Abstract
Impaired gut barrier function has been reported in a wide range of diseases and syndromes and in some functional gastrointestinal disorders. In addition, there is increasing evidence that suggests the gut microbiota tightly regulates gut barrier function and recent studies demonstrate that probiotic bacteria can enhance barrier integrity. Here, we aimed to investigate the effects of Lactobacillus rhamnosus CNCM I-3690 on intestinal barrier function. In vitro results using a Caco-2 monolayer cells stimulated with TNF-α confirmed the anti-inflammatory nature of the strain CNCM I-3690 and pointed out a putative role for the protection of the epithelial function. Next, we tested the protective effects of L. rhamnosus CNCM I-3690 in a mouse model of increased colonic permeability. Most importantly, we compared its performance to that of the well-known beneficial human commensal bacterium Faecalibacterium prauznitzii A2-165. Increased colonic permeability was normalized by both strains to a similar degree. Modulation of apical tight junction proteins expression was then analyzed to decipher the mechanism underlying this effect. We showed that CNCM I-3690 partially restored the function of the intestinal barrier and increased the levels of tight junction proteins Occludin and E-cadherin. The results indicate L. rhamnosus CNCM I-3690 is as effective as the commensal anti-inflammatory bacterium F. prausnitzii to treat functional barrier abnormalities.
Keywords: apical junction proteins, lactobacilli, probiotics
Abbreviations
- AJs
adherence junctions
- CNCM
Collection Nationale de Cultures de Microorganismes
- DNBS
DiNitroBenzene Sulfonic
- EOS
extremely oxygen sensitive
- GVHD
graft-versus-host disease
- IBD
inflammatory bowel diseases
- IBS
irritable bowel syndrome
- LAB
lactic acid bacteria
- L
Lactobacillus
- LGG
Lactobacillus rhamnosus GG
- Lcn-2
Lipocalin-2
- Luc
Luciferase
- MOI
multiplicity of infection
- MPO
Myeloperoxidase
- ON
overnight
- TJs
tight junctions
- TEER
trans-epithelial electrical resistance
- ZO
Zonula occludens
Introduction
The intestinal barrier constitutes the first line of host defense, and is crucial for the maintenance of mucosal homeostasis. Functionally, the intestinal epithelium regulates passage and secretion of solutes and small molecules through the transcellular and paracellular pathways.1 While the first is carried out by specific transporters or channels,2,3 the second relies on the apical junctional complex, mainly composed of tight junctions (TJs) and adherence junctions (AJs).4-6 Although low-grade translocation of luminal antigen is required to prime the immune system,7 impaired gut barrier and increased permeability may lead to uncontrolled translocation. This may contribute to increased local immune activation and inflammation8 as well to a systemic impact such as endotoxemia.9 There is increasing consensus that intestinal barrier dysfunction, also referred to as “leaky gut,” is an important factor in the clinical manifestation of a wide number of disorders and diseases such as irritable bowel syndrome (IBS), food allergies, type-1 diabetes, obesity10-12 as well as may precede chronic inflammation processes such as inflammatory bowel diseases (IBD).13
Lactic acid bacteria (LAB) are used extensively as starter cultures in food fermentation since millennia; furthermore, numerous studies validate the use of LAB as probiotics due to their wide range of health-promoting effects in humans. Composition of microbiota, probiotic strain used, and host genetic background may play an important role in determining the different beneficial effects observed (i.e. anti-inflammatory, pro-inflammatory, anti-pathogen, etc.) by probiotics.14 However, little is known about the molecular mechanisms at the basis of these functions. Several strains belonging to Lactobacillus spp. are used as probiotics and their effects have been and are still investigated in in vitro and in vivo models.15 Besides, some probiotic lactobacilli strains provide important stimuli to the human immune system and influence host homeostasis14 being proposed to restore dysbiosis-mediated diseases.16
Regulation of intestinal barrier function is mediated by endogenous and exogenous factors, such as cytokines, drugs and toxins.8,17,18 Pathogen and commensal bacteria are able to modulate the barrier directly or indirectly.19-21 Increasing evidence indicates that strains of lactic acid bacteria and bifidobacteria regulate gut barrier function.22,23 Improvement of barrier function by such strains has been reported in both in vitro and in vivo studies.5 For instance, the well-known probiotics Escherichia coli Nissle 1917, Lactobacillus rhamnosus GG (LGG) and a mixture of lactobacilli and bifidobacteria prevent the increase in intestinal permeability in vivo.24-27 Notably, fermented milk containing Bifidobacterium lactis CNCM I-2494 and LAB strains present in yogurt prevented the increase of intestinal permeability induced by partial restraint stress in rats.23
Recently, the commensal bacterium Faecalibacterium prausnitzii A2-165, (a major member of the Clostridium leptum group) was shown to display anti-inflammatory and protective effects in both acute28 and chronic colitis models.29 It also showed protective effects on loss of intestinal function in a low-grade inflammation mice model mimicking gut barrier alterations.30 These previous observations prompted us to search for an anti-inflammatory Lactobacillus strain and to test its protective role on epithelial dysfunction in vitro and in vivo. For this we selected L. rhamnosus CNCM I-3690, previously demonstrated to have anti-inflammatory properties,31 and used Caco-2 cells after TNF-α destabilization as well as an animal model of disturbed barrier function induced by a sub-colitic dose of DNBS.
Results
In vitro screening of lactobacilli strains for intestinal barrier function
The in vitro protective effect of 24 selected lactobacilli strains (Table 1) was assayed in Trans-Epithelial Electrical Resistance (TEER) assay (Fig. 1). Lactobacillus rhamnosus GG strain was used as a positive control as previously reported.32 As shown in Figure 1, all strains displayed different levels of protective effect on TNF-α-induced barrier alteration. Strains were then selected on the basis of this screening: a potentially protective strain, L. rhamnosus CNCM I-3690, and a neutral strain, L. paracasei CNCM I-3689. LGG strain, our positive control in this experiment, displays similar effects as those observed with L. rhamnosus CNCM I-3690.
Table 1.
Lactobacilli strains from Danone Collection used in the initial screening. Strains were ranked according to their effect in this model from no effect on the left to positive effect on the right
| Species | Name | Origin |
|---|---|---|
| Lactobacillus (para)casei | CNCM I-1518 | Dairy product |
| Lpp14 | Dairy product | |
| CNCM I-4648 | Dairy product | |
| Lpp46 | Plant | |
| ATCC334 | ATCC 334 | |
| Lpp74 | Dairy product | |
| CNCM I-4270 | Dairy product | |
| CNCM I-3689 | Dairy product | |
| CNCM I-4649 | Dairy product | |
| BL23 | BL23 | |
| Lpp219 | Human | |
| Lactobacillus rhamnosus | Lr9 | Plant |
| CNCM I-3690 | Dairy product | |
| Lr28 | Unknown | |
| Lr32 | Human | |
| LGG | LGG | |
| Lr40 | Unknown | |
| Lr52 | Human | |
| HN001 | HN001 | |
| Lr61 | Dairy product | |
| Lr73 | Human | |
| Lr133 | Human | |
| Lr140 | Dairy product |
Figure 1.
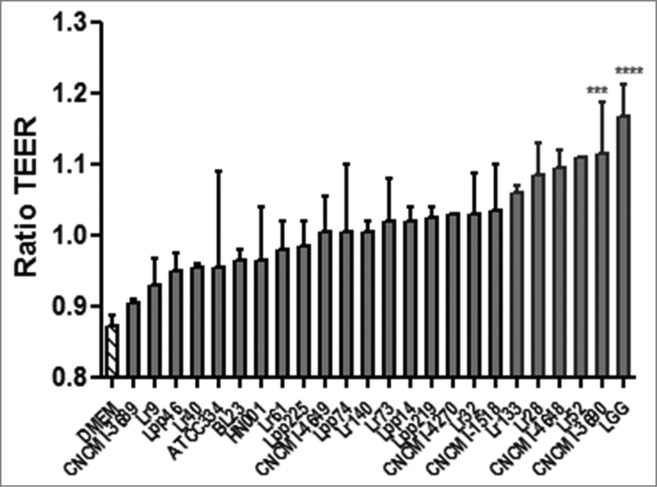
Protective effect of probiotic lactobacilli strains on intestinal barrier integrity measured by Trans-Epithelial Electrical Resistance (TEER) *P < 0.05. TEER was assayed on Caco-2 cells grown on Transwell. TEER was measured before adding 106 CFU of lactobacilli onto the apical surface for 3h prior to treatment of the basolateral medium with TNF-α (100 ng/ml) for 21 h at 37°C and at the end of TNF-α stimulation. Analysis done by ANOVA Test followed by Student-Newman-Keuls multiple comparison post hoc analysis ***p < 0.001, ****p < 0.0001. The resulting data presented as a ratio.
L. rhamnosus CNCM I-3690 blocks NF-κB expression in vitro
The immunomodulatory properties of these 2 strains were further evaluated in a NF-κB luc reporter in vitro model. As shown in Figure 2, the 2 strains display significant different immunomodulation properties (P < 0.05): L. rhamnosus strain CNCM I-3690 reduces NF-κB activation by approximately 40% confirming the previous results,31 whereas the L. paracasei CNCM I-3689 reduces this activation only by 20% (Fig. 2).
Figure 2.
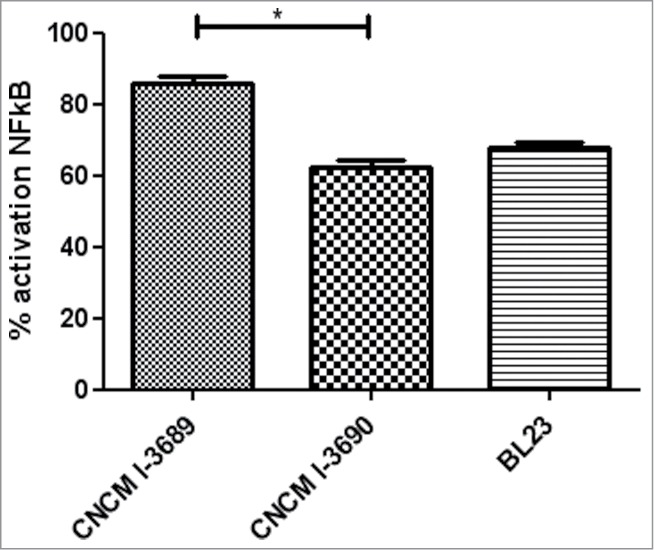
Percentage of inhibition of NF-κB activation by 3 different lactobacilli strains. *p < 0.05. Immunomodulation was assessed in vitro in stable HT-29 pIgK-luciferase plasmid transfectants. Cells were pre-incubated for 2 hours with each bacterial strain using a MOI of 100 bacteria per cell. After 2 hours of pre-incubation with bacteria, cells were stimulated with TNF-α, 25 ng/well, for 6 hours. After incubation, luminescence was determined on cell lysate. Results are expressed as ratio and percentage of NF-κB activation. Analysis done by ANOVA Test followed by Student-Newman-Keuls multiple comparison post hoc analysis *P < 0.05.
Lactobacillus rhamnosus CNCM I-3690 but not L. paracasei CNCM I-3689 protects epithelial barrier function in DNBS-induced increased permeability model
The model of low-grade DNBS inflammation in this study involved a first DNBS injection followed by a recovery period and a reactivation period. The inflammation status after DNBS reactivation was analyzed (macroscopic and histological scores, MPO activity and Lipocalin-2 concentration) (data not shown) confirming the lack of an overt inflammation status in this mouse model.
Barrier function was then assessed using FITC-labeled Dextran tracer at the endpoint. As shown in Figure 3, the FITC-Dextran recovered in serum samples of treated-mice was significantly higher in the DNBS-PBS group compared to the EtOH-PBS control group (P < 0.05). L. rhamnosus CNCM I-3690 administration (1×109 bacteria per day for 10 days) resulted in a significant decrease in DNBS-induced permeability (P < 0.05). This effect was comparable with that observed with the F. prausnitzii strain, known to display protective effects in an acute TNBS-induced colitis28 model and in a chronic DNBS-induced colitis model.29 The DNBS-induced permeability was not modified in the presence of L. paracasei CNCM I-3689 strain, validating the results obtained in the in vitro screening for epithelial function (Fig. 1).
Figure 3.
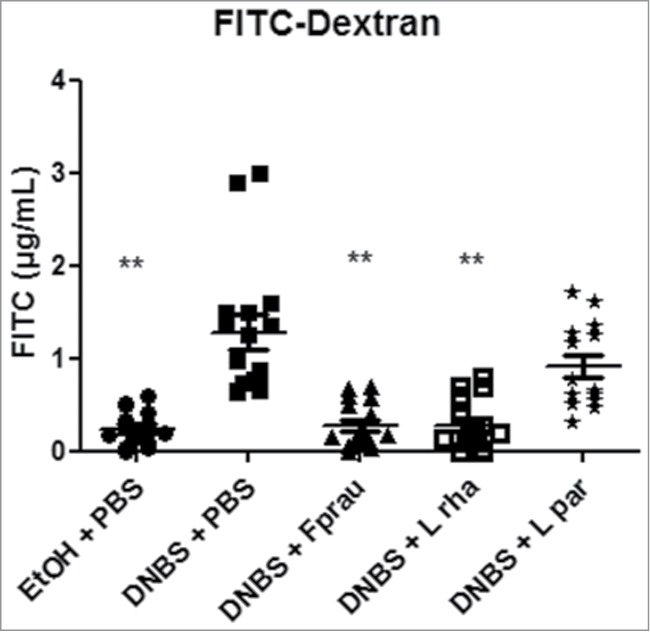
Measurement of in vivo permeability in DNBS-challenged mice treated with several probiotic strains. For in vivo measurements of gut permeability, animals were orally gavaged with FITC-dextran Control non-inflamed (EtOH-PBS), control inflamed (DNBS-PBS), F. prausnitzii A2-165 strain (DNBS-Fprau), L. rhamnosus CNCM I-3690 strain (DNBS-Lrha) , L. paracasei CNCM I-3689 strain (DNBS-Lpar). Analysis done by Kruskal-Wallis followed by a Dunn's Multiple Comparison Test *P < 0.05 versus DNBS-PBS. n = 16 mice per group.
Modulation of the expression of apical junction proteins
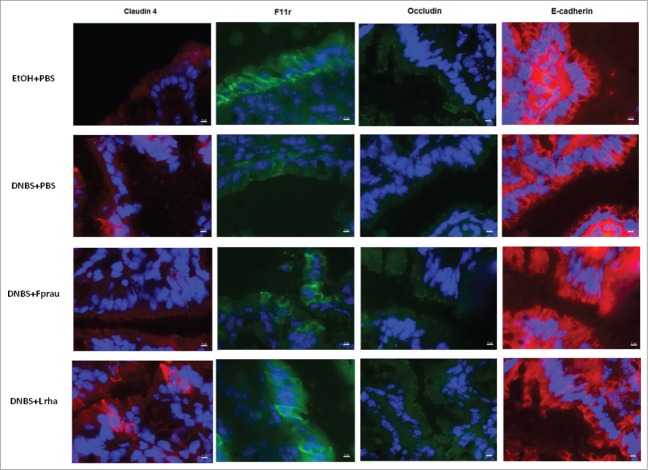
In order to decipher the mechanisms underlying the effects on intestinal barrier function, the modulation of the expression of apical junction proteins was assessed by RT-qPCR on colonic samples (Figs. 4 and 5). DNBS treatment globally reduced the expression of apical junction proteins, except Claudin-2 and Claudin-15 whose expression was not significantly decreased (Fig. 4). Claudin-4, Occludin, F11r, ZO-1 and E-Cadherin were significantly reduced in the DNBS-PBS group (P < 0.05) (Figs. 4 and 5). This reduction was counterbalanced with both L. rhamnosus CNCM I-3690 and F. prausnitzii treatments. Although clear tendencies were observed, statistically significant differences were only found in the DNBS-PBS group with L. rhamnosus CNCM I.3690 for E-cadherin and Occluding and with F. prausnitzii treatment for Claudin-4 and F11r (P < 0.05) (Fig. 4 and Fig. 5). Staining for Claudin-4, F11r, E-cadherin and Occludin- proteins (Fig. 6), according to the qPCR results, showed that L. rhamnosus CNCM I-3690 and F. prausnitzii tends to increase the expression of all these proteins.
Figure 4.
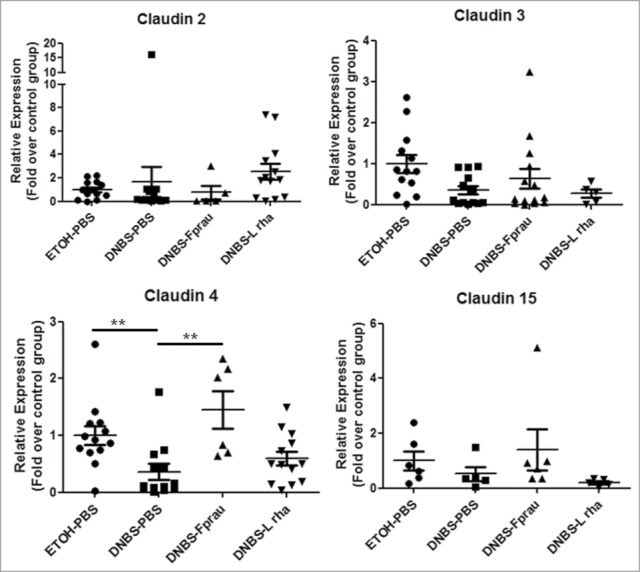
Modulation of Claudin family proteins expression. The expression of tight junction proteins was assessed by RT-qPCR on colonic tissue. Control non-inflamed group (EtOH-PBS), control inflamed group (DNBS-PBS), F. prausnitzii A2-165 strain (DNBS-Fprau), L. rhamnosus CNCM I-3690 strain (DNBS-Lrha) , L. paracasei CNCM I-3689 strain (DNBS-Lpar) Analysis done by Kruskal-Wallis followed by a Dunn's Multiple Comparison Test *P < 0.05 vs. DNBS-PBS. n = 6-13 mice per group.
Figure 5.
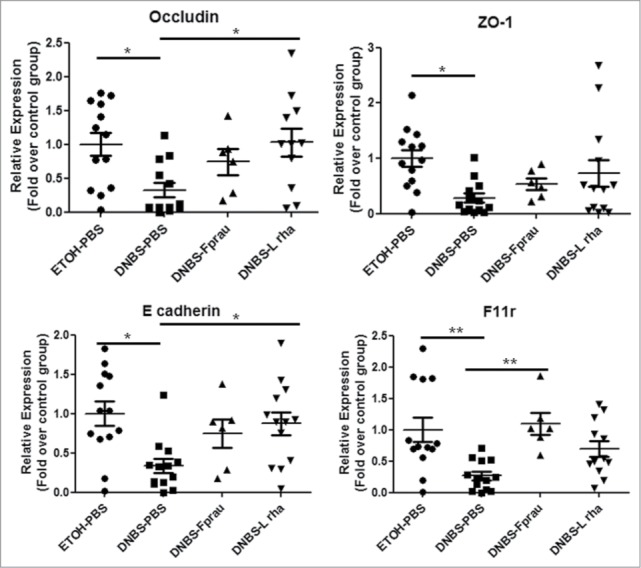
Modulation of apical-junction proteins expression. The expression of tight junction proteins was assessed by RT-qPCR on colonic tissue. Control non-inflamed group (EtOH-PBS), control inflamed group (DNBS-PBS), F. prausnitzii A2-165 strain (DNBS-Fprau), L. rhamnosus CNCM I-3690 strain (DNBS-Lrha) , L. paracasei CNCM I-3689 strain (DNBS-Lpar) Analysis done by Kruskal-Wallis followed by a Dunn's Multiple Comparison Test *P < 0.05 versus DNBS-PBS. n = 6–13 mice per group.
Figure 6.
Effect on apical junction proteins in a DNBS-induced low-grade inflammation model. Sections of the distal colon were stained for Claudin-4 (red), Fr11 (green), occludin (green) and E-cadherin (red) expression. Nuclei (DAPI; blue). Original magnification X60. Representative images control non-inflamed group (EtOH-PBS), control inflamed group (DNBS-PBS), F. prausnitzii A2-165 strain (DNBS-Fprau), L. rhamnosus CNCM I-3690 strain (DNBS-Lrha), L. paracasei CNCM I-3689 strain (DNBS-Lpar).
Lactobacillus rhamnosus CNCM I-3690 counterbalances the production of IL-6, IL-4 and IFN-γ cytokines induced by DNBS-treatment
To further analyze the anti-inflammatory properties of L. rhamnosus CNCM I-3690 in vivo, the cytokines production was assessed in both colonic and serum samples. No cytokine levels were detected in serum samples (data not shown). Compared with healthy controls, DNBS-treated mice showed high cytokines levels in colon samples (IL-13, IL-1α, IL-6, IL-22, IL-2, IL-27, IL-4, IFN-γ, TNF-α) (Fig. 7, data not shown) which suggest a weak local inflammation. L. rhamnosus CNCM I-3690 administration resulted in a reduction in the secretion of some of these cytokines that were induced by the DNBS-treatment. IL-4 and IFN-γ levels were significantly reduced in mice treated with either CNCM I-3690 or F. prausnitzii but not with the L. paracasei CNCM I-3689 strain (P < 0.05) (Fig. 7). A significant reduction in IL-6 levels was observed after treatment with all bacteria (P < 0.05) (Fig. 7).
Figure 7.
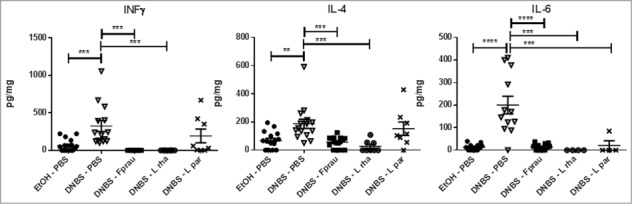
Measurement of colonic cytokine levels. Control non-inflamed group (EtOH-PBS), control inflamed group (DNBS-PBS), F. prausnitzii A2-165 strain (DNBS-Fprau), L. rhamnosus CNCM I-3690 strain (DNBS-Lrha), L. paracasei CNCM I-3689 strain (DNBS-Lpar) Analysis done by Kruskal-Wallis followed by a Dunn's Multiple Comparison Test *P < 0.05 vs. DNBS-PBS. n = 8-16 mice per group.
Discussion
Altered barrier function has been implicated in the pathogenesis of several diseases.33 There is increasing evidence for the presence of increased colonic permeability in inflammatory bowel diseases (IBD), Graft-versus-host disease (GVHD), Type-1 diabetes, human immunodeficiency virus HIV/AIDS, celiac disease and irritable bowel syndrome.8,34-39,40
Several microorganisms have been shown to protect the function of intestinal barrier and to enhance repair.15 Among them, L. acidophilus and Bacteroides thetaiotaomicron prevent the cytokine-induced increase in permeability.41 Other Lactobacilli also protect the intestinal barrier and several mechanisms have been proposed including enhancing membrane translocation of tight junction complex proteins either increasing or stabilizing Trans-Epithelial Electrical Resistance (TEER), and increasing antimicrobial peptide production.27
The L. rhamnosus CNCM I-3690 strain was first selected as an anti-inflammatory strain31 and then tested in an in vitro screening among several L. casei and L. rhamnosus strains for their ability to protect against TNF-α induced permeability increase TEER tests. Its anti-inflammatory profile has been confirmed in vitro in this study. Then, the effect of this strain has been validated in a mice model characterized by an alteration on gut permeability.
L. rhamnosus CNCM I-3690 suppressed TNF-α-induced epithelial permeability impairment on monolayers of Caco-2 cells and inhibited NF-κB signaling. Other lactobacilli have reported similar in vitro effects. Notably, L. rhamnosus GG protects from cytokine mediated alterations through inhibition of NF-κB signaling.26 TNF-α-pro-inflammatory actions are mainly mediated by NF-κB pathways42 having a pivotal role in the disruption of the TEER by TNF-α challenge in Caco-2 monolayers.43 Although, the apoptotic pathway has been pointed out as a major factor mediating barrier dysfunction in Caco-2-TNF-α TEER models, the cytokine concentration used in this study combined to the distinct nature of these 2 parameters (apoptosis and TEER) suggest that this is not the case in our study.26,44,45
To validate in vivo the protective anti-inflammatory effects and the protective role against permeability alteration of this strain, a chronic murine model was used to better mimic the relapsing nature of the symptoms of patients suffering from leaky gut-related diseases. Our results showed no severe or moderate inflammation in the colon as demonstrated by the absence of macro- and microscopic damages as well as by the lack of significant granulocyte infiltrates by MPO activity and of lipocalin 2, an early inflammation maker. However, DNBS-treated mice showed alteration in gut permeability measured by the paracellular tracer FITC-dextran. Treatment with both L. rhamnosus CNCM I-3690 and F. prausnitzii normalized in vivo permeability measurements of FITC-Dextran presence in serum, suggesting that overall total permeability was improved with both bacteria while treatment with L. paracasei CNCM I-3689, dismissing a possible strain-unspecific effect on this parameter.
The intestinal barrier is composed of a mucus layer and a monolayer of epithelial cells closely maintained by tight junctions (TJs), adherence junctions (AJs), desmosomes and gap junctions4,5 and their production and structural assembly are key determinants for intestinal permeability. The AJs are composed of cadherins, such as E-cadherin and is bound to α- and β-catenins. The TJs are composed of transmembrane proteins occludins, claudins and Junctional Adhesion Molecule (JAM/F11r) that are linked to the actin cytoskeleton through Zonula Occludens (ZO) proteins.5 To test the hypothesis of a DNBS-induced permeability due to an alteration of TJs and AJs levels, expression of the most relevant of these proteins was analyzed by RT-qPCR. Our results show that expression of TJ proteins is generally reduced by the DNBS treatment; however, this was protein-specific as not all the proteins studied have been altered due to DNBS challenge.
The intervention with L. rhamnosus CNCM I-3690 restored the expression of Occludin, E-cadherin and tended to restore the expression of F11r (JAM) and Claudin 4 in both qPCR and staining experiments. This result is in agreement with previous studies where a modulation of the expression of TJ proteins by probiotics in both in vitro and in vivo models was shown. L. rhamnosus OLL2838 strain suppressed the increase of intestinal permeability in DSS-treated mice and prevented the loss of ZO-1.46 Mennigen et al. showed that treatment with a mixture of lactic acid bacteria and bifidobacteria prevented changes observed in acute colitis: decreased expression and redistribution of the TJ proteins occludin, ZO-1, and claudin-1, claudin-3, claudin-4 and claudin-525 and Bifidobacterium lactis CNCM I-2494 restored occludin and JAM-A expressions to control levels.23 Furthermore, the effects of L. rhamnosus CNCM I-3690 are comparable to those found for F. prausnitzii-treated mice. Recently, Carlssonn et al. have found and improvement on claudin-1 and claudin-2 expressions in DSS-treated mice with F. prausnitzii supernatant.51,47
Modulation of permeability can induce activation of the mucosal immune system, due to bacterial translocation. We observed increased colonic permeability in association with immune activation and a mild increase in pro-inflammatory cytokines, such as IL-13, IL-1α, IL-6, IL-22, IL-2, IL-27, IL-4, IFN-γ, TNF-α. Both L. rhamnosus and F. prausnitzii treatments restored IFN-γ, IL-6 and IL-4 cytokine levels. Recent studies have linked cytokines to TJ proteins regulation. IFN-γ is known to increase intestinal permeability through the redistribution and expression of TJ proteins.48 IL-6 and IL-4 correlate to increased permeability potentially due to the induction of pore-forming claudin-2 expression.49,50 Furthermore, all of them were restored in both L. rhamnosus and F. prausnitzii-treated mice.
F. prausnitzii is one of the most prevalent commensal bacterium in the human gut51 and the full protection to DNBS-induced barrier impairment effects of this strain in this model is similar to that found for L. rhamnosus CNCM I-3690. Future studies may focus on the possible saturation of the system, as the dose dependent effect can help to discriminate between strains.
The traditional probiotic approach is based on lactic acid bacteria (LAB) strains, however nowadays new candidate bacteria belonging to the intestinal microbiota are being tested without taking into account the extremely difficult process to produce these bacteria at industrial scale and that normally they are have not the Qualified Presumption of Safety (QPS). Hence, the use QPS species with proven technological compliance remains a highly attractive option to deliver health beneficial bacteria to the host remains a feasible option to develop novel probiotic products. In addition, this justifies continuing the screening of LAB strains to look for potential candidates and to develop models better mimicking human symptoms in animals as previously performed by Kechaou et al. (2012).52
In summary, we showed that L. rhamnosus CNCM I-3690 strain partially restores cytokine-induced epithelial dysfunction and increased intestinal permeability caused by a mild inflammatory insult. The beneficial effect was comparable to the improvement detected previously with the commensal bacterium F. prausnitzii A2. Our results indicate the potential of L. rhamnosus CNCM I-3690 to prevent and to treat human pathologies associated with increase of intestinal permeability.
Material and Methods
Bacterial strains, cell lines and culture conditions
Lactobacillus strains from Danone Research collection (Table 1) and Lactobacillus rhamnosus GG (LGG), HN001 and L. casei BL23 were grown at 37°C in MRS medium (Difco, USA). Faecalibacterium prausnitzii A2-165 strain (DSMZ collection, Braunschweig, Germany) (DSM N°17677) was grown at 37°C in LYBHI medium (Brain-heart infusion medium supplemented with 0.5% yeast extract (Difco, Detroit, USA)) supplemented with cellobiose (1 mg/ml; Sigma-Aldrich Chemie GmbH, Buchs, Switzerland), maltose (1 mg/ml; Sigma-Aldrich), and cysteine (0.5 mg/ml; Sigma-Aldrich) in an anaerobic chamber at 37ºC.
Experiments were performed with the human intestinal epithelial cell line Caco-2 (EATCC, Port Down, UK). Cells were prepared as described in Piche et al., 2009.53 Caco-2 were grown in Dulbecco's modified Eagle's minimum essential medium (DMEM, pH 7.4) (Invitrogen) supplemented with 25 mM glucose, 10% inactivated fetal bovine serum (FBS) (Lonza), 1% penicillin streptomycin (PS) and 1% non-essential amino acid solution (Invitrogen). HT-29 (HTB-38) cells were grown in DMEM, supplemented with 10% inactivated FBS, 1% non-essential amino acids solution and 1% PS and gentamicin.
In vitro permeability assay
Caco-2 cells were grown on Transwell semi permeable filter support (12 mm diameter wells, polystyrene membranes with 0.4μm pores, Costar, Corning) and plated at 1×105 cells per well Cells with Trans-Epithelial Electrical Resistance (TEER) readings >900 ohms.cm−2. An overnight (ON) lactobacilli culture was washed in PBS and resuspended in DMEM medium. Strain LGG was used as positive control.54 TEER was measured before adding 1×106 CFU of lactobacilli onto the apical surface for 3 h prior to treatment of the basolateral medium with TNF-α (100 ng/ml) for 21 h at 37°C and at the end of TNF-α stimulation. Experiments were performed at least in duplicate. The resulting data presented as a ratio:
In vitro evaluation of immunomodulation properties of lactobacilli
Immunomodulation properties of lactobacilli were assessed in vitro as described before.31 Briefly, stable HT-29 transfectants containing the Luciferase (Luc) reporter gene were obtained after transfection with pIgK-luciferase plasmid. Forty-eight h before co-incubation, cells were seeded in 12-well plates at a cell density of 1×105/well using a final volume of 2 mL and incubated at 37°C, 10%. The day of the co-culture, bacteria from ON cultures were washed twice with PBS and resuspended in DMEM medium. This medium was renewed and cells were pre-incubated for 2 h with each bacterial strain at a multiplicity of infection (MOI) of 100 bacteria per cell. After 2 h of pre-incubation with bacteria, cells were stimulated with TNF-α (25 ng/well) for 6 h. After incubation, supernatants were collected and cells were rinsed with PBS and immediately lysed with lysis buffer 1X (Tris 25 mM pH7,4; MgCl2 8 mM; Triton 1X, Glycerol 15%; Roche) according to the manufacturer instructions. Cell lysates were frozen at −80°C for further detection of I-κB. Luminescence determination was performed by adding 10 μL of cell lystate to 100 μL of revelation buffer (lusis buffer, DTT 1 mM, ATP 1 mM, Luciferin 2 μM, Roche). Luciferase was quantified in a luminometer Centro LB 960 (Berthold technologies). Experiments were performed at least in triplicate. Results are expressed as ratio and percentage of NFkB activation. L. casei BL23 was used as control.
Animals and experimental design
Male C57BL/6 mice (6 weeks old, Janvier, France) were maintained under standard conditions in the animal facilities of the National Institute of Agricultural Research (UEAR, INRA Jouy-en-Josas, France) for 2 weeks before experimentation. Mice were anesthetized with intraperitoneal injection of 0.1% ketamine and 0.06% xylazine. A tube attached to a tuberculin syringe, was inserted into the colon. Inflammation was induced by intrarectal administration of 100 mg/Kg of DNBS solution (ICN, Biomedical Inc.) in 30% ethanol (EtOH). Control mice (without inflammation) received an equivalent amount of 30% ethanol. Mice were supervised all along the experiment with special attention during the first 3 days after DNBS administration hereafter named “DNBS period.” Ten days following “DNBS period,” bacteria were intragastrically administrated to mice during 10 days. Bacteria was previously grown, harvested, washed and frozen in PBS with 13% glycerol. The bacteria concentrations were determined after de-freezing. Approximately 1×109 CFU or 200 μL PBS (plus 13% glycerol) were daily administrated to each mouse. The study groups were as followed: control non-inflamed group (EtOH-PBS), control inflamed group (DNBS-PBS), F. prausnitzii A2-165 strain (DNBS-Fprau), L. rhamnosus CNCM I-3690 strain (DNBS-Lrha) and L. paracasei CNCM I-3689 strain (DNBS-Lpar). Inflammation was reactivated 21 days after the first DNBS injection with a second administration of 50 mg/Kg of DNBS solution. The severity of the inflammation was determined by determining the weight loss in the first 3 days after the second DNBS injection. Experiments were performed in duplicate. All procedures were carried out according to European Community rules of animal care and approved by the local committees.
To confirm the absence of overt inflammation, macroscopic and histological scores as well as myeloperoxidase (MPO) activity and lipocalin-2 concentration were determined as previously described.29
In vivo permeability assay
Barrier function was assessed at the endpoint (3 days after second DNBS injection) using FITC-labeled dextran tracer. Mice were administrated intragastrically with this permeability tracer (0.6 mg/g of body weight, molecular weight 3000-5000 Da, Sigma-Aldrich). A blood sample was collected from the retro-orbital venous plexus after 3.5 hours and fluorescence intensity was measured in serum (TECAN). The FITC-dextran concentration was determined from a standard curve obtained from serial dilution of FITC-dextran.
Cytokines levels
The colonic sample was homogenized in 400 μl of Tris-HCl buffer containing protease inhibitors (Sigma-Aldrich) in a Tissue Lyser. Samples were centrifuged for 20 min and the supernatant was frozen at 80ºC until assay. Before mice were sacrificed blood samples were obtained from the retro-orbital venous plexus, centrifuged and sera were stored at −80°C until further analysis. Levels of the cytokines IFN-γ, IL-1α, IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, IL-17, IL-21, IL-22, IL-27 and TNF-α) were determined using a cytometric bead array system (Mouse Th1/Th2/Th17/Th22 13plex Kit FlowCytomix, eBioscience).
Apical junctional analysis by quantitative real-time PCR and staining
Total RNA from 20-30 mg colon section was isolated using an RNeasy Mini Kit (Qiagen). Potential DNA contamination was removed by column DNAse treatment (Qiagen). RNA quantity and integrity was checked with NanoDrop (Thermo Scientific) and agarose gel electrophoresis. Only samples with intact RNA were used for subsequent cDNA synthesis with iScript reverse transcriptase (Bio-Rad). An amount of 500 μg of input RNA was used for each sample. Quantitative real-time PCR was performed with diluted cDNA (10x) in triplicate on iQ5 Real-Time Detection System (Bio-Rad). The reaction consisted of SsofastEvagreenSupermix (Bio-Rad), primers at 0.5 μM (Supplemental table S1), and 2 μL of diluted cDNA. Values were expressed as relative fold change normalized to housekeeping gene Gapdh by the 2-ΔΔCTmethod. All procedures were performed according to the manufacturer's instructions.
Protein expression of apical junctional proteins was evaluated also using immunofluorescence. Colon samples were embedded in Tissue-Tek OCT (Sakura, Torrance, CA). Frozen sections were then cut (5 μM), fixed with 3% paraformaldehyde (PFA) for 15 minutes at 20°C, and blocked with phosphate-buffered saline (PBS) / bovine serum albumin at 2% for 1 hour. Samples were immune-stained overnight with E-cadherin antibody (1:1000 dilution, BD Pharmaceutical), Occludin (1:200, Invitrogen), Claudin 4 (1:200, Invitrogen), JAM-A (1:100, R&D) and 1 hour with appropriate secondary antibody (1:250 dilution, Molecular Probes). Representative pictures from each animal were taken with the same exposure time.
Statistical analysis
Statistical analysis was completed using GraphPad software (GraphPad Sofware, La Jolla, CA, USA). Results are presented as bar graphs with means +/− SEM or dot plots with means +/- SEM. Most comparisons were performed by 1-way analysis of variance followed by the Student-Newman-Keuls multiple comparison post hoc analysis. For data sets that were non-Gaussian or based on a score or on a percentage, data was compared using the non-parametric test Kruskal-Wallis followed by a Dunn's Multiple Comparison Test. A p value of less than 0.05 was considered significant.
Disclosure of Potential Conflicts of Interest
LL receives a salary from Danone Nutricia Research in the framework of a CIFRE contract funded by ANRT. RM and SM receive a salary from FPARIS collaborative project. EFV holds a Canada Research Chair.
Acknowledgments
Authors would like to thank Sylvia Le Guin and all UEAR personal (especially Mathilde Bauducel) for their help. A special word to Jean-Jacques Gratadoux who helped us a lot in all microbiological and animal experiments.
Funding
The work was partially funded by CCFC grants to PL and EFV.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1. Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci 2013; 70:631-59; PMID:22782113; http://dx.doi.org/ 10.1007/s00018-012-1070-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Broer S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev 2008; 88:249-86; PMID:18195088; http://dx.doi.org/ 10.1152/physrev.00018.2006 [DOI] [PubMed] [Google Scholar]
- 3. Kiela PR, Ghishan FK. Ion transport in the intestine. Curr Opin Gastroenterol 2009; 25:87-91; PMID:19528875; http://dx.doi.org/ 10.1097/MOG.0b013e3283260900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bradley M D, Sanjay K Nigam. Molecular structure and assembly of the tight junction. Am J Physiol Renal Physiol 1998; 274:1-9. [DOI] [PubMed] [Google Scholar]
- 5. Natividad JM, Verdu EF. Modulation of intestinal barrier by intestinal microbiota: pathological and therapeutic implications. Pharmacol Res 2013; 69:42-51; PMID:23089410; http://dx.doi.org/ 10.1016/j.phrs.2012.10.007 [DOI] [PubMed] [Google Scholar]
- 6. Baum B, Georgiou M. Dynamics of adherens junctions in epithelial establishment, maintenance, and remodeling. J Cell Biol 2011; 192:907-17; PMID:21422226; http://dx.doi.org/ 10.1083/jcb.201009141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Slack E, Hapfelmeier S, Stecher B, Velykoredko Y, Stoel M, Lawson MA, Geuking MB, Beutler B, Tedder TF, Hardt WD, et al. Innate and adaptive immunity cooperate flexibly to maintain host-microbiota mutualism. Science 2009; 325:617-20; PMID:19644121; http://dx.doi.org/ 10.1126/science.1172747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Natividad JM, Huang X, Slack E, Jury J, Sanz Y, David C, Denou E, Yang P, Murray J, McCoy KD, et al. Host responses to intestinal microbial antigens in gluten-sensitive mice. PLoS One 2009; 4:e6472; PMID:19649259; http://dx.doi.org/ 10.1371/journal.pone.0006472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Geurts L, Neyrinck AM, Delzenne NM, Knauf C, Cani PD. Gut microbiota controls adipose tissue expansion, gut barrier and glucose metabolism: novel insights into molecular targets and interventions using prebiotics. Beneficial Microbes 2014; 5:3-17; PMID:23886976; http://dx.doi.org/ 10.3920/BM2012.0065 [DOI] [PubMed] [Google Scholar]
- 10. Camilleri M LK, Zhou W. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 2012; 303:775-85; http://dx.doi.org/ 10.1152/ajpgi.00155.2012 [DOI] [PubMed] [Google Scholar]
- 11. Perrier C CB. Gut permeability and food allergies. Clin Exp Allergy 2011; 41:20-8; PMID:21070397; http://dx.doi.org/ 10.1111/j.1365-2222.2010.03639.x [DOI] [PubMed] [Google Scholar]
- 12. Vaarala O. Is the origin of type 1 diabetes in the gut? Immunol Cell Biol 2012; 90:271-6; PMID:22290506; http://dx.doi.org/ 10.1038/icb.2011.115 [DOI] [PubMed] [Google Scholar]
- 13. Vetrano S, Ploplis VA, Sala E, Sandoval-Cooper M, Donahue DL, Correale C, Arena V, Spinelli A, Repici A, Malesci A, et al. Unexpected role of anticoagulant protein C in controlling epithelial barrier integrity and intestinal inflammation. Proc Natl Acad Sci U S A 2011; 108:19830-5; PMID:22109555; http://dx.doi.org/ 10.1073/pnas.1107140108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Baarlen P, Wells JM, Kleerebezem M. Regulation of intestinal homeostasis and immunity with probiotic lactobacilli. Trends Immunol 2013; 34:208-15; PMID:23485516; http://dx.doi.org/ 10.1016/j.it.2013.01.005 [DOI] [PubMed] [Google Scholar]
- 15. Turpin W, Humblot C, Thomas M, Guyot JP. Lactobacilli as multifaceted probiotics with poorly disclosed molecular mechanisms. Int J Food Microbiol; 143:87-102; PMID:20801536; http://dx.doi.org/ 10.1016/j.ijfoodmicro.2010.07.032 [DOI] [PubMed] [Google Scholar]
- 16. DuPont AW, DuPont HL. The intestinal microbiota and chronic disorders of the gut. Nat Rev Gastroenterol Hepatol 2011; 8:523-31; PMID:21844910; http://dx.doi.org/ 10.1038/nrgastro.2011.133 [DOI] [PubMed] [Google Scholar]
- 17. Madara JL, Stafford J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J Clin Invest 1989; 83:724-7; PMID:2492310; http://dx.doi.org/ 10.1172/JCI113938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jacob C, Yang PC, Darmoul D, Amadesi S, Saito T, Cottrell GS, et al. Mast cell tryptase controls paracellular permeability of the intestine. Role of protease-activated receptor 2 and β-arrestins. J Biol Chem 2005; 280:31936-48; PMID:16027150; http://dx.doi.org/ 10.1074/jbc.M506338200 [DOI] [PubMed] [Google Scholar]
- 19. Smith K, McCoy KD, Macpherson AJ. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Semin Immunol 2007; 19:59-69; PMID:17118672; http://dx.doi.org/ 10.1016/j.smim.2006.10.002 [DOI] [PubMed] [Google Scholar]
- 20. Berkes J, Viswanathan VK, Savkovic SD, Hecht G. Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut 2003; 52:439-51; PMID:12584232; http://dx.doi.org/ 10.1136/gut.52.3.439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science 2001; 292:1115-8; PMID:11352068; http://dx.doi.org/ 10.1126/science.1058709 [DOI] [PubMed] [Google Scholar]
- 22. Distrutti E CS, Mencarelli A, Renga B, Fiorucci S. Probiotics VSL#3 protect against development of visceral pain in murine model of irritable bowel syndrome. PLoS One 2013; 8:e63893; http://dx.doi.org/ 10.1371/journal.pone.0063893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Agostini S GM, Tondereau V, Salvador-Cartier C, Bezirard V, Lévèque M, Keränen H, Theodorou V, Bourdu-Naturel S, Goupil-Feuillerat N, Legrain-Raspaud S, et al. A marketed fermented dairy product containing Bifidobacterium lactis CNCM I-2494 suppresses gut hypersensitivity and colonic barrier disruption induced by acute stress in rats. Neurogastroenterol Motil 2012; 24:376:e178; PMID:22272920; http://dx.doi.org/ 10.1111/j.1365-2982.2011.01865.x [DOI] [PubMed] [Google Scholar]
- 24. Ukena SN SA, Dringenberg U, Engelhardt R, Seidler U, Hansen W, Bleich A, Bruder D, Franzke A, Rogler G, Suerbaum S, et al. Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS One 2007; 2:e1308; PMID:18074031; http://dx.doi.org/ 10.1371/journal.pone.0001308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mennigen R NK, Rijcken E, Utech M, Loeffler B, Senninger N, Bruewer M. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am J Physiol Gastrointest Liver Physiol 2009; 296:1140-9; http://dx.doi.org/ 10.1152/ajpgi.90534.2008 [DOI] [PubMed] [Google Scholar]
- 26. Donato KA GM, Wang YJ, Sherman PM. Lactobacillus rhamnosus GG attenuates interferon-{gamma} and tumour necrosis factor-α-induced barrier dysfunction and pro-inflammatory signalling. Microbiology 2010; 156:3288-97; PMID:20656777; http://dx.doi.org/ 10.1099/mic.0.040139-0 [DOI] [PubMed] [Google Scholar]
- 27. Natividad JM, Hayes CL, Motta JP, Jury J, Galipeau HJ, Philip V, Garcia-Rodenas CL, Kiyama H, Bercik P, Verdu EF. Differential induction of antimicrobial REGIII by the intestinal microbiota and Bifidobacterium breve NCC2950. App Environ Microbiol 2013; 79:7745-54; PMID:24096422; http://dx.doi.org/ 10.1128/AEM.02470-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sokol H PB, Watterlot L, Lakhdari O, Bermúdez-Humarán LG, Gratadoux JJ, Blugeon S, Bridonneau C, Furet JP, Corthier G, Grangette C, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A 2008; 105:16731-6; http://dx.doi.org/ 10.1073/pnas.0804812105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martin R, Chain F, Miquel S, Lu J, Gratadoux JJ, Sokol H, et al. The Commensal Bacterium Faecalibacterium prausnitzii Is Protective in DNBS-induced Chronic Moderate and Severe Colitis Models. Inflammatory Bowel Diseases 2014; 20:417-30. [DOI] [PubMed] [Google Scholar]
- 30. Martín R, Miquel S, Chain F, Natividad JM, Jury J, Lu J, Sokol H, Theodorou V, Bercik P, Verdu EF, Langella P, Bermúdez-Humarán LG. Faecalibacterium prausnitzii prevents physiological damages in a chronic low-grade inflammation murine model. Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grompone G MP, Llopis S, González N, Genovés S, Mulet AP, Fernández-Calero T, Tiscornia I, Bollati-Fogolín M, Chambaud I, Foligné B, et al. Anti-inflammatory Lactobacillus rhamnosus CNCM I-3690 strain protects against oxidative stress and increases lifespan in Caenorhabditis elegans. PLoS One 2012; 7:e52493; PMID:23300685; http://dx.doi.org/ 10.1371/journal.pone.0052493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Prisciandaro LD, Geier MS, Chua AE, Butler RN, Cummins AG, Sander GR, Howarth GS. Probiotic factors partially prevent changes to caspases 3 and 7 activation and transepithelial electrical resistance in a model of 5-fluorouracil-induced epithelial cell damage. Suppor Care Cancer 2012; 20:3205-10; PMID:22526145; http://dx.doi.org/ 10.1007/s00520-012-1446-3 [DOI] [PubMed] [Google Scholar]
- 33. Odenwald MA, Turner JR. Intestinal permeability defects: is it time to treat? Clin Gastroenterol Hepatol 2013; 11:1075-83; http://dx.doi.org/ 10.1016/j.cgh.2013.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Suenaert P, Bulteel V, Lemmens L, Noman M, Geypens B, Van Assche G, Geboes K, Ceuppens JL, Rutgeerts P. Anti-tumor necrosis factor treatment restores the gut barrier in Crohn's disease. Am J Gastroenterol 2002; 97:2000-4; http://dx.doi.org/ 10.1111/j.1572-0241.2002.05914.x [DOI] [PubMed] [Google Scholar]
- 35. Scheinin T, Butler DM, Salway F, Scallon B, Feldmann M. Validation of the interleukin-10 knockout mouse model of colitis: antitumour necrosis factor-antibodies suppress the progression of colitis. Clin Exp Immunol 2003; 133:38-43; http://dx.doi.org/ 10.1046/j.1365-2249.2003.02193.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brown GR, Lindberg G, Meddings J, Silva M, Beutler B, Thiele D. Tumor necrosis factor inhibitor ameliorates murine intestinal graft-versus-host disease. Gastroenterology 1999; 116:593-601. [DOI] [PubMed] [Google Scholar]
- 37. Epple HJ, Schneider T, Troeger H, Kunkel D, Allers K, Moos V, Amasheh M, Loddenkemper C, Fromm M, Zeitz M, et al. Impairment of the intestinal barrier is evident in untreated but absent in suppressively treated HIV-infected patients. Gut 2009; 58:220-7; PMID:18936106; http://dx.doi.org/ 10.1136/gut.2008.150425 [DOI] [PubMed] [Google Scholar]
- 38. Dunlop SP, Hebden J, Campbell E, Naesdal J, Olbe L, Perkins AC, Spiller RC. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol 2006; 101:1288-94; PMID:16771951; http://dx.doi.org/ 10.1111/j.1572-0241.2006.00672.x [DOI] [PubMed] [Google Scholar]
- 39. Smecuol E, Bai JC, Vazquez H, Kogan Z, Cabanne A, Niveloni S, Pedreira S, Boerr L, Mauriño E, Meddings JB. Gastrointestinal permeability in celiac disease. Gastroenterology 1997; 112:1129-36; PMID:9097995; http://dx.doi.org/ 10.1016/S0016-5085(97)70123-9 [DOI] [PubMed] [Google Scholar]
- 40. Ohman L, Simren M. Pathogenesis of IBS: role of inflammation, immunity and neuroimmune interactions. Nat Revi Gastroenterol Hepatol 2010; 7:163-73; PMID:20101257; http://dx.doi.org/ 10.1038/nrgastro.2010.4 [DOI] [PubMed] [Google Scholar]
- 41. Resta-Lenert S, Barrett KE. Probiotics and commensals reverse TNF-α- and IFN-gamma-induced dysfunction in human intestinal epithelial cells. Gastroenterology 2006; 130:731-46; PMID:16530515; http://dx.doi.org/ 10.1053/j.gastro.2005.12.015 [DOI] [PubMed] [Google Scholar]
- 42. Chen F, Castranova V, Shi X, Demers LM. New insights into the role of nuclear factor-kappaB, a ubiquitous transcription factor in the initiation of diseases. Clin Chem 1999; 45:7-17; PMID:9895331 [PubMed] [Google Scholar]
- 43. Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, Said HM. TNF-α-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol 2004; 286:G367-76; PMID:14766535; http://dx.doi.org/ 10.1152/ajpgi.00173.2003 [DOI] [PubMed] [Google Scholar]
- 44. Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, Nusrat A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol 2003; 171:6164-72; PMID:14634132; http://dx.doi.org/ 10.4049/jimmunol.171.11.6164 [DOI] [PubMed] [Google Scholar]
- 45. Yan F, Polk DB. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J Biol Chem 2002; 277:50959-65; PMID:12393915; http://dx.doi.org/ 10.1074/jbc.M207050200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Miyauchi E MH, Tanabe S. Lactobacillus rhamnosus alleviates intestinal barrier dysfunction in part by increasing expression of zonula occludens-1 and myosin light-chain kinase in vivo. J Dairy Sci 2009; 92:2400-8; PMID:19447972; http://dx.doi.org/ 10.3168/jds.2008-1698 [DOI] [PubMed] [Google Scholar]
- 47. Carlsson AH, Yakymenko O, Olivier I, Hakansson F, Postma E, Keita AV, Söderholm JD. Faecalibacterium prausnitzii supernatant improves intestinal barrier function in mice DSS colitis. Scand J Gastroenterol; 48:1136-44; PMID:23971882; http://dx.doi.org/ 10.3109/00365521.2013.828773 [DOI] [PubMed] [Google Scholar]
- 48. Bruewer M UM, Ivanov AI, Hopkins AM, Parkos CA, Nusrat A. Interferon-gamma induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. FASEB J 2005:923-33; PMID:15923402; http://dx.doi.org/ 10.1096/fj.04-3260com [DOI] [PubMed] [Google Scholar]
- 49. Suzuki T YN, Tanabe S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J Biol Chem 2011; 286:31263-71; PMID:21771795; http://dx.doi.org/ 10.1074/jbc.M111.238147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wisner DM HLr, Green CL, Poritz LS. Opposing regulation of the tight junction protein claudin-2 by interferon-gamma and interleukin-4. J Surg Res 2008; 144:1-7; PMID:17640674; http://dx.doi.org/ 10.1016/j.jss.2007.03.059 [DOI] [PubMed] [Google Scholar]
- 51. Tap J, Mondot S, Levenez F, Pelletier E, Caron C, Furet JP, Ugarte E, Muñoz-Tamayo R, Paslier DL, Nalin R, et al. Towards the human intestinal microbiota phylogenetic core. Environ Microbiol 2009; 11:2574-84; PMID:19601958; http://dx.doi.org/ 10.1111/j.1462-2920.2009.01982.x [DOI] [PubMed] [Google Scholar]
- 52. Kechaou N, Chain F, Gratadoux JJ, Blugeon S, Bertho N, Chevalier C, Le Goffic R, Courau S, Molimard P, Chatel JM, et al. Identification of one novel candidate probiotic Lactobacillus plantarum strain active against influenza virus infection in mice by a large-scale screening. Appl Environ Microbiol 2013; 79:1491-9; PMID:23263960; http://dx.doi.org/ 10.1128/AEM.03075-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Piche T, Barbara G, Aubert P, Bruley des Varannes S, Dainese R, Nano JL, Cremon C, Stanghellini V, De Giorgio R, Galmiche JP, et al. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut 2009; 58:196-201; PMID:18824556; http://dx.doi.org/ 10.1136/gut.2007.140806 [DOI] [PubMed] [Google Scholar]
- 54. Donato KA, Gareau MG, Wang YJ, Sherman PM. Lactobacillus rhamnosus GG attenuates interferon-{gamma} and tumour necrosis factor-α-induced barrier dysfunction and pro-inflammatory signalling. Microbiology 2010; 156:3288-97; PMID:20656777; http://dx.doi.org/ 10.1099/mic.0.040139-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.