Abstract
Lamin A/C is part of the nuclear lamina, a meshwork of intermediate filaments underlying the inner nuclear membrane. The lamin network is anchoring a complex set of structural and linker proteins and is either directly or through partner proteins also associated or interacting with a number of signaling protein and transcription factors. During mitosis the nuclear lamina is dissociated by well established phosphorylation- dependent mechanisms. A-type lamins are, however, also phosphorylated during interphase. A recent study identified 20 interphase phosphorylation sites on lamin A/C and explored their functions related to lamin dynamics; movements, localization and solubility. Here we discuss these findings in the light of lamin functions in health and disease.
Keywords: lamin A/C, lamins, intermediate filaments, laminopathy, phosphorylation, signaling
Abbreviations
- EDMD
Emery-Dreifuss muscular dystrophy
- GFP
green fluorescent protein
- IFs
intermediate filaments
- LAP2α
Lamina-associated polypeptide 2 isoform α
Lamins are Intermediate Filament Proteins
Intermediate filaments (IFs) comprise a diverse protein family including more than 70 genes, giving rise to an even higher number of proteins, as they often are alternatively spliced.1 The IFs share a common structure consisting of a structurally conserved rod domain flanked by variable head and tail regions.2 Except for the lamins all IFs are cytoplasmic proteins that are part of the cytoskeleton. In mammals the nuclear IFs are the lamin A (LMNA) gene products lamin A, lamin A Δ10, lamin C and lamin C2 as well as the lamin B1 (LMNB1) and lamin B2 (LMNB2) gene products lamin B1, lamin B2, and the germ cell specific splice variant lamin B3.3,4 In the nucleus the lamins make up the lamina, a thin, fibrous layer inside the inner nuclear membrane, but they are also found in a soluble form inside the nucleoplasm.5-7 The location and solubility of lamins affect their functions.
IFs are found in all metazoan organisms,8 with a variable degree of complexity in the expression patterns. While the more simple organisms express only lamins, the complexity of IFs increase in higher order organisms, with the exception of arthropods, which mostly seem to lack cytoplasmic IFs.9 Invertebrates have in general only one B-type lamin whereas A-type lamins only exist in vertebrates, indicating that lamin A is an evolutionary more recent protein.10 The cytoplasmic IFs found in lower organisms are more closely related to lamins than to cytoplasmic IFs in higher organisms.11 This indicates that a B-type lamin was the first IF to evolve and that this founder IF gradually gave rise to cytoplasmic IFs and later to A-type lamins.
A-and B-Type Lamins
The B-type lamins are found in virtually all cells regardless of their differentiation state. In C. elegans deletion of its single lamin leads to lethality early during development.15Surprisingly, B-type lamins are not required for murine embryogenesis, but mice lacking them die at birth.12-14 However, mouse embryonic stem cells lacking all lamins are able to proliferate and differentiate, demonstrating the tissue specificity in lamin functions.16 The exact role for each lamin, therefore, still remains to be elucidated. A-type lamins have been considered to exist only in differentiated cells, recently, however, lamin A/C has been reported to be expressed, at low levels, in embryonic stem cells and in the inner cell mass of blastocysts.17 The expression of lamin A/C is, however, tightly regulated and correlates with differentiation.3
Lamin A plays a significant role in determining the nuclear shape and stiffness, reflecting the structural properties of IFs. Furthermore, many binding partners of lamin A have been identified, underscoring the diverse functions of this protein.18 Lamin A also binds to DNA, directly and indirectly3, thereby regulating chromatin organization, influencing epigenetic regulations, as well as DNA replication and transcription.3
Phosphorylation is the most extensively studied and the most common posttranslational modification of IFs. It is the basis for the rapid modulation of IF-networks and the highly dynamic properties of this protein family. Phosphorylation of IFs has been shown to play diverse roles at all facets in the life of a cell, determining critical decisions in cellular survival, growth, migration, differentiation, stress responses, and cell death.19
Phosphorylation of Lamins During the Cell Cycle
Lamin A is subjected to numerous post translational modifications, most prominently phosphorylation, but also other modifications have been described, including farnesylation, sumoylation, and acetylation.18,19 Although lamin A is a heavily phosphorylated protein with more than 70 identified unique phosphorylation sites, very little is known about the role of phosphorylation of this protein.18 Phosphorylation of lamins occurs continuously throughout the cell cycle and is present both during interphase and mitosis. The functions of this phosphorylation have, however, mostly been studied in the context of lamin assembly/disassembly or nuclear targeting of lamins.20-22
Cyclin dependent kinase 1 (Cdk1; also known as cdc2) has been identified to directly phosphorylate both A- and B-type lamins during mitosis.23–26 Phosphorylation of N-terminal S22 and C-terminal S392 in human lamin A/C or analogous residues in B-type lamins (often referred to as “mitotic sites”) have been shown to induces the disassembly of the nuclear lamina.23-27 Phosphorylation of the mitotic N-terminal serine (S22 in human lamin A) appears to be more important for lamin head-to-tail de-polymerization, than the C-terminal site28,29 The N-terminal phosphorylation, however, is not considered sufficient enough to cause complete disassembly of the lamina in vivo and Cdk1 might be working collectively with other mitotic related kinase(s) to facilitate this depolymerisation.20 In fact, PKC has been identified as an additional kinase required for lamin B1 disassembly through lipid signaling.30 Also Cdk5 has been shown to phosphorylate the mitotic sites in the context of neuronal apoptosis,31 and S404 has been shown to bean AKT-target.32 Given the numerous identified phosphorylation sites and the variety of contexts during which phosphorylation have been described to take place it is likely that additional kinases still remain to be discovered.
Few of the many identified lamin A/C phosphosites have been studied in detail, as a significant part of the data originates from high-throughput mass-spectrometrical analyses. These analyses have significant limitations, as the sites are typically not validated and there is no information on the relative stoichiometry of these sites. Especially the role of phosphorylation of lamin A during interphase has not been thoroughly investigated. We recently conducted a study in which we identify 20 phosphorylation sites from interphase cells and in detail investigate the structural function of a few of them using single site mutations and advanced imaging techniques.33
Phosphorylation of Lamin A/C During Interphase
In interphase cells we found that the phosphorylation of lamin A/C is concentrated to 3 distinct regions, the head, the beginning of the tail, and the end of the tail.33 (Fig. 1) As some of these sites have been previously identified as mitotic sites23-26, cell synchronization by mitotic shake-off, and a phosphospecific antibody against S22 were employed to validate that this site is phosphorylated also during interphase. Using Western blotting and immunofluorescence, we could quantitatively demonstrate that S22 is indeed phosphorylated in interphase but, as expected, at a much lower rate than during mitosis.
Figure 1.
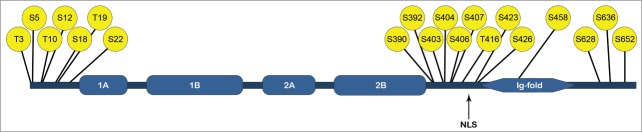
Interphase phosphorylation of lamin A/C. The phosphorylation sites identified from interphase HeLa-cells by Kochin et al.29 The phosphorylation is clustered to the head, the beginning of the tail and the end of the tail. 1A, 1B, 2A and 2B represents the coils of the lamin rod-domain, NLS is the nuclear localization signal.
To study the function of the interphase phosphorylation sites in detail, we utilized GFP-tagged lamin A vectors with mutations of the most occupied phosphorylation sites identified. Substitution of serine for aspartic acid was used to mimic phosphorylation and substitution of serine to alanine as to mimic a phosphorylation-deficient state.
By live cell imaging, we showed that the phosphomimetic mutations of S22, S392 and the double site S404, S407 lead to an increase in nucleoplasmic lamin A and, conversely, phosphodeficent mutations of S390 and S392 lead to increased lamina associated lamin A. The effects of different combinations of phosphomimetic and phosphodeficient mutations of both S22 and S392 showed that phosphorylation of S22 is dominant over S392 and that the double phosphorylation has an additive effect on the localization.
Apart from regulating the localization of lamin A (lamina vs. nucleoplasma), the phosphorylation of S22 and S392 also influences the intranuclear mobility of lamin A, both in the nucleoplasm and in the lamina, as shown by FRAP and FCS. The phosphorylation facilitates faster movements, the functions of which may be associated with the roles of lamin A in the control of signaling and transcription (see the section below). The cellular in vivo observations on increased mobility and dynamics, were reflected in the biochemical properties of the protein, as it was obvious that phosphorylation also affected the solubility of lamin A. Also in this case the phosphomimetic S22 and S392 mutants were more soluble than the wild type lamin A both in the lamina and in the nucleoplasm and S22 was be dominant over S392.
Interestingly, the combined phosphomimetic substitution of S22, S392 and S628 lead to lamin A being found in the cytoplasm in a portion of the cells. S628 is missing from progerin, the splice variant of lamin A, which is expressed in the devastating early aging laminopathy Hutchinson–Gilford progeria syndrome.34 This finding implies that this phosphorylation site has a physiological role in the compartmentalization of lamin A. Conversely, the absence of the site in progerin could be one of the mechanisms underlying the progeria phenotype. Lamin B2 has been reported to have an important role in the cytoplasm where it interacts with mitochondria promoting their function.35 Although a cytoplasmic role for lamin A still remains to be described, the fact that phosphorylation could induce a cytoplasmic shift of lamins indicates the possibility of such cytoplasmic function(s).
Physiological Relevance of Phosphorylation of Lamin A/C
Recently, lamins have emerged as potential mechanosensors that receive information and respond to changes in the structural and mechanical properties of cellular substrates and surroundings36,37 A recent study36 showed that cells grown on softer substrates have softer nuclei and, conversely, that cells grown on stiffer substrates have stiffer nuclei. The changes in nuclear stiffness were related to the levels of lamin A. Interestingly, also phosphorylation of lamin A was affected by substrate stiffness. Apart from lamin A levels, the phosphorylation by itself could be a key factor in determining nuclear stiffness. In light of the study described in the preceeding section, the phosphorylation induced by changes in substrate stiffness will also be consequential for the sequestration, dynamics, mobility, and transport of lamin A. These aspects may, in turn, be consequential for the signaling and transcriptional properties of lamin A. Lamin A interacts with and regulates chromatin organization38 and lamins also directly and indirectly influence transcription by regulating transcription factors.39-42 (Fig. 2).
Figure 2.
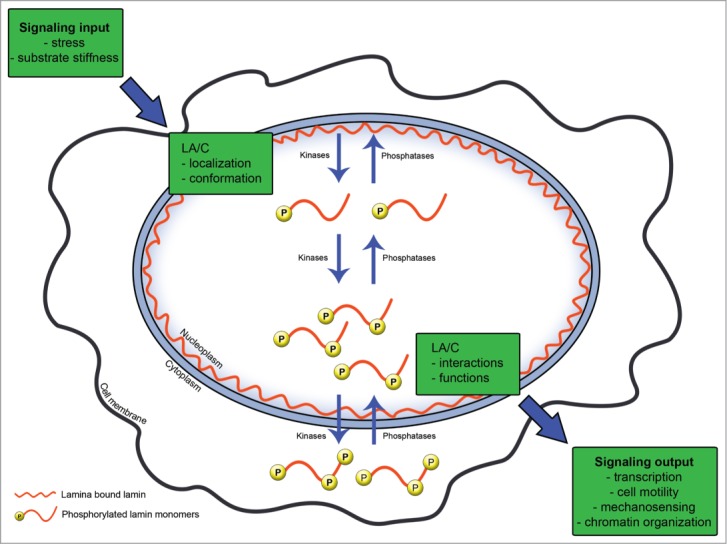
Lamin phosphorylation as a regulator of cell signaling. The information that a cell receives from its environment or from within itself is forwarded to the nucleus where it affects the localization and structure of lamin A/C. The physical distribution of lamin A/C can both be affected by phosphorylation and in itself affect the phosphorylation of lamin A/C. The localization, structure, and phosphorylation in turn regulate the functions of lamin A/C and will influence signaling output such as cell movement and mechanosensing.
One physiological relevant process that depends on nuclear stiffness is cancer metastasis. During migration of tumor cells in solid tissues, the nucleus is subjected to severe mechanical stress. Cells with high levels of lamin A have stiff nuclei that cannot be easily deformed, which will hamper the migration. In contrast, cells with low levels of lamin A have soft nuclei and can easily squeeze through tissue during migration. The soft nuclei, however, are prone to fragmentation leading to cell death.43 The right amount of lamin A is thus a requirement to ensure both successful 3D-migration and survival. Although nothing is known about lamin A phosphorylation during migration, it is tempting to speculate that the increased mobility of phosphorylated lamin A could be one way promote nuclear deformability and thereby facilitate migration. As lamin A has been reported to be both up- and down- regulated in different types of cancers44, growth and cell cycle promoting kinase activity could be a way for the cell to influence nuclear stiffness and promote migration.
Although the nucleoplasmic pool of lamin A has been studied for more than 2 decades, the functions of this pool and what regulates it is still barely known. Regarding its potential signaling functions, eluded to above, lamin A interacts with lamina-associated polypeptide 2 isoform α (LAP2α) in the nucleoplasm and this interaction regulates the activity of retinoblastoma protein (pRb) and cell cycle progression.45 In the absence of LAP2α, nucleoplasmic lamin A is lost,46 indicating that LAP2α stabilizes or sequesters the nucleoplasmic lamin A. Our results, showing that phosphorylation is important for the shuttling of lamin A between lamina and nucleoplasm, offer a new paradigm to understand the mechanisms that drive the lamin A LAP2α interactions. Interestingly, the lamin region interacting with LAP2α is the beginning of the tail and the Ig-fold,47 a region where we identified numerous phosphorylation sites that could potentially affect the binding between lamin A and LAP2α. Thus, phosphorylation could influence both the localization, the opportunity to bind, and the direct binding of LAP2α and lamin A.
Lessons from Laminopathies
To this day almost 20 different diseases or syndromes caused by over 450 different mutations in the LMNA gene have been described.48 These diseases, collectively referred to as laminopathies, are characterized by their diverse but very specific phenotypes, ranging from lipodystrophy, affecting the fat deposition in certain parts of the body, to muscular dystrophies with or without cardiac involvement, to cardiomyophaties affecting either the left or the right ventricle. How mutations in the very same gene of a protein that is expressed in almost all differentiated cell types can give rise to organ and tissue-specific diseases remains a mystery.
The possible connection between phosphorylation and laminopathies has not been studied. However, there are a few cases which highlight the potential role of this post-translational modification in diseases. It has been reported that the total phosphorylation of lamin A is lower in muscle biopsies from Emery-Dreifuss muscular dystrophy (EDMD) patients compared to healthy controls but not which sites are affected or what impact this has on the disease.49 In contrast, S458 has been reported to be specifically phosphorylated in muscle biopsies from myopathy patients with mutations in the Ig-fold of lamin A but not from patients with other lamin A mutations or healthy controls.50 The authors speculate that mutations in the Ig-fold opens the lamin structure and exposes S458 to Akt1 which was shown to target this site.50 Our identification of phosphorylation of the same site from interphase HeLa cells suggests that the phosphorylation also have a non-pathological role and that this modification is regulated in a cell specific manner.
Lamin A R453W, the mutation that gives rise to the above-mentioned S458 phosphorylation, is one of many muscular dystrophy-associated mutations. Interestingly, this specific mutant lamin has also been shown to have significantly lower S390 phosphorylation compared to wild type lamin A.36 This shows that even distant mutations can affect phosphorylation status of the whole protein, but we cannot explain why. The decreased phosphorylation can be due to the mutation changing the assembly properties and/or the mechanical or structural properties of lamin A. The decreased phosphorylation can also change the mechanical or structural properties of lamin A, which could be a way for the cell to compensate for the mutation. The phosphorylation states of different phosphorylation sites can also possibly affect the phosphorylation of others. One site could inhibit or promote the modification of another site, for example, by affecting the assembly properties and/or the conformation of the protein. Whether the change in lamin A phosphorylation in myopathies is a result of the mutations or a compensatory mechanism still remains to be clarified.
Laminopathy mutations could also directly affect the phosphorylation of individual sites. For example, recently, a mutation of P4R (proline at 4 to arginine) of lamin was reported.51,52 This mutation gives rise to a mild progeroid phenotype, including tight, hyperpigmented skin and lipodystrophy. P4 is located between T3 and S5, both sites phosphorylated during interphase33 This mutation disrupts the TP- motif, thereby most likely leading to a deregulation of T4-phosphorylation. The charged side chain of proline is also likely to influence the phosphorylation of S5.
Interestingly, a case of a heterozygous S22L mutation has been reported.53 This patient suffered from dilated cardiomyopathy with premature ventricular beats requiring a heart transplant. However, no molecular data from the case is available. Bearing in mind that the lamin A phosphorylation sites are highly conserved, reflecting low tolerance for mutations, it is not surprising that so few of the large number of reported lamin A mutations directly target known phosphorylation sites.
Conclusions
We propose that the interphase-specific phosphorylation of lamin A provides additional means to regulate the protein function in a cell and tissue specific manner. The identification and validation of interphase-specific in vivo phosphorylation sites on lamin A/C and characterizing the role of these in distribution and dynamics of lamin A is just the beginning of understanding what could be the possible functions of these sites. Given the many diverse functions of lamin A/C, also phosphorylation sites which are seemingly not giving rise to major phenotypes in terms of distribution or dynamics can still have important roles with major impact on the cell during stress or other specific conditions. Understanding how phosphorylation regulates lamin on a molecular level opens up for a deeper understanding on how laminopathies arise and why they are so specific.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We want to acknowledge the other authors of the original study: Takeshi Shimi, Stephen Adam, Anne Goldman, Chan-Gi Pack, Johanna Melo-Cardenas, Susumu Imanishi, and Robert Goldman. We also want to thank Num Wistbacka for the assistance in figure preparation.
Reference
- 1.Hyder CL, Isoniemi KO, Torvaldson ES, Eriksson JE. Insights into intermediate filament regulation from development to ageing. J Cell Sci 2011; 124:1363-72; PMID:21502133; http://dx.doi.org/ 10.1242/jcs.041244 [DOI] [PubMed] [Google Scholar]
- 2.Eriksson JE, Dechat T, Grin B, Helfand B, Mendez M, Pallari H-M, Goldman RD. Introducing intermediate filaments: from discovery to disease. J Clin Invest 2009; 119:1763-71; PMID:19587451; http://dx.doi.org/ 10.1172/JCI38339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dechat T, Pfleghaar K, Sengupta K, Shimi T, Shumaker DK, Solimando L, Goldman RD. Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin. Genes Dev 2008; 22:832-53; PMID:18381888; http://dx.doi.org/ 10.1101/gad.1652708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furukawa K, Hotta Y. cDNA cloning of a germ cell specific lamin B3 from mouse spermatocytes and analysis of its function by ectopic expression in somatic cells. EMBO J 1993; 12:97-106; PMID:8094052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gesson K, Vidak S, Foisner R. Lamina-associated polypeptide (LAP)2? and nucleoplasmic lamins in adult stem cell regulation and disease. Semin Cell Dev Biol 2014; 29:116-24; PMID:24374133; http://dx.doi.org/ 10.1016/j.semcdb.2013.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimi T, Pfleghaar K, Kojima S, Pack C-G, Solovei I, Goldman AE, Adam SA, Shumaker DK, Kinjo M, Cremer T, et al. The A- and B-type nuclear lamin networks: microdomains involved in chromatin organization and transcription. Genes Dev 2008; 22:3409-21; PMID:19141474; http://dx.doi.org/ 10.1101/gad.1735208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dechat T, Gesson K, Foisner R. Lamina-Independent lamins in the nuclear interior serve important functions. Cold Spring Harb Symp Quant Biol 2010; 75:533-43; PMID:21209392; http://dx.doi.org/ 10.1101/sqb.2010.75.018 [DOI] [PubMed] [Google Scholar]
- 8.Erber A, Riemer D, Bovenschulte M, Weber K. Molecular phylogeny of metazoan intermediate filament proteins. J Mol Evol 1998; 47:751-62; PMID:9847417; http://dx.doi.org/ 10.1007/PL00006434 [DOI] [PubMed] [Google Scholar]
- 9.Herrmann H, Strelkov SV. History and phylogeny of intermediate filaments: now in insects. BMC Biol 2011; 9:16; PMID:21356127; http://dx.doi.org/ 10.1186/1741-7007-9-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peter A, Stick R. Evolution of the lamin protein family: what introns can tell. Nucl Austin Tex 2012; 3:44-59; PMID:22156746 [DOI] [PubMed] [Google Scholar]
- 11.Weber K, Plessmann U, Ulrich W. Cytoplasmic intermediate filament proteins of invertebrates are closer to nuclear lamins than are vertebrate intermediate filament proteins; sequence characterization of two muscle proteins of a nematode. EMBO J 1989; 8:3221-7; PMID:2583097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coffinier C, Chang SY, Nobumori C, Tu Y, Farber EA, Toth JI, Fong LG, Young SG. Abnormal development of the cerebral cortex and cerebellum in the setting of lamin B2 deficiency. Proc Natl Acad Sci U S A 2010; 107:5076-81; PMID:20145110; http://dx.doi.org/ 10.1073/pnas.0908790107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JM, Tu Y, Tatar A, Wu D, Nobumori C, Jung H-J, Yoshinaga Y, Coffinier C, de Jong PJ, Fong LG, et al. Reciprocal knock-in mice to investigate the functional redundancy of lamin B1 and lamin B2. Mol Biol Cell 2014; 25:1666-75; PMID:24672053; http://dx.doi.org/ 10.1091/mbc.E14-01-0683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim Y, Sharov AA, McDole K, Cheng M, Hao H, Fan C-M, Gaiano N, Ko MSH, Zheng Y. Mouse B-type lamins are required for proper organogenesis but not by embryonic stem cells. Science 2011; 334:1706-10; PMID:22116031; http://dx.doi.org/ 10.1126/science.1211222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Ben-Shahar TR, Riemer D, Treinin M, Spann P, Weber K, Fire A, Gruenbaum Y. Essential roles for caenorhabditis elegans lamin gene in nuclear organization, cell cycle progression, and spatial organization of nuclear pore complexes. Mol Biol Cell 2000; 11:3937-47; PMID:11071918; http://dx.doi.org/ 10.1091/mbc.11.11.3937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim Y, Zheng X, Zheng Y. Proliferation and differentiation of mouse embryonic stem cells lacking all lamins. Cell Res 2013; 23:1420-3; PMID:23979018; http://dx.doi.org/ 10.1038/cr.2013.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckersley-Maslin MA, Bergmann JH, Lazar Z, Spector DL. Lamin A/C is expressed in pluripotent mouse embryonic stem cells. Nucl Austin Tex 2013; 4:53-60; PMID:23324457; http://dx.doi.org/ 10.4161/nucl.23384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simon DN, Wilson KL. Partners and post-translational modifications of nuclear lamins. Chromosoma 2013; 122:13-31; PMID:23475188; http://dx.doi.org/ 10.1007/s00412-013-0399-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snider NT, Omary MB. Post-translational modifications of intermediate filament proteins: mechanisms and functions. Nat Rev Mol Cell Biol 2014; 15:163-77; PMID:24556839; http://dx.doi.org/ 10.1038/nrm3753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lüscher B, Brizuela L, Beach D, Eisenman RN. A role for the p34cdc2 kinase and phosphatases in the regulation of phosphorylation and disassembly of lamin B2 during the cell cycle. EMBO J 1991; 10:865-75; PMID:1849074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ottaviano Y, Gerace L. Phosphorylation of the nuclear lamins during interphase and mitosis. J Biol Chem 1985; 260:624-32; PMID:3965465 [PubMed] [Google Scholar]
- 22.Rzepecki R, Fisher PA. In vivo phosphorylation of drosophila melanogaster nuclear lamins during both interphase and mitosis. Cell Mol Biol Lett 2002; 7:859-76; PMID:12378269 [PubMed] [Google Scholar]
- 23.Peter M, Nakagawa J, Dorée M, Labbé JC, Nigg EA. In vitro disassembly of the nuclear lamina and M phase-specific phosphorylation of lamins by cdc2 kinase. Cell 1990; 61:591-602; PMID:2188731; http://dx.doi.org/ 10.1016/0092-8674(90)90471-P [DOI] [PubMed] [Google Scholar]
- 24.Heald R, McKeon F. Mutations of phosphorylation sites in lamin A that prevent nuclear lamina disassembly in mitosis. Cell 1990; 61:579-89; PMID:2344612; http://dx.doi.org/ 10.1016/0092-8674(90)90470-Y [DOI] [PubMed] [Google Scholar]
- 25.Dessev GN, Iovcheva-Dessev C, Goldman RD. Lamin dimers. presence in the nuclear lamina of surf clam oocytes and release during nuclear envelope breakdown. J Biol Chem 1990; 265:12636-41; PMID:2373705 [PubMed] [Google Scholar]
- 26.Dessev G, Iovcheva-Dessev C, Bischoff JR, Beach D, Goldman R. A complex containing p34cdc2 and cyclin B phosphorylates the nuclear lamin and disassembles nuclei of clam oocytes in vitro. J Cell Biol 1991; 112:523-33; PMID:1825210; http://dx.doi.org/ 10.1083/jcb.112.4.523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Enoch T, Peter M, Nurse P, Nigg EA. p34cdc2 acts as a lamin kinase in fission yeast. J Cell Biol 1991; 112:797-807; PMID:1999458; http://dx.doi.org/ 10.1083/jcb.112.5.797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goss VL, Hocevar BA, Thompson LJ, Stratton CA, Burns DJ, Fields AP. Identification of nuclear β II protein kinase C as a mitotic lamin kinase. J Biol Chem 1994; 269:19074-80; PMID:8034666 [PubMed] [Google Scholar]
- 29.Peter M, Heitlinger E, Häner M, Aebi U, Nigg EA. Disassembly of in vitro formed lamin head-to-tail polymers by CDC2 kinase. EMBO J 1991; 10:1535-44; PMID:1851086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mall M, Walter T, Gorjánácz M, Davidson IF, Nga Ly-Hartig TB, Ellenberg J, Mattaj IW. Mitotic lamin disassembly is triggered by lipid-mediated signaling. J Cell Biol 2012; 198:981-90; PMID:22986494; http://dx.doi.org/ 10.1083/jcb.201205103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang K-H, Multani PS, Sun K-H, Vincent F, de Pablo Y, Ghosh S, Gupta R, Lee H-P, Lee H, Smith MA, et al. Nuclear envelope dispersion triggered by deregulated Cdk5 precedes neuronal death. Mol Biol Cell 2011; 22:1452-62; PMID:21389115; http://dx.doi.org/ 10.1091/mbc.E10-07-0654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bertacchini J, Beretti F, Cenni V, Guida M, Gibellini F, Mediani L, Marin O, Maraldi NM, de Pol A, Lattanzi G, et al. The protein kinase Akt/PKB regulates both prelamin a degradation and lmna gene expression. FASEB J Off Publ Fed Am Soc Exp Biol 2013; 27:2145-55; PMID:23430973; http://dx.doi.org/ 10.1096/fj.12-218214 [DOI] [PubMed] [Google Scholar]
- 33.Kochin V, Shimi T, Torvaldson E, Adam SA, Goldman A, Pack C-G, Melo-Cardenas J, Imanishi SY, Goldman RD, Eriksson JE. Interphase phosphorylation of lamin A. J Cell Sci 2014; 127:2683-96; PMID:24741066; http://dx.doi.org/ 10.1242/jcs.141820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, Scott L, Erdos MR, Robbins CM, Moses TY, Berglund P, et al. Recurrent de novo point mutations in lamin a cause hutchinson-gilford progeria syndrome. Nature 2003; 423:293-8; PMID:12714972; http://dx.doi.org/ 10.1038/nature01629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoon BC, Jung H, Dwivedy A, O'Hare CM, Zivraj KH, Holt CE. Local translation of extranuclear lamin B promotes axon maintenance. Cell 2012; 148:752-64; PMID:22341447; http://dx.doi.org/ 10.1016/j.cell.2011.11.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PCDP, Pinter J, Pajerowski JD, Spinler KR, Shin J-W, Tewari M, et al. Nuclear lamin-a scales with tissue stiffness and enhances matrix-directed differentiation. Science 2013; 341:1240104; PMID:23990565; http://dx.doi.org/ 10.1126/science.1240104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Isermann P, Lammerding J. Nuclear mechanics and mechanotransduction in health and disease. Curr Biol 2013; 23:R1113-21; PMID:24355792; http://dx.doi.org/ 10.1016/j.cub.2013.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Camozzi D, Capanni C, Cenni V, Mattioli E, Columbaro M, Squarzoni S, Lattanzi G. Diverse lamin-dependent mechanisms interact to control chromatin dynamics: focus on laminopathies. Nucl Austin Tex 2014; 5:427–40; PMID:25482195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ho CY, Jaalouk DE, Vartiainen MK, Lammerding J. Lamin A/C and emerin regulate MKL1-SRF activity by modulating actin dynamics. Nature 2013; 497:507-11; PMID:23644458; http://dx.doi.org/ 10.1038/nature12105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dreuillet C, Tillit J, Kress M, Ernoult-Lange M. In vivo and in vitro interaction between human transcription factor MOK2 and nuclear lamin A/C. Nucleic Acids Res 2002; 30:4634-42; PMID:12409453; http://dx.doi.org/ 10.1093/nar/gkf587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lloyd DJ, Trembath RC, Shackleton S. A novel interaction between lamin A and SREBP1: implications for partial lipodystrophy and other laminopathies. Hum Mol Genet 2002; 11:769-77; PMID:11929849; http://dx.doi.org/ 10.1093/hmg/11.7.769 [DOI] [PubMed] [Google Scholar]
- 42.Markiewicz E, Dechat T, Foisner R, Quinlan RA, Hutchison CJ. Lamin A/C binding protein LAP2α Is required for nuclear anchorage of retinoblastoma protein. Mol Biol Cell 2002; 13:4401-13; PMID:12475961; http://dx.doi.org/ 10.1091/mbc.E02-07-0450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harada T, Swift J, Irianto J, Shin J-W, Spinler KR, Athirasala A, Diegmiller R, Dingal PCDP, Ivanovska IL, Discher DE. Nuclear lamin stiffness is a barrier to 3D migration, but softness can limit survival. J Cell Biol 2014; 204:669-82; PMID:24567359; http://dx.doi.org/ 10.1083/jcb.201308029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Foster CR, Przyborski SA, Wilson RG, Hutchison CJ. Lamins as cancer biomarkers. Biochem Soc Trans 2010; 38:297; PMID:20074078; http://dx.doi.org/ 10.1042/BST0380297 [DOI] [PubMed] [Google Scholar]
- 45.Naetar N, Foisner R. Lamin complexes in the nuclear interior control progenitor cell proliferation and tissue homeostasis. Cell Cycle Georget Tex 2009; 8:1488-93; PMID:19377295; http://dx.doi.org/ 10.4161/cc.8.10.8499 [DOI] [PubMed] [Google Scholar]
- 46.Naetar N, Korbei B, Kozlov S, Kerenyi MA, Dorner D, Kral R, Gotic I, Fuchs P, Cohen TV, Bittner R, et al. Loss of nucleoplasmic LAP2α-lamin a complexes causes erythroid and epidermal progenitor hyperproliferation. Nat Cell Biol 2008; 10:1341-8; PMID:18849980; http://dx.doi.org/ 10.1038/ncb1793 [DOI] [PubMed] [Google Scholar]
- 47.Dechat T, Korbei B, Vaughan OA, Vlcek S, Hutchison CJ, Foisner R. Lamina-associated polypeptide 2alpha binds intranuclear a-type lamins. J Cell Sci 2000; 113 19:3473-84; PMID:10984438 [DOI] [PubMed] [Google Scholar]
- 48.UMD-LMNA at www.umd.be; [Internet].; [cited 2014 Oct 9]; Available from: http://www.umd.be/LMNA/
- 49.Cenni V, Sabatelli P, Mattioli E, Marmiroli S, Capanni C, Ognibene A, Squarzoni S, Maraldi N, Bonne G, Columbaro M, et al. Lamin A N-terminal phosphorylation is associated with myoblast activation: impairment in emery-dreifuss muscular dystrophy. J Med Genet 2005; 42:214-20; PMID:15744034; http://dx.doi.org/ 10.1136/jmg.2004.026112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mitsuhashi H, Hayashi YK, Matsuda C, Noguchi S, Wakatsuki S, Araki T, Nishino I. Specific phosphorylation of Ser458 of A-type lamins in LMNA-associated myopathy patients. J Cell Sci 2010; 123:3893-900; PMID:20980393; http://dx.doi.org/ 10.1242/jcs.072157 [DOI] [PubMed] [Google Scholar]
- 51.Guo H, Luo N, Hao F, Bai Y. p.Pro4Arg mutation in LMNA gene: a new atypical progeria phenotype without metabolism abnormalities. Gene 2014; 546:35-9; PMID:24861648; http://dx.doi.org/ 10.1016/j.gene.2014.05.042 [DOI] [PubMed] [Google Scholar]
- 52.Garg A, Subramanyam L, Agarwal AK, Simha V, Levine B, D'Apice MR, Novelli G, Crow Y. Atypical progeroid syndrome due to heterozygous missense LMNA mutations. J Clin Endocrinol Metab 2009; 94:4971-83; PMID:19875478; http://dx.doi.org/ 10.1210/jc.2009-0472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pethig K, Genschel Peters J. Wilhelmi T. Flemming M. Lochs P. Haverich H. Schmidt A. HH-J. LMNA Mutations in cardiac transplant recipients. Cardiol 2005; 103:57-62; PMID:15539782; http://dx.doi.org/ 10.1159/000082048 [DOI] [PubMed] [Google Scholar]


