alzheimer’s disease–the petrified self. Consciousness and Cognition. 2009;18(4):989–1003. doi: 10.1016/j.concog.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Mol ME, van Boxtel M, Willems D, Jolles J. Do subjective memory complaints predict cognitive dysfunction over time? A six-year follow-up of the Maastricht Aging Study. International Journal of Geriatric Psychiatry. 2006;21(5):432–441. doi: 10.1002/gps.1487. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2411–2413. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Morris R, Hannesdottir K. Loss of “awareness” in Alzheimer’s disease. Cognitive neuropsychology of Alzheimer’s disease. 2004:275–296. [Google Scholar]
- Mousavi-Nasab SMH, Kormi-Nouri R, Nilsson LG. Examination of the bidirectional influences of leisure activity and memory in old people: A dissociative effect on episodic memory. British Journal of Psychology. 2014;105(3):382–398. doi: 10.1111/bjop.12044. [DOI] [PubMed] [Google Scholar]
- Mungas D, Marshall SC, Weldon M, Haan M, Reed BR. Age and education correction of Mini-Mental State Examination for English and Spanish-speaking elderly. Neurology. 1996;46(3):700–706. doi: 10.1212/wnl.46.3.700. [DOI] [PubMed] [Google Scholar]
- Muthen LK, Muthen BO. MPlus User’s Guide. 6. Los Angeles: Muthen & Muthen; 2010. [Google Scholar]
- O’Connor DW, Pollitt PA, Roth M, Brook CPB, Reiss BB. Memory complaints and impairment in normal, depressed, and demented elderly persons identified in a community survey. Archives of General Psychiatry. 1990;47(3):224. doi: 10.1001/archpsyc.1990.01810150024005. [DOI] [PubMed] [Google Scholar]
- Orfei MD, Blundo C, Celia E, Casini AR, Caltagirone C, Spalletta G, Varsi AE. Anosognosia in mild cognitive impairment and mild Alzheimer’s disease: frequency and neuropsychological correlates. The American Journal of Geriatric Psychiatry. 2010;18(12):1133–1140. doi: 10.1097/JGP.0b013e3181dd1c50. [DOI] [PubMed] [Google Scholar]
- Pannu JK, Kaszniak AW. Metamemory experiments in neurological populations: A review. Neuropsychology Review. 2005;15(3):105–130. doi: 10.1007/s11065-005-7091-6. [DOI] [PubMed] [Google Scholar]
- Parisi JM, Gross AL, Rebok GW, Saczynski JS, Crowe M, Cook SE, Unverzagt FW. Modeling change in memory performance and memory perceptions: findings from the ACTIVE study. Psychology and Aging. 2011;26(3):518. doi: 10.1037/a0022458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrig-Chiello P, Perrig W, Stahelin H. Differential aspects of memory self-evaluation in old and very old people. Aging & Mental Health. 2000;4(2):130–135. [Google Scholar]
- Perrotin A, Desgranges B, Duval C, La Joie R, Mézenge F, Landeau B, Eustache F. The IMAP project: How does the awareness of memory deficits evolve in the course of Alzheimer’s disease? Insights from its relationships to PET β-amyloid and metabolism measurements. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association. 2011;7(4):S742–S743. [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Archives of Neurology. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Purser JL, Fillenbaum GG, Wallace RB. Memory Complaint Is Not Necessary for Diagnosis of Mild Cognitive Impairment and Does Not Predict 10-Year Trajectories of Functional Disability, Word Recall, or Short Portable Mental Status Questionnaire Limitations. Journal of the American Geriatrics Society. 2006;54(2):335–338. doi: 10.1111/j.1532-5415.2005.00589.x. [DOI] [PubMed] [Google Scholar]
- Reid LM, Maclullich AMJ. Subjective memory complaints and cognitive impairment in older people. Dementia & Geriatric Cognitive Disorders. 2006;22(5–6):471–485. doi: 10.1159/000096295. [DOI] [PubMed] [Google Scholar]
- Reisberg B, Shulman MB, Torossian C, Boksay I, Khan S, Zhu W. Which comes first: Subjective cognitive impairment (SCI) or cognitive change? and is SCI a frequently occurring stage in the evolution of Alzheimer-associated cognitive change? Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association. 2010;6(4):S177–S177. [Google Scholar]
- Reisberg B, Shulman MB, Torossian C, Leng L, Zhu W, Reisberg B, Zhu W. Outcome over seven years of healthy adults with and without subjective cognitive impairment. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Alzheimer’s & Dementia. 2010;6(1):11–24. doi: 10.1016/j.jalz.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J, Clare L, Woods R. Subjective memory complaints and awareness of memory functioning in mild cognitive impairment: a systematic review. Dementia and Geriatric Cognitive Disorders. 2009;28(2):95–109. doi: 10.1159/000234911. [DOI] [PubMed] [Google Scholar]
- Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G, Villemagne VL. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiology of Aging. 2010;31(8):1275–1283. doi: 10.1016/j.neurobiolaging.2010.04.007. http://dx.doi.org/10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Schroeder DH, Ferrer E. Estimating retest effects in longitudinal assessments of cognitive functioning in adults between 18 and 60 years of age. Developmental Psychology. 2004;40(5):813. doi: 10.1037/0012-1649.40.5.813. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Wishart HA, Rabin LA, Santulli RB, Flashman LA, West JD, Mamourian AC. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67(5):834–842. doi: 10.1212/01.wnl.0000234032.77541.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield PW, Marder K, Dooneief G, Jacobs DM, Sano M, Stern Y. Association of subjective memory complaints with subsequent cognitive decline in community-dwelling elderly individuals with baseline cognitive impairment. American Journal of Psychiatry. 1997;154(5):609–615. doi: 10.1176/ajp.154.5.609. [DOI] [PubMed] [Google Scholar]
- Shaked D, Farrell M, Huey E, Metcalfe J, Cines S, Karlawish J, Cosentino S. Cognitive correlates of metamemory in alzheimer’s disease. 2014 doi: 10.1037/neu0000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small BJ, Dixon RA, McArdle JJ, Grimm KJ. Do changes in lifestyle engagement moderate cognitive decline in normal aging? Evidence from the Victoria Longitudinal Study. Neuropsychology. 2012;26(2):144. doi: 10.1037/a0026579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snitz BE, Morrow LA, Rodriguez EG, Huber KA, Saxton JA. Subjective memory complaints and concurrent memory performance in older patients of primary care providers. Journal of the International Neuropsychological Society. 2008;14(06):1004–1013. doi: 10.1017/S1355617708081332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snitz BE, Yu L, Crane PK, Chang CCH, Hughes TF, Ganguli M. Subjective Cognitive Complaints of Older Adults at the Population Level: An Item Response Theory Analysis. Alzheimer Disease and Associated Disorders. 2012;26(4):344–351. doi: 10.1097/WAD.0b013e3182420bdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John P, Montgomery P. Are cognitively intact seniors with subjective memory loss more likely to develop dementia? International Journal of Geriatric Psychiatry. 2002;17(9):814–820. doi: 10.1002/gps.559. [DOI] [PubMed] [Google Scholar]
- Unverzagt FW, Morgan OS, Thesiger CH, Eldemire DA, Luseko J, Pokuri S, Hendrie HC. Clinical utility of CERAD neuropsychological battery in elderly Jamaicans. Journal of the International Neuropsychological Society. 1999;5(03):255–259. doi: 10.1017/s1355617799003082. [DOI] [PubMed] [Google Scholar]
- Vogel A, Stokholm J, Gade A, Andersen BB, Hejl AM, Waldemar G. Awareness of deficits in mild cognitive impairment and Alzheimer’s disease: do MCI patients have impaired insight? Dementia and Geriatric Cognitive Disorders. 2004;17(3):181–187. doi: 10.1159/000076354. [DOI] [PubMed] [Google Scholar]
- Wang L, van Belle G, Crane PK, Kukull WA, Bowen JD, McCormick WC, Larson EB. Subjective memory deterioration and future dementia in people aged 65 and older.[see comment] Journal of the American Geriatrics Society. 2004;52(12):2045–2051. doi: 10.1111/j.1532-5415.2004.52568.x. [DOI] [PubMed] [Google Scholar]
- Weaver Cargin J, Collie A, Masters C, Maruff P. The nature of cognitive complaints in healthy older adults with and without objective memory decline. Journal of Clinical and Experimental Neuropsychology. 2008;30(2):245–257. doi: 10.1080/13803390701377829. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale Revised. The Psychological Corporation; 1987. [Google Scholar]
- Wilson RS, Segawa E, Boyle PA, Bennett DA. Influence of late-life cognitive activity on cognitive health. Neurology. 2012;78(15):1123–1129. doi: 10.1212/WNL.0b013e31824f8c03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimprich D, Martin M, Kliegel M. Subjective cognitive complaints, memory performance, and depressive affect in old age: a change-oriented approach. The International Journal of Aging and Human Development. 2003;57(4):339–366. doi: 10.2190/G0ER-ARNM-BQVU-YKJN. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.