Abstract
Nucleoli in mammalian oocytes and zygotes, sometimes referred to as nucleolus precursor bodies (NPBs), are compact and morphologically different from nucleoli in somatic cells. We applied a unique NPB analyzing method “enucleolation” technique to zygotes to remove the NPBs. It has been reported that oocyte NPBs are essential for embryonic development; in their absence, the oocytes complete maturation and can be fertilized, but no nucleoli are formed in the zygotes and embryos, leading to developmental failure. However, we found that when NPBs were removed from zygotes, the zygotes developed successfully to live-born pups. These results indicated that oocyte NPBs are essential for embryonic development, but zygote NPBs are not. In addition, the enucleolated zygotes formed somatic-type nucleoli during early embryonic development, demonstrating that somatic-type nucleoli do not originate from zygote NPBs. We summarize our recent investigation on NPBs, and provide additional comments and findings.
Keywords: embryo, mouse, NPB, nucleolus, oocyte
Introduction
The oocyte nucleolus was first reported in 1835 in the jellyfish,1 and in mammals it was first documented in the rabbit in 1842.2 In 1964, Brown and Gurdon showed that anucleolate (no nucleoli) mutant Xenopus embryos exhibited arrested development due to their inability to synthesize new ribosomes, suggesting that the nucleolus is the site of rRNA (rRNA) synthesis and nascent ribosome assembly.3 Since then, the nucleoli in somatic cells have been considered as the site of rRNA synthesis and ribosomal subunit assembly.4 Apart from those nucleolus studies, extensive studies on in vitro maturation and fertilization of mammalian oocytes have greatly advanced our fundamental knowledge of mammalian reproduction since the mid-20th century.5 Thus it is well established that mammalian oocytes grow in the ovary, mature after periodic gonadotropic stimulation from the pituitary, and are ovulated into the oviduct and fertilized by the spermatozoon. During the growth phase, the oocyte nucleolus is engaged in rRNA synthesis and ribosome assembly much like the nucleolus in somatic cells; however, in oocytes the nucleolus changes its morphology and decreases its rRNA synthetic activity as the cells approach full-size.6 Finally, in the fully-grown oocytes, a single large nucleolus, which does not contain DNA, is formed in the nucleus (germinal vesicle: GV).7 This nucleolus is transcriptionally inactive and structurally distinct from the nucleoli in somatic cells, and has been termed a nucleolus precursor body (NPB).8 A single large and morphologically distinguishable nucleolus is seen in oocytes from various species, such as starfish9,10 and sea urchins,11 although it is not clear whether the nucleoli in invertebrate oocytes is similar in character to mammalian NPBs. In contrast, the Xenopus oocyte contains as many as 1,500 nucleoli.12 And in spite of these advances in understanding, the actual reason that the NPB evolved in mammals is unknown and remains a topic of great interest.
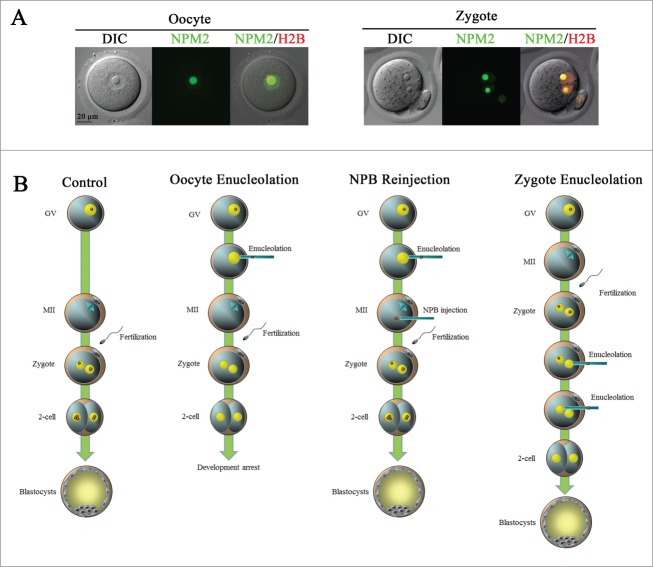
During the maturation of mammalian oocytes, the NPBs disappear, and upon fertilization the NPBs are formed again in male and female pronuclei of zygotes (Fig. 1A). The zygote NPBs are transcriptionally inactive and morphologically similar to the oocyte NPBs.8 Our recent studies showed that enucleolated mammalian oocytes, whose NPBs had been removed micro-surgically at the GV-stage, were able to mature to metaphase II (MII) and to be fertilized.13-15 However, the enucleolated oocytes neither formed NPBs in zygotes nor developed to blastocysts after fertilization (Fig. 1B). When oocyte NPBs were re-injected into previously enucleolated oocytes at MII and these oocytes were fertilized, they formed pronuclei with NPBs and developed to full term.13 Thus, zygotes inherit their NPBs from oocytes, and oocyte NPBs are essential for embryonic development. We have also shown that zygote NPBs are not required for embryonic development. When zygote NPBs were removed, the enucleolated zygotes formed new nucleoli after several divisions and developed to live pups. Here, we summarize these studies on oocyte and zygote NPBs, and briefly discuss the role of NPBs in early embryonic development.
Figure 1.
(A). The morphology of oocyte nucleolus precursor bodies (NPBs) is similar to that of zygote NPBs. Confocal imaging was performed after an injection with NPM2-EGFP (green) and H2B-mCherry (red) mRNAs. (B). Experimental summary of oocyte and zygote enucleolation. NPBs were microsurgically aspirated from germinal vesicles (GVs) to produce enucleolated oocytes (Oocyte Enucleolation). Oocyte NPBs were re-injected into previously enucleolated oocytes at MII (NPB Re-injection). The NPBs of zygotes were aspirated from both pronuclei (Zygote Enucleolation).
The Dynamics of NPBs
During oogenesis in mammals, oocytes become arrested at the diplotene stage of the first meiotic prophase and begin growing. Dynamic changes in nucleolar morphology during oogenesis and embryogenesis in mammals have been observed by electron microscopy from the 1960s.16 The reticulated nucleoli of non-growing and growing oocytes are composed of fibrillar centers, dense fibrillar components, and granular components.17-19 The oocyte nucleolus is the site of active rRNA synthesis and ribosome production.20 The nucleoli contain chromatin, although the chromatin is moved out of the nucleoli during oocyte growth, and finally in fully grown oocytes the nucleoli are transformed into a single compacted NPB, which no longer contains DNA, and has no rRNA synthetic activity (Fig. 2).7,18After the GV breakdown of oocytes, NPB materials diffuse into the oocyte cytoplasm, and upon fertilization, NPBs are formed again in male and female pronuclei of zygotes.8 The compact NPBs remain as the core in the process of nucleolus formation during the development of embryos,8 and reticulated nucleoli are gradually formed around the NPBs. Then, the nucleoli become transcriptionally active (at the end of the 2-cell stage in mouse embryos) to synthesize rRNA, and at the morula stage the somatic-type nucleoli are formed and the original NPBs disappear.22 Although the dynamics of NPBs during oocyte maturation and early embryonic development has been well characterized, their function has not been elucidated.
Figure 2.
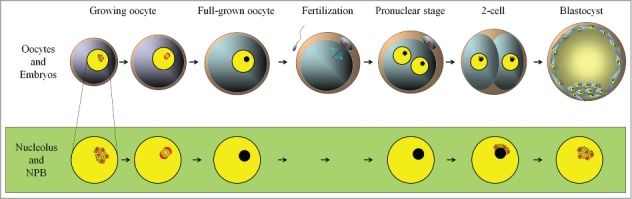
The nucleoli of growing oocytes in mammals are composed of fibrillar centers (red), dense fibrillar components, and granular components (black dots). At the end of the growth phase, nucleoli are inactivated to become NPBs (black). During oocyte maturation, the nucleoli (NPBs) disappear, and upon fertilization, NPBs (black) are formed in pronuclei. At the end of the 2-cell stage, fibrillar centers and fibrillar components (red), and granular components (dots) develop on the surface of NPBs. This figure is based on Figure 2 from the study by Hyttel (2011; reference (21)) with some modifications.
The Enucleolation Technique
About 10 years ago, a new method for analyzing the NPB was introduced by Fulka and his colleagues.23 They showed that oocyte NPBs can be microsurgically removed (enucleolation) in a process akin to enucleation in animal cloning.24 In our recent experiments, we applied this enucleolation method to zygotes, from which we were thereby able to remove the NPBs.14
The enucleolation method is technically impressive, but its effectiveness is sometimes questioned by other researchers. That is because the perinucleolar chromatin rings are tightly associated with NPBs in oocytes and zygotes, as shown by the staining of DNA and epigenetic markers such as HP1 β and H3K9me. 15,25,26 Thus a question arises as to how NPBs could be selectively removed without any damage to the DNA. A previous report established that there is no DNA damage during enucleolation.13 This report showed that when oocyte NPBs were re-injected into previously enucleolated oocytes at MII, the oocytes were fertilized and developed to full term. Moreover, the chromosome spreads of enucleolated oocytes showed undamaged chromosomes.27
The procedure used to enucleolated oocytes without sucking out the DNA is outlined in Figure 3. First, the NPBs of oocytes or zygotes were aspirated from outside of the nuclear envelopes. Oocytes and zygotes were treated with an inhibitor of actin polymerization, cytochalasin B, to soften the cell membrane. Using a square-ended injection pipette, the zona pellucida was punctured by a PIEZO pulse, and then the tip of the pipette was pushed into the cytoplasm to reach the nuclear envelope near the NPB, while leaving the cell membrane intact. Thereafter, gentle suction was applied through the cell membrane and the nuclear envelope. As a result of this suction, the NPB penetrated the nuclear envelope and moved into the mouth of the pipette with the surrounding cell membrane. In this process, the nuclear envelope seemed to work as a filter, such that all the chromatin remained inside the nuclear envelope. In other words, the NPB was able to pass through the nuclear envelope without breaking it, perhaps due to the NPB behaving as a liquid-like droplet.12 Moreover, when NPBs were injected into the cytoplasm of enucleolated GV oocytes, the injected NPBs were disassembled in the oocyte cytoplasm and gradually reassembled in the GV.28 The NPB materials dispersed in the cytoplasm by the NPB disassembly may pass through the nuclear envelope into the nucleoplasm. However, the details of how NPBs pass through the nuclear membrane remain to be determined.
Figure 3.
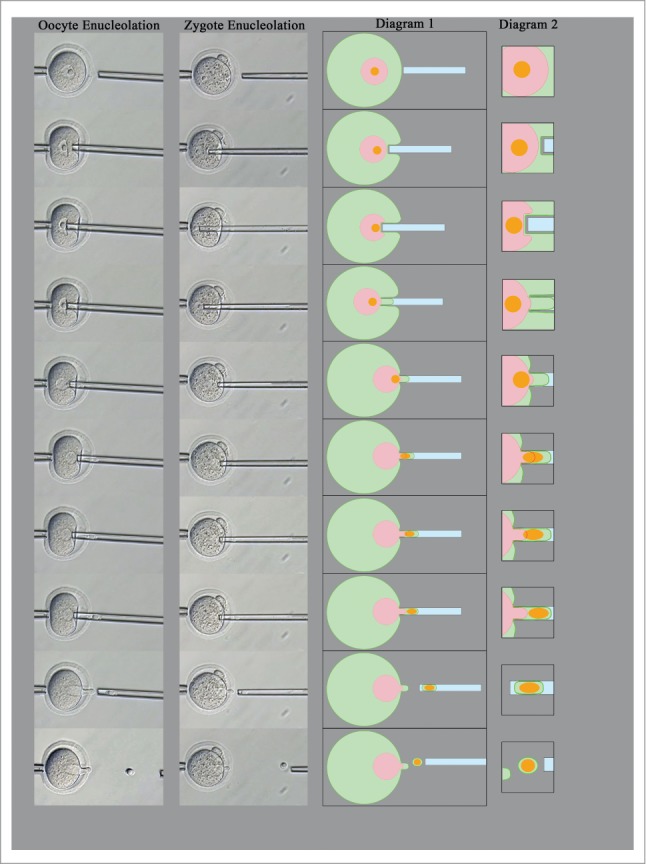
Enucleolation of oocytes and zygotes. An injection pipette is used to penetrate the zona pellucida, and its tip is pushed against the nuclear envelope. Due to the mild suction from the outside cell membrane and nuclear envelope, the NPB is preferentially aspirated into the injection pipette through the nuclear envelope. The NPB can then be detached from the zygote. Diagrams show the cytoplasm (green), nucleoplasm (red), and NPBs (orange).
De novo Formation of Nucleoli in Developing Embryos
In our recent report, we applied this enucleolation method to zygotes and yielded interesting results. After removal of NPBs from zygotes, the embryos originating from enucleolated zygotes formed new somatic-type nucleoli after several divisions. This result contradicts a classical dogma in developmental biology (embryology), since it is commonly accepted that nucleoli in developing embryos originate from zygote NPBs that are then, as the embryos develop, gradually transformed into fully differentiated nucleoli.29
In mouse embryos, proteins required for nucleolar function, such as upstream binding factor (UBF), fibrillarin and B23, begin to assemble at the periphery of NPBs before rDNA transcription resumes.30 During embryonic development, the differentiation of reticulated nucleoli is strictly limited to the periphery of NPBs, while the core of NPBs remains compact and detectable in the following cleavage stages up to the morula.31 At the blastocyst stage, reticulated somatic-type nucleoli are formed in every nucleus (Fig. 2). Thus, the nucleolar material originating from zygote NPBs has been thought to be required for the assembly of fully functional somatic-type nucleoli at a later stage of embryonic development.22 However, our study showed that reticulated nucleoli develop in enucleolated zygotes; we observed nucleolus formation in enucleolated zygotes during early embryonic development by immunostaining against nucleolus markers for somatic cells (B23 and UBF). The embryos derived from enucleolated zygotes had no visible nucleoli at the 2-cell stage, but directly formed somatic-type nucleoli at the 4-cell stage. The nucleoli were B23- and UBF-positive and did not have compact cores like those observed in embryos from intact zygotes. It has not been determined whether nucleoli in the embryos derived from enucleolated zygotes are fully functional in rRNA synthesis; however, at least the embryos developed to live-born pups at a rate similar to sham-operated zygotes. These results indicate that reticulated somatic-type nucleoli develop even without NPBs in early embryos.
De novo nucleolus formation in embryos is also observed in NPM2 (nucleoplasmin 2)-knockout mice (unpublished data). NPM2 is an oocyte-specific nuclear protein and a component of oocyte and zygote NPBs in mouse oocytes and zygotes.32,33 NPM2−/− mouse oocytes and embryos never form NPBs in the nuclei. However, some embryos develop beyond the 2-cell stage, and a few develop to blastocysts after fertilization.33 We examined nucleolus formation in developing NPM2−/− mouse embryos after an injection with B23-EGFP mRNA. B23 is one of the proteins in reticulated somatic-type nucleoli in mouse embryos.27 In NPM2+/+ embryos, at the end of the 2-cell stage, robust B23-EGFP signals were detected around the NPBs (Fig. 4), and then reticulated somatic-cell type nucleoli were gradually formed as the embryos developed (unpublished data). The NPM2−/− embryos had no visible NPBs throughout embryonic development to blastocysts. However, robust EGFP signals were observed from the 2-cell stage and somatic-cell type nucleoli were formed directly at the end of the 2-cell stage (Fig. 4). These findings also indicate that zygotes without NPBs are able to form somatic-type nucleoli, and that zygote NPBs do not contribute to the nucleoli of somatic cells.
Figure 4.
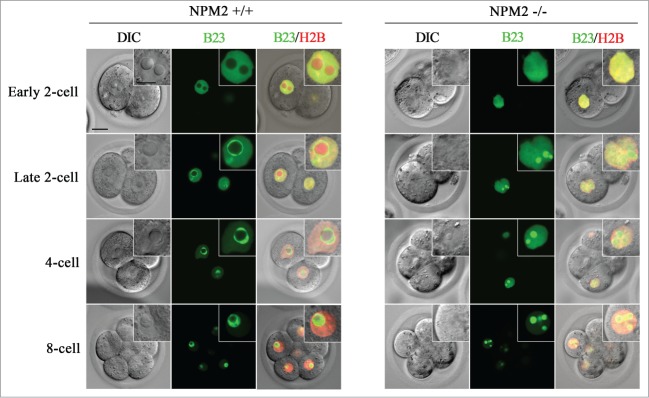
Nucleolus formation in the embryos originating from NPM2−/− zygotes. Confocal imaging was performed after an injection with B23-EGFP (green) and H2B-mCherry (red) mRNAs into the oocytes at MII. There is no NPB formation in NPM2−/− embryos after fertilization. At the late 2-cell stage, both types of embryos formed nucleoli that showed robust EGFP signals. Bars in the figures and insets indicate 20 μm and 10 μm, respectively.
What is The Role of NPB?
When NPBs were removed from oocytes, the enucleolated oocytes matured to MII, but the enucleolated and matured oocytes did not develop to blastocysts after fertilization.13,14 Moreover, when 2-cell embryos derived from enucleolated oocytes were transferred into recipients’ oviducts, they never developed to live-born pups. These results show that oocyte NPBs are essential for embryonic development after fertilization (Fig. 1B). However, when NPBs were removed from zygotes, the zygotes cleaved and developed to blastocysts.14 After the transfer of 2-cell embryos derived from enucleolated zygotes, live-born pups were obtained. All of these offspring grew into adults with full fertility. These findings suggest that oocyte NPBs are essential for embryonic development,13 whereas zygote NPBs are not.14 This paradox could be explained by the difference of components between oocyte and zygote NPBs. However, when NPBs from 2-cell stage embryos were transferred into enucleolated oocytes at the MII stage, the resulting oocytes restored the developmental ability to blastocysts.34 This result implies that NPBs from 2-cell stage embryos support the embryonic development instead of oocyte NPBs, and NPB materials function between fertilization and the first cleavage of embryos.
To determine the stage at which the NPB materials are required, a previous study investigated the timing of NPB re-injection into MII oocytes or zygotes originating from enucleolated oocytes.15 When the NPBs were re-injected into zygotes at the pronuclear stage, both blastocyst development and full-term development were severely retarded compared to those when the NPBs were re-injected into oocytes at the MII stage. This finding again indicates that NPB materials are essential only between MII and the pronuclear stage of zygotes. During this step, male and female chromosomes are dynamically changed and NPBs are associated with heterochromatin. The NPBs (or NPB materials) may be important for decondensation and modification (reprogramming) of chromatin. In cloned embryos produced by somatic cell nuclear transfer, multiple NPB formation in pseudo-pronuclei is one of the apparent abnormalities.24 This abnormality indicates that NPBs may have a functional role in chromosome decondensation or genome reprogramming.
NPM2 has been suggested to be the factor responsible for sperm chromatin decondensation (SCD).35 However, male pronucleus formation following SCD has been shown to occur in enucleolated oocytes,13,35 as well as in Npm2−/− oocytes35 after fertilization. These results suggest that other factors are also involved in SCD, and that SCD is not the main function of NPBs. A recent report showed that NPBs were important for centromere satellite maintenance during pronucleus organization.27 This study reported that the embryos derived from enucleolated oocytes showed abnormal chromatin remodeling, replication and expression of centric and pericentric satellite DNA accompanied by developmental arrest. The role of nucleoli in cell cycle regulation has been documented in yeasts36-38 and somatic cells.39 In surf clam oocytes, the nucleolinus, an RNA-rich structure inside the nucleolus, was associated with spindle formation and cell division.40,41 However, the critical role of NPBs in mammals remains to be determined.
Conclusion
Zygote NPBs are required neither for formation of somatic-type nucleoli during embryonic development nor for the full-term development of embryos, but oocyte NPB materials appear to be required at the early step of pronucleus organization in zygotes. The NPBs may have specific functions which are completely different from those of somatic nucleoli. Elucidating the function of various molecules in NPBs and the role of NPBs in embryonic development remain future challenges.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank M. M. Matzuk (Baylor College of Medicine) for providing the NPM2-KO mice; and K. Hamada (RIKEN Center for Developmental Biology) for her generous support; and we are grateful to the Laboratory for Animal Resources and Genetic Engineering for housing the mice.
Funding
This work was supported by a Research Fellowship for Young Scientists (to HK) and a Grant-in-Aid (KAKENHI Grant Number 25660253 to TM) from the Japan Society for the Promotion of Science.
References
- 1. Wanger R. Einige bemerkungen und fragen über das keimbläschen (vesicular germinativa). Muller's Archiv Anat Physiol Wissenschaft Med 1835; 373-377. [Google Scholar]
- 2. Bischoff TLW. Entwicklungsgeschichte des Kaninchen-Eies. Druck und Verlag von Friendrich Vieweg und Sohn 1842. [Google Scholar]
- 3. Brown D, Gurdon J. Absence of rRNA synthesis in the anucleolate mutant of X. laevis. Proc Natl Acad Sci USA 1964; 51:139-146; PMID:14106673; http://dx.doi.org/ 10.1073/pnas.51.1.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Olson MOJ. Introduction. In:Olson MOJ. (ed.), The nucleolus. New York: Landes Bio-science/ Kluwer Academic; 2004:1-9. [Google Scholar]
- 5. Yanagimachi R. Fertilization studies and assisted fertilization in mammals:their development and future. J Reprod Dev 2012; 58:25-32; PMID:22450281; http://dx.doi.org/ 10.1262/jrd.11-015 [DOI] [PubMed] [Google Scholar]
- 6. Crozet N, Motlik J, Szollosi D. Nucleolar fine structure and RNA synthesis in porcine oocytes during early stages of antrum formation. Biol Cell 1984; 72:323-328. [Google Scholar]
- 7. Chouinard LA. A light- and electron-microscope study of the nucleolus during growth of the oocyte in the prepubertal mouse. J Cell Sci 1971; 9:637-663; PMID:4112474 [DOI] [PubMed] [Google Scholar]
- 8. Flechon JE, Kopecny V. The nature of the 'nucleolus precursor body' in early preimplantation embryos:a review of fine-structure cytochemical, immunocytochemical and autoradiographic data related to nucleolar function. Zygote 1998; 6:183-191; PMID:9770784; http://dx.doi.org/ 10.1017/S0967199498000112 [DOI] [PubMed] [Google Scholar]
- 9. Santella L1, Kyozuka K. Association of calmodulin with nuclear structures in starfish oocytes and its role in the resumption of meiosis. Eur J Biochem 1997; 246:602-610; PMID:9219515; http://dx.doi.org/ 10.1111/j.1432-1033.1997.t01-1-00602.x [DOI] [PubMed] [Google Scholar]
- 10. Terasaki M, Campagnola P, Rolls MM, Stein PA, Ellenberg J, Hinkle B, Slepchenko B. A new model for nuclear envelope breakdown. Mol Biol Cell 2001; 12:503-510; PMID:11179431; http://dx.doi.org/ 10.1091/mbc.12.2.503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anderson E. Oocyte differentiation in the sea urchin, Arbacia punctulata, with particular reference to the origin of cortical granules and their participation in the cortical reaction. J Cell Sci 1968; 37:514-539; http://dx.doi.org/ 10.1083/jcb.37.2.514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brangwynne CP, Mitchison TJ, Hyman AA. Active liquid-like behavior of nucleoli determines their size and shape in Xenopus laevis oocytes. Proc Natl Acad Sci USA 2011. 15; 108:4334-4339; http://dx.doi.org/ 10.1073/pnas.1017150108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ogushi S, Palmieri C, Fulka H, Saitou M, Miyano T, Fulka J, Jr. The maternal nucleolus is essential for early embryonic development in mammals. Science 2008; 319:613-616; PMID:18239124; http://dx.doi.org/ 10.1126/science.1151276 [DOI] [PubMed] [Google Scholar]
- 14. Kyogoku H, Fulka J, Jr, Wakayama T, Miyano T. De novo formation of nucleoli in developing mouse embryos originating from enucleolated zygotes. Development 2014; 141:2255-2259; PMID:24803589; http://dx.doi.org/ 10.1242/dev.106948 [DOI] [PubMed] [Google Scholar]
- 15. Ogushi S, Saitou M. The nucleolus in the mouse oocyte is required for the early step of both female and male pronucleus organization. J Reprod Dev 2010; 56:495-501; PMID:20519829; http://dx.doi.org/ 10.1262/jrd.09-184H [DOI] [PubMed] [Google Scholar]
- 16. Parsons DF. An electron microscope study of radiation damage in the mouse oocyte. J Cell Biol 1962; 14:31-48; PMID:14484088; http://dx.doi.org/ 10.1083/jcb.14.1.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Olson MO, Dundr M, Szebeni A. The nucleolus:an old factory with unexpected capabilities. Trends Cell Biol 2000; 10:189-196; PMID:10754561; http://dx.doi.org/ 10.1016/S0962-8924(00)01738-4 [DOI] [PubMed] [Google Scholar]
- 18. Crozet N. Nucleolar structure and RNA synthesis in mammalian oocytes. J Reprod Fertil Suppl 1989; 38:9-16; PMID:2477544 [PubMed] [Google Scholar]
- 19. Carmo-Fonseca M, Mendes-Soares L, Campos I. To be or not to be in the nucleolus. Nat Cell Biol 2000; 2:E107-E112; PMID:10854340; http://dx.doi.org/ 10.1038/35014078 [DOI] [PubMed] [Google Scholar]
- 20. Borsuk E, Vautier D, Szollosi MS, Besombes D, Debey P. Development-dependent localization of nuclear antigens in growing mouse oocytes. Mol Reprod Dev 1996; 43:376-386; PMID:8868251; http://dx.doi.org/ 10.1002/(SICI)1098-2795(199603)43:3%3c376::AID-MRD12%3e3.0.CO;2- [DOI] [PubMed] [Google Scholar]
- 21. Hyttel P. Electron microscopy of mammalian oocyte development, maturation and fertilization. In:Tosti E, Boni R, editors. Oocyte Maturation and Fertilization:A Long History for a Short Event. Belgium:Bentham Science Publishers; 2011; 1-37. [Google Scholar]
- 22. Geuskens M, Alexandre H. Ultrastructural and autoradiographic studies of nucleolar development and rDNA transcription in preimplantation mouse embryos. Cell Differ 1984; 14:125-134; PMID:6467377; http://dx.doi.org/ 10.1016/0045-6039(84)90037-X [DOI] [PubMed] [Google Scholar]
- 23. Fulka J, Jr, Moor RM, Loi P, Fulka J. Enucleolation of porcine oocytes. Theriogenology 2003; 59:1879-1885; PMID:12566159; http://dx.doi.org/ 10.1016/S0093-691X(02)01226-8 [DOI] [PubMed] [Google Scholar]
- 24. Wakayama T, Perry AC, Zuccotti M, Johnson KR, Yanagimachi R. Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature 1998; 23:369-374. [DOI] [PubMed] [Google Scholar]
- 25. Greda P, Karasiewicz J, Modlinski JA. Mouse zygotes as recipients in embryo cloning. Reproduction 2006; 132:741-748; PMID:17071775; http://dx.doi.org/ 10.1530/rep.1.01204 [DOI] [PubMed] [Google Scholar]
- 26. Probst AV, Santos F, Reik W, Almouzni G, Dean W. Structural differences in centromeric heterochromatin are spatially reconciled on fertilisation in the mouse zygote. Chromosoma 2007; 116:403-415; PMID:17447080; http://dx.doi.org/ 10.1007/s00412-007-0106-8 [DOI] [PubMed] [Google Scholar]
- 27. Fulka H, Langerova A. The maternal nucleolus plays a key role in centromere satellite maintenance during the oocyte to embryo transition. Development 2014; 141:1694-1704; PMID:24715459; http://dx.doi.org/ 10.1242/dev.105940 [DOI] [PubMed] [Google Scholar]
- 28. Kyogoku H, Ogushi S, Miyano T, Fulka J, Jr. Nucleoli from growing oocytes inhibit the maturation of enucleolated, full-grown oocytes in the pig. Mol Reprod Dev 2011; 78:426-435; PMID:21542050; http://dx.doi.org/ 10.1002/mrd.21320 [DOI] [PubMed] [Google Scholar]
- 29. Tesarik J, Kopecny V, Plachot M, Mandelbaum J. High-resolution autoradiographic localization of DNA-containing sites and RNA synthesis in developing nucleoli of human preimplantation embryos:a new concept of embryonic nucleologenesis. Development 1987; 101:777-791; PMID:2460303 [DOI] [PubMed] [Google Scholar]
- 30. Zatsepina O, Baly C, Chebrout M, Debey P. The step-wise assembly of a functional nucleolus in preimplantation mouse embryos involves the cajal (coiled) body. Dev Biol 2003; 253:66-83; PMID:12490198; http://dx.doi.org/ 10.1006/dbio.2002.0865 [DOI] [PubMed] [Google Scholar]
- 31. Maddox-Hyttel P, Bjerregaard B, Laurincik J. Meiosis and embryo technology:renaissance of the nucleolus. Reprod Fertil Dev 2005; 17:3-14; PMID:15745627; http://dx.doi.org/ 10.1071/RD04108 [DOI] [PubMed] [Google Scholar]
- 32. Inoue A, Aoki F. Role of the nucleoplasmin 2 C-terminal domain in the formation of nucleolus-like bodies in mouse oocytes. FASEB J 2010; 24:485-494; PMID:19805576; http://dx.doi.org/ 10.1096/fj.09-143370 [DOI] [PubMed] [Google Scholar]
- 33. Burns KH, Viveiros MM, Ren Y, Wang P, DeMayo FJ, Frail DE, Eppig JJ, Matzuk MM. Roles of NPM2 in chromatin and nucleolar organization in oocytes and embryos. Science 2003; 300:633-636; PMID:12714744; http://dx.doi.org/ 10.1126/science.1081813 [DOI] [PubMed] [Google Scholar]
- 34. Kyogoku H, Ogushi S, Miyano T. Nucleoli from two-cell embryos support the development of enucleolated germinal vesicle oocytes in the pig. Biol Reprod 2012; 87:113; PMID:22976279; http://dx.doi.org/ 10.1095/biolreprod.112.103119 [DOI] [PubMed] [Google Scholar]
- 35. Inoue A, Ogushi S, Saitou M, Suzuki MG, Aoki F. Involvement of mouse nucleoplasmin 2 in the decondensation of sperm chromatin after fertilization. Biol Reprod 2011; 85:70-77; PMID:21415138; http://dx.doi.org/ 10.1095/biolreprod.110.089342 [DOI] [PubMed] [Google Scholar]
- 36. Cockell MM, Gasser SM. The nucleolus:nucleolar space for RENT. Curr Biol 1999; 9:R575-576; PMID:10469557; http://dx.doi.org/ 10.1016/S0960-9822(99)80359-5 [DOI] [PubMed] [Google Scholar]
- 37. Visintin R, Amon A. The nucleolus:the magician's hat for cell cycle tricks. Curr Opin Cell Biol 2000; 12:752; PMID:11063944; http://dx.doi.org/ 10.1016/S0955-0674(00)00165-4 [DOI] [PubMed] [Google Scholar]
- 38. Visintin R, Hwang ES, Amon A. Cfi1 prevents premature exit from mitosis by anchoring Cdc14 phosphatase in the nucleolus. Nature 1999; 398:818-823; PMID:10235265; http://dx.doi.org/ 10.1038/19775 [DOI] [PubMed] [Google Scholar]
- 39. Hudson JW, Kozarova A, Cheung P, Macmillan JC, Swallow CJ, Cross JC, Dennis JW. Late mitotic failure in mice lacking Sak, a polo-like kinase. Curr Biol 2001; 11:441-446; PMID:11301255; http://dx.doi.org/ 10.1016/S0960-9822(01)00117-8 [DOI] [PubMed] [Google Scholar]
- 40. Alliegro MA, Henry JJ, Alliegro MC. Rediscovery of the nucleolinus, a dynamic RNA-rich organelle associated with the nucleolus, spindle, and centrosomes. Proc Natl Acad Sci USA 2010; 107:13718-13723; http://dx.doi.org/ 10.1073/pnas.1008469107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Alliegro MC, Hartson S, Alliegro MA. Composition and dynamics of the nucleolinus, a link between the nucleolus and cell division apparatus in surf clam (Spisula) oocytes. J Biol Chem 2012; 287:6702-6713; PMID:22219192; http://dx.doi.org/ 10.1074/jbc.M111.288506 [DOI] [PMC free article] [PubMed] [Google Scholar]