Abstract
The size and composition of the circulating bile acid (BA) pool are important factors in regulating the human gut microbiota. Disrupted regulation of BA metabolism is implicated in several chronic diseases. Bile salt hydrolase (BSH)-active Lactobacillus reuteri NCIMB 30242, previously shown to decrease LDL-cholesterol and increase circulating BA, was investigated for its dose response effect on BA profile in a pilot clinical study. Ten otherwise healthy hypercholesterolemic adults, recruited from a clinical trial site in London, ON, were randomized to consume delayed release or standard release capsules containing L. reuteri NCIMB 30242 in escalating dose over 4 weeks. In another aspect, 4 healthy normocholesterolemic subjects with LDL-C below 3.4 mmol/l received delayed release L. reuteri NCIMB 30242 at a constant dose over 4 weeks. The primary outcome measure was the change in plasma BA profile over the intervention period. Additional outcomes included circulating fibroblast growth factor (FGF)-19, plant sterols and LDL-cholesterol as well as fecal microbiota and bsh gene presence. After one week of intervention subjects receiving delayed release L. reuteri NCIMB 30242 increased total BA by 1.13 ± 0.67 μmol/l (P = 0.02), conjugated BA by 0.67 ± 0.39 μmol/l (P = 0.02) and unconjugated BA by 0.46 ± 0.43 μmol/l (P = 0.07), which represented a greater than 2-fold change relative to baseline. Increases in BA were largely maintained post-week 1 and were generally correlated with FGF-19 and inversely correlated with plant sterols. This is the first clinical support showing that a BSH-active probiotic can significantly and rapidly influence BA metabolism and may prove useful in chronic diseases beyond hypercholesterolemia.
Keywords: bile salt hydrolase, bile acid metabolism, cholesterol, fibroblast growth factor-19, probiotic
Introduction
Bile acids (BA) are involved in a variety of metabolic processes such as energy regulation,1 cholesterol metabolism,2 and modulation of inflammation.3,4 Further, the size and composition of the circulating BA pool are important factors in regulating gut microbial community structure in humans.5 Disrupted BA metabolism is associated with an expanding list of chronic diseases, including metabolic syndrome,6 obesity,7 and disorders of the liver8 and gastrointestinal (GI) tract.9,10 Synthesized in the liver from cholesterol, BA are then secreted into the small intestine where they undergo several enzymatic bacterial transformations. BA deconjugation, carried out by the enzyme bile salt hydrolase (BSH), is the gateway reaction in the GI tract. Additionally, BA deconjugation is ubiquitous within the healthy gut microbiota and an important process in the host's health.5 In particular, the release of unconjugated BA by BSH function as mediators in the regulation of cholesterol synthesis as well as in energy management through nuclear farnesoid X receptor (FXR) in enterocytes and hepatocytes11 or G-protein coupled receptor (TGR5) in various tissues.12
Microbiota dysbiosis associated with metabolic or inflammatory disorders including obesity,13 type 2 diabetes14 or inflammatory bowel disease,15 have been correlated with lower levels of bsh gene presence and enzymatic activity in the gut.16 Further, a phylum level abundance analysis showed a significant reduction in Firmicute-derived bsh gene in ulcerative colitis and type 2 diabetes, relative to healthy controls.17 BSH positively influences lipid metabolism, weight gain, and cholesterol levels in the host.18,19 Moreover, probiotic bacteria with elevated BSH activity have been clinically shown to improve LDL-cholesterol (LDL-C).20-22 Ooi et al. demonstrated reduced LDL-C in subjects receiving 4 capsules daily containing 109 colony forming units (CFU) of L. gasseri CHO-220 as well as the prebiotic inulin for 12 weeks.22 Our group has reported reduced LDL-C and increased circulating BA with 3 × 109 CFU of L. reuteri NCIMB 30242 twice daily for 9 weeks.21 In particular, it was shown that individual changes in LDL-C were inversely correlated with circulating BA over the entire intervention period. However, the BA time and dose response to L. reuteri NCIMB 30242 was not investigated. Similarly, the dose response in fibroblast growth factor (FGF)-19, an FXR target gene activated by intestinal BA stimulation,23 and plant sterols, surrogate markers of cholesterol absorption,24 were previously unknown. Further, the release profile of the probiotic was investigated in terms of the above parameters. As deconjugation should be targeted between BA release in the duodenum and active absorption in the terminal ileum, it was postulated that standard or delayed release of the probiotic may provide a differential response.
The primary objective of this study was to investigate the effect of standard or delayed release L. reuteri NCIMB 30242, taken in escalated dose, on the BA profile in otherwise healthy hypercholesterolemic subjects. We subsequently evaluated L. reuteri NCIMB 30242 in affecting BA profile in a cohort of healthy normocholesterolemic adults.
Results
Hypercholesterolemic study population
Ten otherwise healthy hypercholesterolemic subjects were randomized, with 5 subjects receiving standard capsules and 5 subjects receiving delayed release capsules. The two groups showed largely homogeneous baseline characteristics, including age, sex, body weight, BMI and blood pressure (Table S1). Antibiotics were not consumed by any subject during the study period. BA profile was not different between groups at baseline, with mean total BA of 1.04 ± 0.88 μmol/l and 1.06 ± 0.35μmol/l for the delayed and standard release capsule groups, respectively. No significant differences in mean baseline LDL-C was observed for delayed release (4.65 ± 0.24 mmol/l) or standard release (4.38 ± 0.55 mmol/l) capsule groups.
BA and lipid profile in hypercholesterolemic adults
The distribution and mean response of total, conjugated and unconjugated BA over the 4-week intervention and 2-week post-intervention are shown in Figure 1. After week one of the intervention period (NLT 3.0 x 109 CFU daily at dinner), subjects receiving delayed release L. reuteri increased mean total BA by 1.13 ± 0.67 μmol/l (P = 0.02), conjugated BA by 0.67 ± 0.39 μmol/l (P = 0.02) and unconjugated BA by 0.46 ± 0.43 μmol/l (P = 0.07), which represented mean changes, relative to baseline of 178%, 179% and 179%, respectively. Increases in BA profile were largely maintained or showed further increases after weeks 2, 3, and 4. In fact, the mean Tmax, calculated by taking the mean time from baseline to maximal effect for each individual subject, was 4.0 weeks, 4.2 weeks, and 2.6 weeks for total, conjugated, and unconjugated BA, respectively. The mean Cmax, calculated by taking the mean of the maximal concentration for each individual subject, was 3.84 ± 0.92 μmol/l, 2.49 ± 0.73 μmol/l, and 1.74 ± 0.71 μmol/l, for total, conjugated, and unconjugated BA, respectively. The increases observed in total and conjugated BA over time were predominantly due to increased glyco-conjugates, as shown in Figure 2. Further, in subjects consuming delayed release L. reuteri, mean LDL-C post-intervention was significantly changed from baseline by – 0.39 ± 0.21 mmol/l (P = 0.02), with all subjects displaying decreased LDL-C.
Figure 1.
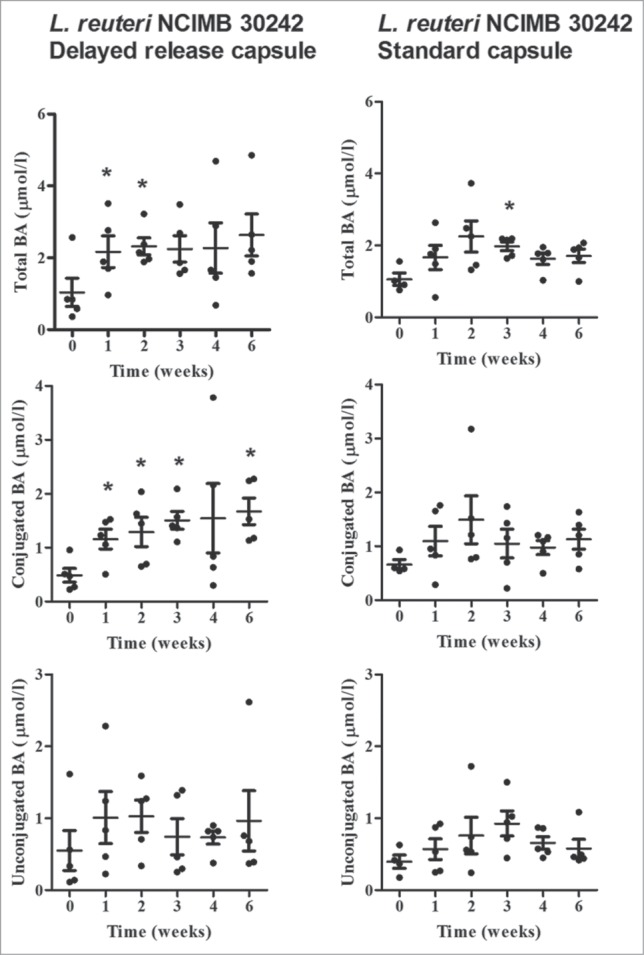
Plasma total bile acids (BA), conjugated BA and unconjugated BA at baseline, during 4-week dose escalation period and after 2-week follow-up in otherwise healthy hypercholesterolemic subjects consuming L. reuteri NCIMB 30242 in delayed release capsules (left column) or standard capsules (right column). Each timepoint is represented by individual values and mean ± SEM. Significantly different from baseline (*P ˂ 0.05).
Figure 2.
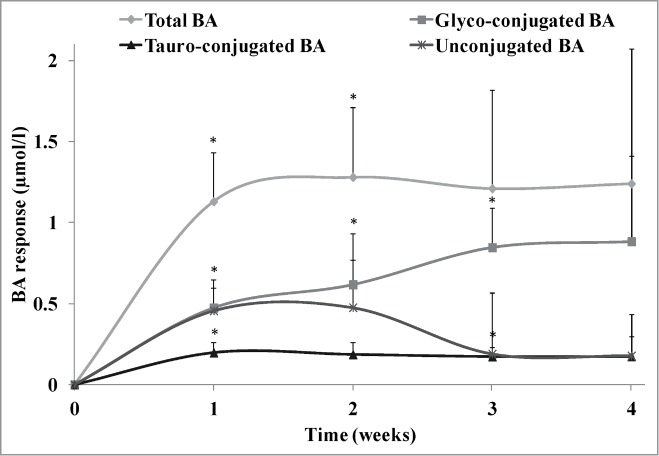
Bile acid (BA) profile response over the 4-week dose escalation period in otherwise healthy hypercholesterolemic subjects consuming L. reuteri NCIMB 30242 in delayed release capsules. BA profile consists of glyco-conjugated BA, tauro-conjugated BA and unconjugated BA. Each timepoint is represented by mean ± SEM. Significantly different from baseline (*P < 0.05).
Subjects consuming standard release L. reuteri did not show appreciable differences in total, conjugated or unconjugated BA over time. With the exception of one subject with baseline BA levels >2.5 SD from the mean, all other subjects within the first week increased total BA by 0.65 ± 1.21 μmol/l, conjugated BA by 0.47 ± 0.86 μmol/l and unconjugated BA by 0.18 ± 0.50 μmol/l, which represented mean changes, relative to baseline of 91%, 94%, and 107%, respectively (Fig. 1). However, these differences were not significant. Increases above baseline remained variable after weeks 2, 3, and 4. Mean LDL-C post-intervention in subjects consuming standard release L. reuteri was non-significantly changed from baseline by –0.09 ± 0.27, with 4 of 5 subjects displaying decreased LDL-C. Between capsule groups, a trend to a greater reduction in LDL-C was observed in subjects consuming delayed release L. reuteri as compared to subjects consuming standard release capsules (P = 0.09).
FGF-19 and plant sterol profile in hypercholesterolemic adults
The mean response of fasting plasma FGF-19 and plant sterol profile are shown in Figure 3. Among subjects taking delayed release L. reuteri, no appreciable differences in FGF-19 were observed at any of the timepoints. However, one subject presented baseline FGF-19 levels ˃2.5 SD from the mean. A sub-analysis of all other subjects demonstrated increased mean FGF-19 of 84.9 ± 52.3 pg/ml, 80.7 ± 21.6 pg/ml, 58.6 ± 46.8 pg/ml, and 90.0 ± 48.5 pg/ml after weeks 1, 2, 3, and 4 respectively. However, in the sub-analysis only the 2 week timepoint reached statistical significance (P = 0.04). Subjects consuming standard release L. reuteri showed no significant differences in FGF-19. Examining the between group response over time, a trend was observed to higher FGF-19 in subjects consuming delayed release L. reuteri as compared to standard release L. reuteri (P = 0.08).
Figure 3.
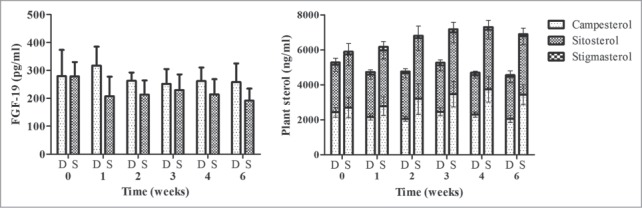
Plasma FGF-19 (left) and plant sterol profile (right) at baseline, during 4-week dose escalation period and after 2-week follow-up in otherwise healthy hypercholesterolemic subjects consuming L. reuteri NCIMB 30242 in delayed release capsules (D) or standard capsules (S). Each timepoint is represented by mean ± SEM. Plant sterol profile consists of campesterol, sitosterol and stigmasterol.
After four weeks, subjects consuming delayed released L. reuteri showed non-significant decreases in mean total plant sterols of 591.0 ± 717.1 ng/ml while subjects consuming standard release L. reuteri showed significant increases in mean total plant sterols of 1415.6 ± 595.0 ng/ml (P < 0.01). Examining the between group effect over time, a trend was observed toward lower total plant sterols in subjects consuming delayed release L. reuteri as compared to standard release L. reuteri (P = 0.05).
Association between BA, FGF-19 and plant sterols in hypercholesterolemic adults
A heat map visualization is shown in Figure 4, demonstrating the association between individual changes in BA and changes in FGF-19 and plant sterols over the 4-week intervention period. An analysis of all subjects consuming L. reuteri NCIMB 30242 showed significant positive associations between unconjugated BA and FGF-19 (P < 0.05). As well, individual changes in total and conjugated BA were shown to be inversely associated with changes in the primary plant sterol (campesterol) as well as total plant sterols (P < 0.05).
Figure 4.
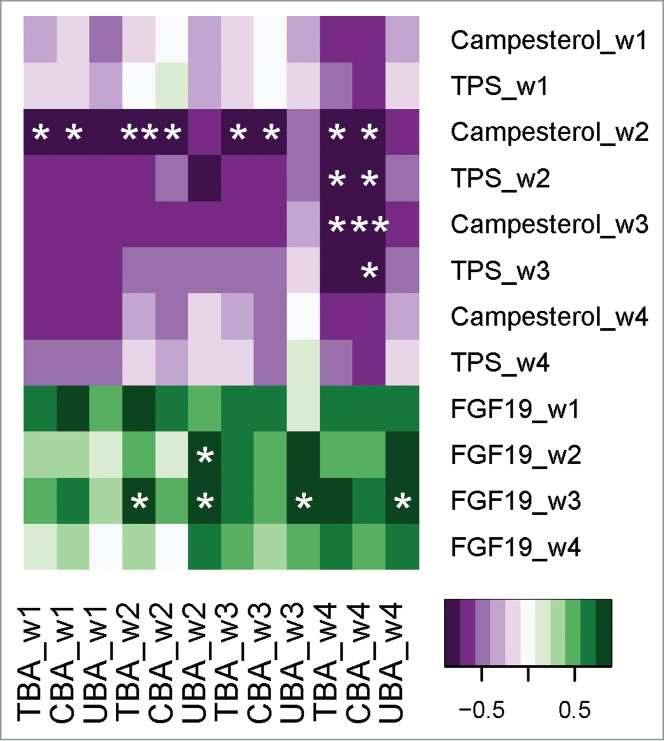
Heat map visualization of changes in bile acids over the 4-week dose escalation period and associations with changes in FGF-19 and plant sterols in otherwise healthy hypercholesterolemic subjects consuming L. reuteri NCIMB 30242. Changes in total bile acids (TBA), conjugated bile acids (CBA) and unconjugated bile acids (UBA) are ordered on the x-axis while changes in campesterol, total plant sterols (TPS) and FGF-19 are ordered on the y-axis. Significant positive or negative associations (*P < 0.05, **P < 0.01).
Effect of L. reuteri on fecal microbiome and bsh copy numbers
L. reuteri NCIMB 30242 bsh gene copies were not detectable in any subject at baseline. In contrast, L. reuteri NCIMB 30242 bsh gene copies were recovered from all individuals after week 4 of intervention (P < 0.05), including subjects taking delayed release capsules (Median: 69.2 copies/ng DNA; Range: 16.7–226.4 copies/ng DNA) and subjects taking standard capsules (Median: 92.4 copies/ng DNA; Range: 6.1–960.0 copies/ng DNA). Total firmicute-derived bsh gene copies after week 4 of intervention were not significantly different to baseline. However, subjects taking delayed release capsules showed a trend (P = 0.07) to increased Firmicutes to Bacteroidetes ratio post-intervention (Median: 1.91; Range 1.16–5.02) as compared to baseline (Median: 1.36; Range: 0.77–4.01), representing a mean increase of 43.1% (Fig. 5). No other differences in bacterial phyla were observed.
Figure 5.
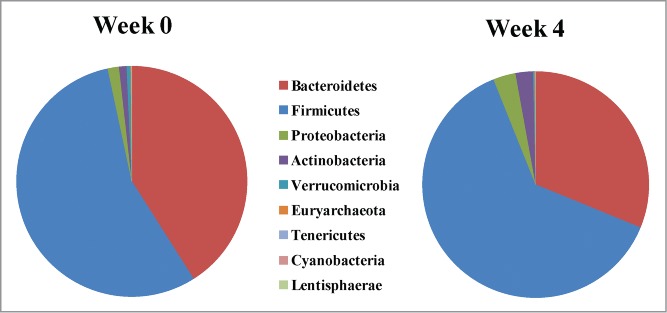
Fecal abundance of bacterial populations (phylum-level) in otherwise healthy hypercholesterolemic subjects consuming L. reuteri NCIMB 30242 in delayed release capsules at baseline (Week 0) and after the 4-week dose escalation period (Week 4).
BA profile in normocholesterolemic adults
After observing improved BA profile and LDL-C response in otherwise healthy hypercholesterolemic adults, delayed release L. reuteri NCIMB 30242 was further evaluated for its effect on BA profile in healthy normocholesterolemic adults. Demographic data is shown in Table S2. Antibiotics were not consumed by any subject during the study period. The distribution and mean response of BA profile in the normocholesterolemic cohort receiving a constant dose of delayed release L. reuteri is shown in Figure 6. Three out of 4 subjects exhibited 3-fold increases from baseline in total, conjugated and unconjugated BA through 3 weeks before normalizing during week 4. FGF-19 profile responded similarly to BA profile, with normocholesterolemic subjects displaying an approximate 2-fold increase from baseline to week 4 of intervention before normalizing during the post-intervention period. The mean Cmax for total BA, conjugated BA, unconjugated BA and FGF-19 was 5.06 ± 2.13 μmol/l, 3.50 ± 2.18 μmol/l, 2.23 ± 0.89 μmol/l and 501.9 ± 199.1 pg/ml, respectively. This compared to baseline concentrations of 1.42 ± 0.70 μmol/l, 0.60 ± 0.18 μmol/l, 0.83 ± 0.75 μmol/l and 204.7 ± 123.5 pg/ml, for total BA, conjugated BA, unconjugated BA and FGF-19, respectively. However, the mean changes in BA profile and FGF-19 did not reach statistical significance. Among normocholesterolemic subjects, plant sterols did not appreciably change during the intervention period or after the post-intervention period.
Figure 6.
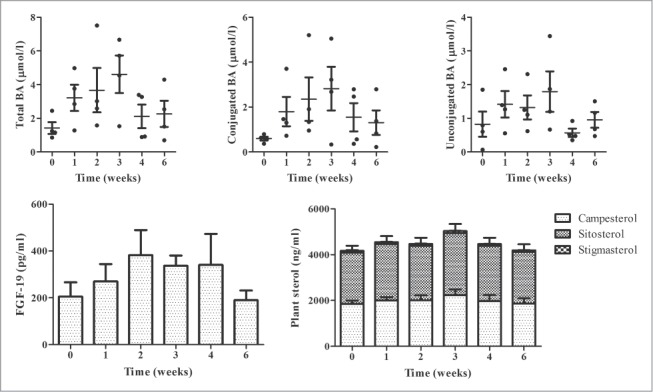
Plasma total bile acids (BA), conjugated BA and unconjugated BA (upper row, left to right) and FGF-19 and plant sterol profile (bottom row, left to right) at baseline, during 4-week constant dose intervention period and after 2-week follow-up in normocholesterolemic subjects consuming L. reuteri NCIMB 30242 in delayed release capsules. Plant sterol profile consists of campesterol, sitosterol and stigmasterol. Each timepoint is represented by individual values and mean ± SEM for total BA, conjugated BA and unconjugated BA and by mean ± SEM for FGF-19 and plant sterol profile.
Discussion
A therapeutic dose of 3.0 × 109 CFU, taken once daily at dinner, was established to have a greater than 2-fold increase in circulating BA within the first week of treatment, with all subjects receiving delayed release L. reuteri NCIMB 30242 responding to intervention. Delivery using delayed release capsules appeared to ameliorate the response in circulating BA as compared to standard capsules. It is postulated that delayed release capsules promoted a greater release of viable cells and BSH activity at the site of interest. Nevertheless, both capsule delivery systems resulted in significant recovery of strain specific bsh gene copies in all individuals, with no differences between groups. In previous randomized controlled trials, BSH-active L. reuteri NCIMB 30242 was shown to increase circulating BA levels with changes inversely correlated with LDL-C.21 In contrast, L. reuteri NCIMB 30242 did not increase BA excretion in the feces of otherwise healthy hypercholesterolemic adults.20 This is interesting in the context of increased fecal bsh gene copies shown herein and could be explained by the efficient absorption of BA, both conjugated and unconjugated, via a combination of passive and active transport mechanisms.25
In subjects consuming delayed release L. reuteri, total circulating BA concentrations were largely maintained following the initial week, however increases in circulating unconjugates were replaced by glyco-conjugates with ascending dose. This increase in circulating glyco-conjugates was observed despite the fact that the L. reuteri NCIMB 30242 BSH enzyme is more efficient at hydrolyzing glyco-conjugates than tauro-conjugates. Substrate recognition for the BSH enzyme is suggested to be primarily at the amino acid moiety, with most BSH's, including that of L. reuteri NCIMB 30242, showing preference for glycine.26 While glycine is readily available in the human liver, taurine is primarily dietary and thus limiting in its ability to conjugate BA.27 It is therefore postulated that increased unconjugated BA as a result of intestinal BA deconjugation were preferentially and efficiently conjugated by glycine, resulting in a shifted BA profile with dose escalation.
Variation in community BSH profiles, including overall number, structure, substrate specificity and preferred pH26,28 have important physiological implications for the host. Recently, Joyce et al. reported on cloned and expressed bsh genes from L. salivarius JCM1046 and L. salivarius UCC118, referred to as BSH types 1 and 2 respectively.19 Of these BSH types, BSH1 displayed dramatically increased activity and differential substrate selection as compared to BSH2, attributable to an 8-amino acid region that was absent in the latter.26 Sequence alignment of L. reuteri NCIMB 30242 and L. salivarius JCM 1046 homologues encoding highly active BSH show this region to be largely conserved (Fig. S1). BA deconjugation with BSH has been implicated in several key transcriptional changes in circulation, liver and the GI tract, including genes encoding effectors of lipid and cholesterol metabolic pathways.19 Mice colonized with the more active BSH type demonstrated reduced weight gain, serum LDL-C and liver triglycerides19 as well as increased intestinal gene expression of adenosine triphosphate-binding cassette (ABC) G5/G8 transporters known to contribute to cholesterol efflux29 and promote neutral sterol excretion.30 In context of the current results and sequence similarities, these comparisons are of potential interest despite well documented differences in human and murine BA metabolism.
Our group has previously reported on reduced concentrations of plasma plant sterols, surrogate markers of cholesterol absorption,24 with L. reuteri NCIMB 30242 intervention as compared to placebo.21 Specifically, campesterol, sitosterol, stigmasterol and total plant sterols were decreased by 41.5%, 34.2%, 40.7%, and 38.9%, respectively from baseline to 9-week endpoint in subjects receiving L. reuteri NCIMB 30242 relative to placebo.21 The current study shows that the release profile of L. reuteri NCIMB 30242 may play a key role in the observed effect. While a placebo comparator was not available in the current pilot study, it is postulated that a non-treated group would have shown increased circulating plant sterols over the autumn study period. In a previous randomized controlled trial, a significant increase in plant sterol concentrations was observed in subjects taking placebo over a similar seasonal period21 and could be explained in part by seasonal variation in plasma volume and lipid levels in northern climates.31 Further, individual changes in BA were inversely associated with plant sterols, potentially indicative of increased cholesterol efflux due to increased intestinal BA deconjugation in response to intervention. ABCG5/G8 transporters, which play established roles in limiting the accumulation of cholesterol and other dietary sterols.32 are expressed in locations where BA are present in high concentrations.33 In particular, BA have been shown to increase ABCG5/G8 specific cholesterol efflux through increased ATPase activity, with unconjugated BA supporting the highest ATPase activity in ABCG5/G8.33 As well, mice colonized with active BSH type show increased intestinal gene expression of ABCG5/G8,19 further supporting the inverse associations observed herein. The possibility, however, that the observed changes in plant sterols are a result of decreased absorption, due to an inhibition of Niemann-Pick C1-like 1 transporter or a combination of mechanisms, should be considered as well. Independent of the mechanism, subsequent studies examining the excretion of neutral sterols, including cholesterol, would be of interest to further clarify the purported effect.
Comparatively, FGF-19 increased in normocholesterolemic subjects as well as in 4 of 5 hypercholesterolemic subjects receiving L. reuteri in delayed released capsules. Further, individual changes in BA were positively associated with FGF-19, suggesting increased BA deconjugation as a mechanism. FGF-19 is produced by the small intestine through BA stimulation and is an FXR target gene.23 Ileal FXR activation enhances gene transcription of FGF-19 in humans, as well as its ortholog FGF-15 in mice, ultimately acting on the liver to repress BA synthesis.34 Postprandial increases in intestinal BA concentrations activate FXR, inducing expression and secretion of FGF-19.34,35 Similarly, increasing intestinal unconjugated BA through supplementary BA deconjugation is postulated to increase FGF-19., Unconjugated BA, notably CDCA, have been shown to be the most potent stimulators of FGF-19 expression.36 In agreement with this, Kuribayashi et al. showed enhanced ileal FXR signaling through bacteria-mediated deconjugation of taurocholic acid.37 In the current study, the FGF-19 response in the normocholesterolemic cohort receiving L. reuteri was similar to that observed with obeticholic acid, a semi-synthetic FXR agonist with 100-times greater potency than CDCA administered to subjects with primary BA diarrhea.38 A potentially higher FGF-19 response in normocholesterolemics as compared to hypercholesterolemics is of interest and should be investigated further. Unconjugated BA display greater passive diffusion than conjugated BA, but the location of which, in particular the jejunum as compared to the colon, could be impacted by the site of BA deconjugation as well as diet, transit time and intestinal pH. Differences in absorption profile could well contribute to differences observed in BA stimulation of FGF-19. Also of note is the difference in mean age and BMI between the hypercholesterolemic and normocholesterolemic populations, indicating that they may be a cofactors of interest in the context of a large randomized controlled trial. The current result is also of interest when compared to previous data showing down-regulation of the FXR-FGF-15 axis in mice due to increased BA deconjugation with the probiotic VSL#3.39 In particular, the murine conjugated BA profile comprises a predominance of tauro-conjugated BA as compared to glyco-conjugates as well as several BA, including tauromuricholic acids, not common to humans.40
BA have both direct anti-microbial effects on gut microbes, and indirect effects through FXR-induced antimicrobial peptides.5 BA pool size and composition both regulate and are regulated by the gut microbiota and in turn, show striking differences between germ-free and conventional murine models.5 Dysbiosis in the gut microbiota, demonstrated in several chronic diseases, is often characterized by alteration in the Bacteroides:Firmicutes ratio and increase in potentially pathogenic taxa such as Enterobacteriaceae.8 It has also been reported that Bacteroidetes are expanded in mice fed a high-fat diet, irrespective of the type of fat.41 Moreover, the ratios of Bacteroidetes to Firmicutes as well as Bacteroides-Prevotella to C. coccoides-E. rectale appear to be correlated with plasma glucose concentration.14 Administration of L. reuteri NCIMB 30242 showed a trend to increased Firmicutes, at the expense of Bacteroidetes in hypercholesterolemic subjects. Increased Firmicutes:Bacteroidetes was previously reported in mice receiving the probiotic VSL#3, as a result of enhanced BA deconjugation and fecal excretion.39 In contrast, oral antibiotic treatment was shown to reduce insulin sensitivity as well as microbial diversity, with a decrease in Firmicutes and a compensatory increase in Proteobacteria.42 Altered Firmicutes was associated with decreased fecal secondary BA.42 Short term administration of BSH-active L. reuteri NCIMB 30242 therefore appears to affect fecal microbiota composition in the opposing direction to oral antibiotic treatment. Whether the associated changes in microbiota result in or are a result of changes in BA metabolism are unclear and should be investigated further. Also, while no antibiotics were consumed during the study period,
This study shows the potential of a BSH-active probiotic to significantly influence BA profile, FGF-19 and sterol absorption while improving LDL-C. Administration of L. reuteri NCIMB 30242 in a delayed release formulation was shown to increase circulating BA by greater than 2-fold, with responses observed in both hypercholesterolemic and normocholesterolemic adults. Individual increases in circulating BA, as a result of increased intestinal BA deconjugation, were associated with increased activation of FGF-19, a downstream effector of BA, and inversely associated with markers of cholesterol absorption. As a pilot study with a small sample size, the observed effects should be substantiated with a well-powered randomized controlled study. A greater understanding of fasting and postprandial BA response in the context of differential diet, transit time and intestinal pH would also be of interest. However, our data provide the first clinical support of BSH activity taken in ascending dose and its ability to affect BA over time. In addition to hypercholesterolemia, the observed effect has the potential to address metabolic or inflammatory disorders that have been linked to deficiencies in BSH gene presence and enzymatic activity in the GI tract.
Subjects and Methods
Subjects
The present study was conducted according to the principles of the Declaration of Helsinki. Otherwise healthy hypercholesterolemic adults were recruited from KGK Synergize Inc. (London, ON). The study protocol and informed consent form were approved by IRB Services (FDA/OHRP IORG Registration #IORG0000456, Aurora, ON) and Health Canada (Notice of Authorization).
Ten otherwise healthy hypercholesterolemic males and females between 20 and 75 y (inclusive) with LDL-C above 3.4 mmol/l, TG below 4.0 mmol/l, body mass index (BMI) of 23.0–32.5 kg/m2 and total BA below 10 μmol/l were included. Females who were pregnant, breast feeding or with an intent to get pregnant were excluded. Additional exclusion criteria included previously diagnosed diabetes, high Framingham risk score, history of chronic use of alcohol or heavy smoking, current intake of systemic antibodies, corticosteroids, androgens or phenytoin, history of angina, congestive heart failure, inflammatory bowel disease, pancreatitis, gastrointestinal, renal, pulmonary, hepatic or biliary disease, or cancer. In another aspect, 4 healthy normocholesterolemic male subjects with LDL-C below 3.4 mmol/l and an average age of 37.75 ± 8.26 y were included. The study protocol was carefully explained and all subjects provided written informed consent prior to inclusion. The study was registered under identifier NCT02216825 on clinicaltrials.gov.
Preparation of capsules
Lactobacillus reuteri NCIMB 30242 was propagated using current Good Manufacturing Practices (cGMP) at Dupont Nutrition and Health (Madison, US). White, size one, delayed release (DRcap®) or standard vegetarian (Vcap®) capsules (Capsugel, Colmar, FR) contained a blend of L. reuteri NCIMB 30242 in a base of microcrystalline cellulose, silicon dioxide and magnesium stearate. Capsules were produced under cGMP at Ropack Inc. (Montreal, CA) and PNP Pharmaceuticals Inc. (Burnaby, CA). Microbiological analyses and culture purity were confirmed following production. Capsules were identical in taste and appearance and bottled in white screw-cap bottles with polyethylene shell and integrated desiccant plastic sleeve (CSP Technologies Inc., Auburn, US).
Study design
The study was a pilot, randomized, dose-escalation design lasting 6 weeks. Otherwise healthy hypercholesterolemic subjects were assigned to receive delayed or standard release L. reuteri NCIMB 30242 over a 4-week intervention period. During week one, subjects received Not Less Than (NLT) 3.0 × 109 CFU L. reuteri once daily at dinner. During weeks 2, 3, and 4, subjects received NLT 3.0 × 109 CFU, 6.0 × 109 CFU and 9.0 × 109 CFU respectively, twice daily with lunch and dinner. Capsule potency was confirmed, with no significant differences between or within groups over the intervention period (P > 0.05). The intervention period was followed by a 2-week post-intervention period in which subjects did not consume L. reuteri NCIMB 30242. Normocholesterolemic subjects received delayed release L. reuteri NCIMB 30242 at a dose of NLT 6.0 × 109 CFU twice daily with lunch and dinner over a 4-week intervention period followed by a 2-week post-intervention period.
Blood collection and analyses
Twelve-hour fasting blood samples were obtained by venipuncture at baseline, weeks 1 through 4 of intervention and 2 weeks post-intervention. Baseline specimens were transported immediately to a central laboratory (LifeLabs Medical Laboratory Services, London, CA) for analysis. Serum total cholesterol (TC), HDL-cholesterol (HDL-C), triglycerides (TG), biochemistry, hematology and total BA were analyzed (Advia Chemistry Systems 1200/1800/2400) using appropriate reagent kits and controls. Plasma BA profile, FGF-19 and plant sterols (campesterol, sitosterol, and stigmasterol) were assessed at baseline, weeks one through 4 of intervention and 2 weeks post-intervention at University Hospital Regensburg (Regensburg, DE) by liquid chromatography-tandem mass spectrometry,43 ELISA and gas chromatography-tandem mass spectrometry,44 respectively. LDL-C was assessed by electrospray ionization tandem mass spectrometry at baseline and endpoint of intervention at University Hospital Regensburg.
Fecal collection and analyses
DNA was isolated from fecal samples at baseline and endpoint of intervention. Fecal samples were frozen and stored at −80°C until DNA isolation. A 0.2-to-0.3g aliquot of the fecal sample was extracted with the Powersoil DNA isolation kit (MO BIO laboratories, Carlsbad, US), following the manufacturer's instructions. The DNA was quantified and stored at -20°C in TE buffer.
Quantification of bsh copy number
Quantification of L. reuteri NCIMB 30242 and firmicute derived bsh gene copy number was performed by qPCR using the Microbiome SenseIT bsh kit (Micropharma Limited, Montreal, CA) on a CFX96 thermal cycler (Bio-Rad Laboratories Inc.., Hercules, US) following manufacturer's instructions. The PCR reactions were prepared using iTAQtm universal probes mastermix (Bio-Rad Laboratories Inc.., Hercules, US).
16s. sequencing for assessment of bacterial diversity
The 16S rRNA gene V4 variable region PCR primers 515/806 were used to assess bacterial diversity. Sequencing was performed on an Ion Torrent Personal Genome Machine with data processing at Molecular Research LP (Shallowater, US). In summary, sequences were depleted of barcodes and primers, then sequences <150bp as well as sequences with ambiguous base calls and with homopolymer runs exceeding 6bp were removed. Sequences were denoised, operational taxonomic units (OTUs) generated and chimeras removed. OTUs were defined by clustering at 3% divergence (97% similarity). Final OTUs were taxonomically classified using BLASTn against a curated GreenGenes database.
Statistical analysis
The primary null hypothesis was that BSH-active L. reuteri NCIMB 30242, delivered in a delayed release or standard capsule, would not alter plasma BA profile over a 4-week dose escalation intervention period. Descriptive statistics are presented as mean ± SD or as median and range for continuous variables or as a percentage for categorical variables. Differences between groups for baseline characteristics were analyzed using a one-way analysis of variance (ANOVA) or a non-parametric Mann-Whitney Wilcoxon test for continuous variables, or Fisher's exact test for categorical variables. Within group changes over time in BA, FGF-19, plant sterols and LDL-C was assessed by paired t-test. Between group changes over the intervention period for delayed release and standard capsule groups were assessed by repeated measures ANOVA. A Pearson correlation coefficient was used to assess correlations between BA, FGF-19 and plant sterols. Changes in bsh gene copy number and phylum-level diversity were analyzed by Wilcoxon signed-rank test. Statistical significance was determined at P < 0.05 for all analyses. All data analyses were performed using SPSS software package version 19.0 (SPSS Inc., Chicago, US).
Disclosure of Potential Conflicts of Interest
MLJ and SP acknowledge a conflict of interest as they are co-founders and shareholders of Micropharma. CJM, AL and JGG are employed by Micropharma.
Acknowledgments
We thank M Evans, G Schmitz, S Matysik and P McDonald for their contribution to the study. We also thank all volunteers who participated in the study. GS and SM oversaw the analysis of bile acids, cholesterol, FGF-19 and plant sterols. ME and PM oversaw the clinical study.
Funding
This work was supported by Micropharma Limited.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
Reference List
- 1. Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 2006; 439:484-9; PMID:16400329; http://dx.doi.org/ 10.1038/nature04330 [DOI] [PubMed] [Google Scholar]
- 2. Watanabe M, Houten SM, Wang L, Moschetta A, Mangelsdorf DJ, Heyman RA, Moore DD, Auwerx J. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest 2004; 113:1408-18; PMID:15146238; http://dx.doi.org/ 10.1172/JCI21025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Houten SM, Watanabe M, Auwerx J. Endocrine functions of bile acids. EMBO J 2006; 25:1419-25; PMID:16541101; http://dx.doi.org/ 10.1038/sj.emboj.7601049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pols TW, Noriega LG, Nomura M, Auwerx J, Schoonjans K. The bile acid membrane receptor TGR5 as an emerging target in metabolism and inflammation. J Hepatol 2011:54: 1263-72; PMID:21145931; http://dx.doi.org/ 10.1016/j.jhep.2010.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Bile acids and the gut microbiome. Curr Opin Gastroenterol 2014; 30:332-8; PMID:24625896; http://dx.doi.org/ 10.1097/MOG.0000000000000057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of Bile Acids and Bile Acid Receptors in Metabolic Regulation. Physiol Rev 2009; 89:147-91; PMID:19126757; http://dx.doi.org/ 10.1152/physrev.00010.2008 [DOI] [PubMed] [Google Scholar]
- 7. Patti ME, Houten SM, Bianco AC, Bernier R, Larsen PR, Holst JJ, Badman MK, Maratos-Flier E, Mun EC, Pihlajamaki J, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity 2009; 17:1671-7; PMID:19360006; http://dx.doi.org/ 10.1038/oby.2009.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ridlon JM, Alves JM, Hylemon PB, Bajaj JS. Cirrhosis, bile acids and gut microbiota: unraveling a complex relationship. Gut Microbes 2013; 4:382-7; PMID:23851335; http://dx.doi.org/ 10.4161/gmic.25723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Duboc H, Rajca S, Rainteau D, Benarous D, Maubert MA, Quervain E, Thomas G, Barbu V, Humbert L, Despras G, et al. Connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut 2012; 62:531-9; PMID:22993202; http://dx.doi.org/ 10.1136/gutjnl-2012-302578 [DOI] [PubMed] [Google Scholar]
- 10. Duboc H, Rainteau D, Rajca S, Humbert L, Farabos D, Maubert M, Grondin V, Jouet P, Bouhassira D, Seksik P, et al. Increase in fecal primary bile acids and dysbiosis in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil 2012; 24:513-7; PMID:22356587; http://dx.doi.org/ 10.1111/j.1365-2982.2012.01893.x [DOI] [PubMed] [Google Scholar]
- 11. Matsubara T, Li F, Gonzalez FJ. FXR signaling in the enterohepatic system. Mol Cell Endocrinol 2013; 368:17-29; PMID:22609541; http://dx.doi.org/ 10.1016/j.mce.2012.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab 2009; 10:167-77; PMID:19723493; http://dx.doi.org/ 10.1016/j.cmet.2009.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto JM, Kennedy S, et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013; 500:541-6; PMID:23985870; http://dx.doi.org/ 10.1038/nature12506 [DOI] [PubMed] [Google Scholar]
- 14. Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, Al-Soud WA, Sorensen SJ, Hansen LH, Jakobsen M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One 2010; 5:e9085; PMID:20140211; http://dx.doi.org/ 10.1371/journal.pone.0009085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Peterson DA, Frank DN, Pace NR, Gordon JI. Metagenomic approaches for defining the pathogenesis of inflammatory bowel diseases. Cell Host Microbe 2008; 3:417-27; PMID:18541218; http://dx.doi.org/ 10.1016/j.chom.2008.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ogilvie LA, Jones BV. Dysbiosis modulates capacity for bile acid modification in the gut microbiomes of patients with inflammatory bowel disease: a mechanism and marker of disease? Gut 2012; 61:1642-3; PMID:22490526; http://dx.doi.org/ 10.1136/gutjnl-2012-302137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Labbé A, Ganopolsky JG, Martoni CJ, Prakash S, Jones ML. Bacterial bile metabolising gene abundance in the Crohn's, ulcerative colitis and type 2 diabetes metagenome. PLoS One 2014; 9:e115175; PMID: 2551711518757757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jones BV, Begley M, Hill C, Gahan CG, Marchesi JR. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc Natl Acad Sci U S A 2008; 105:13580-5; PMID:18757757; http://dx.doi.org/ 10.1073/pnas.0804437105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Joyce SA, MacSharry J, Casey PG, Kinsella M, Murphy EF, Shanahan F, Hill C, Gahan CG. Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut. Proc Natl Acad Sci U S A 2014; 111:7421-6; PMID:24799697; http://dx.doi.org/ 10.1073/pnas.1323599111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jones ML, Martoni CJ, Parent M, Prakash S. Cholesterol-lowering efficacy of a microencapsulated bile salt hydrolase-active Lactobacillus reuteri NCIMB 30242 yoghurt formulation in hypercholesterolaemic adults. Br J Nutr 2012; 107:1505-13; PMID:22067612; http://dx.doi.org/ 10.1017/S0007114511004703 [DOI] [PubMed] [Google Scholar]
- 21. Jones ML, Martoni CJ, Prakash S. Cholesterol lowering and inhibition of sterol absorption by Lactobacillus reuteri NCIMB 30242: a randomized controlled trial. Eur J Clin Nutr 2012; 66:1234-41; PMID:22990854; http://dx.doi.org/ 10.1038/ejcn.2012.126 [DOI] [PubMed] [Google Scholar]
- 22. Ooi LG, Ahmad R, Yuen KH, Liong MT. Lactobacillus gasseri CHO-220 and inulin reduced plasma total cholesterol and low-density lipoprotein cholesterol via alteration of lipid transporters. J Dairy Sci 2010; 93: 5048-58:PMID:20965319; http://dx.doi.org/ 10.3168/jds.2010-3311 [DOI] [PubMed] [Google Scholar]
- 23. Holt JA, Luo G, Billin AN, Bisi J, McNeill YY, Kozarsky KF, Donahee M, Wang DY, Mansfield TA, Kliewer SA, et al. Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev 2003; 17:1581-91 PMID:12815072; http://dx.doi.org/ 10.1101/gad.1083503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miettinen TA, Gylling H, Nissinen MJ. The role of serum non-cholesterol sterols as surrogate markers of absolute cholesterol synthesis and absorption. Nutr Metab Cardiovasc Dis 2011; 21:765-9; PMID:21899991; http://dx.doi.org/ 10.1016/j.numecd.2011.05.005 [DOI] [PubMed] [Google Scholar]
- 25. Schiff ER, Small NC, Dietschy JM. Characterization of the kinetics of the passive and active transport mechanisms for bile acid absorption in the small intestine and colon of the rat. J Clin Invest 1972; 51:1351-62; PMID:5024036; http://dx.doi.org/ 10.1172/JCI106931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fang F, Li Y, Bumann M, Raftis EJ, Casey PG, Cooney JC, Walsh MA, O'Toole PW. Allelic variation of bile salt hydrolase genes in Lactobacillus salivarius does not determine bile resistance levels. J Bacteriol 2009; 191:5743-57; PMID:19592587; http://dx.doi.org/ 10.1128/JB.00506-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gaull GE, Pasantes-Morales H, Wright CE. Taurine in human nutrition: overview. Prog Clin Biol Res 1985; 179:3-21; PMID:3903756 [PubMed] [Google Scholar]
- 28. Grill JP, Cayuela C, Antoine JM, Schneider F. Isolation and characterization of a Lactobacillus amylovorus mutant depleted in conjugated bile salt hydrolase activity: relation between activity and bile salt resistance. J Appl Microbiol 2000; 89:553-63; PMID:11054157; http://dx.doi.org/ 10.1046/j.1365-2672.2000.01147.x [DOI] [PubMed] [Google Scholar]
- 29. Abumrad NA, Davidson NO. Role of the gut in lipid homeostasis. Physiol Rev 2012; 92:1061-85; PMID:22811425; http://dx.doi.org/ 10.1152/physrev.00019.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yu L, Li-Hawkins J, Hammer RE, Berge KE, Horton JD, Cohen JC, Hobbs HH. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J Clin Invest 2002; 110: 671-80:PMID:12208868; http://dx.doi.org/ 10.1172/JCI0216001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ockene IS, Chiriboga DE, Stanek EJ, III, Harmatz MG, Nicolosi R, Saperia G, Well AD, Freedson P, Merriam PA, Reed G, et al. Seasonal variation in serum cholesterol levels: treatment implications and possible mechanisms. Arch Intern Med 2004; 164:863-70; PMID:15111372; http://dx.doi.org/ 10.1001/archinte.164.8.863 [DOI] [PubMed] [Google Scholar]
- 32. Kidambi S, Patel SB. Cholesterol and non-cholesterol sterol transporters: ABCG5, ABCG8 and NPC1L1: a review. Xenobiotica 2008; 38:1119-39; PMID:18668442; http://dx.doi.org/ 10.1080/00498250802007930 [DOI] [PubMed] [Google Scholar]
- 33. Johnson BJ, Lee JY, Pickert A, Urbatsch IL. Bile acids stimulate ATP hydrolysis in the purified cholesterol transporter ABCG5/G8. Biochemistry 2010; 49:3403-11; PMID:20210363; http://dx.doi.org/ 10.1021/bi902064g [DOI] [PubMed] [Google Scholar]
- 34. Potthoff MJ, Kliewer SA, Mangelsdorf DJ. Endocrine fibroblast growth factors 15/19 and 21: from feast to famine. Genes Dev 2012; 26:312-24; PMID:22302876; http://dx.doi.org/ 10.1101/gad.184788.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu X, Ge H, Baribault H, Gupte J, Weiszmann J, Lemon B, Gardner J, Fordstrom P, Tang J, Zhou M, et al. Dual actions of fibroblast growth factor 19 on lipid metabolism. J Lipid Res 2013; 54:325-32; PMID:23204296; http://dx.doi.org/ 10.1194/jlr.M027094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Styer AM, Roesch SL, Argyropoulos G. Modulation of fibroblast growth factor 19 expression by bile acids, meal replacement and energy drinks, milk, and coffee. PLoS One 2014; 9:e85558; PMID:24465600; http://dx.doi.org/ 10.1371/journal.pone.0085558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kuribayashi H, Miyata M, Yamakawa H, Yoshinari K, Yamazoe Y. Enterobacteria-mediated deconjugation of taurocholic acid enhances ileal farnesoid X receptor signaling. Eur J Pharmacol 2012; 697:132-8; PMID:23051670; http://dx.doi.org/ 10.1016/j.ejphar.2012.09.048 [DOI] [PubMed] [Google Scholar]
- 38. Vassie C, Nolan JD, Johnston IM, Shapiro D, Walters JR. Obeticholic acid, a farnesoid X receptor agonist, reduces bile acid synthesis in patients with primary bile acid diarrhea. Gastroenterology 2014; 146:S-797; PMID:25329562; http://dx.doi.org/ 10.1016/S0016-5085(14)62881-X25329562 [DOI] [Google Scholar]
- 39. Degirolamo C, Rainaldi S, Bovenga F, Murzilli S, Moschetta A. Microbiota modification with probiotics induces hepatic bile acid synthesis via downregulation of the Fxr-Fgf15 axis in mice. Cell Rep 2014; 7:12-8; PMID:24656817; http://dx.doi.org/ 10.1016/j.celrep.2014.02.032 [DOI] [PubMed] [Google Scholar]
- 40. Begley M, Gahan CGM, Hill C. The interaction between bacteria and bile. Fems Microbiology Reviews 2005; 29:625-51; PMID:16102595; http://dx.doi.org/ 10.1016/j.femsre.2004.09.003 [DOI] [PubMed] [Google Scholar]
- 41. Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, Antonopoulos DA, Jabri B, Chang EB. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature 2012; 487:104-8; PMID:22722865; http://dx.doi.org/ 10.1038/nature11225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vrieze A, Out C, Fuentes S, Jonker L, Reuling I, Kootte RS, Van NE, Holleman F, Knaapen M, Romijn JA, et al. Impact of oral vancomycin on gut microbiota, bile acid metabolism, and insulin sensitivity. J Hepatol 2014; 60:824-31; PMID:24316517; http://dx.doi.org/ 10.1016/j.jhep.2013.11.034 [DOI] [PubMed] [Google Scholar]
- 43. Scherer M, Gnewuch C, Schmitz G, Liebisch G. Rapid quantification of bile acids and their conjugates in serum by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2009; 877:3920-5; PMID:19819765; http://dx.doi.org/ 10.1016/j.jchromb.2009.09.038 [DOI] [PubMed] [Google Scholar]
- 44. Matysik S, Klunemann HH, Schmitz G. Gas chromatography-tandem mass spectrometry method for the simultaneous determination of oxysterols, plant sterols, and cholesterol precursors. Clin Chem 2012; 58:1557-64; PMID:22997279; http://dx.doi.org/ 10.1373/clinchem.2012.189605 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


