Abstract
Despite substantial progress, global polio eradication has remained elusive. Indigenous wild poliovirus (WPV) transmission in four endemic countries (Afghanistan, India, Nigeria, and Pakistan) persisted into 2010 and outbreaks from imported WPV continued. By 2013, most outbreaks in the interim were promptly controlled. The number of polio-affected districts globally has declined by74% (from 481 in 2009 to 126 in 2013), including a 79% decrease in the number of affected districts in endemic countries (from 304 to 63). India is now polio-free. The challenges to success in the remaining polio-endemic countries include 1) threats to the security of vaccinators in each country and a ban on polio vaccination in areas of Afghanistan and Pakistan; 2) a risk of decreased government commitment; and 3) remaining surveillance gaps. Coordinated efforts under the International Health Regulations and efforts to mitigate the challenges provide a clear opportunity to soon secure global eradication.
Keywords: Poliovirus, polio, poliomyelitis, surveillance, outbreak control, eradication, international health regulations
INTRODUCTION
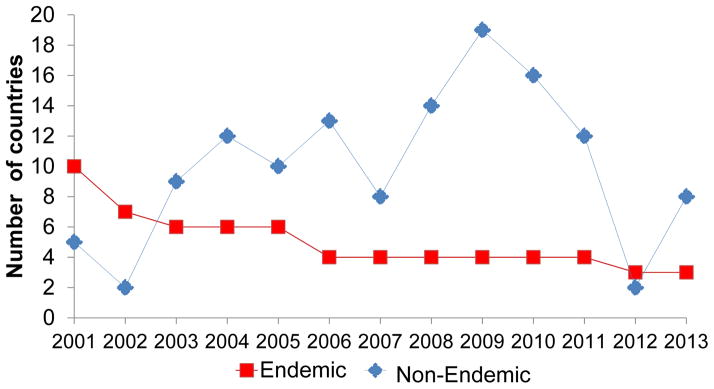
Following the 1988 World Health Assembly (WHA) resolution to eradicate polio worldwide by 2000 [1], Global Polio Eradication Initiative (GPEI) efforts led to a 99% reduction from 350,000 estimated polio cases in 1988 to fewer than 1000 confirmed cases in 2001 [2, 3]. The number of endemic countries that had never interrupted indigenous wild poliovirus (WPV) transmission was reduced to 10 by 2001 (Figure 1; see Box 1 for definitions). The World Health Organization (WHO) regions of the Americas and the Western Pacific were certified polio-free in 1994 and 2000 respectively, and the European Region in 2002. No WPV type 2 (WPV2) cases have been detected since 1999 [4]. By 2006, the number of endemic countries decreased to four—Pakistan, Afghanistan, India, and Nigeria (Figure 1) [3, 5]; transmission had been interrupted in countries experiencing civil conflict and social disruption, such as Angola and Somalia. The remaining endemic countries had limited health infrastructure and suboptimal implementation of supplementary immunization activities (SIAs). More oral poliovirus vaccine (OPV) doses appeared to be needed to raise population immunity where malnutrition and enteric diseases were highly prevalent [6, 7].
Figure 1.
Box 1. Definitions Used by the Global Polio Eradication Initiative.
| Active transmission | Detection of ≥1 WPV case or of WPV isolated from ≥2 environmental samples collected >1 month apart. The end of active transmission in a previously polio- free country is the lack of isolation of WPV from environmental samples or humans for 6 months; in a country with endemic or reestablished transmission, the end of active transmission is no WPV cases/isolation for 12 months. |
| Endemic transmission | Circulation of indigenous WPV without interruption. |
| Importation event | Detection of ≥1 WPV case or ≥1 isolation from sewage in a country previously polio-free for which genomic sequences most closely match WPV actively circulating in another country (exporting WPV). |
| Importation outbreak | Detection of ≥1 WPV case secondary to ≥1 importation event. |
| Indigenous WPV | WPV that has historically been circulating in a defined geographic area of a unique genotype (>15% nucleotide difference) or cluster (>5% nucleotide difference). |
| Polio-free country | No evidence of indigenous WPV transmission for ≥1 year and subsequent WPV cases are determined to be due to WPV of external origin by genomic sequence analysis. |
| Prompt outbreak control | Status when the last identified genetically-linked WPV case is detected within 6 months of laboratory confirmation of the outbreak. |
| Reestablished transmission | Detection in a previously polio-free country of transmission of the same WPV lineage persisting for ≥12 months from onset of the first case following WPV importation to onset of the most recent case. |
Reintroduction of monovalent oral poliovirus vaccines (mOPV) against types 1 (mOPV1) and 3 (mOPV3) improved per-dose effectiveness against the relevant serotype compared with trivalent OPV (tOPV) [8–10]. Predominant mOPV1 use in SIAs in some endemic countries beginning in 2005–2006—to preferentially target WPV type 1 (WPV1)—had substantial impact on WPV1 transmission but did not interrupt circulation [3, 11]. Resurgence in WPV type 3 (WPV3) transmission in those countries, along with WPV1 and WPV3 importation outbreaks, increased the total annual number of reported cases to more than 1000 until 2011 [3, 11]. During 2001–2009, polio outbreaks were reported in 38 previously polio-free countries; WPV transmission persisted for ≥12 months in some of these countries because of health infrastructure weaknesses [12–17]. Repeated WPV exportation from reservoir countries into polio-free countries posed a major threat to the GPEI in 2009 (Figure 1). The GPEI Strategic Plan for 2010–2012 focused on bivalent OPV (types 1 and 3, bOPV) as the preferred SIA vaccine, improved speed in outbreak confirmation and control, and enhanced community engagement [18, 19].
Progress during 2010–2011 was limited; the Independent Monitoring Board convened to assess progress warned in October 2011 that the revised target for interrupting global WPV transmission by the end of 2012 was unlikely to be reached [20]. In 2012, the WHA declared completion of polio eradication a “programmatic emergency for global public health” [21, 22]. The GPEI Emergency Action Plan for 2012–2013 [23] focused on improved commitment of governments down to the lowest levels, further enhanced community engagement, and a surge of technical support by the major GPEI partners for planning, implementation, supervision, and monitoring of GPEI activities. By early 2012, the 1-year absence of reported WPV cases in India underscored the technical feasibility of eradication.
Despite enhancements to routine immunization (RI) delivery systems in each endemic country, coverage had not improved substantially by 2012 [24]. As the risk for outbreaks of circulating type 2 vaccine-derived polioviruses (cVDPV2) [25] increased and the strategic path to eventual cessation of all use of OPVs was needed, the Polio Eradication and Endgame Strategic Plan 2013–2018 [26] provided comprehensive, long-term strategies to deliver a polio-free world by 2018.
This report summarizes progress made toward interruption of WPV transmission during 2010–2013 and presents strategic considerations to address remaining challenges.
DETECTION OF WPV TRANSMISSION
Although paralysis is a rare outcome of WPV (and cVDPV) infections (<1%) [27], acute flaccid paralysis (AFP) surveillance has been the proven means of tracing WPV transmission [28]. AFP surveillance consists of detecting and investigating acute paralysis in children, including timely collection of fecal specimens and prompt testing in accredited laboratories of the Global Polio Laboratory Network (GPLN) [29–31]. Standardized GPEI performance indicators evaluate the quality of AFP surveillance and identify where WPV transmission might go undetected [30, 32]. The indicator used to assess whether surveillance is sufficiently sensitive to promptly detect WPV circulation is the annual proportion of AFP cases excluding WPV and VDPV cases and cases classified as polio-compatible, among children <15 years of age (nonpolio AFP [NPAFP]). Countries in WHO regions not certified as polio-free—the African and Eastern Mediterranean regions—should achieve annual NPAFP rates of ≥2 cases per 100,000 [32, 33]. To ensure ample and reliable laboratory analysis, ≥80% of AFP cases should have two stool specimens collected ≤14 days of paralysis onset, ≥24 hours apart, arriving in good condition at an accredited GPLN laboratory (“adequate” specimens). National data can mask heterogeneous subnational performance; we applied performance indicators to subnational areas [19] and assessed the proportion of the national population residing in subnational areas where both indicator targets were met.
Table 1 indicates the trends in countries meeting performance indicators at national and subnational levels during 2010–2013 among 29 countries in the two remaining endemic regions with WPV cases during 2009–2013 and nine selected neighboring countries. Although most countries detect ≥2 NPAFP cases/100,000 children aged <15 years nationally and in a large proportion of first administrative subnational units (province/state), some exceptions occur in small countries (such as Burundi, Equatorial Guinea, and Djibouti), and in conflict-affected Central African Republic (CAR) and Syria. Inadequate specimen collection is a larger problem. Many countries fail to meet this indicator nationally or in a substantial proportion of subnational areas, including countries of Central Africa, and in the Horn of Africa. In 13 of the 38 countries reviewed, less than half of the population lives in areas that met both primary AFP surveillance indicators in 2013. Alternatively, some countries experienced recent improvements in performance indicators, most notably the Republic of the Congo (Congo) and the Democratic Republic of the Congo (DRC). In large countries, performance is better assessed at lower administrative levels (districts), since gaps can be masked when aggregated at a province/state level.
TABLE 1.
National and subnational acute flaccid paralysis (AFP) surveillance indicators, among countries with wild poliovirus transmission during 2004–2013 and selected neighboring countries within the African and Eastern Mediterranean regions of the World Health Organization (WHO), 2010–2013.*
| WHO region/country | 2010
|
2011
|
2012
|
2013
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nat. NPAFP Rate |
% Prov. NPAFP ≥2 |
Nat. % Adeq. Spec. |
% Prov. ≥80% Adeq. Spec. |
% Pop. Both Met |
Nat. NPAFP Rate |
% Prov. NPAFP ≥2 |
Nat. % Adeq. Spec. |
% Prov. ≥80% Adeq. Spec. |
% Pop. Both Met |
Nat. NPAFP Rate |
% Prov. NPAFP ≥2 |
Nat. % Adeq. Spec. |
% Prov. ≥80% Adeq. Spec. |
% Pop. Both Met |
Nat. NPAFP Rate |
% Prov. NPAFP ≥2 |
Nat. % Adeq. Spec. |
% Prov. ≥80% Adeq. Spec. |
% Pop. Both Met |
|
| African | ||||||||||||||||||||
| Angola | 3.1 | 94 | 88 | 89 | 79 | 2.3 | 56 | 91 | 89 | 43 | 3.1 | 94 | 92 | 100 | 98 | 2.9 | 89 | 92 | 94 | 79 |
| Benin | 2.3 | 67 | 93 | 83 | 55 | 2.5 | 83 | 86 | 83 | 64 | 3.6 | 92 | 92 | 92 | 87 | 4.3 | 100 | 91 | 92 | 95 |
| Burkina Faso | 3.7 | 92 | 89 | 77 | 71 | 3.7 | 100 | 78 | 38 | 41 | 4.0 | 93 | 89 | 100 | 100 | 3.7 | 86 | 85 | 71 | 65 |
| Burundi | 3.1 | 53 | 83 | 67 | 44 | 2.6 | 65 | 93 | 76 | 67 | 2.9 | 71 | 98 | 100 | 72 | 2.4 | 59 | 91 | 88 | 49 |
| Cameroon | 2.4 | 70 | 84 | 80 | 65 | 2.6 | 50 | 80 | 80 | 18 | 2.8 | 100 | 79 | 60 | 56 | 4.3 | 100 | 77 | 30 | 25 |
| CAR | 7.2 | 100 | 91 | 86 | 87 | 6.0 | 100 | 81 | 71 | 68 | 6.0 | 100 | 85 | 86 | 88 | 2.6 | 57 | 90 | 71 | 36 |
| Chad | 4.5 | 94 | 87 | 89 | 84 | 5.7 | 100 | 75 | 39 | 33 | 6.7 | 100 | 82 | 67 | 67 | 8.6 | 100 | 82 | 56 | 56 |
| Rep. Congo | 5.1 | 100 | 25 | 45 | 18 | 3.1 | 60 | 75 | 55 | 20 | 2.7 | 64 | 84 | 64 | 19 | 5.2 | 100 | 79 | 64 | 78 |
| Côte d’Ivoire | 3.3 | 95 | 80 | 53 | 47 | 5.1 | 95 | 64 | 0 | 0 | 4.3 | 100 | 83 | 83 | 85 | 4.9 | 100 | 88 | 83 | 87 |
| DRC | 5.5 | 100 | 82 | 73 | 65 | 4.9 | 100 | 79 | 27 | 34 | 4.4 | 100 | 86 | 91 | 86 | 4.8 | 100 | 83 | 73 | 70 |
| Eritrea | 4.3 | 80 | 97 | 83 | 72 | 4.7 | 100 | 97 | 100 | 100 | ||||||||||
| Ethiopia | 2.8 | 90 | 87 | 55 | 92 | 2.7 | 90 | 81 | 55 | 33 | 2.8 | 91 | 85 | 55 | 69 | 2.8 | 64 | 71 | 9 | 0 |
| Eq. Guinea | ||||||||||||||||||||
| Gabon | 2.7 | 50 | 88 | 70 | 33 | 2.9 | 0 | 60 | 33 | 10 | 2.5 | 63 | 76 | 75 | 16 | 0.6 | 67 | 17 | 0 | 0 |
| Gambia | 5.5 | 100 | 100 | 100 | 100 | 3.6 | 100 | 78 | 20 | 5 | ||||||||||
| Ghana | 1.8 | 50 | 87 | 90 | 40 | 2.5 | 80 | 83 | 60 | 45 | ||||||||||
| Guinea | 4.0 | 100 | 96 | 100 | 100 | 3.7 | 100 | 68 | 0 | 0 | 3.3 | 100 | 97 | 100 | 100 | 4 | 100 | 54 | 0 | 0 |
| Guinea-Bissau | 2.2 | 67 | 53 | 25 | 16 | 2.5 | 33 | 56 | 29 | 0 | ||||||||||
| Kenya | 2.2 | 88 | 89 | 100 | 74 | 3.1 | 88 | 83 | 75 | 49 | 4.2 | 100 | 92 | 100 | 100 | 3.5 | 88 | 84 | 88 | 65 |
| Liberia | 2.8 | 60 | 96 | 93 | 42 | 3.3 | 40 | 85 | 80 | 34 | 3.2 | 73 | 100 | 100 | 70 | 2.9 | 80 | 98 | 100 | 86 |
| Mali | 2.2 | 50 | 94 | 100 | 53 | 2.7 | 100 | 84 | 67 | 64 | 3.4 | 75 | 94 | 88 | 92 | 3.1 | 88 | 88 | 88 | 96 |
| Mauritania | 4.4 | 86 | 98 | 100 | 90 | 3.8 | 100 | 92 | 92 | 90 | 5.7 | 100 | 95 | 100 | 100 | 4.2 | 100 | 93 | 85 | 90 |
| Mozambique | 2.5 | 90 | 86 | 60 | 51 | 3.1 | 100 | 87 | 80 | 88 | 3.1 | 100 | 89 | 100 | 100 | 3.3 | 100 | 89 | 80 | 85 |
| Namibia | 3.2 | ?? | 86 | 67 | 25 | 3.6 | 33 | 92 | 85 | 63 | ||||||||||
| Niger | 4.4 | 100 | 82 | 75 | 76 | 3.9 | 88 | 73 | 25 | 20 | 4.3 | 88 | 80 | 50 | 55 | 4.1 | 100 | 75 | 25 | 8 |
| Nigeria | 7.9 | 100 | 95 | 100 | 100 | 8.1 | 100 | 93 | 100 | 100 | 8.7 | 100 | 95 | 97 | 96 | 10.5 | 100 | 96 | 100 | 100 |
| Senegal | 5.3 | 100 | 63 | 27 | 26 | 2.1 | 55 | 79 | 55 | 38 | 2.7 | 73 | 81 | 55 | 50 | 3.7 | 91 | 68 | 18 | 7 |
| Sierra Leone | 6.3 | 100 | 89 | 75 | 77 | 6.6 | 100 | 83 | 50 | 57 | 6.3 | 75 | 95 | 100 | 79 | 6.4 | 60 | 92 | 100 | 79 |
| South Sudan | 4.0 | 100 | 93 | 100 | 100 | 4.5 | 100 | 93 | 100 | 100 | 4.3 | 100 | 95 | 90 | 97 | 3.8 | 90 | 94 | 90 | 87 |
| Togo | 2.5 | 83 | 95 | 100 | 82 | 2.5 | 83 | 88 | 100 | 88 | 2.9 | 100 | 97 | 100 | 100 | 4.7 | 100 | 85 | 83 | 87 |
| Uganda | 2.9 | 61 | 87 | 75 | 45 | 3.2 | 53 | 87 | 71 | 41 | 3.2 | 65 | 88 | 71 | 52 | 3.3 | 71 | 87 | 77 | 51 |
|
| ||||||||||||||||||||
| Eastern Mediterranean | ||||||||||||||||||||
| Afghanistan | 8.8 | 100 | 93 | 97 | 95 | 10.0 | 100 | 92 | 91 | 91 | 10.2 | 100 | 92 | 94 | 91 | 10.8 | 100 | 94 | 97 | 97 |
| Djibouti | 1.2 | 0 | 33 | 0 | 0 | 2.5 | 0 | 67 | 50 | 13 | ||||||||||
| Pakistan | 6.7 | 88 | 88 | 100 | 99 | 7.0 | 100 | 88 | 88 | 95 | 5.6 | 88 | 89 | 88 | 98 | 5.2 | 88 | 90 | 100 | 99 |
| Somalia | 3.2 | 94 | 99 | 100 | 82 | 3.3 | 94 | 99 | 95 | 81 | 2.8 | 79 | 98 | 100 | 56 | 6.4 | 100 | 88 | 89 | 93 |
| Sudan | 3.2 | 100 | 95 | 100 | 100 | 3.1 | 92 | 95 | 100 | 89 | ||||||||||
| Syria | 1.1 | 15 | 85 | 62 | 10 | 1.3 | 15 | 64 | 38 | 4 | ||||||||||
| Yemen | 3.9 | 100 | 96 | 100 | 100 | 3.4 | 100 | 92 | 95 | 93 | 4.0 | 100 | 93 | 95 | 98 | 5.2 | 100 | 92 | 91 | 84 |
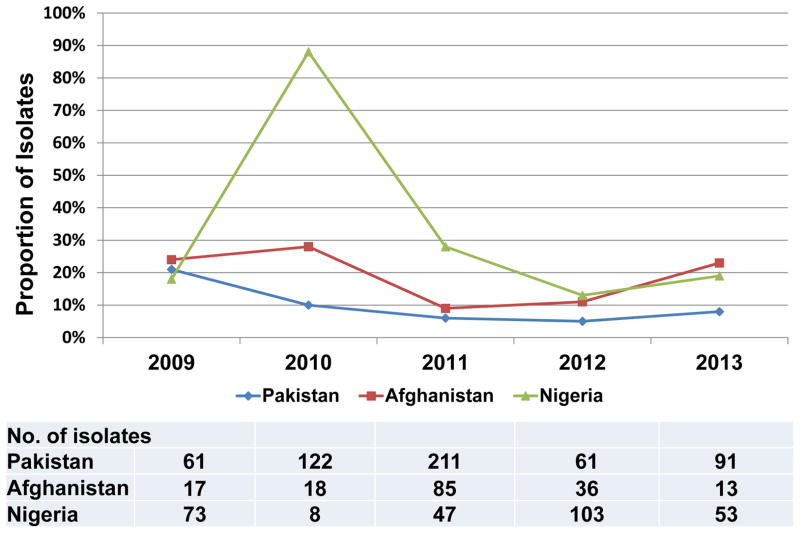
A high rate of evolution of polioviruses occurs at the nucleotide level (~1% per year) [34]. GPEI and GPLN take advantage of this rapid evolution rate by determining the sequence of the genomic region encoding the VP1 capsid protein for all non-Sabin-like polioviruses isolated (i.e., suspected of being WPV or VDPV), regardless of source [24]. The sequences provide molecular epidemiologic evidence for linkages among cases even over long geographic distances. Similarly, the VP1 sequences can be used to detect AFP surveillance gaps by identifying so-called “orphan” viruses, which differ from all other sequenced polioviruses by ≥1.5% in VP1 sequence coding. A difference of 1.5% implies 0.75–1.5 years of transmission/evolution between the two viruses and that expected intervening paralytic cases were missed by the surveillance system. With a high proportion of orphans among WPV1 isolated from AFP cases, sequence analysis indicated that WPV1 cases were likely being missed by AFP surveillance as recently as during 2012–2013 in Afghanistan and Nigeria (Figure 2), as well as in Cameroon, Chad, Niger and Syria (S. Oberste, personal communication 2014).
Figure 2.
Environmental surveillance [ES] (testing sewage samples for polioviruses) can be more sensitive than AFP surveillance, detecting WPV transmission that might occur in the absence of detected WPV-confirmed AFP cases if specimens are taken from converging drainage serving high-risk populations [35]. ES has been established in three currently polio-endemic countries: Pakistan since 2009 (currently 29 sites in five provinces), Afghanistan since 2013 (currently nine sites in four provinces), and Nigeria since 2011 (currently 26 sites in eight states), as well as in one recent outbreak country (Kenya, 4 sites) and at least 24 countries without active WPV transmission: India (24 sites in six states), Egypt (34 sites in 11 cities), China (10 sites in 10 provinces), Australia (3 sites in 2 states) and multiple sites in 20 countries of the European Region.
Active WPV1 transmission without detection of paralytic polio cases was identified by an extensive ES system in Israel, the West Bank, and Gaza in 2013 [36, 37]. Genomic sequence analyses indicate that the WPV1 originated in Pakistan and was closely linked to WPV1 isolated from two sewage specimens collected in December 2012 in Cairo, Egypt [38] and to WPV1 cases detected in 2013 in Syria [39, 40], signifying widespread circulation in the Middle East during the end-2012–early-2013 (S. Oberste, S. Sharif, personal communication 2014).
REPORTED WPV CASES
Overview
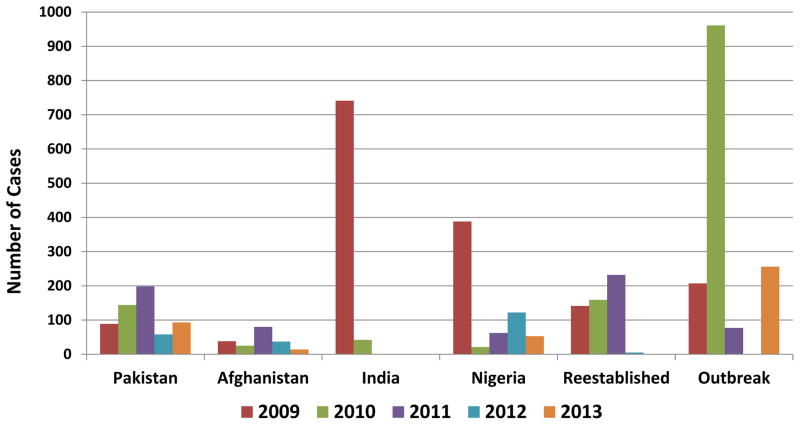
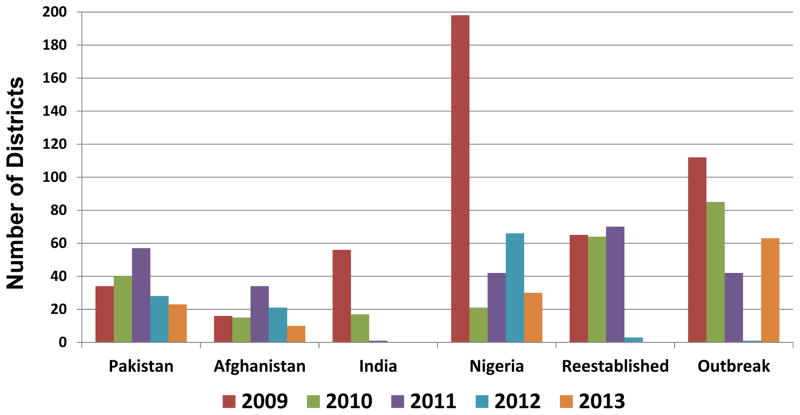
In 2009, 1604 WPV cases were reported by 23 countries (Figure 3). During 2010–2012, the number of reported WPV cases and the number of affected countries progressively declined compared with 2009 (Figures 1 and 3): 1352 reported cases in 20 countries in 2010, 650 cases in 16 countries in 2011, and 223 cases in five countries in 2012. In 2013, cases increased to 416 and affected countries increased to 13. These numbers reflect different trends in transmission by WPV serotype among each endemic country and variability in the annual number and size of outbreaks after WPV importation (Table 2). The effects of implementation of national emergency plans were seen in Pakistan in 2012 and in Nigeria in 2013. The number of reported polio-affected districts decreased globally by 74% from 481 in 2009 to 126 in 2013 and among endemic countries, decreased 79%, from 304 to 63 (Figure 4). In Pakistan, Afghanistan and Nigeria, the number of affected districts decreased by 75% from 248 in 2009 to 63 in 2013.
Figure 3.
Table 2.
Number of Confirmed Wild Poliovirus Cases in Affected Countries by World Health Organization Region and by Serotype, 2010–2013
| WHO Region/Country | 2010 | 2011 | 2012 | 2013 | |||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| WPV1 | WPV3 | WPV1 | WPV3 | WPV1 | WPV3 | WPV1 | WPV3 | WPV Origin | |
| African | |||||||||
| Angola | 33 | 5 | Indiaa | ||||||
| Cameroon | 4 | Nigeriab | |||||||
| CAR | 4 | Nigeriab | |||||||
| Chad | 11 | 15 | 129 | 3 | 5 | Nigeriaa | |||
| Côte d’Ivoire | 36 | Nigeria | |||||||
| DRC | 100 | 93 | Indiac | ||||||
| Ethiopia | 9 | Nigeria | |||||||
| Gabon | 1 | Indiad | |||||||
| Guinea | 3 | Nigeriae | |||||||
| Kenya | 1 | 14 | Nigeriaf | ||||||
| Liberia | 2 | Nigeriag | |||||||
| Mali | 3 | 1 | 7 | Nigeriah | |||||
| Mauritania | 5 | Nigeriae | |||||||
| Niger | 2 | 5 | 1 | Nigeria | |||||
| Nigeria | 8 | 13 | 47 | 15 | 103 | 19 | 53 | Indigenous | |
| Republic of the Congo | 382 | 1 | Indiai | ||||||
| Senegal | 18 | Nigeriaj | |||||||
| Sierra Leonef | 1 | Nigeria | |||||||
| Ugandak | 4 | Nigeria | |||||||
|
| |||||||||
| Eastern Mediterranean | |||||||||
| Afghanistan | 17 | 8 | 80 | 37 | 14 | Indigenous | |||
| Pakistan | 120 | 24 | 196 | 2 | 56 | 2l | 93 | Indigenous | |
| Somalia | 194 | Nigeria | |||||||
| Syria | 35 | Pakistan | |||||||
|
| |||||||||
| Southeast Asian | |||||||||
| India | 18 | 24 | 1 | Indigenous | |||||
| Nepal | 6 | India | |||||||
|
| |||||||||
| European | |||||||||
| Kazakhstan | 1 | Indiam | |||||||
| Russian Federation | 14 | Indial | |||||||
| Tajikistan | 460 | India | |||||||
| Turkmenistan | 3 | Indial | |||||||
|
| |||||||||
| Western Pacific | |||||||||
| China | 21 | Pakistan | |||||||
Abbreviations: CAR, Central African Republic; DRC, Democratic Republic of the Congo; WHO, World Health Organization; WPV1, wild poliovirus type 1; WPV3, wild poliovirus type 3.
Reestablished transmission; some cases secondary to WPV most recently circulating in DRC
Most recently circulating in Chad
Most recently circulating in Angola and the Republic of the Congo; reestablished transmission and outbreaks
Most recently circulating in the Republic of the Congo
Most recently circulating in Côte d’Ivoire
2011 case WPV most recently circulating in Uganda and South Sudan; 2013 cases most recently circulating in Somalia
Most recently circulating in Guinea
Prior to 2011, WPV1 most recently circulating in Guinea, Mauritania and Burkina Faso, WPV3 most recently circulating in Niger; in 2011, WPV3 most recently circulating in Nigeria
Most recently circulating in Angola
Most recently circulating in Mauritania and Guinea
Most recently circulating in Kenya
An additional case, counted as WPV1 case, was associated with coinfection of WPV1 and WPV3
Most recently circulating in Tajikistan
Figure 4.
WPV1
In 2010, WPV1 cases were detected in 19 countries, including the four countries with endemic transmission of indigenous WPV (Table 2). Large outbreaks occurred in 2010 in Tajikistan (and other former Soviet republics) and Congo. In 2011, WPV1 cases were detected in 16 countries. The last detected case in India occurred in January 2011. In 2012, WPV1 cases were detected in five countries and in 2013, in eight countries, including Pakistan, Afghanistan and Nigeria. In.
WPV3
In 2010, WPV3 cases were detected in seven countries, including the four endemic countries. As bOPV use increased during 2010–2012, WPV3 circulation decreased markedly. The last reported WPV3 case in Afghanistan had onset of paralysis April 11, 2010; the last reported case in India occurred October 22, 2010. In 2011, WPV3 cases were detected in six countries, including Pakistan and Nigeria. In 2012, WPV3 cases were detected only in Pakistan and Nigeria; the latest reported case in Pakistan was associated with a WPV1 and WPV3 co-infection and occurred April 18, 2012. The most recent WPV3 case detected globally was in Nigeria with onset November 10, 2012. The latest WPV3 detected from any source was from a sewage sample collected in Lagos November 11, 2012.
WPV Cases in Endemic Countries
Pakistan
In 2010, 120 WPV1 cases and 24 WPV3 cases were reported (Table 2), increased from 61 WPV1 cases (one WPV3 co-infection) and 28 WPV3 cases reported in 2009. In 2011, a 63% increase in WPV1 cases and a 97% decrease in WPV3 cases were reported. In 2012, a 71% decrease in WPV1 cases and no change in WPV3 cases were reported. In 2013, a 71% decrease of WPV1 cases and no WPV3 case (100% decrease) were reported. Compared to an overall 35% decrease in the number of reported cases during 2010–2013, the number of affected districts fell by 43% from 40 in 2010 to 23 in 2013 (Figure 4). The remaining areas of transmission are in Federally Administered Tribal Areas (FATA), central and southern Khyber Pakhtunkhwa (KP) province, and Karachi.
Afghanistan
In 2010, 17 WPV1 cases and 8 WPV3 cases were reported, compared with 16 WPV1 cases (one WPV3 co-infection) and 22 WPV3 cases reported in 2009. In 2011, a 370% increase in WPV1 cases and no WPV3 cases (100% decrease) were reported. In 2012, a 54% decrease in WPV1 cases was reported. In 2013, a further 62% decrease was reported. A 33% decrease occurred in the number of affected districts from 15 in 2010 to 3 in 2013. Although cross-border and indigenous transmission of WPV1 occurred through 2012 in the South Region bordering the Quetta reservoir in Pakistan, all WPV1 cases in 2013 in Afghanistan but one represent importations from Pakistan into the Eastern Region.
India
In 2010, 17 WPV1 cases and 24 WPV3 cases were reported, decreased from 80 WPV1 cases and 661 WPV3 cases in 2009. A single WPV1 case was reported in 2011 with onset January 13. No WPV cases were detected in India in 2012 and 2013. India was removed from the WHO list of WPV-endemic countries in February 2012.
Nigeria
In 2010, 8 WPV1 cases and 13 WPV3 cases were reported, decreased from 75 WPV1 cases and 313 WPV3 cases reported in 2009. In 2011, a 487% increase in reported WPV1 cases and a 15% increase in reported WPV3 cases occurred. In 2012, reported WPV1 cases increased 119% compared to 2011 and WPV3 cases increased 27%. In 2013, reported WPV1 cases decreased 49% from 2012 and no WPV3 cases (100% decrease) were reported. Although three reservoir areas remained during 2010–2012, no WPV cases were detected in the northwest reservoir in 2013 nor was WPV isolated from sewage sampled in the northwestern city of Sokoto. The other reservoir areas are north-central states (primarily Kano) and the northeast (primarily Borno).
WPV Cases in Countries with Reestablished Transmission
Chad
After outbreaks of WPV3 imported from Nigeria in 2007, ongoing WPV3 transmission was reestablished in Chad. Additionally, transmission continued after WPV1 was imported from Nigeria in September 2010. In 2010, 11 WPV1 cases and 15 WPV3 cases were reported, decreased from 64 WPV3 cases reported in 2009. In 2011, a 10.7-fold increase in WPV1 cases and a 95% decrease in WPV3 cases were reported. In 2012, reported WPV1 cases decreased by 96%, the most recent case having onset June 14, 2012; no WPV3 case was reported.
Angola
During 2005–2007, three separate WPV importations into Angola (one WPV3, two WPV1) WPV originated from India. WPV1 transmission was reestablished after the latest importation in 2007; when transmission became widespread in 2010, subsequent exportations occurred into DRC and Congo. During 2010, 33 WPV1 cases were detected, including in central Angola and at the eastern border with DRC, leading to spread to DRC. In 2011, WPV1 cases decreased 85% from 2010. The last indication of ongoing reestablished transmission was a cluster of four WPV1 cases in the southern province of Kuando-Kubango during January–March 2011. With local transmission occurring after a new importation from DRC, the latest WPV1 case occurred in the northern province of Uige on July 7, 2011.
DRC
WPV1 cases occurred during 2006–2008 after WPV1 was introduced from Angola; no WPV1 case was detected in 2009 (although a linked case was detected in Burundi). During 2010, six genetically-linked WPV1 cases were identified in Katanga Province in southeastern DRC in addition to 94 other cases in outbreaks secondary to new importations. Two genetically distinct outbreaks occurred in 2011; 79 WPV1 cases reported in western provinces resulted from importations from Angola and Congo, and 14 WPV1 cases in the eastern provinces of Katanga and Maniema represented ongoing reestablished transmission. The last reported case occurred December 20, 2011.
WPV Outbreaks following Importation into Polio-Free Countries
Outbreaks that began in 2009 continued into 2010 in Mali, Mauritania, and Sierra Leone. Nine new outbreaks occurred in 2010 related to multiple importation events. Additional importation events occurred in Mali in 2010, and new outbreaks with WPV of Nigeria origin also occurred in Liberia, Niger, and Senegal. Large WPV1 outbreaks occurred in Tajikistan (with transmission to the Russian Federation, Kazakhstan, Turkmenistan and Uzbekistan) and in Congo; both of these were associated with a substantial proportion of cases in persons aged ≥15 years [14, 42]. The Uganda outbreak was related to the 2009 transmission in Kenya. The Mali WPV3 2010 outbreak that continued into 2011 was not interrupted within 6 months after confirmation. Aside from Kenya-Uganda cross-border transmission and the outbreak in Mali, all other 2010 outbreaks were controlled promptly.
In 2011, 11 WPV outbreaks occurred worldwide, including nine new importation events in eight countries and two outbreaks representing ongoing transmission from 2010 into 2011 (WPV3 in Mali and WPV1 in Congo). The 2011 outbreak in Kenya represented transmission from Uganda of the WPV1 previously isolated in Kenya in 2009. The other importation events in 2011 occurred in China and in seven countries in Africa, including the last identified outbreaks of WPV3. The WPV outbreak in China was the first reported in the WHO Western Pacific Region since 1997 [43]. All new outbreaks in 2011 were controlled promptly.
In 2012, a single case in Niger was the only outbreak. The number of WPV cases in outbreaks after importation increased to 256 in five countries in 2013 (Figure 1). In Horn of Africa countries, the first identified case had onset in Somalia in April 2013 and spread resulted in 217 cases in Somalia, Kenya, and Ethiopia in 2013. More than 400,000 children aged <5 years reside in areas in Somalia controlled by antigovernment elements and inaccessible to vaccination efforts. Cases have since occurred in Ethiopia in January 2014 and in Somalia in June 2014, indicating that the outbreak was not controlled promptly there and that transmission has been reestablished in Somalia. Four WPV1 cases were reported in Cameroon in 2013; the outbreak was not controlled promptly, with transmission continuing into 2014. Importation of WPV of Pakistan origin into Syria resulted in 35 cases in 2013 and one case to date in 2014 (and spread to Iraq in 2014) but transmission tentatively controlled.
DISCUSSION
Despite setbacks, substantial progress toward global polio eradication has occurred in recent years. Reestablished transmission in four countries has been interrupted (the last case in Sudan was in 2009). As of July 22, 2014, no WPV3 case has been identified since November 2012, raising the possibility that both WPV2 and WPV3 transmission have been interrupted globally. In March 2014, the WHO South-East Asian Region joined the American, Western Pacific, and European Regions as being certified free of indigenous WPV. With this achievement, 80% of the world’s population now lives in WHO Regions certified as polio-free [44].
Indigenous WPV transmission appears to be restricted to fewer geographical areas within each of the three remaining endemic countries compared with any time previously. The reservoirs of indigenous WPV1 in Quetta in Balochistan, Pakistan, and in Sokoto and Zamfara states in northwest Nigeria, have not been active in 2013 or 2014 to date. The substantial decreases in the number of WPV cases and of affected states and districts in Nigeria were preceded by markedly improved SIA quality indicators in most areas [41, 45]. WPV transmission in Nigeria, as of July 2014, has been detected only in Kano and Borno states. As of July 22 2014, 99 cases have been reported in Pakistan compared with 21 cases at this time in 2013; five cases in Nigeria, compared with 35 cases, and eight cases in Afghanistan compared with three, respectively. Despite the 329% increase in case numbers in Pakistan in 2014, only 12 districts are polio-affected to date compared with 10 at this time in 2013.
One of the key tools contributing to this progress has been the introduction and widespread use of bOPV in SIAs since late 2009. Another contributing factor was the implementation of emergency action plans in WPV-affected countries. Key elements of national emergency plans included activities to 1) enhance government commitment to polio eradication and increase accountability at all administrative levels; 2) increase vaccination coverage through RI and SIAs (e.g., improved micro-planning, implementation of strategies to vaccinate nomadic and remote populations, enhanced monitoring of SIA quality); 3) improve partner coordination (e.g., polio control rooms at national and state levels); and 4) implement innovative approaches (e.g., implementation of short-interval additional dose SIAs, enhanced efforts to vaccinate children in transit and migrating). Since 2012, priority countries received considerably increased technical support and human resources, including the support provided by the international and national “Stop Transmission of Polio (STOP)” programs [46, 47].
Sensitive AFP surveillance is the “gold standard” to track WPV transmission and to allow early detection of and response to WPV importation into polio-free areas. Performance of AFP surveillance across polio-affected countries is varied. The “orphan” virus proportion of recent WPV isolates in several countries provides evidence of undetected transmission caused by deficiencies in AFP surveillance; the quality of AFP surveillance needs to be enhanced. ES is becoming an increasingly important supplemental tool for monitoring end-stage progress in endemic countries and the maintenance of polio-free status [35].
In 2014, the major challenge to the success of global polio eradication is an ongoing inability to access and vaccinate children in areas because of physical threats to the security of vaccinations teams. The increase in cases in 2013 and 2014 in Pakistan is the result of a ban on polio vaccination in North and South Waziristan, FATA; local community leaders have prevented vaccination of more than 350,000 children since June 2012. Ongoing military operations in Khyber Agency also limit access. Targeted attacks against polio workers and police officers assigned to protect them have compromised the implementation of SIAs in parts of FATA, KP province, and Karachi. Directed violence against vaccinators in Kano, Yobe, and Borno in early 2013 curtailed SIA implementation. Antigovernment elements continue to cause insecurity in Borno state and elsewhere in northern Nigeria. However, in Borno 100% were of children were inaccessible in March 2012 declining to 16% in March 2014 [41]). The active conflict in Syria led to the immunity gaps that allowed the outbreak to occur and impeded early comprehensive outbreak response. WPV continues to be reimported from FATA into the Eastern Region of Afghanistan where access and security problems continue to affect the implementation of quality SIAs. During 2010–2012, the conflict in Afghanistan prevented vaccinators from safely accessing children in many areas of the Southern Region of Afghanistan. Systematic negotiations improved access to children during 2012–2013, which, together with successful efforts to improve the quality of SIAs, virtually interrupted indigenous WPV1 transmission. Despite this, antigovernment elements have again prevented vaccination of all children in Helmand province thus far in 2014. Limitations of access for SIAs into parts of south-central Somalia remain. The ongoing WPV1 circulation in Cameroon (that affected Equatorial Guinea in early 2014) poses a serious risk for renewed spread into populations affected by insecurity, civil unrest and displacement in the CAR.
Complacency is the other major threat to GPEI’s success: gaps in program performance remain in some secure areas of Nigeria and Pakistan; serious potential consequences can result from decreased program performance during elections; and limited political commitment can result in low effectiveness of outbreak response performance. These risks are compounded by gaps in surveillance in polio-free areas and the continued threat of new international spread of WPV.
Managing these risks requires full national ownership of the eradication program in all affected countries, including full engagement of all relevant line ministries to local authorities. Urgently needed improvements in accessing and vaccinating children in insecure and conflict-affected areas will require enhancing the engagement of international bodies, religious leaders, and humanitarian actors to implement area-specific plans, generate greater community demand and participation, and adapt activities to local contexts.
On 5 May, 2014, under authority of the International Health Regulations (2005), the WHO Director-General declared renewed international spread of WPV in early 2014 a ‘Public Health Emergency of International Concern’ [48]. She issued Temporary Recommendations to reduce international exportation by having affected countries ensure/encourage that all residents and long-term visitors traveling from polio-affected countries receive an officially documented booster dose of polio vaccine within a year before departure. WHO appealed to all member states to enhance surveillance and immunization activities and fully implement immunization recommendations for of travelers [49].
If international and national importation of WPV into polio-free areas can be prevented (or controlled quickly, if not), effective activities continued during political change, access to all children increased through negotiation and innovation, and activities not curtailed from insufficient funding or complacency, polio eradication can be completed in the near future. All evidence points to the technical and operational feasibility of eradication. At no time in the past has this been so close.
Acknowledgments
The authors would like to thank Ajay Goel, Christine Lamoureux, and Paul Chenoweth of WHO, and Cara Burns, Beth Henderson, Jane Iber, Jean Baptiste Kamgang and Eric Mast of CDC for their kind assistance. We also would like to express appreciation for the sincere efforts of the immunization and surveillance field workers and country program counterparts at all levels working in the Global Polio Eradication Initiative, the regional coordinators, laboratory scientists, and other workers of the GPLN, the massive contributions of the GPEI international partnership to the effort, including the core partners WHO, Rotary International, CDC, UNICEF, and the Bill and Melinda Gates Foundation.
Funding statement: This work is publically funded. CDC is a U.S. Government agency within the Department of Human Services. The World Health Organization is funded by Member States and by voluntary donations.
Footnotes
Conflict of interest statement: The authors report no conflict of interest.
Meetings where the information has previously been presented: This work is in the public domain and portions of the information have been presented in several informal and scientific meetings, but at no scientific conferences in 2013.
Copyright disclaimer: Some of the authors are employees of the U.S. Federal Government and as such cannot assign copyright as this is a work in the public domain and therefore there is no copyright to assign.
References
- 1.World Health Assembly. Global eradication of poliomyelitis by the year 2000: resolution of the 41st World Health Assembly. Geneva, Switzerland: World Health Organization; 1988. (WHA resolution no. 41.28) [Google Scholar]
- 2.Pallansch MA, Sandhu HS. The eradication of polio--progress and challenges. N Engl J Med. 2006;355:2508–11. doi: 10.1056/NEJMp068200. [DOI] [PubMed] [Google Scholar]
- 3.Moturi EK, Porter KA, Wassilak SGF, et al. Progress Toward Polio Eradication — Worldwide, 2013–2014. MMWR Morb Mortal Wkly Rep. 2014;63:468–72. [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Apparent global interruption of wild poliovirus type 2 transmission. MMWR Morb Mortal Wkly Rep. 2001;50:222–4. [PubMed] [Google Scholar]
- 5.Aylward RB, Maher C. Interrupting poliovirus transmission – New solutions to an old problem. Biologicals. 2006;34:133–9. doi: 10.1016/j.biologicals.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 6.Posey DL, Linkins RW, Oliveria MJC, Monteiro D, Patriarca PA. The effect of diarrhea on oral poliovirus vaccine failure in Brazil. J Infect Dis. 1997;175:S258–63. doi: 10.1093/infdis/175.supplement_1.s258. [DOI] [PubMed] [Google Scholar]
- 7.Grassly NC1, Fraser C, Wenger J, et al. New strategies for the elimination of polio from India. Science. 2006;314:1150–3. doi: 10.1126/science.1130388. [DOI] [PubMed] [Google Scholar]
- 8.El-Sayed N, El-Gamal Y, Abassy AA, et al. Monovalent type 1 oral poliovirus vaccine in newborns. N Eng J Med. 2008;359:1655–65. doi: 10.1056/NEJMoa0800390. [DOI] [PubMed] [Google Scholar]
- 9.Waggie Z1, Geldenhuys H, Sutter RW, et al. Randomized trial of type 1 and type 3 oral monovalent poliovirus vaccines in newborns in Africa. J Infect Dis. 2012;205:228–36. doi: 10.1093/infdis/jir721. [DOI] [PubMed] [Google Scholar]
- 10.Grassly NC, Wenger J, Durrani S, et al. Protective efficacy of a monovalent oral type 1 poliovirus vaccine: a case-control study. Lancet. 2007;369:1356–62. doi: 10.1016/S0140-6736(07)60531-5. [DOI] [PubMed] [Google Scholar]
- 11.Cochi SL, Linkins RW. The Final Phase of Polio Eradication: New Vaccines and Complex Choices. J Infect Dis. 2012;205:169–71. doi: 10.1093/infdis/jir727. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Resurgence of wild poliovirus type 1 transmission and consequences of importation-21 countries, 2002–2005. MMWR Morb Mortal Wkly Rep. 2006;55:145–50. [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Wild poliovirus type 1 and type 3 importations—15 countries, Africa, 2008–2009. MMWR Morb Mortal Wkly Rep. 2009;58:357–62. [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Outbreaks Following Wild Poliovirus Importations --- Europe, Africa, and Asia, January 2009–September 2010. MMWR Morb Mortal Wkly Rep. 2010;59:1393–9. [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Progress Toward Interrupting Wild Poliovirus Circulation in Countries With Reestablished Transmission --- Africa, 2009—2010. MMWR Morb Mortal Wkly Rep. 2011;60:306–11. [PubMed] [Google Scholar]
- 16.Mach O, Tangermann R, Wassilak SG, et al. Outbreaks of Paralytic Poliomyelitis during 1996–2012: The Changing Epidemiology of a Disease in the Final Stages of Eradication. J Infect Dis. 2014 doi: 10.1093/infdis/jit454. THIS SUPPLEMENT. [DOI] [PubMed] [Google Scholar]
- 17.O’Reilly KM, Chauvin C, Aylward RB, et al. A Statistical Model of the International Spread of Wild Poliovirus in Africa Used to Predict and Prevent Outbreaks. PLoS Med. 2011;8(10):e1001109. doi: 10.1371/journal.pmed.1001109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sutter RW, John TJ, Jain H, et al. Immunogenicity of bivalent types 1 and 3 oral poliovirus vaccine: a randomised, double-blind, controlled trial. Lancet. 2010;376:1682–8. doi: 10.1016/S0140-6736(10)61230-5. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. Global Polio Eradication Initiative: Strategic plan 2010–2012. Geneva, Switzerland: World Health Organization; 2010. Available at http://www.polioeradication.org/content/publications/gpei.strategicplan.2010-2012.eng.may.2010.pdf. [Google Scholar]
- 20.Independent Monitoring Board of the Global Polio Eradication Initiative. October 2011 report. Geneva, Switzerland: World Health Organization; 2011. Available at http://www.polioeradication.org/portals/0/document/aboutus/governance/imb/4imbmeeting/imbreportoctober2011.pdf. [Google Scholar]
- 21.World Health Organization Executive Board. Poliomyelitis: intensification of the global eradication initiative. Geneva, Switzerland: World Health Organization; 2012. (Executive Board Resolution EB130.R10). Available at http://apps.who.int/gb/ebwha/pdf_files/eb130/b130_r10-en.pdf. [Google Scholar]
- 22.World Health Assembly. Poliomyelitis: intensification of the global eradication initiative. Geneva, Switzerland: World Health Organization; 2012. (WHA Resolution WHA65.5 p10). Available at http://apps.who.int/gb/ebwha/pdf_files/WHA65-REC1/A65_REC1-en.pdf#page=25. [Google Scholar]
- 23.The Global Polio Eradication Emergency Action Plan 2012–2013. Geneva, Switzerland: World Health Organization; 2012. Available at. http://www.polioeradication.org/Portals/0/Document/Resources/StrategyWork/EAP_201205.pdf. [Google Scholar]
- 24.World Health Organization, UNICEF. WHO/UNICEF estimates of national immunization coverage. Available at: http://www.who.int/immunization/monitoring_surveillance/routine/coverage/en/index4.html.
- 25.Kew O, Sutter R, et al. Vaccine-derived polioviruses: State-of-the-Art. J Infect Dis. 2014 doi: 10.1093/infdis/jiu295. THIS SUPPLEMENT. [DOI] [PubMed] [Google Scholar]
- 26.Global Polio Eradication Initiative. Polio Eradication and Endgame Strategic Plan (2013–2018) Geneva, Switzerland: World Health Organization; 2013. Available at http://www.polioeradication.org/Portals/0/Document/Resources/StrategyWork/PEESP_EN_US.pdf. [Google Scholar]
- 27.Sutter RW, Kew OM, Cochi SL, Aylward RB. Poliovirus vaccine—live. In: Plotkin SA, Orenstein WA, Offit P, editors. Vaccines. 6. Saunders Elsevier; 2013. pp. 598–645. [Google Scholar]
- 28.Birmingham ME, Linkins RW, Hull BP, Hull HF. Poliomyelitis surveillance: The compass for eradication. J Infect Dis. 1997;175:S146–50. doi: 10.1093/infdis/175.supplement_1.s146. [DOI] [PubMed] [Google Scholar]
- 29.Hull BP, Dowdle WR. Poliovirus surveillance: Building the global polio laboratory network. J Infect Dis. 1997;175:S113–S116. doi: 10.1093/infdis/175.supplement_1.s113. [DOI] [PubMed] [Google Scholar]
- 30.Banerjee K, Hlady WG, Andrus JK, Sarkar S, Fitzsimmons J, Abeykoon P. Poliomyelitis surveillance: the model used in India for polio eradication. Bull WHO. 2000;78:321–9. [PMC free article] [PubMed] [Google Scholar]
- 31.Evolution of the Global Polio Laboratory Network. Diop O, Sanders R, Hull B, De Gourville E. J Infect Dis. 2014 THIS SUPPLEMENT. [Google Scholar]
- 32.World Health Organization. . Conclusions and recommendations of the Advisory Committee on Poliomyelitis Eradication, Geneva, 11–12 October 2005. Wkly Epidemiol Rec. 2005;80:410–6. [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention. Surveillance Systems to Track Progress Toward Global Polio Eradication — Worldwide, 2012–2013. MMWR Morb Mortal Wkly Rep. 2014;63:356–61. [PMC free article] [PubMed] [Google Scholar]
- 34.Jorba J, Campagnoli R, De L, Kew O. Calibration of multiple poliovirus molecular clocks covering an extended evolutionary range. J Virol. 2008;82:4429–40. doi: 10.1128/JVI.02354-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asghar H, Diop OM, Weldegebriel G, et al. Environmental Surveillance for Polioviruses in the Global Polio Eradication Initiative. J Infect Dis. 2014 doi: 10.1093/infdis/jiu384. THIS SUPPLEMENT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tulchinsky TH, Ramlawi A, Abdeen Z, Grotto I, Flahault A. Polio lessons 2013: Israel, the West Bank, and Gaza. Lancet. 2013;382:1611–2. doi: 10.1016/S0140-6736(13)62331-4. [DOI] [PubMed] [Google Scholar]
- 37.Shulman LM, Gavrilin LM, Jorba EJ, et al. Molecular epidemiology of silent introduction and sustained transmission of wild poliovirus type 1, Israel, 2013. Eurosurveillance. 2014;19(7) doi: 10.2807/1560-7917.es2014.19.7.20709. pii=20709. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20709. [DOI] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention. Evaluating surveillance indicators supporting the Global Polio Eradication Initiative, 2011–2012. MMWR Morb Mortal Wkly Rep. 2013;62:270–4. [PMC free article] [PubMed] [Google Scholar]
- 39.Mohammadi D. Middle Eastern countries scramble to stop spread of polio. Lancet. 2013;382:1621–2. doi: 10.1016/s0140-6736(13)62289-8. [DOI] [PubMed] [Google Scholar]
- 40.Aylward RB, Alwanb A. Polio in Syria. Lancet. 2014;383:489–91. doi: 10.1016/S0140-6736(14)60132-X. [DOI] [PubMed] [Google Scholar]
- 41.World Health Organization, Rotary International, Centers for Disease Control and Prevention, UNICEF. Global Polio Eradication Initiative (GPEI) status report April 30, 2014. Partners’ report to the Independent Monitoring Board Meeting of May 2014; Available at http://www.polioeradication.org/Portals/0/Document/Aboutus/Governance/IMB/10IMBMeeting/2.2_10IMB.pdf. [Google Scholar]
- 42.Patel MK, Konde MK, Didi-Ngossaki BH, et al. An outbreak of wild poliovirus in the Republic of Congo, 2010–2011. Clin Infect Dis. 2012;55:1291–8. doi: 10.1093/cid/cis714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hui-Ming Luo HMm, Zhang Y, Xin-Qi Wang XQ, et al. Identification and Control of a Poliomyelitis Outbreak in Xinjiang, China. N Engl J Med. 2013;369:1981–90. doi: 10.1056/NEJMoa1303368. [DOI] [PubMed] [Google Scholar]
- 44.World Health Organization Regional Office for South-East Asia. WHO South-East Asia Region certified polio-free. World Health Organization; New Delhi, India: 2014. Available at http://www.searo.who.int/mediacentre/releases/2014/pr1569/en/ [Google Scholar]
- 45.Centers for Disease Control and Prevention. Progress Toward Poliomyelitis Eradication — Nigeria, January 2012–September 2013. MMWR Morb Mortal Wkly Rep. 2013;62:1009–13. [PMC free article] [PubMed] [Google Scholar]
- 46.Centers for Disease Control and Prevention. The Global Polio Eradication Initiative Stop Transmission of Polio (STOP) Program — 1999–2013. MMWR Morb Mortal Wkly Rep. 2013;62:501–3. [PMC free article] [PubMed] [Google Scholar]
- 47.Centers for Disease Control and Prevention. Polio Field Census and Vaccination of Underserved Populations — Northern Nigeria, 2012–2013. MMWR Morb Mortal Wkly Rep. 2013;62:663–5. [PMC free article] [PubMed] [Google Scholar]
- 48.World Health Organization. WHO statement on the meeting of the International Health Regulations Emergency Committee concerning the international spread of wild poliovirus. Geneva, Switzerland: World Health Organization; 2014. Available at http://www.who.int/mediacentre/news/statements/2014/polio-20140505/en/ [Google Scholar]
- 49.World Health Organization. International Travel and Health. Geneva, Switzerland: World Health Organization; 2014. 2014 Updates. Available at http://www.who.int/ith/en/ [Google Scholar]