Abstract
Chikungunya virus (CHIKV) is a mosquito-transmitted alphavirus that can cause fever and chronic arthritis in humans. CHIKV that is generated in mosquito or mammalian cells differs in glycosylation patterns of viral proteins, which may affect its replication and virulence. Herein, we compare replication, pathogenicity, and receptor binding of CHIKV generated in Vero cells (mammal) or C6/36 cells (mosquito) through a single passage. We demonstrate that mosquito cell-derived CHIKV (CHIKVmos) has slower replication than mammalian cell-derived CHIKV (CHIKVvero), when tested in both human and murine cell lines. Consistent with this, CHIKVmos infection in both cell lines produce less cytopathic effects and reduced antiviral responses. In addition, infection in mice show that CHIKVmos produces a lower level of viremia and less severe footpad swelling when compared with CHIKVvero. Interestingly, CHIKVmos has impaired ability to bind to glycosaminoglycan (GAG) receptors on mammalian cells. However, sequencing analysis shows that this impairment is not due to a mutation in the CHIKV E2 gene, which encodes for the viral receptor binding protein. Moreover, CHIKVmos progenies can regain GAG receptor binding capability and can replicate similarly to CHIKVvero after a single passage in mammalian cells. Furthermore, CHIKVvero and CHIKVmos no longer differ in replication when N-glycosylation of viral proteins was inhibited by growing these viruses in the presence of tunicamycin. Collectively, these results suggest that N-glycosylation of viral proteins within mosquito cells can result in loss of GAG receptor binding capability of CHIKV and reduction of its infectivity in mammalian cells.
Author Summary
Chikungunya virus (CHIKV) is a chronic arthritis-causing pathogen in humans, for which no licensed vaccine or specific antiviral drug is currently available. Due to the global spread of its mosquito vectors, CHIKV is now becoming a public health threat worldwide. CHIKV can replicate in both mammalian and mosquito cells, however it does not cause apparent damage to mosquito cells, yet it rapidly kills mammalian cells within a day after infection. In addition, mosquito and mammalian cells have different mechanism of protein glycosylation, which can result in different glycan structures of viral glycoproteins. In this study, we report that mosquito cell-generated CHIKV has lower infectivity in cell culture and causes less severe disease in mice, when compared to mammalian cell-generated CHIKV. We demonstrate that only mammalian cell-generated CHIKV, but not mosquito-cell generated CHIKV, binds to mammalian cell surface glycosaminoglycan receptors. Interestingly, mosquito-cell generated CHIKV can re-acquire glycosaminoglycan receptor binding capability after a single passage in mammalian cells and replicate at similar levels with mammalian cell-generated CHIKV, suggesting that passage of CHIKV in mosquito cells can reduce its infectivity.
Introduction
Chikungunya virus (CHIKV) is a mosquito-transmitted, single-stranded RNA virus belonging to the genus Alphavirus of the family Togaviridae. In humans, CHIKV infection can cause fever, headache, maculopapular rashes, myalgia, acute joint swelling, persistent arthritis, and even life-threatening neurological or cardiovascular complications [1–5]. CHIKV was first identified in Africa in 1952 and has been endemic in the tropical Indian Ocean countries for decades [6]. In recent years, this virus has caused more widespread and noticeable outbreaks. From 2004 to 2011, approximately six million cases of CHIKV infection were reported from nearly forty countries in Africa, Asia, and Europe [6–9]. In recent years, CHIKV mosquito transmission vectors Aedes aegypti and Ae. albopictus have spread from tropical to temperate climates, making CHIKV an emerging pathogen within these climate zones [10,11]. In line with this, CHIKV cases have been recently reported from more than twenty-five countries in the Caribbean islands, thereby posing a potential threat to North America [12]. Unfortunately, CHIKV pathogenesis is not well understood, and there is no vaccine or specific antiviral treatment currently available for CHIKV infection [13–15].
CHIKV circulates between mammalian and mosquito hosts and this cyclical transmission may provide a suitable environment for increased viral fitness and the emergence of more pathogenic strains [16,17]. Interestingly, re-emergence of CHIKV during the 2005–2006 epidemic on Reunion Island was associated with a single point mutation in its genome, which increased CHIKV fitness within its mosquito vector Ae. albopictus [18]. Additionally, CHIKV and other alphaviruses differ in their ability to infect mammalian and mosquito cells. For example, alphaviruses can cause cytopathic effects in mammalian cells and can also shut-down the mammalian macromolecular machinery involved in cellular protein synthesis at both the transcription and translational levels [19–21]. In contrast, alphavirus infection of mosquito cells causes little to no cytopathic effects and does not affect the cellular transcription and translational processes [21–24].
Mammalian and mosquito cells have distinct cellular enzymatic systems for protein glycosylation; therefore, different post-translational processing of viral surface proteins are possible in these host cells [25], which can influence replication [26–28], pathogenesis [28,29], transmission [30], and evolution [17] of mosquito-transmitted viruses. In line with this, mammalian- and mosquito-generated arboviruses can bind to different receptors expressed on the surface of host cells. For instance, differential glycosylation of viral receptor-binding proteins in mammalian- and mosquito-generated Sindbis virus [31], West Nile virus (WNV) [32], and dengue virus [33], can affect binding of these virus to host cell receptors. Similarly, mammalian cell-generated Ross River virus (RRV), Venezuelan equine encephalitis virus (VEEV), and WNV can induce more potent interferon responses compared to their mosquito cell-generated counterparts [34,35]. However, it remains unclear whether CHIKV generation in mosquito and mammalian cells can affect its infectivity and virulence.
Glycosaminoglycans (GAGs) are highly sulfated polysaccharides that are ubiquitously expressed on the cell surface and the extracellular matrix of mammalian cells [36,37]. Many viruses including CHIKV can utilize GAGs as receptors to infect host cells [38]. However, research on the role of GAG receptor binding in CHIKV and other alphaviruses has been inconclusive. The GAG receptor binding of CHIKV and other alphaviruses can be acquired through acquisition of basic amino acids in viral receptor-binding proteins via mutations during their continuous passage in cell culture [36,38]. Although such dependence on GAG receptor binding increases viral infectivity in vitro, it can potentially decreases viral fitness in vivo [39]. In contrast, GAG binding properties have also been described in non-cell culture adapted alphaviruses, including a clinical strain of CHIKV [40] and a wild-type strain of eastern equine encephalitis virus (EEEV) [41], suggesting that some other mechanisms that are independent of cell culture adaptation may also control GAG binding and virulence of CHIKV and other alphaviruses. Biochemically, GAG receptors possess a negative charge that enables virus-GAG receptor interaction [40–42]. In addition, mosquito and mammalian cells have different N-glycosylation mechanisms that can generate different configurations of viral glycoproteins [43] and modulate charge dependent interaction of viruses to host cell receptors. Thus, virus generation in these different host cells can potentially influence receptor binding and infectivity of CHIKV. However, the role of mosquito- and mammalian cells on GAG binding capability of CHIKV and other alphaviruses is unclear.
Herein, we report that CHIKV generated in mammalian cells replicates more efficiently during its subsequent infection of both human and murine cells in vitro and is more virulent in a mouse model of CHIKV arthritis, when compared to its mosquito cell-generated counterpart. We further demonstrate that the reduced replication of mosquito cell-generated CHIKV is associated with its failure to bind to cell surface GAG receptors on mammalian cells due to differential glycosylation of viral proteins in mosquito cells.
Materials and Methods
Ethics statement
This study was carried out in strict accordance with the recommendations described in the Guide for the Care and Use of Laboratory Animals of the National Research Council of The National Academies. The Institutional Animal Care and Use Committee at the University of Southern Mississippi (Animal Welfare Assurance # A3851-01) reviewed and approved all the animal care and use procedures under the protocol #12041201.
Biosafety
All in vitro experiments and animal studies involving CHIKV were performed by certified personnel in biosafety level 3 (BSL3) laboratories, following standard biosafety protocols approved by the University of Southern Mississippi Institutional Biosafety Committee.
Viruses, cell culture, and chemicals
Low-passaged, Vero cell-generated CHIKV Ross strain (provided by Dr. John F. Anderson, Connecticut Agricultural Experiment Station) and LR OPY1 2006 strain (provided by Dr. Robert B. Tesh, University of Texas Medical Branch) were used as parental viral stocks in this study. Majority of experiments were performed using the Ross strain and to test strain specificity, some experiments were repeated using the LR OPY1 2006 strain. The viral stocks used in this study were prepared by a single passage of parental viruses in C6/36 (ATCC, CRL-1660) or Vero cells (ATCC, CCL-81) and designated as CHIKVmos and CHIKVvero, respectively. All viral stocks were titered in Vero cells by a plaque-forming assay. C6/36 cells were cultured at 28°C with 5% CO2 in Eagle’s minimum essential media (EMEM, ATCC) supplemented with 10% fetal bovine serum (FBS). Vero cells were cultured at 37°C with 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM, Life Technologies) supplemented with 10% FBS. L929 (ATCC, CCL-1), NIH3T3 (ATCC, CRL-1658), Raw 264.7 (ATCC, TIB-71), human foreskin fibroblasts (HFF, ATCC, CRL-2522), human THP-1 cells (ATCC, TIB-202) and human dermal fibroblasts were cultured at 37°C with 5% CO2 in DMEM supplemented with 10% FBS.
To generate murine bone marrow-derived dendritic cells (mBMDC), healthy C57BL/6J mice (7 weeks old) were euthanized and bone marrow cells were recovered from both femurs. After red blood cells were lysed, the bone marrow cells were cultured in R10 medium supplemented with 10% J558L cell supernatant (as a source of granulocyte-macrophage colony-stimulating factor) at a final density of 1 × 106 cells/ml at 37°C with 5% CO2. The medium was changed every 3 days and mBMDCs were ready for infection at day 10.
Dermatan sulfate (from porcine intestinal mucosa), chondroitin sulfate A (from bovine trachea), heparin (from porcine intestinal mucosa), sepharose CL-4B, heparin-sepharose, yeast mannan, tunicamycin, and neutral red were all purchased from Sigma.
In vitro infection
Cells were plated 24 h before infection in 6-, 12- or 24-well plates to 60–80% confluence. CHIKVmos or CHIKVvero (MOI = 1) were added to the cells and incubated at 37°C for 1 h to allow for viral adsorption and penetration. The inoculation medium was then replaced with fresh medium to remove unadsorbed viruses. Cells were washed once with fresh medium and further incubated at 37°C with 5% CO2 and collected at selected time points for analysis of viral genome replication and host’s gene expression.
Microscopy and cell viability assay
Cells were infected with CHIKV (MOI = 1 or 5) for 48 h and fixed with 4% paraformaldehyde (PFA, Electron Microscopy Science). Phase contrast images were acquired using Zeiss LSM510 META confocal imaging system (Carl Zeiss Microscopy, NY). Cell viability was quantified by toluidine blue (TB) staining, according to the previously published method [44]. Viable cells were assayed by measuring the absorbance of TB at 630 nm using a microplate reader (BIO-TEC). Percentage of viable cells was calculated after normalization to uninfected controls.
Real time-quantitative PCR (RT-qPCR)
CHIKV infected cells were subjected to total RNA extraction using TRI-reagent (Molecular Research Center, Inc.). For RNA isolation from mouse blood samples, RNeasy mini kit (Qiagen) was used. The first-strand complementary DNA (cDNA) was synthesized using the iSCRIPT cDNA synthesis kit (Bio-Rad). RT-qPCR assays were performed in a CFX96 Real-Time system (Bio-Rad) using SYBR Green supermix (Bio-Rad). Viral RNA copy numbers were expressed as the ratio of CHIKV envelope-1 (CHIKV E1) to cellular β-actin. For cytokine RT-qPCR assay, data were presented as relative fold change (RFC) in expression by the ΔΔCT method after normalized to cellular β-actin. Primer sequences for β-actin of mice [45] and human [46] were previously described. Primers for CHIKV E1 gene (Forward: TCC GGG AAG CTG AGA TAG AA; Reverse: ACG CCG GGT AGT TGA CTA TG), and Ae. albopictus ribosomal protein 7 gene (Forward: CTC TGA CCG CTG TGT ACG AT; Reverse: CAA TGG TGG TCT GCT GGT TC) were designed using NCBI online primer designing tool. Primer sequences for host immune genes (Ifn-α, Ifn-β, Tlr3, Rig-I, Mda-5, and Il-1β) were described in a previous report [44]. All primers were synthesized by Integrated DNA Technologies.
Plaque forming assay and viral genome copy quantification
Plaque assays were performed according to our previous report with some modifications [47]. Briefly, Vero, L929 or NIH3T3 cells were plated at 5 × 105 cells/well in 6-well plates one day before infection. Virus-containing samples were added to cell monolayers to allow viral adsorption/penetration at 37°C with 5% CO2 for 1 h. After removing unadsorbed viruses, cells were overlaid with 1% SeaPlaque agarose (Lonza) containing medium and further incubated at 37°C with 5% CO2 for an additional 48 h. Plaques were counted after staining with 0.3% neutral red.
CHIKV particles in viral stocks were also quantified by RT-qPCR, as described previously [38]. Briefly, 200 μl of viral stocks were treated with 50 units (U) of RNase A (Affymetrix) for 1 h at 37°C. TRI-reagent was added to inactivate RNase and lyse viral particles, and viral RNA was isolated after adding 5 μg of tRNA as carrier. First strand cDNA synthesis and CHIKV E1 gene quantification by RT-qPCR were performed as described above.
Animal studies
Five week old, sex-matched C57BL/6J mice (The Jackson Laboratory) were subcutaneously inoculated on the ventral side of the right hind footpad toward the ankle with 105 plaque forming units (PFUs) of CHIKVvero or CHIKVmos (Ross strain or LR 2006 OPY 1) in 50 μl phosphate buffer saline (PBS), or with 50 μl PBS for mock controls, according to previous publications [48–50]. Blood samples were collected in 0.5M EDTA by retro-orbital bleeding and viral RNA in these samples were quantified by RT-qPCR. The height (thickness) and breath (width) of the perimetatarsal area of inoculated feet were measured daily from day 0 to day 10 post infection (d.p.i.) by using a digital caliper (Electron Microscopy Science), and the relative increase in swelling was calculated as previously described [50]. Briefly, footpad swelling was expressed as the relative increase in swelling compared to pre-infection (x d.p.i.– 0 d.p.i.)/0 d.p.i.).
Histology
Mice were euthanized and inoculated footpad tissues were collected at 6 d.p.i. and fixed overnight in 4% PFA, followed by decalcification in 10% EDTA for over 10 days. Tissues were then dehydrated, paraffin embedded, and sectioned (10 μm) with a microtome (American Optical Spencer 820), followed by staining with hematoxylin and eosin (H&E). The images were acquired using a bright-field microscope (Olympus BH2).
Attachment assays
CHIKVs (MOI = 1 or 5) were added to cell monolayer for attachment at 4°C for 1 h followed by washing with fresh medium to remove unattached viruses. The attached viruses were quantified either by RT-qPCR or by a plaque assay in the same cells, as described above. In some experiments, media containing the unattached viruses were also collected and the unattached viruses were quantified by a plaque assay in Vero cells to confirm equal numbers of virions were added to each sample.
In addition, CHIKV attachment was analyzed by flow cytometry. CHIKVvero or CHIKVmos (MOI = 2.5) were added to NIH3T3 cells in triplicates in PBS supplemented with 2% FBS (staining buffer) and were incubated at 4°C for 45 min. Unbound viruses were removed by washing twice with the staining buffer and the cells were fixed with 2% PFA (Electron Microscopy Science) for 15 min at room temperature (RT). After washing, the cells were probed with mouse monoclonal anti-CHIKV antibody (Abcam) and Cy5 conjugated goat anti-mouse IgG (KPL) secondary antibody, both for 1 h at RT. The cells were then washed twice and re-suspended in the staining buffer and analysed in a BD LSRFortessa (BD Biosciences) using FACSDiva version 6.0 software (BD Biosciences).
Virus entry assays
To assess CHIKVvero and CHIKVmos entry into the host cells, we blocked the endosome acidification process using a lysomotrophic agent alone or in combination with a low pH medium (pH 5.5), the latter mediated viral envelope and cytoplasmic membrane fusion, as previously described [51,52]. Briefly, infection was carried out in the medium containing 20 mM NH4Cl to block endosomal acidification. For the direct membrane fusion assay, viruses were allowed to attach onto cells and then immediately treated with low pH medium for 2 minutes. The internalized viruses were quantified by RT-qPCR and plaque assays.
Blocking assays for virus attachment
To analyze virus binding to GAG receptors, we performed a GAG neutralization assay, in which viruses were pre-incubated with soluble GAGs to inhibit their attachment to cell surface GAG receptors. Briefly, viruses (2.5 x 106 PFU/ml) were pre-incubated with heparin, chondroitin sulfate A or dermatan sulfate (concentration indicated in figures) in DMEM containing 2% FBS at 37°C for 1 h. The virus-GAGs mixtures were then added to cells (MOI = 1) at 4°C for 1 h to allow attachment. The unattached virus-GAGs mixtures were removed and cells were washed once with fresh culture medium. The viruses attached to cells were quantified by RT-qPCR and plaque assays. In some experiments, the effect of heparin-pretreatment on viral replication was measured at 24 hours post-infection (h.p.i.) by RT-qPCR.
To measure virus binding to lectin receptors such as DC-SIGN and L-SIGN, we performed a blocking assay in the presence of yeast mannan that disrupts interaction of viruses to cell surface lectin receptors. Briefly, cells were pretreated with different concentrations of yeast mannan for 30 minutes at room temperature. Viruses were then added to the cells (MOI = 1) and incubated at 4°C for 1 h. The viruses attached on cells were quantified by RT-qPCR.
For flow cytometric analysis of GAG neutralization, CHIKVvero or CHIKVmos (2.5 × 106 PFU/ml) were pre-incubated with different concentrations of GAGs in DMEM containing 2% FBS at 37°C for 1 h. Virus-GAGs mixtures were added to NIH3T3 cells in PBS supplemented with 2% FBS (MOI = 2.5) and incubated at 4°C for 45 min. Cells were washed twice at 4°C to remove unbound virus and immediately fixed with 2% PFA for 15 min. Cells were then probed with anti-CHIKV antibody and analyzed by flow cytometry, as described above.
Heparin sepharose bead binding assays
Heparin-conjugated sepharose beads or unconjugated control beads were purchased from Sigma. The beads (60 μl) were washed twice in 200 μl DMEM, and mixed with 105 PFUs of CHIKV in a total of 60 μl DMEM containing 2% FBS, and incubated at 4°C for 30 min. The beads were then washed three times in DMEM containing 2% FBS and the washed solution was collected for subsequent plaque assays to quantify the unbound viruses. Viruses bound to beads were lysed in 50 μl of Laemmli sample buffer (Bio- Rad), and viral proteins were separated by 10% SDS-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane (Bio-Rad). After blocked with 5% bovine serum albumin (BSA) for 1 h at RT, the membranes were probed with mouse monoclonal anti-CHIKV primary antibody (Abcam) at 4°C for overnight on a rocker. The membranes were then washed five times (5 min each) with Tris-buffered saline with Tween 20 (TBS-T) buffer and reacted with horseradish peroxidase conjugated goat anti-mouse IgG secondary antibody (Jackson Immunoresearch) for 1 h at RT. The membranes were then washed and developed using SuperSignal West Pico Chemiluminiscence Substrate (Thermo Scientific) and images were acquired using a ChemiDoc MP system (Bio-Rad).
Sequence analysis
Parental CHIKV viruses (original stocks received from suppliers), single-passaged CHIKV in Vero cells (CHIKVvero) or mosquito cells (CHIKVmos), and single-passaged CHIKVvero and CHIKVmos in NIH3T3 cells were subjected to RNA isolation using RNeasy Mini Kit (Qiagen). cDNA was prepared using the iScript cDNA synthesis kit (Bio-Rad). Complete CHIKV E2 gene was amplified using a Q5 high fidelity polymerase (New England Biolab). The PCR primers were used according to a previous report [38]. The PCR fragments were purified by PureLink quick PCR Purification Kit (Life Technologies) and sequenced by Functional Biolab.
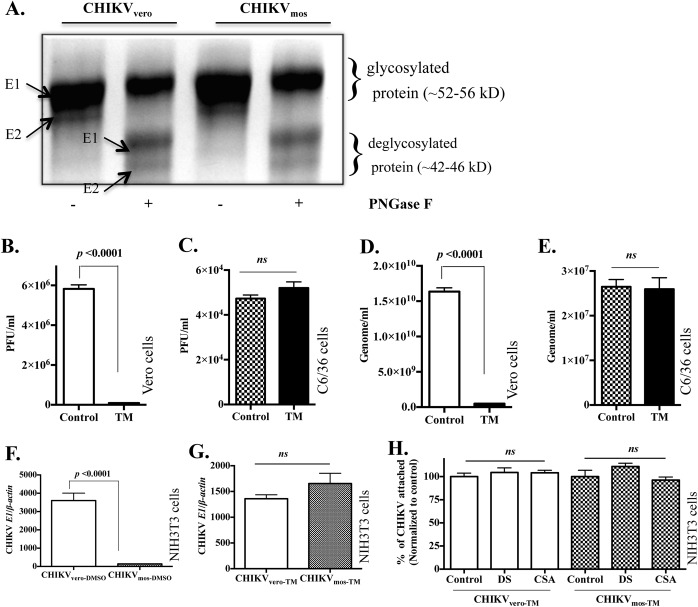
Concentration of CHIKV and protein glycosylation assays
Stocks of CHIKV (Ross strain) were prepared in Vero and C6/36 cells, UV-inactivated, and viruses were concentrated by pelleting with 20% sucrose at 28,000 rpm for 2 h in an ultracentrifuge (Beckman Coulter). Deglycosylation of viral proteins were carried out using peptide-N-glycosidase F (PNGase F, Sigma) treatment following the manufacturer’s instruction. Viral proteins were separated in a 10% Mini-PROTEAN Precast Gels (Bio-Rad) and imaged in a ChemiDoc MP system (Bio-Rad) after coomassie brilliant blue staining.
Tunicamycin treatment assays
Tunicamycin (TM) was purchased from Sigma and dissolved (10 mg/ml) in cell culture grade dimethyl sulfoxide (DMSO, ATCC). Vero cells and C6/36 cells were plated for 24 h and infected with a 0.1 MOI of parental CHIKV (Ross strain). Viruses were allowed to adsorb and penetrate for 1 h at 37°C. After unadsorbed viruses were removed, the cells were further cultured with medium containing 0.1 μg/ml of TM or the same final concentration of DMSO (0.001%) as vehicle controls. Cell culture media were collected at 24 h for virus quantification by plaque assays and RT-qPCR assays. Virus stocks generated in Vero and C6/36 cells in the presence of TM or DMSO were used to infect NIH3T3 cells (MOI = 0.1) and viral genome replication was analyzed at 24 h by RT-qPCR.
Statistical analysis
Data were analyzed using GraphPad Prism (version 6.0, GraphPad software) and p < 0.05 was considered statistically significant. Data were compared using the two-tailed student's t-test or analysis of variance (ANOVA).
Results
CHIKVmos has a lower level of replication in murine and human cells
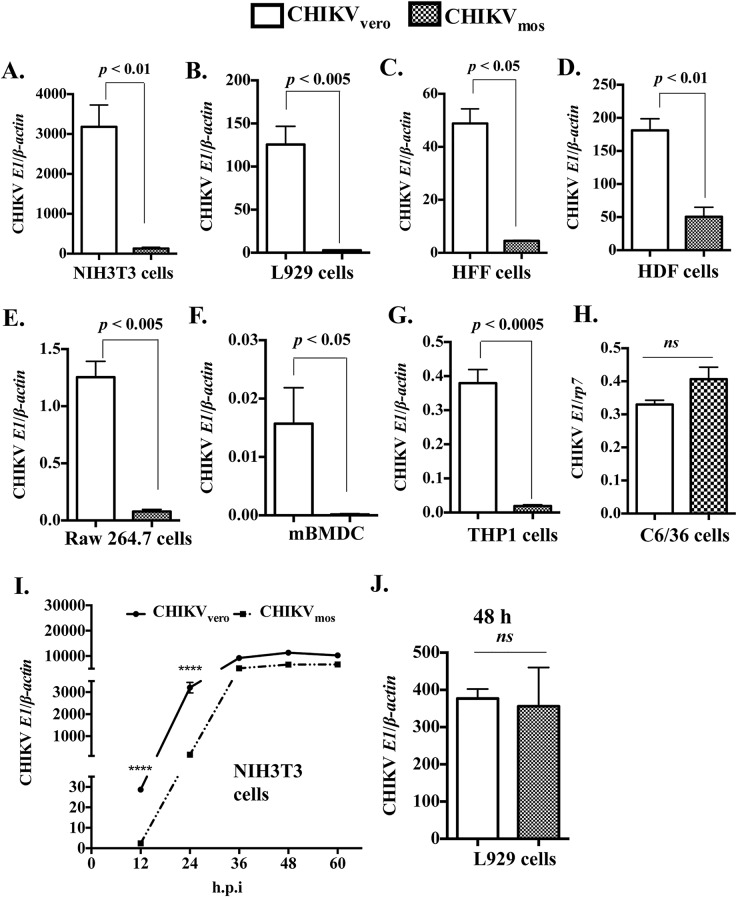
Previous reports have suggested that passage of virus through mosquito and mammalian cells can modulate arboviral infectivity [29,31,43]. To investigate the difference between mosquito and mammalian cells generated CHIKV, we prepared CHIKV stocks (Ross strain) by infecting African green monkey (mammal) kidney cell line (Vero cells) or an Ae. albopictus (mosquito) cell line (C6/36 cells). Thus generated CHIKV stocks in Vero or C6/36 cells were titered by plaque assay in Vero cells and designed as CHIKVvero and CHIKVmos, respectively. CHIKV replicates more efficiently in fibroblastic cells compared to hematopoietic cells [53,54], therefore we infected mouse embryonic fibroblasts (NIH3T3 cells) with CHIKVmos or CHIKVvero at a multiplicity of infection (MOI) of 1. At 24 h.p.i., the cells were collected for total RNA extraction and the first-strand complementary DNA (cDNA) synthesis. CHIKV envelope-1 (E1) gene RNA copy numbers were quantified by reverse transcription quantitative polymerase chain reaction (RT-qPCR) and cellular β - actin was used as an internal control. The RT-qPCR results showed that the level of CHIKVmos replication was significantly lower (approximately 25-folds) than CHIKVvero at 24 h.p.i. (Fig 1A, p < 0.01). In addition, we also confirmed that CHIKVmos had lower replication in mouse subcutaneous fibroblasts (L929 cells, Fig 1B, p < 0.05), human foreskin fibroblastic cells (HFF cells, Fig 1C, p < 0.05) and human dermal fibroblasts (HDF cells, Fig 1D, p < 0.01) at 24 h.p.i. by RT-qPCR assay. To further test whether CHIKVmos had lower replication over CHIKVvero in cells other than fibroblasts, we compared their replication in a mouse macrophage cell line (Raw 264.7 cells), primary mouse bone marrow derived dendritic cells (mBMDC), and a human monocyte cell line (THP-1). Although CHIKV replication levels were relatively lower in these immune cells when compared to fibroblasts, similar reduction of CHIKVmos replication over CHIKVvero was also observed in Raw 264.7 cells (Fig 1E, p < 0.005), mBMDC (Fig 1F, p < 0.05), and THP-1 cells (Fig 1G, p < 0.0005). In contrast to murine and human cells, both CHIKVvero and CHIKVmos replicated similarly when their gene copy numbers were compared in mosquito (C6/36) cells (Fig 1H).
Fig 1. CHIKVmos has reduced infectivity than CHIKVvero.
(A-H) Indicated cells were infected with CHIKVvero or CHIKVmos (Ross strain, MOI = 1) for 24 h to measure gene expression of CHIKV E1 and cellular β-actin by RT-qPCR. (I) NIH3T3 cells were infected with CHIKVvero and CHIKVmos (Ross strain, MOI = 1) for 60 h and CHIKV E1 expression was measured at indicated time points by RT-qPCR. (J) L929 cells were infected with CHIKVvero or CHIKVmos (Ross strain, MOI = 1) for 48 h to measure gene expression of CHIKV E1 by RT-qPCR. Results were shown as the ratio (mean ± SEM) copy number of CHIKV E1 to cellular β-actin. Ribosomal protein 7 (rp7) was used as a housekeeping gene control for C6/36 cells. All data represent at least two independent experiments performed in triplicates with similar results. Replication between CHIKVvero and CHIKVmos were compared using student’s t test (**** denotes p < 0.0001, and ns denotes p ≥ 0.05).
To further study the kinetics of CHIKVvero and CHIKVmos replication over a longer infection period, we infected NIH3T3 cells with CHIKVvero or CHIKVmos (MOI = 1) and cells were collected at various time points to analyze CHIKV E1 gene replication by RT-qPCR. In NIH3T3 cells, levels of CHIKVmos replication was about 30-fold lower than CHIKVvero at 12 and 24 h.p.i (Fig 1I, p < 0.0001), but both viruses replicated at comparable levels at the later time points (36, 48 and 60 h.p.i). Both CHIKVvero and CHIKVmos also replicated at comparable levels at 48 h when assayed in L929 cells (Fig 1J). These observations suggest that CHIKVvero replicates more efficiently than CHIKVmos in murine and human cells during the early time points. However, over the course of infection in mammalian cells, CHIKVmos may gain its infectivity and replicates similarly to CHIKVvero.
Besides using PFU/ml as a standard titer for infection assays, we also determined viral titers by measuring viral genome copies in our viral stocks using RT-qPCR. We infected NIH3T3 cells with equal amounts of genome copies of CHIKVvero and CHIKVmos, and compared their replication levels by RT-qPCR. Similarly, we observed a significantly lower replication of CHIKVmos compared to CHIKVvero (S1A Fig), which suggests that the lower replication of CHIKVmos over CHIKVvero was not due to the difference in unencapsidated viral genome present in our viral stocks. To test whether our results were specific to the CHIKV Ross strain, we also compared the replication levels of Vero cell-generated and C6/36 cell-generated CHIKV-LR OPY1 strain. Similarly, we observed a lower replication of C6/36 cell-generated CHIKV-LR OPY1 when NIH3T3 cells were infected (S1B Fig). Collectively, these results demonstrate that mosquito cell-generated CHIKV has reduced levels of replication in both murine and human cells during early stage of infection, when compared to Vero cell-generated CHIKV.
CHIKVmos induces moderate cytopathic effects in both murine and human cells
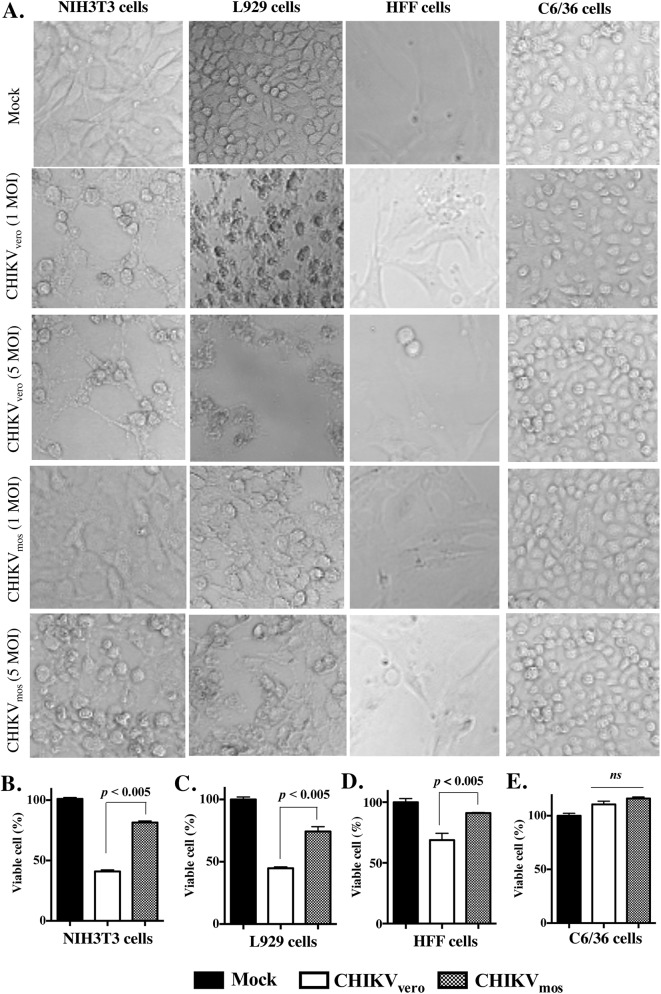
CHIKV infection can cause cytopathic effects and lysis of mammalian cells [21]. Since CHIKVmos has a much slower replication than CHIKVvero in both mouse and human cells at the early time points post infection, we expected that CHIKVmos might also cause less cytopathic effects in these cells. To test this, we infected NIH3T3, L929, HFF, and C6/36 cells with CHIKVmos and CHIKVvero (Ross strain, MOI = 1 or 5) for 48 h, a time point when cytopathic effects were clearly visible under a microscope. The microscopy results showed that CHIKVvero caused more morphological distress and cell death than CHIKVmos in both human and mouse fibroblasts, but no cytopathic effect was observed in C6/36 cells (Fig 2A). This observation was further confirmed by a cell viability assay using toluidine blue staining, which showed that CHIKVmos only caused moderate cytopathic effects compared to CHIKVvero in NIH3T3 (Fig 2B, p < 0.005), L929 (Fig 2C, p < 0.005) and HFF cells (Fig 2D, p < 0.005). In contrast to murine and human cells, both CHIKVmos and CHIKVvero did not cause any apparent cytopathic effects in C6/36 cells (Fig 2E). To rule out the possibility that the differences in cytopathic effects were not due to Vero and mosquito cell-specific proteins that could be released in culture supernatant and might be present in our virus stocks, we examined the cytopathic effects of UV-inactivated CHIKVvero and CHIKVmos stocks in L929 cells. We did not observe any cytopathic effects until 72 h post-treatment (S1C Fig), suggesting that the observed cytopathic effects were specific to CHIKVvero and CHIKVmos infection.
Fig 2. CHIKVmos induces minimal cytopathic effects than CHIKVvero.
NIH3T3, L929, HFF, and C6/36 cells were infected with CHIKVmos or CHIKVvero (Ross strain, MOI as indicated) for 48 h and fixed with 4% PFA after removing culture medium. (A) Phase contrast images (100X) were acquired using a Zeiss LSM510 META microscope. The cell viability data of NIH3T3 (B), L929 (C), HFF (D) and C6/36 (E) cells infected with CHIKVmos or CHIKVvero (Ross strain, MOI = 1) for 48 h are presented as the percentage of viable cell as determined by toluidine blue staining. The controls represent cells without viral infection (Mock). Error bars indicate mean ± SEM. Cell viability data represent two independent experiments performed in triplicates with similar results.
CHIKVmos induces low levels of antiviral responses
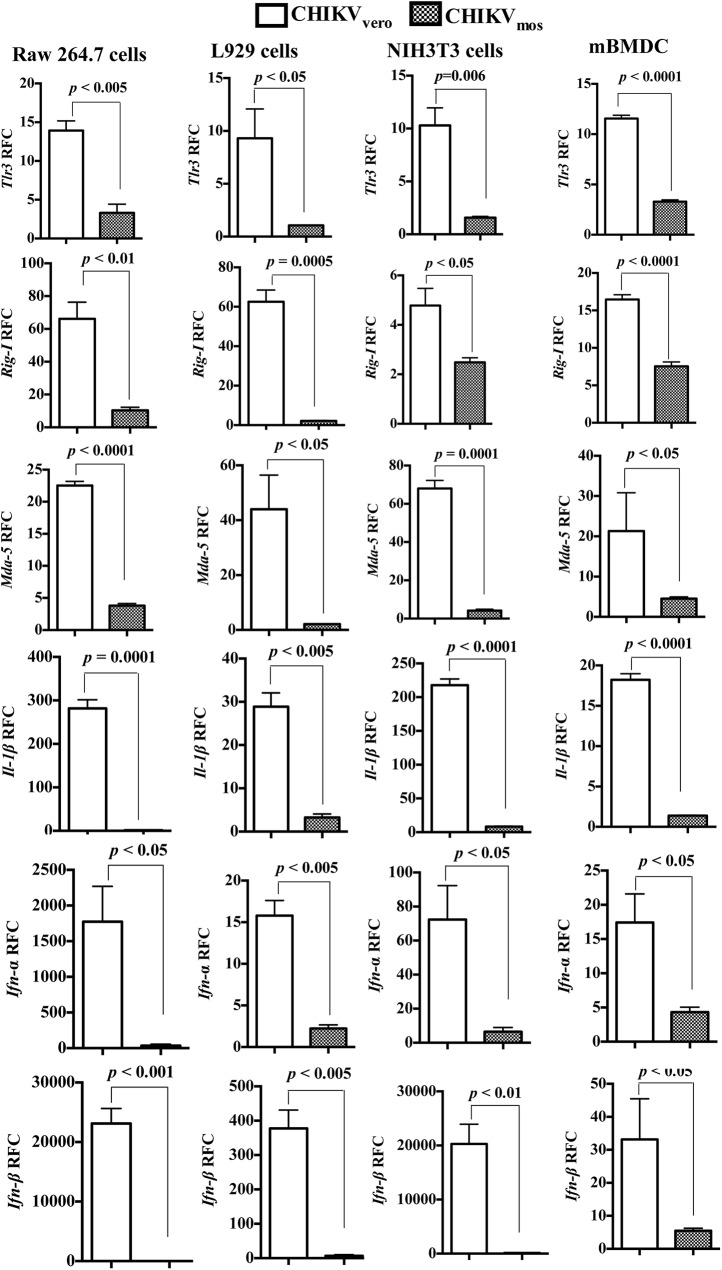
Some mosquito cell-derived viruses including RRV, VEEV and WNV have been reported to exhibit enhanced infection in primary myeloid dendritic cells due to their inhibition of type I interferon production when compared to corresponding mammalian cell-derived viral preparations [34,35,55]. While our results of CHIKVvero and CHIKVmos were opposite to those of RRV, VEEV and WNV in terms of replication [34,35,55], we asked whether the difference in CHIKVmos and CHIKVvero replication was due to differential induction of cellular antiviral or inflammatory responses by these viruses. To test this, we measured the expression profiles of selected pattern recognition receptors (PRRs) and inflammatory cytokines in CHIKVmos or CHIKVvero (Ross strain) infected Raw 264.7, L929, NIH3T3, and mBMDC (MOI = 1) by RT-qPCR assay. In consistent with its lower replication, CHIKVmos induced significantly lower levels of antiviral cytokines (Ifn-α and Ifn-β), proinflammatory cytokine (Il-1β), and PRRs (Tlr3, Rig-I, and Mda-5) in all of the tested cell types at 24 h.p.i. (Fig 3, p < 0.05). Difference in expression of these genes in CHIKVvero and CHIKVmos infected cells correspond with replication levels of these viruses in respective cells, suggesting that higher replication of CHIKVvero over CHIKVmos may not be due to an inhibition of antiviral or inflammatory cytokine expression by these cells. Thus, the slower replication of CHIKVmos in the early stage of infection might be due to a mechanism that is independent of the host cell antiviral responses.
Fig 3. CHIKVmos induces lower antiviral responses than CHIKVvero.
Raw 264.7 cells, L929 cells, NIH3T3 cells, and mouse bone marrow derived dendritic cells (mBMDC) were infected with CHIKVvero or CHIKVmos (Ross strain, MOI = 1) for 24 h. Gene expressions of Tlr3, Rig-I, Mda-5, Il-1β, and type I IFNs (Ifn-α and Ifn-β) were analyzed by RT-qPCR. The gene copy numbers were normalized to cellular β-actin and the data are presented as relative fold changes in expression of respective gene compared to the mock-infected control designated as 1 (not indicated in figures). All data sets represents two independent experiments performed in triplicates with similar results.
CHIKVmos causes less severe disease in mice
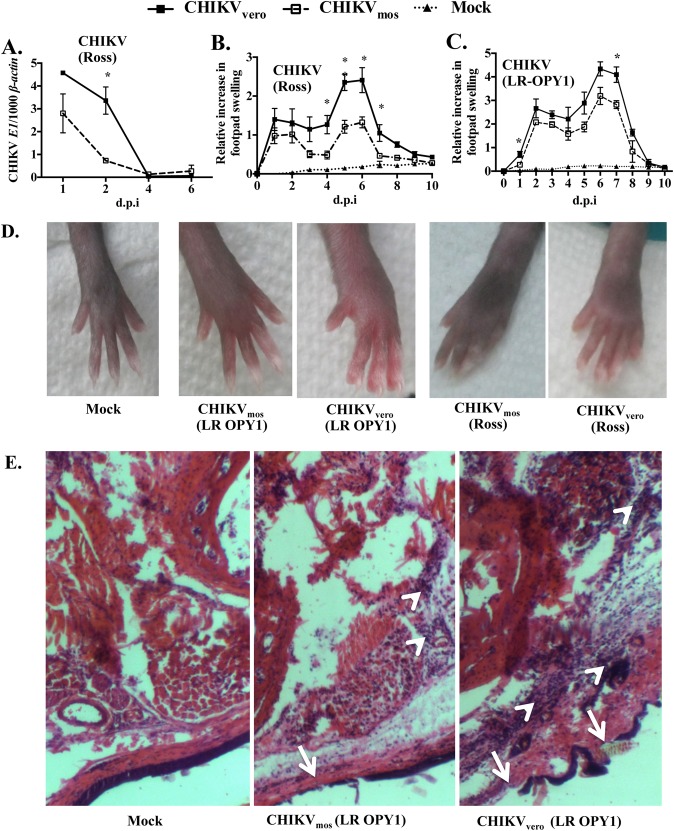
Differences in in vitro replication of mammalian and mosquito-generated viruses may not always produce the similar clinical symptoms in a mouse model, as previously reported with WNV infection [56]. Therefore, we asked whether CHIKVmos and CHIKVvero also differed in their virulence in vivo. To test this, we infected five-week-old, sex-matched C57BL/6J mice subcutaneously via footpad inoculations with 1 × 105 PFUs of CHIKVmos or CHIKVvero (Ross strain) or PBS as a vehicle control (mock), according to the previous reports [48–50]. Blood samples were collected on day 1, 2, 4 and 6 post-infection (d.p.i.) for viremia measurement by RT-qPCR, and footpad swelling was measured daily from 0 to 10 d.p.i.. CHIKVmos produced lower viremia (presented as CHIKV E1 / β-actin) in mice over the course of infection when compared to CHIKVvero, which reached statistical significance at 2 d.p.i. (Fig 4A, p < 0.05). These results suggest that CHIKVmos displays reduced infectivity in mice. Consistent with the viremia results, CHIKVmos induced milder footpad swelling than CHIKVvero throughout the experiment (Fig 4B and 4D). Similar results were also obtained when footpad swelling was compared in mice infected with mosquito cell- and Vero cell-generated CHIKV LR OPY1 strain (Fig 4C and 4D). To further test whether CHIKVmos causes less pathology in mice compared to CHIKVvero, we collected inflamed footpad tissue at 6 d.p.i. and performed a histological analysis. We found that CHIKVmos induced less leukocyte infiltration and limited subcutaneous necrosis in the inflamed foot when compared to CHIKVvero (Fig 4E). Since type I interferons have been shown to play important roles in CHIKV pathogenesis [53,57], we also measured expression of Ifn-α and Ifn-β in the blood of CHIKVvero and CHIKVmos infected mice at 1, 2 and 4 d.p.i. by RT-qPCR.. No significant difference in expression of these genes was observed between mice infected with CHIKVvero and CHIKVmos (S1D and S1E Fig), suggesting that lower level of swelling in mice infected with CHIKVmos may not be due to difference in IFN expression, but due to lower infectivity of this virus. All these in vivo data suggest that CHIKVmos displays lower virulence than CHIKVvero in a mouse model of footpad swelling.
Fig 4. CHIKVmos produces lower levels of viremia and footpad swelling in mice.
Wild-type C57BL/6J mice were subcutaneously infected with 1 × 105 PFUs of CHIKVvero, CHIKVmos or mock infected with PBS and monitored daily for 10 days. (A) Viral load in blood at day 1, 2, 4 and 6-post infection (d.p.i) with CHIKV (Ross strain) is presented as ratio of CHIKV E1 copy number per 1000 copy of cellular β-actin. Swelling of hind footpad (perimetatarsal area) of mice (n = 5/group) infected with CHIKV Ross (B) and LR-OPY1 (C) are presented as relative increase in swelling that were calculated by measuring height (thickness) and breadth (width) of inoculated footpad. (D) Representative image showing footpad swelling after infection with CHIKVmos or CHIKVvero at 6 d.p.i. (E) H&E stained histological images (100X) of foot tissue at 6 d.p.i displays subcutaneous necrosis (arrow) and infiltrated leukocytes (arrowhead). CHIKVvero and CHIKVmos data were compared using student’s t test (** denotes p < 0.005, * denotes p < 0.05, and ns denotes p ≥ 0.05).
CHIKVmos has lower ability to attach to host cell surface receptors
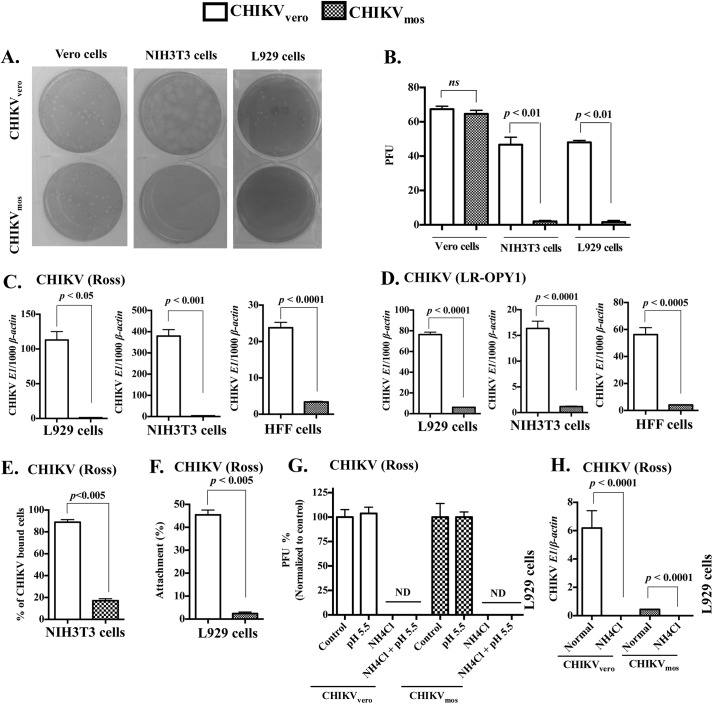
To further dissect the mechanism by which CHIKVmos has reduced replication and virulence, we next compared the plaque-forming phenotypes of CHIKVvero and CHIKVmos in various cells by plaque assays and counted plaques at 48 h post infection. Consistent with RT-qPCR results (Fig 1), the plaque assays showed that CHIKVmos had an approximately 25-fold reduction in PFUs over CHIKVvero in both NIH3T3 and L929 cells (Fig 5A and 5B, p < 0.01) when equal amounts of virus (~70 PFUs) were used for plaque development. In contrast to the numbers of PFUs, both CHIKVvero and CHIKVmos formed plaques at 48 h and no difference in plaque size was observed in all the tested cells (Fig 5A). These results suggest that the lower replication of CHIKVmos in murine and human cells may be due to its reduced ability to attach or enter into these cells.
Fig 5. CHIKVmos has reduced attachment to host cells than CHIKVvero.
(A), Equal PFUs of CHIKVvero or CHIKVmos (Ross strain) were added to Vero cells (left), NIH3T3 cells (middle) and L929 cells (right) for plaque development. (B), Plaque counts of CHIKVvero and CHIKVmos in the indicated cells were quantified. (C) L929, NIH3T3 and HFF cells were inoculated with CHIKVvero or CHIKVmos (Ross strain, MOI = 1) at 4°C for 1 h and attached viruses were quantified by RT-qPCR and presented as the ratio of CHIKV E1 copy number per 1000 copy of cellular β-actin. (D) L929, NIH3T3 and HFF cells were inoculated with CHIKVvero or CHIKVmos (LR OPY1, MOI = 1) at 4°C for 1 h and viruses attached to cells were quantified by RT-qPCR. (E) NIH3T3 cells were infected with CHIKVvero or CHIKVmos (Ross strain, MOI = 2.5) at 4°C for 45 min and the virus-bound cells were quantified by flow cytometry. (F) CHIKVvero or CHIKVmos (100 PFUs) were added to monolayer of L929 cells at 4°C for 1 h and both attached and unattached viruses were quantified by plaque assay to calculate percentage attachment. (G) CHIKVvero or CHIKVmos (Ross strain, 100 PFUs) were added to the L929 cell monolayer at 4°C for 1 h. After removing unattached virus, cells were replaced with fresh medium (control), or treated with acidic medium (pH 5.5) for 2 minutes before adding fresh medium, or replaced with NH4Cl (20 mM) containing media. Viruses that entered into cells were analyzed by allowing plaque development for 48 h and normalized to controls. (H) Viral RNA copies in L929 cells infected with CHIKVvero or CHIKVmos (Ross strain, MOI = 1) with or without NH4Cl (20 mM) were measured by RT-qPCR at 24 h.p.i. Data represent at least two independent experiments performed in triplicates with the similar results. ND denotes not detected, and ns denotes not significant (p ≥ 0.05).
To test whether CHIKVmos attaches to cell receptors at a lower affinity compared to CHIKVvero, we measured the attachment of CHIKVmos and CHIKVvero (Ross strain, MOI = 5) on L929, NIH3T3 and HFF cells. Viruses were allowed to bind to the target cells for 1 h at 4°C, a condition at which most of the viruses attach to cell surfaces but do not enter into cells [58]. Unattached viruses were removed by washing with fresh medium and the viruses attached to cells were quantified by measurement of CHIKV E1 RNA copies by RT-qPCR. The results showed that CHIKVmos had significantly reduced attachment to L929, NIH3T3 and HFF cells (Fig 5C) when compared to CHIKVvero. Similar results were also obtained when mosquito- and Vero cell-generated CHIKV-LR OPY1 viruses were assayed for their attachment to these cells by RT-qPCR (Fig 5D). In contrast to murine and human cells, no difference in attachment between CHIKVvero and CHIKVmos was observed in C6/36 cells (S1F Fig).
To further confirm lower attachment of CHIKVmos, we incubated NIH3T3 cells with CHIKVvero or CHIKVmos (Ross strain, MOI = 2.5) at 4°C for 45 min, immediately fixed the cell surface bound viruses with 4% PFA, probed these cells with anti-CHIKV monoclonal antibody, and analyzed them by flow cytometry. The results showed that CHIKVvero attached to 95% of these target cells while CHIKVmos attached to only 18% (Fig 5E, p < 0.005), which confirmed our hypothesis that CHIKVmos has a reduced attachment to host cells, when compared to CHIKVvero. To further test whether the reduced attachment of CHIKVmos correspond to its lower infectivity, the viruses attached to these cells were also visualized by plaque development. For this, we incubated 100 PFUs of CHIKVmos and CHIKVvero (Ross strain) with L929 cells at 4°C for 1 h and washed away unattached viruses. The attached viruses were allowed to develop plaques for 48 h in a 37°C incubator. To ensure that equal amounts of viruses were used during this experiment, unattached viral particles were also quantified by a plaque assay in Vero cells. As expected, the sum of attached and unattached virus matched between CHIKVvero and CHIKVmos. The attachment results are expressed as percentage using the sum of attached viruses and unattached viruses as denominator (Fig 5F, p < 0.005). These results showed that only 2% of CHIKVmos, compared to 45% of CHIKVvero, developed plaques in L929 cells, suggesting a 22.5-fold reduction in plaque development, when viruses were allowed to attach at 4°C. These results were in agreement with the RT-qPCR and plaque assay results showing that CHIKVmos had approximately 40-fold reduced replication (Fig 1C, p < 0.005) and approximately 23-fold reduction in plaque numbers (Fig 5A, L929 cells, p < 0.01) in L929 cells when compared to CHIKVvero. Collectively, these attachment assay results measured by RT-qPCR, flow cytometry and plaque assays, all suggest that lower replication of CHIKVmos is due to its reduced attachment to the host cells.
Alphaviruses, including CHIKV, primarily use receptor-mediated endocytosis to enter into host cells, in which viruses enter the endosome through clathrin-independent endocytosis followed by a low pH dependent viral uncoating process in the endosome to gain entry into the cytoplasm [59,60]. Besides the receptor-mediated endocytosis pathway, direct viral membrane fusion with the cytoplasmic membrane has also been described as a possible mechanism that mediates the entry of some alphavirus (e.g. Semliki Forest virus) [61]. To test the possibility that whether CHIKVvero or CHIKVmos may use different pathways to enter into host cells, we performed plaque assays in the presence or absence of a lysomotrophic agent (NH4Cl) in growth media that blocks endosomal acidification and inhibits viral entry through endosomes. To test direct viral membrane fusion with cytoplasmic membrane, we induced a low pH mediated viral fusion with the plasma membrane and further cultured cells with or without NH4Cl. The plaque assay results showed that both CHIKVmos and CHIKVvero fail to develop plaques in L929 cells (Fig 5G) and Vero cells (S1G Fig) when endosomal acidification was blocked, suggesting that both types of viruses use receptor-mediated endocytosis, but not direct viral-plasma membrane fusion, to enter into cells. To further confirm that the receptor-mediated entry of CHIKV occurs through the endosome, we infected L929 cells (MOI = 1) with CHIKVvero and CHIKVmos in the presence or absence of NH4Cl and analyzed the expression of CHIKV E1 at 24 h by RT-qPCR. These results further demonstrated that both CHIKVvero and CHIKVmos enter cells via the endosome pathway (Fig 5H, p < 0.0001).
CHIKVvero, but not CHIKVmos, binds to mammalian cell surface glycosaminoglycan receptors
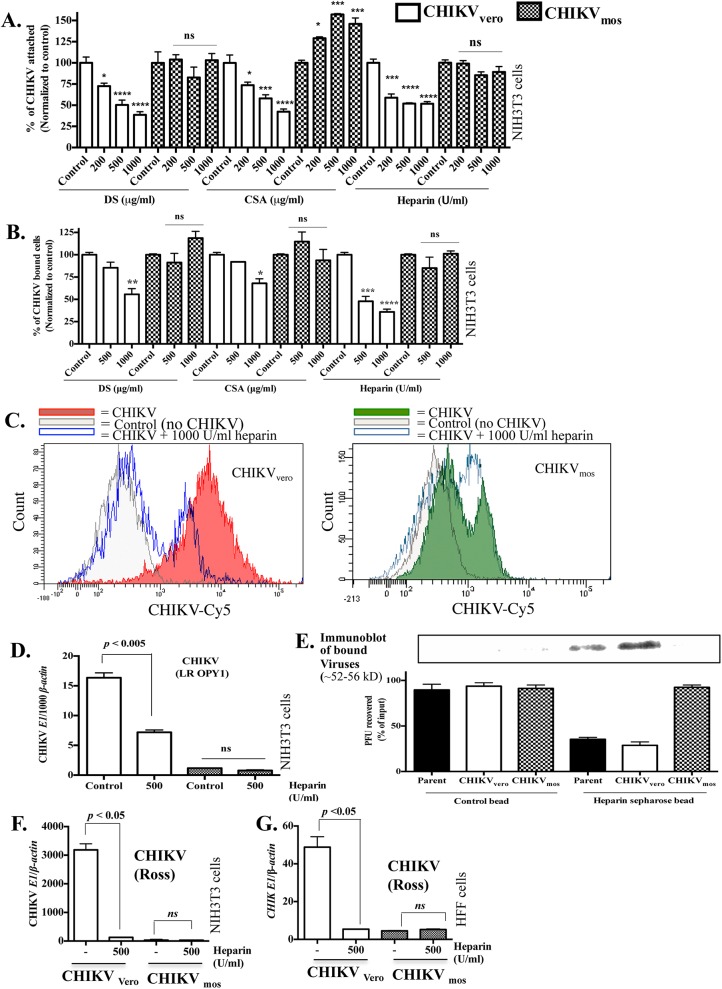
CHIKV E2 is the major viral protein that mediates binding of CHIKV to host cell surface receptors [62]. Although a number of cell surface receptors have been described for CHIKV and other alphaviruses [59,60], the cell surface GAG receptors have been suggested to play a role in alphavirus infectivity [40–42,63,64]. We asked whether lower attachment of CHIKVmos was due to its failure to bind to the GAG receptors on host cells. To test this, we performed a GAG neutralization assay, in which we pre-incubated CHIKVmos or CHIKVvero (Ross strain) with a range of soluble GAGs (dermatan sulfate and heparin from porcine intestinal mucosa and chondroitin sulfate A from bovine trachea) at 37°C for 1 h. The virus-GAG mixtures were added to NIH3T3 cells and cells were further incubated at 4°C for 1 h for viral attachment. After removal of unattached viruses, the cells were washed once with fresh medium and the attached viruses were quantified by RT-qPCR. The results showed that pre-treatment with all the tested GAGs reduced CHIKVvero attachment to NIH3T3 cells in a concentration-dependent manner, but did not affect the attachment of CHIKVmos (Fig 6A). These results suggest that only CHIKVvero, but not CHIKVmos, may utilize GAG receptors to enter into cells. It has been previously shown that mosquito cell-generated alphaviruses preferentially bind to lectin receptors to enter into cells [31]. Thus, it is possible that CHIKVmos uses lectin receptor to enter into cells. To test this, we performed mannan-blocking assay, in which pre-incubation of cells with yeast mannan blocks the binding of viruses to lectin receptors, such as DC-SIGN, L-SIGN and mannose receptors [31,59]. However, preincubation of both NIH3T3 and HFF cells with yeast mannan (up to 200 μg/ml) did not affect attachment of CHIKVmos or CHIKVvero in these cells (S1H Fig).
Fig 6. CHIKVvero, but not CHIKVmos, binds to cell surface glycosaminoglycan receptors.
(A) Equal PFUs of CHIKVvero or CHIKVmos (Ross strain) were pre-incubated with the indicated soluble GAGs at 37°C for 1 h. The virus-GAG complexes were added to NIH3T3 cell monolayer (MOI = 1) and incubated at 4°C for 1 h and the GAGs neutralization of viral attachment was measured by RT-qPCR. Data were normalized to controls without GAG treatment. (B) Equal PFUs of CHIKVvero or CHIKVmos (Ross strain) were pre-incubated at 37°C for 1 h with indicated GAGs and added to NIH3T3 cells (MOI = 2.5) at 4°C for 45 min. The virus-bound cells were quantified by flow cytometry and the representative histograms are shown in (C). (D) Heparin (500U/ml) neutralization of CHIKVvero and CHIKVmos (LR-OPY1 strain) attachment was performed in NIH3T3 cells and measured by RT-qPCR. (E) CHIKVvero, CHIKVmos or their parental stock (105 PFU, Ross strain) were mixed with heparin-conjugated sepharose beads or unconjugated sepharose beads (control) at 4°C for 45 min and unbound viruses recovered from beads were quantified by plaque assay in Vero cells (shown in bottom). Input viruses without beads (control) were also quantified by a plaque assay and viruses recovered from the beads were expressed as percentage of the input. Viruses bound to the respective heparin-conjugated and control beads were lysed and also analyzed by immunoblotting assays (shown in top). The effect of heparin pre-treatment (1000 U/ml) on CHIKVvero and CHIKVmos (Ross strain) replication was measured in NIH3T3 (F) and HFF (G) cells at 24 h by RT-qPCR. GAG treated samples were normalized to their respective controls and analyzed using a one-way ANOVA (**** denotes p < 0.0001, *** denotes p < 0.0005, ** denotes p < 0.005, * denotes p < 0.05, and ns denotes p ≥ 0.05). DS, dermatan sulfate; CSA, chondroitin sulfate A.
To further confirm GAG receptor binding of CHIKVvero, we also assayed GAG neutralization by flow cytometry. Similarly, we observed a concentration-dependent reduction of CHIKVvero attachment to NIH3T3 cells after GAGs pre-treatment, while attachment of CHIKVmos was not affected (Fig 6B and 6C). In addition, pretreatment with GAGs also reduced the plaque development of CHIKVvero but not CHIKVmos (S1I Fig). Similar results were also obtained when CHIKVmos and CHIKVvero (LR OPY1 strain) were tested for heparin neutralization in NIH3T3 cells by RT-qPCR (Fig 6D, p < 0.005). These results suggest that CHIKVvero, but not CHIKVmos, binds to the cell surface GAG receptors.
To further confirm differential effects of pre-incubation with soluble GAGs in CHIKVvero and CHIKVmos attachment to host cells, we performed a direct GAG binding assay using heparin-conjugated sepharose beads. Equal PFUs of CHIKVvero or CHIKVmos or their parental viruses (Ross strain, all generated in Vero cells) were incubated with heparin-conjugated sepharose beads or unconjugated control beads at 4°C for 30 min. The beads were washed three times in DMEM containing 2% FBS, and the unbound viruses in the wash solution were quantified by a plaque assay. Approximately 95% of CHIKVvero, CHIKVmos and their parental viruses were recovered in the wash solution after incubation with the control beads (Fig 6E, bottom), suggesting that none of the tested viruses bound onto the control beads. While approximately 95% of CHIKVmos were recovered in the wash solution after incubation with the heparin sepharose beads, only about 25% of CHIKVvero, and the parental viruses were recovered (Fig 6E, bottom), suggesting that only Vero cell-generated CHIKV (CHIKVvero and their parental viruses) bound to heparin. These results were also confirmed by western blot by measuring viruses bound to the heparin sepharose and control beads (Fig 6E, top). Collectively, the results of GAG neutralization by RT-qPCR, flow cytometry, and direct heparin-conjugated sepharose bead binding assays demonstrate that only CHIKV generated in Vero cells, but not in mosquito cells, can attach to cell surface GAG receptors.
To test whether the difference in GAG receptor binding contributes to the different levels of replication of CHIKVmos and CHIKVvero, we infected NIH3T3 and HFF cells with heparin pre-treated (1000 U/ml) or untreated CHIKVmos and CHIKVvero (Ross strain) at 37°C and measured viral replication at 24 h by RT-qPCR. Significant reduction in replication of CHIKVvero (Fig 6G, p < 0.05), but not CHIKVmos (Fig 6F, p > 0.05), was measured in both NIH3T3 cells and HFF cells (Fig 6G) after heparin pre-treatment. Of note, CHIKVvero pre-incubated with heparin had comparable replication to CHIKVmos in both mouse (Fig 6F) and human cells (Fig 6G), suggesting that the GAG receptor binding of CHIKVvero contributes to its higher replication, when compared to CHIKVmos.
Mosquito cells eliminate GAG receptor binding of CHIKV
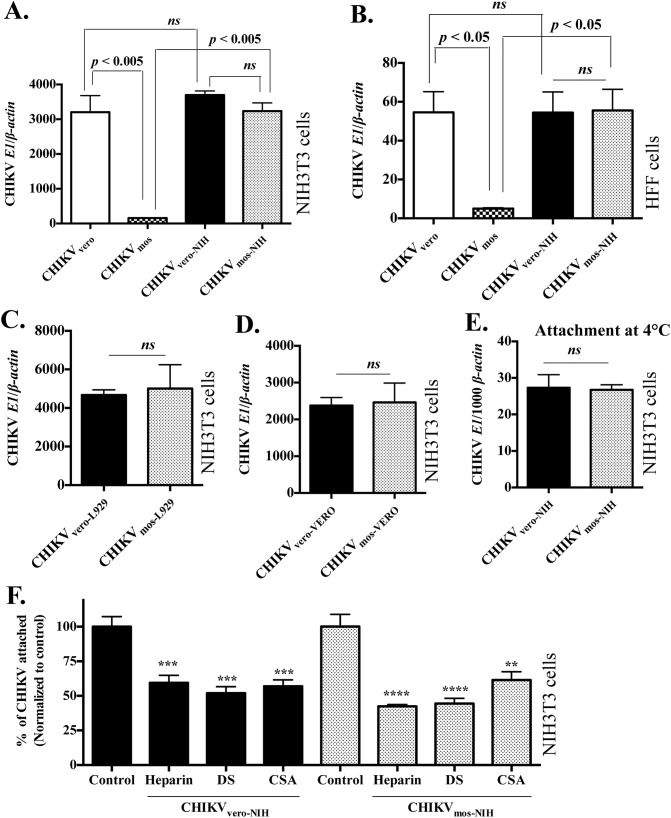
CHIKV E2 is the receptor binding protein of CHIKV. It has been suggested that charge-dependent interaction between the conserved basic amino acid residues in the alphavirus E2 domains and the negatively charged GAG receptors on mammalian host cell surface facilitate viral attachment [16,60]. In line with this, GAG receptor binding has been previously reported in cell-culture adapted alphaviruses, including CHIKV, whereby continuous cell-culture passages of virus result in acquisition of basic amino acid(s) in the viral E2 glycoprotein via mutations [38,64]. To test the possibility of potential mutations that could be acquired during CHIKVvero and CHIKVmos generation through a single passage, we sequenced the receptor binding protein-encoding gene E2 of CHIKVvero, CHIKVmos, and their parental viral stocks (both Ross and LR-OPY1 strains). The sequence alignment showed that the E2 gene sequences were identical among CHIKVvero, CHIKVmos, and their parental stock within each strain (S2 Fig), which suggested no mutations were acquired in the E2 gene of our CHIKVvero and CHIKVmos stocks after a single cell passage. In addition, the heparin-sepharose bead assays showed that the parental viruses, also generated in Vero cells, bound to soluble GAGs (Fig 6E), which indicated that Vero cell-generated CHIKV lost its GAG binding capability after a single passage in mosquito cells. To further confirm this, we generated CHIKVmos-NIH and CHIKVvero-NIH by growing CHIKVmos and CHIKVvero (Ross strain) in NIH3T3 cells for 48 h (single passage) and compared their replication levels in NIH3T3 and HFF cells at 24 h.p.i. by RT-qPCR. The results showed that both CHIKVmos-NIH and CHIKVvero-NIH produced a comparable amount of viral RNA and replicated similarly to CHIKVvero in NIH3T3 cells (Fig 7A) and HFF cells (Fig 7B). We similarly passaged CHIKVmos and CHIKVvero once for 48 h in L929 to generate CHIKVmos-L929 and CHIKVvero-L929, or in Vero cells to generate CHIKVmos-VERO and CHIKVvero-VERO. No differences in replication were observed when CHIKVmos and CHIKVvero passaged once in L929 cells (Fig 7C) or in Vero cells (Fig 7D) were used to infect NIH3T3 cells for 24 h. These data demonstrate that CHIKVvero and CHIKVmos no longer differ in replication after their single passage in mammalian cells, suggesting that mosquito cell-mediated reduction of CHIKVmos replication can be regained after a single passage of CHIKVmos in mammalian cells.
Fig 7. CHIKVmos infectivity can be enhanced after replication in mammalian cells.
The viral stocks of CHIKVvero-NIH3T3 and CHIKVmos-NIH3T3 were prepared after CHIKVvero or CHIKVmos (Ross strain, MOI = 1) replicated in NIH3T3 for 48 h. (A) NIH3T3 and HFF cells (B) were infected with CHIKVvero, CHIKVmos, CHIKVvero-NIH3T3 and CHIKVmos-NIH3T3 (MOI = 1), and viral RNA copy numbers were measured at 24 h by RT-qPCR. Data represents ratio of copy number of CHIKV E1 to β-actin. (C) NIH3T3 cells were infected with CHIKVvero-L929 and CHIKVmos-L929 (MOI = 1), and viral RNA copy numbers were measured by RT-qPCR at 24 h. (D) NIH3T3 cells were infected with CHIKVvero-VERO and CHIKVmos-VERO (MOI = 1), and viral RNA copy numbers were measured at 24 h by RT-qPCR. (E) Attachment of CHIKVvero-NIH and CHIKVmos-NIH (MOI = 1) were performed in NIH3T3 cells at 4°C for 1 h and attached viruses were measured by RT-qPCR. (F) GAGs neutralization of CHIKVvero-NIH3T3 and CHIKVmos-NIH3T3 attachment was performed with heparin (1000 U/mL), chondroitin sulfate A (CSA, 1000 μg/mL) and dermatan sulfate (DS, 1000 μg/mL) in NIH3T3 cells, and the attached viruses were quantified by RT-qPCR and normalized to the respective untreated controls. All data sets represent two independent experiments performed in triplicates. GAG-treated samples were compared to the respective controls (without GAGs) and analyzed using a one-way ANOVA (**** denotes p < 0.0001, *** denotes p < 0.0005, ** denotes p < 0.005, and ns denotes p ≥ 0.05).
To further test whether CHIKVvero and CHIKVmos after a single passage in mammalian cells have a similar level of attachment and GAG receptor binding, we performed attachment assays and GAG receptor neutralization assays of CHIKVmos-NIH and CHIKVvero-NIH, and measured the CHIKV E1 gene by RT-qPCR, as described above. The RT-qPCR data showed that both CHIKVmos-NIH and CHIKVvero-NIH had similar levels of attachment (Fig 7E). In addition, the RT-qPCR results confirmed that pre-treatment with heparin, dermatan sulfate and chondroitin sulfate A significantly inhibited attachment of both CHIKVmos-NIH and CHIKVvero-NIH onto NIH3T3 cells at similar levels, confirming that CHIKVmos had regained GAG receptor binding after a single passage through the mammalian cells (Fig 7F). To ensure this was not due to viral mutation, we also sequenced and compared the E2 gene in CHIKVmos-NIH and CHIKVvero-NIH, which showed that no mutation occurred in CHIKV E2 gene (S2 Fig). Collectively, these results indicate that a single passage within mosquito cells can reduce CHIKV infectivity by eliminating its GAG receptor binding capability.
Mosquito cell glycosylation reduces CHIKV infectivity in mammalian cells
Mosquito and mammalian cells use different cellular enzymes for N-glycosylation of proteins, and generate different carbohydrate residues in viral glycoproteins that influence receptor binding and virulence of viruses [31,43,65]. CHIKV E2, the receptor binding protein, is comprised of 423 amino acids with two putative N-linked glycosylation sites at positions 263 and 345 [66]. Therefore, we hypothesized that differential glycosylation of CHIKV receptor binding protein in mammalian and mosquito cells might lead to differential GAG receptor binding and therefore influence replication of CHIKVvero and CHIKVmos. To confirm that N-glycosylation occurs in CHIKV proteins, we treated CHIKVvero and CHIKVmos with PNGase F, an enzyme that removes N-linked glycan from glycoproteins [67], and analyzed the viral proteins by SDS-PAGE. We observed shifts in protein bands in the gel after PNGase treatment (Fig 8A), suggesting that both E1 and E2 proteins are glycosylated in CHIKVvero and CHIKVmos. However, the composition of glycan at these glycosylation sites differs because the post-translational modifications within insect cells generate high-mannose oligosaccharides at all glycosylation sites of viral proteins, while such modifications in vertebrate cells generate both complex and high-mannose carbohydrates chains at similar glycosylation sites [43,65,68].
Fig 8. Mosquito cell-specific glycosylation reduces CHIKV infectivity.
(A) SDS-PAGE image for CHIKVvero and CHIKVmos proteins that were treated with PNGase F to remove N-glycans from viral glycoproteins. (B-E) Vero cells or C6/36 cells were infected for 20 h with parental stocks of CHIKV (Ross) in the presence of tunicamycin (TM, 0.1 μg/ml), or with dimethyl sulfoxide (DMSO) as vehicle controls to measure infectious virus particles released in culture media by plaque-forming assay (B and C) and the viral genome copies in the culture media by RT-qPCR (D and E). NIH3T3 cells were infected with 0.1 MOI of CHIKVvero-DMSO and CHIKVmos-DMSO (F), or CHIKVvero-TM and CHIKVmos-TM (G), and viral replication was measured at 24 h by RT-qPCR. (H) GAGs neutralization of CHIKVvero-TM and CHIKVmos-TM binding was performed with chondroitin sulfate A (CSA, 500 μg/mL) and dermatan sulfate (DS, 500 μg/mL) in NIH3T3 cells, and the attached viruses were quantified by RT-qPCR. A two-tailed student’s t-test was used for statistical analysis. Error bars indicate mean ± SEM. ns denotes not significant (p ≥ 0.05).
To test whether removal of N-glycosylation from CHIKVvero and CHIKVmos affect replication of these viruses, we infected C6/36 and Vero cells for 20 h with the parental stocks of CHIKV (Ross strain) in the presence of tunicamycin (TM, 0.1μg/ml), an antibiotic that specifically inhibits N-glycosylation of proteins [26], or with DMSO as a vehicle control. We analyzed the effect of TM on virus production in mosquito cells by plaque assays and RT-qPCR. Treatment with TM reduced infectious viral particle production in Vero cells (Fig 8B, p < 0.001), but not in C6/36 cells (Fig 8C), when measured by plaque assay. Similar to the plaque assay results, genome quantification in culture media by RT-qPCR also showed that TM treatment similarly reduced CHIKV production from Vero cells (Fig 8D, p < 0.001), but not in C6/36 cells (Fig 8E). In addition, CHIKV generated in C6/36 and Vero cells in the presence of DMSO or TM has comparable genome to PFU ratio, suggesting that our observations are not due to presence of defective viral particles between groups. These results suggest that N-glycosylation of CHIKV protein can influence its replication in mammalian cells but not in mosquito cells.
We further compared the replication of non-glycosylated CHIKVvero and CHIKVmos that were generated after TM treatment. For this, we infected NIH3T3 cells and measured viral RNA at 24 h by RT-qPCR. As expected, CHIKVmos has approximately 30-fold lower replication than CHIKVvero when these viruses are generated in respective cells treated with DMSO (designated as CHIKVvero-DMSO and CHIKVmos-DMSO, respectively) as vehicle controls (Fig 8F, p < 0.001). Interestingly, CHIKVvero and CHIKVmos generated in the presence of TM (designated as CHIKVvero-TM and CHIKVmos-TM, respectively) no longer differed in their replication in NIH3T3 cells at 24 h (Fig 8G). To further test whether CHIKVvero-TM and CHIKVmos-TM differ in GAG receptor binding, we performed GAG receptor neutralization assays of these viruses by RT-qPCR as described above. The RT-qPCR data showed that GAGs pre-treatment did not inhibit binding of both CHIKVmos-TM and CHIKVvero-TM to NIH3T3 cells (Fig 7H). These results collectively indicate that mosquito cell-specific N-glycosylation of viral protein does not favor binding of CHIKV to cell surface GAG receptor and reduces its infectivity in murine and human cells.
Discussion
Since viruses utilize host cell enzymatic systems to replicate their genome and synthesize functional proteins, viral infection may interrupt normal cellular functions and cause death of the host cells. However, mosquito-transmitted viruses replicate efficiently in mosquito cells, but do not cause apparent cellular damage to these cells [22,24]. Therefore, viruses may have adapted a mechanism to minimize their harmful effects within mosquito cells while replicating in mosquito vectors. This may be a viral fitness strategy, whereby a virus can efficiently replicate and generate a high titer in mosquito vectors, which is necessary for its efficient transmission to mammalian hosts. Since viral replication uses mammalian and mosquito cellular enzymatic systems to modify viral structural components [43,69], it could be possible that this fitness advantage developed in mosquito cells may reduce viral infectivity in mammalian cells [70]. To test whether mosquito or mammalian cell passages can influence CHIKV infectivity, we compared the replication levels of CHIKVmos and CHIKVvero generated respectively in C6/36 (mosquito, CHIKVmos) or Vero (mammal, CHIKVvero) cells through a single passage. We observed that CHIKVmos had a significantly lower replication than CHIKVvero in both human and murine cells, and it also induced moderate cytopathic effects and lower antiviral immune responses. In contrast, both CHIKVmos and CHIKVvero replicated similarly in C6/36 cells and did not caused apparent cytopathic effects to these cells (for up to 72 h.p.i.). In a mouse model of CHIKV-induced footpad swelling, CHIKVmos caused lower viremia, footpad swelling, and milder histological profiles within the inoculated foot when compared to CHIKVvero. These results suggest that generation through mosquito cells reduces CHIKV replication in both human and murine cells and cause a less severe disease in mice.
Previous studies have demonstrated that arboviruses (e.g. Sindbis virus, WNV and dengue virus) generated in mammalian or mosquito cells can bind to different cell surface receptors [31–33]. In this study, CHIKVmos replicated at lower levels at the early stages of infection (6 to 24 h) and also developed fewer plaques in both human and murine fibroblasts, compared to CHIKVvero. However, both CHIKVvero and CHIKVmos produced plaques at the same time point (approximately 48 h.p.i.) and no difference in plaque size was observed, suggesting only a portion of inoculated CHIKVmos may initiate productive infection in these cells. Moreover, the cytokine expression profiles suggest that the higher replication of CHIKVvero over CHIKVmos may not be due to suppression of antiviral cytokines, but rather an intrinsic property of the viral particles generated in two different cell lines. Therefore, we hypothesized that CHIKVmos might have a lower binding capability to murine and human cell surface receptors compared to CHIKVvero, which may account for lower replication of CHIKVmos at an early phase of infection. This hypothesis was proven by the attachment assay and viral entry assay results, which showed that CHIKVmos had reduced receptor binding to murine and human cells. Although the cellular receptors for CHIKV and other alphaviruses remain elusive, mammalian cell surface glycosaminoglycan (GAG) receptors are the most extensively studied receptors for alphaviruses [64,71,72]. Interestingly, it has been previously demonstrated that a single passage of RRV (T48 strain) in C6/36 cells resulted in loss of GAG receptor-binding capability, while RRV derived from mammalian cells bound to GAG receptors [73]. Consistent with this report, our GAG receptor neutralization and heparin-sepharose bead binding assays showed that CHIKVmos does not bind to GAG receptors. These results provided evidence for reduced receptor binding and infectivity of CHIKVmos compared to CHIKVvero. In addition, we also demonstrated that GAG receptor binding of CHIKVmos could be regained after a single passage in mammalian cells, indicating that mosquito cells can reduce infectivity of CHIKV by eliminating its ability to bind to GAG receptors on murine and human cells. It is likely that CHIKVmos may use some other receptors to enter into cells, and acquire GAG receptor-binding capability after replication in mammalian cells, which could eventually enhance its infectivity.
Viruses that are generated in different host cells can acquire host cell-specific modifications on their structural components. For instance, during the viral budding process, enveloped viruses acquire a portion of the host cell membrane as the viral envelope membrane, resulting in variable carbohydrate and lipid compositions of these viruses, depending on the cell types in which they are generated [43,69,74]. In addition, mosquito and mammalian post-translational modifications, particularly the N-glycosylation, uses different cellular enzymes to modify the viral glycoproteins [31,65]. Our PNGase F treatment assay results confirm that CHIKV E2, the receptor binding protein of CHIKV, has N-linked glycosylation sites. However, the composition of the carbohydrate residues at the glycosylation sites of E2 may vary depending on the host cell types used for CHIKV generation. Insect cells, including mosquitoes, are deficient in enzymes for carbohydrate synthesis, including N-acetylglucosaminyl-, galactosyl-, and sialyltransferases, resulting in high-mannose oligosaccharides at all glycosylation sites. In contrast, vertebrate cells can generate both complex and high-mannose carbohydrates chains at these glycosylation sites [43,65,68]. Glycosylations of Sindbis virus (SINV) E2 at positions 196 and 318 have been documented to play critical roles in receptor binding and infectivity in both cell culture and in a mouse model [28]. Thus, we hypothesized that differential glycosylation patterns in mammalian and mosquito cells may play a role in differential infectivity of CHIKV generated in these cell lines. TM is a potent inhibitor of N-glycosylation and it has been previously shown that viral stocks prepared in the presence of TM lose glycan at their glycolysation sites [26]. We demonstrated that CHIKVvero and CHIKVmos no longer differed in their replication when these viruses were prepared in the presence of TM, suggesting that mosquito and mammalian cell-specific glycosylation can affect the replication of CHIKVvero and CHIKVmos in murine and human cells. Although we could not completely exclude the possibility that mosquito cell-specific processing of other structural and nonstructural viral proteins might also influence the infectivity of CHIKVmos, our data provided several lines of evidence to support that mosquito cell-specific glycosylation of E2 does not favor GAG receptor binding and reduces CHIKV infectivity.
Previous studies suggested that mammalian cell-generated RRV, VEEV, and WNV induce potent IFN responses, but these viruses generated in mosquito cells inhibit IFN production and replicate more efficiently in mammalian cells in vitro [34,35]. It has been suggested that mosquito cell-generated WNV has higher infectivity in vitro [32], however, it produced significantly lower viral load in mice compared to mammalian cell-generated WNV during an early phase of infection, indicating greater infectivity of mammalian cell-generated WNV in vivo [56]. We found that mammalian (Vero) cell-generated CHIKV possessed greater infectivity in both in vitro and in vivo conditions. It is possible that the lack of GAG receptor binding of mosquito-generated CHIKV may favor its replication in mosquito cells, however this can potentially reduce its infectivity in mammalian cells. This hypothesis is also supported by a recent report, which showed that RRV had increased fitness in mosquito cells but produced less severe disease in a mouse model [70].
The role of GAG receptors in viral pathogenesis has been extensively studied in human immunodeficiency virus [75], herpes simplex virus [76], Echovirus [77], and human papillomavirus [78]. However, the role of GAG receptor binding in alphavirus pathogenicity remains controversial. Several reports document that cell-culture adapted alphaviruses (about 10–20 generations) can acquire GAG receptor binding capability in vitro via mutations in the E2 gene that leads to acquisition of basic amino acids [38,64,71,72]. Such GAG receptor dependence, often described as a cell-culture adapted property, has been suggested to reduce viral pathogenicity in vivo, presumably because of rapid clearance of these viruses from circulation, as shown in VEEV [39] and SFV [79]. In contrast, GAG receptor binding of cell-culture adapted SINV has been shown to enhance its infectivity and neurovirulence in mice [42]. It has been believed that wild-type (non cell-culture adapted) alphaviruses generally do not use heparan sulfate (HS) receptors, a prototype of GAG receptors, for host cell attachment but depend on other cell surface receptors including DC-SIGN, L-SIGN, and C-type lectin molecules [31,59]. However, it has been demonstrated that wild-type isolates of eastern equine encephalomyelitis virus (EEEV) and VEEV that bound to GAG receptors were neurovirulent in mice [41,80]. Unlike other cell culture adapted alphaviruses, the GAG receptor binding capability of wild-type EEEV does not occur through acquisition of additional basic amino acids in E2 [41]. Similarly, GAG receptor binding property has been also described in a non cell-culture adapted clinical CHIKV strain, which also occurred independent of additional basic amino acids acquisition in E2 [40]. However, a non cell-culture adapted CHIKV LR strain does not bind to GAG receptors [57], suggesting that GAG receptor binding may not be a property of all wild-type CHIKV strains. Herein, we report that mosquito cell-generated CHIKV does not bind on GAG receptors when compared to Vero cell generated counterpart, but it can acquire GAG receptors binding capability after a single passage in mammalian cells, such as NIH3T3 and L929 cells. CHIKV E2 sequencing results further confirmed that the GAG binding ability of mammalian cell-generated CHIKV was not due to mutation. It is noteworthy that we did not detect any previously published E2 amino acids in our CHIKV strains that were described to facilitate GAGs binding of the tissue-culture adapted CHIKV strain [38], suggesting that GAG receptor binding in our mammalian cell generated CHIKV does not reflect cell-culture adaptation and occurs without acquisition of basic amino acids. Thus, our results support the notion that the GAG receptor binding capability in CHIKV and other alphavirus can also be acquired through mechanisms independent of basic amino acid acquisition in E2 and such GAG binding capability can also enhance CHIKV infectivity both in vitro and in vivo.
Attenuation of viruses through a continuous cell-culture passage has been used as a strategy to develop live-attenuated arboviral vaccines [81–84]. Live-attenuated yellow fever virus vaccine (17D strain) is one of the most successful vaccines generated through this approach. Unfortunately, uses of this approach in an attempt to generate similar vaccines to other viruses were not successful. Although the detailed molecular mechanisms for attenuation of 17D vaccine strain remain elusive, it has been proposed that viral dependence on GAG receptor binding, particularly HS receptors, which can be acquired during continuous cell-culture passage, might play a role [82,84,85]. Recently, GAG-receptor binding CHIKV strains generated through continuous cell-culture passage has been evaluated as potential vaccine candidates [38,86,87]. Although GAG receptor dependence has been believed to attenuate alphaviruses in vivo, some of these CHIKV vaccine strains have been shown to be pathogenic [38]. For example, CHIKV vaccine strain (181/25) has been reported to cause transient arthralgia in clinical trials [88]. Although the selection of GAG receptor dependence has been proposed as a vaccine developmental strategy, accumulating evidence [38,40,41,89] suggests that other mechanisms are also possible in attenuation of cell-culture adapted arboviruses. Therefore, strategies to generate and/or evaluate CHIKV and other arboviral vaccine candidates should not be solely based on selection of GAG receptor dependence. In addition, viruses may have different affinity of GAG receptor binding or they may bind to different types of GAG receptors that may affect GAG receptor-dependent CHIKV infectivity in vitro and in vivo, which requires further investigation.
The mechanisms by which mosquito-transmitted viruses cause minimal or no damage to mosquito vectors, yet can cause cell death and diseases in mammalian hosts, are not well understood. The cellular machinery of mosquitos, which prevents CHIKV to bind to GAG receptors, may favor viral replication in mosquito cells without causing significant cytopathic effects. Our observation of reduced infectivity of mosquito-generated CHIKV puts forward an intriguing question of how CHIKV manages to infect hosts during natural infection, when viruses are inoculated into human skin by a mosquito bite. It has been previously reported that the mosquito saliva facilitates pathogenicity of some arboviruses, including CHIKV, La Crosse, dengue, and WNV by inducing CD4+ T helper-2 (Th2) cell dominant anti-inflammatory responses [90–95]. It is likely that CHIKV may take advantage of mosquito salivary proteins to initiate early infection in humans. A recent report showed that an Ae. aegypti saliva serine protease enhanced dengue virus infectivity by increasing the attachment of viruses to HS proteoglycans in mammalian hosts [96], suggesting that the reduction in viral GAG receptor binding by mosquito cells can be compensated by mosquito saliva during natural infections. After inoculation into the human skin through a mosquito bite, the mosquito cell-derived CHIKV may use alternative receptors to initiate a low level of infection in human cells. During the course of infection, CHIKV replicates using human cellular machinery and acquires GAG receptor binding, resulting in its enhanced infectivity.
In conclusion, this study demonstrates that the infectivity of CHIKV is reduced when generated in mosquito cells. We show that mosquito cell-generated CHIKV does not bind to GAG receptors and has reduced attachment to mammalian cells. Furthermore, we provide several lines of evidence to support that N-glycosylation within mosquito cells may lower infectivity of CHIKV by removing its ability to bind GAG receptors on murine and human cells. This new understanding of how mosquito and mammalian host cells alter CHIKV receptor binding and infectivity may help with the development of effective therapeutics or vaccines against CHIKV and other mosquito-transmitted viruses.
Supporting Information
(A) NIH3T3 cells were infected with CHIKVvero or CHIKVmos (Ross strain, 100 viral particles/cell) for 24 h and expression of CHIKV E1 (normalized to cellular β-actin) was measured by RT-qPCR. (B) NIH3T3 cells were infected with CHIKVvero and CHIKVmos (LR OPY1 strain, MOI = 1) for 24 h and CHIKV E1 expression was measured by RT-qPCR. (C) L929 cells were infected with UV-inactivated CHIKVmos or CHIKVvero (Ross strain) for 72 h and phase contrast images (100X) were acquired using a LSM510 META microscope (Zeiss). Expressions of Ifn-α (D) and Ifn-β (E) in the blood of wild-type C57BL/6J mice infected with CHIKVvero and CHIKVmos (Ross strain, 105 PFUs) were measured in blood by RT-qPCR at day 1, 2, 4 and 6-post infection (d.p.i). (F) C6/36 cells were inoculated with CHIKVvero or CHIKVmos (Ross strain, MOI = 1) at 4°C for 1 h and the viruses attached to cells were quantified by RT-qPCR. (H) NIH3T3 and HFF cells were pre-incubated with the indicated concentration of yeast mannan for 1 h at room temperature. CHIKVvero or CHIKVmos (Ross strain, MOI = 1) were then added to the cells and further incubated at 4°C for 1 h to allow attachment of viruses. The blocking of viral attachment by yeast mannan was measured by RT-qPCR. Data were normalized to the control cells without yeast mannan treatment. (I) One hundred PFUs of CHIKVvero or CHIKVmos (Ross strain) were pre-incubated with heparin at 37°C for 1 h and then added to Vero cells at 4°C for 1 h for virus attachment. The neutralization of plaque development in Vero cells by heparin was measured by plaque assay.
(TIF)
E2 glycoprotein sequences of different CHIKV stocks used in this study were shown. Ross strain (black color) and LR OPY1 strain (blue color).
(TIF)
Acknowledgments
We thank Dr. Robert B. Tesh (University of Texas Medical Branch) for providing CHIKV strain LR OPY1 2006, Ms. Baobin Kang for assistance with phase contrast imaging, and Dr. Kenneth Curry and Dr. Douglas Masterson for their technical support. We also thank Mississippi-IDeA Network of Biomedical Research Excellence (MS-INBRE) for the use of a research facility.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by a new faculty startup fund (DE01363 to FB) from The University of Southern Mississippi (www.usm.edu). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lee VJ, Chow A, Zheng X, Carrasco LR, Cook AR, et al. (2012) Simple clinical and laboratory predictors of Chikungunya versus dengue infections in adults. PLoS Negl Trop Dis 6: e1786 10.1371/journal.pntd.0001786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schilte C, Staikowsky F, Couderc T, Madec Y, Carpentier F, et al. (2013) Chikungunya virus-associated long-term arthralgia: a 36-month prospective longitudinal study. PLoS Negl Trop Dis 7: e2137 10.1371/journal.pntd.0002137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gauri LA, Ranwa BL, Nagar K, Vyas A, Fatima Q (2012) Post chikungunya brain stem encephalitis. J Assoc Physicians India 60: 68–70. [PubMed] [Google Scholar]
- 4. Tandale BV, Sathe PS, Arankalle VA, Wadia RS, Kulkarni R, et al. (2009) Systemic involvements and fatalities during Chikungunya epidemic in India, 2006. J Clin Virol 46: 145–149. 10.1016/j.jcv.2009.06.027 [DOI] [PubMed] [Google Scholar]
- 5. Pellot AS, Alessandri JL, Robin S, Samperiz S, Attali T, et al. (2012) [Severe forms of chikungunya virus infection in a pediatric intensive care unit on Reunion Island]. Med Trop (Mars) 72 Spec No: 88–93. [PubMed] [Google Scholar]
- 6. Thiberville SD, Moyen N, Dupuis-Maguiraga L, Nougairede A, Gould EA, et al. (2013) Chikungunya fever: epidemiology, clinical syndrome, pathogenesis and therapy. Antiviral Res 99: 345–370. 10.1016/j.antiviral.2013.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gerardin P, Guernier V, Perrau J, Fianu A, Le Roux K, et al. (2008) Estimating Chikungunya prevalence in La Reunion Island outbreak by serosurveys: two methods for two critical times of the epidemic. BMC Infect Dis 8: 99 10.1186/1471-2334-8-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mavalankar D, Shastri P, Bandyopadhyay T, Parmar J, Ramani KV (2008) Increased mortality rate associated with chikungunya epidemic, Ahmedabad, India. Emerg Infect Dis 14: 412–415. 10.3201/eid1403.070720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Suhrbier A, Jaffar-Bandjee MC, Gasque P (2012) Arthritogenic alphaviruses—an overview. Nat Rev Rheumatol 8: 420–429. 10.1038/nrrheum.2012.64 [DOI] [PubMed] [Google Scholar]
- 10. Benedict MQ, Levine RS, Hawley WA, Lounibos LP (2007) Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vector Borne Zoonotic Dis 7: 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ruiz-Moreno D, Vargas IS, Olson KE, Harrington LC (2012) Modeling dynamic introduction of Chikungunya virus in the United States. PLoS Negl Trop Dis 6: e1918 10.1371/journal.pntd.0001918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leparc-Goffart I, Nougairede A, Cassadou S, Prat C, de Lamballerie X (2014) Chikungunya in the Americas. Lancet 383: 514 10.1016/S0140-6736(14)60185-9 [DOI] [PubMed] [Google Scholar]
- 13. Abdelnabi R, Neyts J, Delang L (2015) Towards antivirals against chikungunya virus. Antiviral Res 121: 59–68. 10.1016/j.antiviral.2015.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lum FM, Ng LF (2015) Cellular and molecular mechanisms of chikungunya pathogenesis. Antiviral Res 120: 165–174. 10.1016/j.antiviral.2015.06.009 [DOI] [PubMed] [Google Scholar]
- 15. Weaver SC, Osorio JE, Livengood JA, Chen R, Stinchcomb DT (2012) Chikungunya virus and prospects for a vaccine. Expert Rev Vaccines 11: 1087–1101. 10.1586/erv.12.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Strauss JH, Strauss EG (1994) The alphaviruses: gene expression, replication, and evolution. Microbiol Rev 58: 491–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weaver SC, Brault AC, Kang W, Holland JJ (1999) Genetic and fitness changes accompanying adaptation of an arbovirus to vertebrate and invertebrate cells. J Virol 73: 4316–4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S (2007) A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog 3: e201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garmashova N, Gorchakov R, Volkova E, Paessler S, Frolova E, et al. (2007) The Old World and New World alphaviruses use different virus-specific proteins for induction of transcriptional shutoff. J Virol 81: 2472–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gorchakov R, Frolova E, Frolov I (2005) Inhibition of transcription and translation in Sindbis virus-infected cells. J Virol 79: 9397–9409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li YG, Siripanyaphinyo U, Tumkosit U, Noranate N, An A, et al. (2013) Chikungunya virus induces a more moderate cytopathic effect in mosquito cells than in mammalian cells. Intervirology 56: 6–12. 10.1159/000339985 [DOI] [PubMed] [Google Scholar]
- 22. Mims CA, Day MF, Marshall ID (1966) Cytopathic effect of Semliki Forest virus in the mosquito Aedes aegypti. Am J Trop Med Hyg 15: 775–784. [DOI] [PubMed] [Google Scholar]
- 23. Raghow RS, Davey MW, Dalgarno L (1973) The growth of Semliki Forest virus in cultured mosquito cells: ultrastructural observations. Arch Gesamte Virusforsch 43: 165–168. [DOI] [PubMed] [Google Scholar]
- 24. Raghow RS, Grace TD, Filshie BK, Bartley W, Dalgarno L (1973) Ross River virus replication in cultured mosquito and mammalian cells: virus growth and correlated ultrastructural changes. J Gen Virol 21: 109–122. [DOI] [PubMed] [Google Scholar]
- 25. Heidner HW, Knott TA, Johnston RE (1996) Differential processing of sindbis virus glycoprotein PE2 in cultured vertebrate and arthropod cells. J Virol 70: 2069–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leavitt R, Schlesinger S, Kornfeld S (1977) Tunicamycin inhibits glycosylation and multiplication of Sindbis and vesicular stomatitis viruses. J Virol 21: 375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mondotte JA, Lozach PY, Amara A, Gamarnik AV (2007) Essential role of dengue virus envelope protein N glycosylation at asparagine-67 during viral propagation. J Virol 81: 7136–7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Knight RL, Schultz KL, Kent RJ, Venkatesan M, Griffin DE (2009) Role of N-linked glycosylation for sindbis virus infection and replication in vertebrate and invertebrate systems. J Virol 83: 5640–5647. 10.1128/JVI.02427-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vigerust DJ, Shepherd VL (2007) Virus glycosylation: role in virulence and immune interactions. Trends Microbiol 15: 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moudy RM, Zhang B, Shi PY, Kramer LD (2009) West Nile virus envelope protein glycosylation is required for efficient viral transmission by Culex vectors. Virology 387: 222–228. 10.1016/j.virol.2009.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Klimstra WB, Nangle EM, Smith MS, Yurochko AD, Ryman KD (2003) DC-SIGN and L-SIGN can act as attachment receptors for alphaviruses and distinguish between mosquito cell- and mammalian cell-derived viruses. J Virol 77: 12022–12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Davis CW, Nguyen HY, Hanna SL, Sanchez MD, Doms RW, et al. (2006) West Nile virus discriminates between DC-SIGN and DC-SIGNR for cellular attachment and infection. J Virol 80: 1290–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Alen MM, Dallmeier K, Balzarini J, Neyts J, Schols D (2012) Crucial role of the N-glycans on the viral E-envelope glycoprotein in DC-SIGN-mediated dengue virus infection. Antiviral Res 96: 280–287. 10.1016/j.antiviral.2012.10.007 [DOI] [PubMed] [Google Scholar]
- 34. Shabman RS, Morrison TE, Moore C, White L, Suthar MS, et al. (2007) Differential induction of type I interferon responses in myeloid dendritic cells by mosquito and mammalian-cell-derived alphaviruses. J Virol 81: 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Silva MC, Guerrero-Plata A, Gilfoy FD, Garofalo RP, Mason PW (2007) Differential activation of human monocyte-derived and plasmacytoid dendritic cells by West Nile virus generated in different host cells. J Virol 81: 13640–13648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu J, Thorp SC (2002) Cell surface heparan sulfate and its roles in assisting viral infections. Med Res Rev 22: 1–25. [DOI] [PubMed] [Google Scholar]
- 37. Olofsson S, Bergstrom T (2005) Glycoconjugate glycans as viral receptors. Ann Med 37: 154–172. [DOI] [PubMed] [Google Scholar]
- 38. Gardner CL, Hritz J, Sun C, Vanlandingham DL, Song TY, et al. (2014) Deliberate attenuation of chikungunya virus by adaptation to heparan sulfate-dependent infectivity: a model for rational arboviral vaccine design. PLoS Negl Trop Dis 8: e2719 10.1371/journal.pntd.0002719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bernard KA, Klimstra WB, Johnston RE (2000) Mutations in the E2 glycoprotein of Venezuelan equine encephalitis virus confer heparan sulfate interaction, low morbidity, and rapid clearance from blood of mice. Virology 276: 93–103. [DOI] [PubMed] [Google Scholar]
- 40. Silva LA, Khomandiak S, Ashbrook AW, Weller R, Heise MT, et al. (2014) A single-amino-acid polymorphism in Chikungunya virus E2 glycoprotein influences glycosaminoglycan utilization. J Virol 88: 2385–2397. 10.1128/JVI.03116-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gardner CL, Ebel GD, Ryman KD, Klimstra WB (2011) Heparan sulfate binding by natural eastern equine encephalitis viruses promotes neurovirulence. Proc Natl Acad Sci U S A 108: 16026–16031. 10.1073/pnas.1110617108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ryman KD, Gardner CL, Burke CW, Meier KC, Thompson JM, et al. (2007) Heparan sulfate binding can contribute to the neurovirulence of neuroadapted and nonneuroadapted Sindbis viruses. J Virol 81: 3563–3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hsieh P, Rosner MR, Robbins PW (1983) Host-dependent variation of asparagine-linked oligosaccharides at individual glycosylation sites of Sindbis virus glycoproteins. J Biol Chem 258: 2548–2554. [PubMed] [Google Scholar]
- 44. Wang R, Wang J, Paul AM, Acharya D, Bai F, et al. (2013) Mouse embryonic stem cells are deficient in type I interferon expression in response to viral infections and double-stranded RNA. J Biol Chem 288: 15926–15936. 10.1074/jbc.M112.421438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bai F, Wang T, Pal U, Bao F, Gould LH, et al. (2005) Use of RNA interference to prevent lethal murine west nile virus infection. J Infect Dis 191: 1148–1154. [DOI] [PubMed] [Google Scholar]
- 46. Kozaci DL, Chernajovsky Y, Chikanza IC (2007) The differential expression of corticosteroid receptor isoforms in corticosteroid-resistant and -sensitive patients with rheumatoid arthritis. Rheumatology (Oxford) 46: 579–585. [DOI] [PubMed] [Google Scholar]
- 47. Paul AM, Shi Y, Acharya D, Douglas JR, Cooley A, et al. (2014) Delivery of antiviral small interfering RNA with gold nanoparticles inhibits dengue virus infection in vitro. J Gen Virol 95: 1712–1722. 10.1099/vir.0.066084-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gardner J, Anraku I, Le TT, Larcher T, Major L, et al. (2010) Chikungunya virus arthritis in adult wild-type mice. J Virol 84: 8021–8032. 10.1128/JVI.02603-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Teo TH, Lum FM, Claser C, Lulla V, Lulla A, et al. (2013) A pathogenic role for CD4+ T cells during Chikungunya virus infection in mice. J Immunol 190: 259–269. 10.4049/jimmunol.1202177 [DOI] [PubMed] [Google Scholar]
- 50. Teng TS, Foo SS, Simamarta D, Lum FM, Teo TH, et al. (2012) Viperin restricts chikungunya virus replication and pathology. J Clin Invest 122: 4447–4460. 10.1172/JCI63120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Marsh M, Wellsteed J, Kern H, Harms E, Helenius A (1982) Monensin inhibits Semliki Forest virus penetration into culture cells. Proc Natl Acad Sci U S A 79: 5297–5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Helenius A, Marsh M, White J (1982) Inhibition of Semliki forest virus penetration by lysosomotropic weak bases. J Gen Virol 58 Pt 1: 47–61. [DOI] [PubMed] [Google Scholar]
- 53. Schilte C, Couderc T, Chretien F, Sourisseau M, Gangneux N, et al. (2010) Type I IFN controls chikungunya virus via its action on nonhematopoietic cells. J Exp Med 207: 429–442. 10.1084/jem.20090851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sourisseau M, Schilte C, Casartelli N, Trouillet C, Guivel-Benhassine F, et al. (2007) Characterization of reemerging chikungunya virus. PLoS Pathog 3: e89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shabman RS, Rogers KM, Heise MT (2008) Ross River virus envelope glycans contribute to type I interferon production in myeloid dendritic cells. J Virol 82: 12374–12383. 10.1128/JVI.00985-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lim PY, Louie KL, Styer LM, Shi PY, Bernard KA (2010) Viral pathogenesis in mice is similar for West Nile virus derived from mosquito and mammalian cells. Virology 400: 93–103. 10.1016/j.virol.2010.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gardner CL, Burke CW, Higgs ST, Klimstra WB, Ryman KD (2012) Interferon-alpha/beta deficiency greatly exacerbates arthritogenic disease in mice infected with wild-type chikungunya virus but not with the cell culture-adapted live-attenuated 181/25 vaccine candidate. Virology 425: 103–112. 10.1016/j.virol.2011.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rue CA, Ryan P (2003) A role for glycoprotein C in pseudorabies virus entry that is independent of virus attachment to heparan sulfate and which involves the actin cytoskeleton. Virology 307: 12–21. [DOI] [PubMed] [Google Scholar]
- 59. Leung JY, Ng MM, Chu JJ (2011) Replication of alphaviruses: a review on the entry process of alphaviruses into cells. Adv Virol 2011: 249640 10.1155/2011/249640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kielian M, Chanel-Vos C, Liao M (2010) Alphavirus Entry and Membrane Fusion. Viruses 2: 796–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Marsh M, Bron R (1997) SFV infection in CHO cells: cell-type specific restrictions to productive virus entry at the cell surface. J Cell Sci 110 (Pt 1): 95–103. [DOI] [PubMed] [Google Scholar]
- 62. Bernard E, Solignat M, Gay B, Chazal N, Higgs S, et al. (2010) Endocytosis of chikungunya virus into mammalian cells: role of clathrin and early endosomal compartments. PLoS One 5: e11479 10.1371/journal.pone.0011479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gardner CL, Choi-Nurvitadhi J, Sun C, Bayer A, Hritz J, et al. (2013) Natural variation in the heparan sulfate binding domain of the eastern equine encephalitis virus E2 glycoprotein alters interactions with cell surfaces and virulence in mice. J Virol 87: 8582–8590. 10.1128/JVI.00937-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Klimstra WB, Ryman KD, Johnston RE (1998) Adaptation of Sindbis virus to BHK cells selects for use of heparan sulfate as an attachment receptor. J Virol 72: 7357–7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hsieh P, and Robbins P. W. (1984) Regulation of asparagine-linked oligosaccharide processing. Oligosaccharide processing in Aedes albopictus mosquito cells. J Biol Chem 259: 2375–2382. [PubMed] [Google Scholar]
- 66. Voss JE, Vaney MC, Duquerroy S, Vonrhein C, Girard-Blanc C, et al. (2010) Glycoprotein organization of Chikungunya virus particles revealed by X-ray crystallography. Nature 468: 709–712. 10.1038/nature09555 [DOI] [PubMed] [Google Scholar]
- 67. Mishin VP, Novikov D, Hayden FG, Gubareva LV (2005) Effect of hemagglutinin glycosylation on influenza virus susceptibility to neuraminidase inhibitors. J Virol 79: 12416–12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Butters TD, Hughes RC, Vischer P (1981) Steps in the biosynthesis of mosquito cell membrane glycoproteins and the effects of tunicamycin. Biochim Biophys Acta 640: 672–686. [DOI] [PubMed] [Google Scholar]
- 69. Strauss JH Jr., Burge B. W., and Darnell J. E.. (1970) Carbohydrate content of the membrane protein of Sindbis virus. J Mol Biol 47: 437–448. [DOI] [PubMed] [Google Scholar]
- 70. Jupille HJ, Medina-Rivera M, Hawman DW, Oko L, Morrison TE (2013) A tyrosine-to-histidine switch at position 18 of the Ross River virus E2 glycoprotein is a determinant of virus fitness in disparate hosts. J Virol 87: 5970–5984. 10.1128/JVI.03326-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Heil ML, Albee A, Strauss JH, Kuhn RJ (2001) An amino acid substitution in the coding region of the E2 glycoprotein adapts Ross River virus to utilize heparan sulfate as an attachment moiety. J Virol 75: 6303–6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Smit JM, Waarts BL, Kimata K, Klimstra WB, Bittman R, et al. (2002) Adaptation of alphaviruses to heparan sulfate: interaction of Sindbis and Semliki forest viruses with liposomes containing lipid-conjugated heparin. J Virol 76: 10128–10137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. La Linn M, Eble JA, Lubken C, Slade RW, Heino J, et al. (2005) An arthritogenic alphavirus uses the alpha1beta1 integrin collagen receptor. Virology 336: 229–239. [DOI] [PubMed] [Google Scholar]
- 74. Mitsuhashi J, Nakasone S., and Horie Y (1983) Sterol-free eukaryotic cells from continuous cell lines of insects. Cell Biol Int Rep 7: 1057–1062. [DOI] [PubMed] [Google Scholar]
- 75. Connell BJ, Lortat-Jacob H (2013) Human Immunodeficiency Virus and Heparan Sulfate: From Attachment to Entry Inhibition. Front Immunol 4: 385 10.3389/fimmu.2013.00385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Trybala E, Roth A, Johansson M, Liljeqvist JA, Rekabdar E, et al. (2002) Glycosaminoglycan-binding ability is a feature of wild-type strains of herpes simplex virus type 1. Virology 302: 413–419. [DOI] [PubMed] [Google Scholar]
- 77. Goodfellow IG, Sioofy AB, Powell RM, Evans DJ (2001) Echoviruses bind heparan sulfate at the cell surface. J Virol 75: 4918–4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Richards KF, Bienkowska-Haba M, Dasgupta J, Chen XS, Sapp M (2013) Multiple heparan sulfate binding site engagements are required for the infectious entry of human papillomavirus type 16. J Virol 87: 11426–11437. 10.1128/JVI.01721-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Glasgow GM, Sheahan BJ, Atkins GJ, Wahlberg JM, Salminen A, et al. (1991) Two mutations in the envelope glycoprotein E2 of Semliki Forest virus affecting the maturation and entry patterns of the virus alter pathogenicity for mice. Virology 185: 741–748. [DOI] [PubMed] [Google Scholar]
- 80. Wang E, Brault AC, Powers AM, Kang W, Weaver SC (2003) Glycosaminoglycan binding properties of natural venezuelan equine encephalitis virus isolates. J Virol 77: 1204–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. McKinney RW, Berge TO, Sawyer WD, Tigertt WD, Crozier D (1963) Use of an Attenuated Strain of Venezuelan Equine Encephalomyelitis Virus for Immunization in Man. Am J Trop Med Hyg 12: 597–603. [DOI] [PubMed] [Google Scholar]
- 82. Roukens AH, Visser LG (2008) Yellow fever vaccine: past, present and future. Expert Opin Biol Ther 8: 1787–1795. 10.1517/14712598.8.11.1787 [DOI] [PubMed] [Google Scholar]
- 83. Hoke CH Jr., Pace-Templeton J, Pittman P, Malinoski FJ, Gibbs P, et al. (2012) US Military contributions to the global response to pandemic chikungunya. Vaccine 30: 6713–6720. 10.1016/j.vaccine.2012.08.025 [DOI] [PubMed] [Google Scholar]
- 84. Lee E, Lobigs M (2002) Mechanism of virulence attenuation of glycosaminoglycan-binding variants of Japanese encephalitis virus and Murray Valley encephalitis virus. J Virol 76: 4901–4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lee E, Lobigs M (2008) E protein domain III determinants of yellow fever virus 17D vaccine strain enhance binding to glycosaminoglycans, impede virus spread, and attenuate virulence. J Virol 82: 6024–6033. 10.1128/JVI.02509-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gorchakov R, Wang E, Leal G, Forrester NL, Plante K, et al. (2012) Attenuation of Chikungunya virus vaccine strain 181/clone 25 is determined by two amino acid substitutions in the E2 envelope glycoprotein. J Virol 86: 6084–6096. 10.1128/JVI.06449-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Roy CJ, Adams AP, Wang E, Plante K, Gorchakov R, et al. (2014) Chikungunya Vaccine Candidate Is Highly Attenuated and Protects Nonhuman Primates Against Telemetrically Monitored Disease Following a Single Dose. J Infect Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Edelman R, Tacket CO, Wasserman SS, Bodison SA, Perry JG, et al. (2000) Phase II safety and immunogenicity study of live chikungunya virus vaccine TSI-GSD-218. Am J Trop Med Hyg 62: 681–685. [DOI] [PubMed] [Google Scholar]
- 89. Lee E, Wright PJ, Davidson A, Lobigs M (2006) Virulence attenuation of Dengue virus due to augmented glycosaminoglycan-binding affinity and restriction in extraneural dissemination. J Gen Virol 87: 2791–2801. [DOI] [PubMed] [Google Scholar]
- 90. Fontaine A, Diouf I, Bakkali N, Misse D, Pages F, et al. (2011) Implication of haematophagous arthropod salivary proteins in host-vector interactions. Parasit Vectors 4: 187 10.1186/1756-3305-4-187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Schneider BS, Higgs S (2008) The enhancement of arbovirus transmission and disease by mosquito saliva is associated with modulation of the host immune response. Trans R Soc Trop Med Hyg 102: 400–408. 10.1016/j.trstmh.2008.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Cox J, Mota J, Sukupolvi-Petty S, Diamond MS, Rico-Hesse R (2012) Mosquito bite delivery of dengue virus enhances immunogenicity and pathogenesis in humanized mice. J Virol 86: 7637–7649. 10.1128/JVI.00534-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Styer LM, Lim PY, Louie KL, Albright RG, Kramer LD, et al. (2011) Mosquito saliva causes enhancement of West Nile virus infection in mice. J Virol 85: 1517–1527. 10.1128/JVI.01112-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Osorio JE, Godsey MS, Defoliart GR, Yuill TM (1996) La Crosse viremias in white-tailed deer and chipmunks exposed by injection or mosquito bite. Am J Trop Med Hyg 54: 338–342. [DOI] [PubMed] [Google Scholar]
- 95. Thangamani S, Higgs S, Ziegler S, Vanlandingham D, Tesh R, et al. (2010) Host immune response to mosquito-transmitted chikungunya virus differs from that elicited by needle inoculated virus. PLoS One 5: e12137 10.1371/journal.pone.0012137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Conway MJ, Watson AM, Colpitts TM, Dragovic SM, Li Z, et al. (2014) Mosquito saliva serine protease enhances dissemination of dengue virus into the mammalian host. J Virol 88: 164–175. 10.1128/JVI.02235-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) NIH3T3 cells were infected with CHIKVvero or CHIKVmos (Ross strain, 100 viral particles/cell) for 24 h and expression of CHIKV E1 (normalized to cellular β-actin) was measured by RT-qPCR. (B) NIH3T3 cells were infected with CHIKVvero and CHIKVmos (LR OPY1 strain, MOI = 1) for 24 h and CHIKV E1 expression was measured by RT-qPCR. (C) L929 cells were infected with UV-inactivated CHIKVmos or CHIKVvero (Ross strain) for 72 h and phase contrast images (100X) were acquired using a LSM510 META microscope (Zeiss). Expressions of Ifn-α (D) and Ifn-β (E) in the blood of wild-type C57BL/6J mice infected with CHIKVvero and CHIKVmos (Ross strain, 105 PFUs) were measured in blood by RT-qPCR at day 1, 2, 4 and 6-post infection (d.p.i). (F) C6/36 cells were inoculated with CHIKVvero or CHIKVmos (Ross strain, MOI = 1) at 4°C for 1 h and the viruses attached to cells were quantified by RT-qPCR. (H) NIH3T3 and HFF cells were pre-incubated with the indicated concentration of yeast mannan for 1 h at room temperature. CHIKVvero or CHIKVmos (Ross strain, MOI = 1) were then added to the cells and further incubated at 4°C for 1 h to allow attachment of viruses. The blocking of viral attachment by yeast mannan was measured by RT-qPCR. Data were normalized to the control cells without yeast mannan treatment. (I) One hundred PFUs of CHIKVvero or CHIKVmos (Ross strain) were pre-incubated with heparin at 37°C for 1 h and then added to Vero cells at 4°C for 1 h for virus attachment. The neutralization of plaque development in Vero cells by heparin was measured by plaque assay.
(TIF)
E2 glycoprotein sequences of different CHIKV stocks used in this study were shown. Ross strain (black color) and LR OPY1 strain (blue color).
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.