Abstract
Neurotechnology is broadly defined as a set of devices used to understand neural processes and applications that can potentially facilitate the brain’s ability to repair itself. In the past decade, an increasingly explicit understanding of basic biological mechanisms of brain-related illnesses has produced applications that allow a direct yet noninvasive method to index and manipulate the functioning of the human nervous system. Clinical scientists are poised to apply this technology to assess, treat, and better understand complex socioemotional processes that underlie many forms of psychopathology. In this review, we describe the potential benefits and hurdles, both technical and methodological, of neurotechnology in the context of clinical dysfunction. We also offer a framework for developing and evaluating neurotechnologies that is intended to expedite progress at the nexus of clinical science and neural interface designs by providing a comprehensive vocabulary to describe the necessary features of neurotechnology in the clinic.
Keywords: neurotechnology, personalized medicine, translational science, emotion regulation, psychopathology
Recent advances in mobile computing have changed the way we communicate and go about our daily lives. Wireless technology, once the realm of science-fiction, is now nearly ubiquitous, supporting, for example, online banking, social networking and home monitoring. It can even be a challenge to connect with a human when one tries (think of the last time you tried to reach a live operator though a large company’s customer service number). Above and beyond the provision of speed and new conveniences, many aspects of technology have materially impacted the dynamics of human social interaction by redefining the geographic and temporal scales of communication. In short, technology both affects and is affected by the way our society operates. Yet, there has been little concerted effort to bring its considerable potential to bear on the field of mental health in the form of neurotechnology. Broadly speaking, neurotechnology refers to devices and applications used to understand, assess, and manipulate processes within the neural system (Giordano, 2012; McDowell & Ries, 2013).
Neurotechnologies can be used for assessment purposes (e.g., neuroimaging and thermography) as well as intervention (e.g., transcranial stimulation, implants, brain-machine interfacing) (Giordano, 2012). Some emerging technologies such as real-time fMRI provide utility in both areas by enabling brain computer interface capabilities (Papageorgiou et al., 2013; LaConte et al., 2007) that simultaneously image the neurobiology of cognitive and psychiatric states as well as hold promise for neurofeedback-based rehabilitation and therapy (Stoeckel et al., 2014; deCharms, 2008; Sulzer et al., 2013; LaConte, 2011). In this paper, we define the state of the relationship and the probable progeny, in the form of neurotechnologies to understand and remediate mental illness, of the inevitable ‘marriage’ of the fields of computer science and clinical psychology. We primarily discuss Magnetic Resonance Imaging (MRI)/functional MRI (fMRI) imaging technologies and Electroencephalography (EEG)/Brain-Computer Interface (BCI) cranial surface measurement technologies due to their more widespread use and availability. However, for the most part, the presented findings and suggestions could be applicable to other imaging (Positron Emission Tomography, Computed Tomography), cranial surface measurements (Magnetoencephalography), transcranial stimulation (Transcranial Magnetic Stimulation, Transcranial Direct Current Stimulation), implants and related technologies.
Neurotechnology does not focus solely on technology per se, but more directly on the utilization of that technology to gain insight into the functioning of the human nervous system, particularly for the purpose of understanding processes involved in health and disease (National Institutes of Health, 2014). For example, BCI devices have expanded human capacity for physical motion (c.f. Nicolas-Alonso & Gomez-Gil, 2012) and in some cases have restored aspects of cognitive functions by merging the electrical readout of the human brain with the principles of machine learning (Lee et al., 2013). Along similar lines, more invasive bioengineering technologies such as microwire array implants (e.g., Jackson & Fetz, 2007; Prasad et al., 2012) and programmable physical prosthetics (Santhanam et al., 2006) overcome the limited regenerative capacity of the brain to repair nerve cells damaged by combat or disease.
Although neurotechnology in its broadest sense has existed for almost fifty years (e.g., The Society for Neuroscience was founded in 1969), the last two decades have witnessed an astonishing increase in research, design and marketing of neurotechnology applications. The Decade of the Brain (http://www.loc.gov/loc/brain/) started in 1990 with the explicit goal of enhancing public awareness of the benefits to be derived from brain research. The Decade of the Mind Project followed (Albus et al., 2007), creating an international initiative with the stated goal of advancing trans-disciplinary understanding of how the mind and complex behaviors are related to the intrinsic activity of the brain. In 2013, the Obama administration announced the BRAIN Initiative (Brain Research through Advancing Innovative Neurotechnologies), with the ambitious goal of mapping the activity of every neuron in the human brain. In June of 2014, the National Institutes of Health (NIH) BRAIN working group’s final report was issued (National Institutes of Health, 2014). In that report, use of new technologies and approaches to understand neural activity involved in mental illness was specifically identified as the most important of the seven areas of high priority for future research under this initiative. Given these initiatives, along with documented shortcomings in our current mental health care system, such as the fact that one in four adults and about one in five adolescents in the United States experiences mental illness in any given calendar year (National Institute of Mental Health, 2012a, b) and only up to one-half of people with mental illness receive effective treatment with sustained benefit (National Institute of Mental Health, 2012c), the emphasis on neuroscience-based therapeutics is likely to stimulate development of entirely new classes of interventions for psychiatric disorders (Insel & Sahakian, 2012). This may be especially true for disorders involving difficulties with processes that cannot be introspected with absolute precision, such as socioemotional processing, including emotion awareness, recognition, and expression. Socioemotional difficulties are present in multiple disorders including schizophrenia, depression, anxiety, and autism spectrum disorders. Neurotechnologies may also be useful in evidence-based, clinically informative evaluation, since effective assessment tools are lacking. We anticipate that neurotechnologies will, within the next decade, emerge as the ‘third leg’ in our cadre of treatment approaches for mental illness. As such, they are not to replace pharmacological and psychosocial approaches (the first two ‘legs’), but rather complement them and offer a viable alternative for patients and providers.
Although BCI devices are currently most often used in gaming and consumer electronics (for example, MindRDR for Google Glass enables a user to take and share pictures just by thinking), they are also used for mental wellbeing and therapy (http://myndplay.com). They are basically lower cost EEG devices with smaller numbers (1–16) of (dry) electrodes. BCI devices have clinical utility and have been used to assist in stroke recovery, paralysis, and degenerative conditions such as amyotrophic lateral sclerosis (e.g., Moghimi, Kushki, Guerguerian, & Chau, 2013). Research on the clinical application of such neurotechnologies to mental health issues is now emerging.
Indeed, examples of success of neurotherapeutics have recently been highlighted by Stoeckel et al. (2014). Specifically in evaluating the potential impact of real-time fMRI, the authors illustrate the breadth of technology-based successes such as i) the preliminary promising efficacy of treating depression with deep-brain stimulation of the subgenual cingulate (Mayberg et al., 2005), ii) randomized controlled trials of EEG-based feedback in attention-deficit/hyperactivity disorder (ADHD; Arns et al., 2009; Hirshberg et al., 2005), and iii) the application of real-time fMRI for neurofeedback therapy of chronic pain (deCharms et al., 2005). Following a brief synthesis of the extant research on neurotechnologies in clinical science, our goals herein are to provide a framework to guide development and critical evaluation of neurotechnologies for clinical dysfunction and offer suggestions about how we might expand on this exciting, though limited, research base.
Clinical Neuroscience and Neurotechnology: A Logical Union
The symbiotic relationship between design and application, where user requirements drive design and design exposes new applications, is exemplified by the recent logical union between clinical psychology/psychiatry and neural interface designs. In this context, questions of clinical relevance have the potential to inform software and hardware applications that do not yet exist, while on the other hand some existing interfaces have the potential to stimulate work on clinical constructs that have not been well explored. Despite the nascent synergy between these fields, they advance at different rates and with dramatically different end goals in mind, resulting in an uneven terrain between the bench (i.e., development of the technology) and bedside (i.e., application of the technology to a clinical problem), which increasingly lacks a common vocabulary. Accordingly, in this section we outline a set of general guidelines that can be used to evaluate the problem space and simultaneously provide a vocabulary to facilitate a common understanding between the engineers who develop the technology and clinicians who seek to apply it. In a review of real-time fMRI, Stoeckel and colleagues (2014) proposed guidelines for establishing real-time fMRI as a neurotherapeutic tool. In a parallel manner, we broadly outline seven distinct principles, or attributes, that describe as comprehensively as possible the features of an effective ‘bench’ system (or neural interface design) in the context of a ‘bedside’ or general clinical application, while deemphasizing specific technological details. Specifically, we propose that neurotechnology applications should be: (1) verifiable, (2) useful, (3), consistent, (4) reproducible, (5) mechanism-driven, (6) complete and (7) deployable. Collectively, these characteristics represent a rational framework, which allows one to formalize parameters in the problem space between the requirements of a user and the deliverable output of any neurotechnology system (Table 1).
Table 1.
Principles for the Development of Clinical Neurotechnology Systems.
| Principle | Explanation |
|---|---|
| 1. Verifiable | Intended function, or target, of the system is testable and falsifiable |
| 2. Useful | Fulfills an unmet clinical need or offers a reasonable alternative to an existing, established approach |
| 3. Consistent | Alignment between clinical need and deliverable product of the technical design |
| 4. Reproducible | Produces consistent effects for a well-defined, fairly homogeneous user group |
| 5. Mechanism-driven | Targets a known or hypothesized pathophysiological process |
| 6. Complete | Has parameters that are sufficient to produce expected result, yet parsimonious enough to eliminate alternative explanations for its effect |
| 7. Deployable | Has promise for wide-scale adoption and use |
Principle 1: Verifiable
The most crucial aspect of any neurotechnology system is that its revealed functionality can be evaluated against its planned functionality, which in essence makes it possible to evaluate (as true or false) whether the system has met the user’s requirement. Placed in the broader context of hypothesis-driven science, a ‘good’ neurotechnology system is one whose expected behavior can be tested against a prediction and falsified. As an example, consider the following proposal: “We will produce a system that is user-friendly.” While this is a laudable goal of any human-computer interaction, it is difficult to verify and greater specification may be useful; for example, the system can be used by a specific category of users, rather than all users in general. Some users may feel that a system is user-friendly whereas others, for a variety of reasons, may not. This end-user variability introduces noise into a design that will make downstream evaluation of clinical efficacy more difficult. Extending this simplified example to a more complex system allows us to ask (and answer) the question “how will we explicitly know if the design delivers the expected function?” This is a simple but non-trivial issue, because the answer to this question allows one to tease apart two critical but completely separate design features: (1) whether the system has done its job (Principle 1) and (2) whether the output of the system produces movement in the clinical construct of interest (corresponding to Principle 5: Mechanism-driven, below).
Our concrete suggestion is that specific steps should be taken to understand the principle output of a system in a process of “build-verification” (or build verification testing, also called smoke testing; Kaner, Bach & Pettichord, 2001). This is an early but crucial step that is intended to exercise the complete program in order to reveal failures severe enough to reject a prospective system design. Importantly, build verification endorses a formal comprehensive method of assessing fundamental instability or key failures, and will therefore reveal unanticipated problems more efficiently and earlier than ad hoc testing, which relies on tester skill and intuition. Build verification, at its essence, also involves the establishment of ground-truth, or objective data against which subsequent results are compared. For example, a finger tapping pattern could be used to activate the motor cortex and establish accuracy of a machine learning classifier based on EEG. We recommend early and, if possible, automated build-verification as a cost-effective and time-saving functional test, chiefly because it minimizes wasted time in the form of unusable collected data. Build verification therefore has the immediate benefit of finding important ‘bugs’ fast, and long-term benefit of allowing differentiation between verifiability and clinical efficacy.
Principle 2: Useful
The utility principle underscores the notion that if any neurotechnology design is to produce a sustained, positive influence on the field, it will ideally fill a gap for which an established, well-understood or simpler solution does not already exist or augment (improve upon) some established approach. Alternatively, the technology system should offer some other benefit (e.g., more readily available or economical to the end-user) than an existing protocol that is already established. Although we argue herein that utility of the system must be considered, we do not argue for its necessity. It is well-accepted that treatments are not equivalently useful across all recipients (e.g., Weisz et al., 2013) and end-user preference ought to be considered. Some users (clients) might prefer use of technology, for instance, to a traditional therapeutic approach and, as such, respond better to the technology than they would to a human therapist.
Consider that in the case of most phobias, exposure-based treatment is able to capitalize on habituation and fear-extinction mechanisms as a relatively straightforward and brief method of intervention (cf. Seligman, Swedish, & Ollendick, 2014). There is always the possibility, however, of developing technology-based interventions around these well-understood processes. For certain feared stimuli (animals, insects), it is almost certainly simpler to use the actual phobic stimuli in vivo. However, there are some problems for which this is not practical, such as scenarios that are impossible to reproduce in the clinic (battlefields, heights, large bodies of water). In these cases, the necessity of a technology-supplemented treatment is evident when considering that virtual reality (VR) could faithfully recreate certain conditions that are practically impossible to attain in the clinic. We therefore recommend that technology-enhanced treatment systems should be critically evaluated in terms of their actual or incremental value-added necessity, and not in terms of their aesthetic appeal or superfluous properties that may only serve to pleasantly surprise the user (the ‘wow’ factor).
Principle 3: Consistent
We operationalize the consistency attribute as the degree of alignment between bench capabilities and bedside requirements. Misalignment (inconsistency) can occur when either (1) the requirements of a bedside application exceed the capabilities of the available technology, or (2) the available technology manipulates processes that are not relevant to the clinician or target clinical problem. Inconsistency may result from lack of clear communication between the two sides of the house, namely development (engineering) and application (clinicians). We believe that inconsistency, and the wasted effort that would result, can be avoided if there is a clearly established link between what is achievable within the constraints of neurotechnology designs and what is needed to test a hypothesized effect and ultimately resolve a clinical problem.
A mismatch scenario may not be obvious during project development, and for this reason we specifically note that consistency should be evaluated within the crucible of construct validation. As an example, in the case of cochlear implants, a detailed knowledge of the tonotopic organization of the basilar membrane informed the development of prosthetic designs that stimulate the cochlear nerve according to the same principles, bypassing damaged or degraded hair cells completely and allowing the brain to interpret frequency signals as sound. Such a feat could not have occurred unless hardware design was able to deliver functionality that acted directly upon the known physiology of the inner ear. In this example, consistency is evident when considering that the neural interface design braids into a metamodel that relates the intervention to the problem via logically supportable statements about electrophysiology, electrical engineering and anatomy. Inconsistencies would be exposed in earnest during this process, because estimates of discriminant and convergent validity (as two examples among many) would reveal that a prosthetic acts upon a theoretically distinct construct from what was intended. In clinical psychology, a conceptually similar example is found in the literature on BCI-based neurofeedback for ADHD. In a study by Lim and colleagues (2012), youth with ADHD completed an attention training game in which a user controls an avatar in a ‘race’ using a dry-fit EEG headband. In this game, the higher the concentration level, the greater the speed of the avatar’s movement. This is an excellent example of consistency between the bioengineered device and the clinical application, because the construct of attentional focus is both measureable within the constraints of the device and also directly relevant to the clinical phenomenon of ADHD.
A corollary here is that fabrication details only matter to the extent that they operate on testable psychological phenomena. The power of a digital intervention is therefore not derived from its basis in a symbol-manipulating processor, but rather by the fact that it delivers functionality that meshes well with the existing nomological net (conforming to predictions without contradicting what is already known). In the broadest interpretation, any intervention could be held to this standard; however, we highlight it as particularly important in the domain of technology-enabled intervention or assessment because critical evaluation of consistency is meant to inform the central question: how can we effectively interface what is deliverable from an engineering standpoint with what is known from behavioral and cognitive research? To clarify, this principle refers not to the recommendation that an intervention produces consistent results (see Principle 4: Reproducible below), but rather that it conforms to a nomological network that is defined in the traditional terms: by the relations among the constructs it measures.
Principle 4: Reproducible
A reproducible neurotechnology design is one that distributes the same effect upon all, or almost all, cases of a particular user type. This is intuitive on one hand, mainly because most of us are accustomed to human-computer interfaces that work for all or almost all users (e.g., LCD monitor, two-axis mouse). On the other hand, there are certain characteristics of psychiatric disorders that could conceivably dramatically lower the reproducibility of effects in neurotechnology systems. For instance, heterogeneity in DSM-V diagnoses, lack of robust endophenotypes and documented developmental changes in pathophysiological mechanisms themselves must be taken into account when considering the efficacy of a digitally based intervention. Reproducibility could be construed as similar to the traditional notion of test-retest reliability, with the special exception that given the same inputs, deterministic algorithms will always return the same result. Our conceptualization of reproducibility recasts the traditional focus of reliability onto sources of variability that conspire to reduce the similarity of the effect across users.
Charles Babbage, the English philosopher and inventor, noted in his memoirs: “On two occasions I have been asked: ‘Mr. Babbage, if you put into the machine the wrong figures, will the right answers come out?’ I am not rightly able to apprehend the kind of confusion of ideas that could provoke such a question” (Babbage, 1864, p. 67). This quotation is intimately tied to our notion of reproducibility and highlights the “garbage-in-garbage-out” principle, which proposes that computers will process nonsensical data (garbage in) according to the same logical rules as any other data, and produce nonsensical output (garbage out) as a result. Even the ubiquitous ‘qwerty’ keyboard could be blamed for irreproducible effects (i.e., high variability in typing speed or spelling errors across heterogeneous user types), even though the input/output and transfer characteristics of the keyboard are unchanged for each user. Conversely, given precisely homogeneous user types (e.g., 10 groups, each populated by users with exactly the same degree of typing skill), the reproducibility of the keyboard’s effect will approach 1.0 within that group because the error rate (garbage in) for the group remains constant.
In keeping with this truism, we challenge the assumption that reproducibility is the sole domain of the engineering design and argue that in fact, the burden for ensuring reproducibility lies at the opposite end of the translational bridge. Undocumented clinical heterogeneity will introduce noise (garbage in) to an otherwise reproducible effect. Undocumented variation along axes of affect, cognition and motivation that cut across diagnostic categories (Insel et al., 2010) adds to existing variability within diagnostic classes (e.g., two individuals with antisocial personality disorder may have no overlap in symptoms, as only three of seven possible symptoms are required for a diagnosis; APA, 2013), along with developmental principles such as heterotypic continuity. These sources of variation should be taken into account because they each decrease the reproducibility of a system’s effect. Along these lines, most of these problems can be resolved by precisely defining the cases for whom an intervention is likely to be successful. When identifying a set of clinical requirements that will eventually be addressed by an engineered system design, clinicians must be clear on which processes will be manipulated by the intervention. Ultimately, this informs the larger question: “For whom will this intervention be effective?”
Principle 5: Mechanism-driven
This principle highlights the fact that technology-enabled intervention designs should be anchored to a pathophysiological mechanism that can be logically explained and tested. When the clinician or any end-user asks, “How does this intervention work?” according to this principle a response of “I don’t know” indicates a shortcoming in design. To confront this problem, we endorse a policy of ‘no magic,’ which specifies that during the design phase of a project, no unjustifiable decisions should be made (Larman, 2004), and that design should halt precisely before that frontier is crossed. Adherence to this policy ought to lead to a scenario wherein the choice of neurotechnology components and their relationship to a pathophysiological mechanism can be rationally explained and learned. A ‘no magic’ approach is not orthogonal to a hypothesis-testing approach. To the contrary, the ability to concretely explain every aspect of the logical flow of ideas, including one’s hypothesis, prevents abstraction of meaning from logical relations that are not (or cannot be) sufficiently established. The alternative is that the design indexes or manipulates some empty conceptual space, which leads to a black box scenario in which the inner logic of a mechanism can only be inferred by its input and output characteristics. A particularly undesirable characteristic of a black box scenario is that it explicitly requires a process of reverse engineering, or going ‘backwards’ in the development cycle in order to understand the process by which the intervention achieves its effects.
We consider cognitive bias modification (CBM) procedures, an increasingly popular and direct cognitive intervention for various forms of anxiety disorders in children (e.g., Eldar et al., 2012; Shechner et al., 2014) and adults (e.g., Schmidt, Richey, Buckner, & Timpano, 2009), an example of this backward cycling in intervention development. Most forms of CBM (with some exceptions in interpretation [Grey & Mathews, 2000, 2009] and recall training [c.f. MacLeod & Mathews, 2004]) are premised on the notion that a fast-latency attentional bias toward environmental threat stimuli plays a causal role in the development and maintenance of the disorders and that by extension, removal of the cause by systematically training attention away from threat should eliminate the symptoms (e.g., MacPherson & Fristad, 2014). Although these general principles have been upheld by several dozen studies (c.f. MacLeod & Mathews, 2012, for an integrative review), and three meta analyses (Beard, Sawyer & Hofmann, 2012; Hakamata et al., 2010; Hallion & Ruscio, 2011), the neurobiological mechanism by which CBM interventions achieve their effect is still unclear. As a consequence, recent work in this area is increasingly focused on revealing mechanisms that underlie the observed effects (Clarke et al., 2014; Browning et al., 2010), which is a predictable but necessary reaction when the precise mechanism of action cannot yet be completely explained or directly tested.
Principle 6: Complete
An axiom that is frequently attributed to Albert Einstein (probably paraphrased from Einstein, 1934, p. 165) states that “Everything should be made as simple as possible, but no simpler.” The completeness of neural interface design refers to the desirable tension between parsimony and sufficiency in meeting the clinical objective, or the reasonable expectation that the presence of the system will meet the user’s requirement. Any intervention or medical device that is necessary but not sufficient to produce change is not maximally useful, because it is incomplete. However, there is an opposing force in our conceptualization here, to the extent that parsimony should constrain the development of systems that are unnecessarily complex. Excessively complex system designs may exceed the user’s requirements, and perhaps capabilities, by providing features that are redundant or unnecessary, thus making it difficult to disentangle the precise cause of any observed benefits or lack thereof. When establishing some initial common vision for a project, the tension between sufficiency and parsimony should be explicitly considered in light of the proposition that a system that has complex design features will, by definition, obscure the true source of its observed effect. Ultimately, this means that a neural interface model should not even be created unless it is deemed likely that the precise scope (no more, no less) will believably produce the expected change, without generating alternative explanations.
Principle 7: Deployable
Deployability, as a general property, refers to the ability of the intervention concept to accommodate needs of users without compromising functionality. In an idealized sense, this also means that the intervention concept should be able to tolerate distribution on a large scale with perfect fidelity. The long-term impact of neurotechnology is closely related to the ability to scale-up, whereas neurotechnologies that produce an effect only if a set of idealized conditions is met are unlikely to be beneficial in real-world settings. However, deployability, like the other principles, is an aspirational goal. An intervention is not to be construed as inherently inferior or fatally flawed if distribution is not immediately (or perhaps even ever) possible. For example, there may be instances in which ease of deployability of a system may be necessarily low, such as in the case of gamma knife capsulotomy for OCD (e.g., Kondziolka, Flickinger & Hudak, 2011; Sheehan et al., 2013), where an extremely unique confluence of hardware, software and intellectual expertise is required to deploy a targeted treatment. In these cases, the utility (Principle 2) of the intervention should be high enough to offset concerns about deployability (e.g., because no other viable alternative is available). Thus, there may be cases in which hardware and software designs are unique; however, this ostensible weakness is offset by the impact of the intervention. All else being equal, it is desirable for the intervention concept to survive distribution. If only one working group in the world has the required expertise to make a system work, all other principles that we have outlined here may have been met, but the engineering design may not confer the desired clinical or societal benefit because it cannot be distributed with any effect.
Summary of Principles
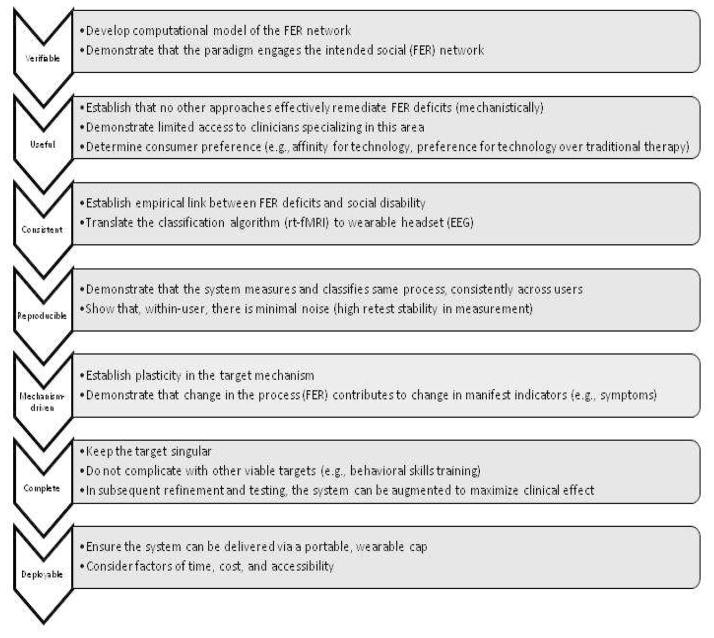
In this section we presented a set of standards that can be used both in the development and critical evaluation of neural interface designs. The logical and perhaps inevitable courtship between clinical psychology and biomedical engineering has proceeded quickly enough that its union has outpaced the available guidelines for development, and we consider these principles to be timely design considerations that, when fulfilled, are likely to lead to neurotechnologies that will produce a lasting benefit. Although inter-related, these principles can be thought of somewhat sequentially hierarchically (e.g., falsifiability is critical in early development phases, but deployability can be a later consideration). In Figure 1, we apply these principles to a project our group is undertaking to develop and evaluate the feasibility of a real-time neurofeedback system to remediate deficits in facial emotion recognition, using synchronizing fMRI and EEG, with adolescents and adults who have ASD. As depicted, for each principle we propose specific benchmarks or goals related to this system.
Figure 1.
Model of the application of the seven principles to an example of neurotechnology development.
It is unlikely that any system will meet all of these principles, and we do not contend that an intervention system is inherently ‘poor’ if it fails to meet all seven. Rather, these principles are meant to comprise an aspirational heuristic for understanding the variables in the problem space that exists between clinical psychology and neural interface designs, and in particular, for critical evaluation of such designs in the context of peer review. Because we conceptualize these principles as benchmarks toward an ideal course of the development of neural interfaces for clinical psychology, caution should be taken not be too rigid in evaluation of new neurotechnology, lest we risk impeding progress. Virtually all technology starts out as expensive, complicated, and clunky to use. However, given sufficient demand coupled with innovativeness of the core idea, the ultimate application can exceed expectations (consider the early start of the personal computer, for example).
Applying Neurotechnology to Socioemotional Processes of Psychopathology
Neurotechnology can be applied to a vast range of psychopathological processes and risk factors. In this section, we narrow our focus on the application of neurotechnology to the assessment and rehabilitation of socioemotional processes broadly related to psychopathology transdiagnostically. In particular, we discuss several promising areas that may be ready to benefit from the synthesis of cyber and physical systems, most notably because such systems provide information that is, by the definition of the problem, difficult or in some cases impossible to introspect.
Emotion Recognition, Expression, and Regulation: Transdiagnostic Processes
At the most basic level, socioemotional processes involve mental routines and their biological substrates that collectively support normative social interaction. Socioemotional competence can therefore be regarded as the capacity to correctly identify the subjective, internal states of other people as well as intentionally monitor and regulate one’s own behavior and affect in real-time. Problems with emotion recognition and emotion regulation have been implicated in many forms of psychopathology (Gross & Jazaieri, 2014) and can be expressed in various ways, including a lack of awareness or appreciation of other people and their feelings, not responding appropriately to others’ emotional needs, not changing behavior to fit a social situation, and letting one’s own emotions negatively affect a social interaction. In particular, recognition and expression of emotions are inter-related processes that influence probability of successful social interactions (Nuske, Vivanti, & Dissanayake, 2013).
The ability to discriminate certain expressions develops early on in childhood. Babies as young as 3 months of age show the ability to distinguish happy, sad, and surprised emotions from static cues (Young-Browne et al., 1977) and by 7 months, they are able to discriminate dynamic happy and angry faces (Soken & Pick, 1999). By 4 years of age, typically developing children can accurately verbally label most basic, prototypical emotions (Taylor et al., 1999). As previously mentioned, these processes are sometimes impaired, making social interactions and self-regulation difficult for affected individuals. Emotion regulation refers to an ability to modify one’s emotions in order to respond to situational demands and to meet one’s goals (Thompson, 1994). Problems with emotion regulation are expressed in many ways, including low frustration tolerance and reactive aggression.
Impaired emotion recognition and theory of mind abilities (i.e., inferring others’ thoughts and intentions) has been associated with childhood-onset conduct disorder (Donno, Parker, Gilmour, & Skuse, 2010; Fairchild et al., 2009), and is present among children with high levels of psychopathic traits (Dadds, Masry, Wimalaweera, & Guastella, 2008). Developmentally, socioemotional competence has been found to moderate the relationship between oppositionality in early childhood and later delinquency, such that young children with poorer socioemotional competence are more likely to progress to conduct disorder (Mandy, Skuse, Steer, St Purcain, & Oliver, 2013). Impaired recognition of others’ facial emotions, particularly the expression of fear, has been found to be associated with criminal behaviors among people with schizophrenia (Weiss et al., 2006) and bipolar disorder (Demirel et al., 2014), and there is a fairly robust association between inability to perceive fear via facial cues and antisocial behavior (Marsh & Blair, 2008). For many people with ADHD, poor social perspective-taking, insensitive interpersonal approaches, emotion regulation difficulties, and difficulty forming stable social relationships are life-course persistent problems (Kristensen et al., 2014; Marton et al., 2009). Impaired emotion recognition, especially in response to more subtle or fleeting cues (Rump, Giovannelli, Minshew, & Strauss, 2009; Tell, Davidson, & Camras, 2014; Uljarevic & Hamilton, 2013) and atypical cognitive processing of emotional stimuli (Eack, Mazefsky, & Minshew, 2014) has been found among children and adults with ASD. Impaired access to, and outward expression of, one’s own emotions (e.g., Shalom et al., 2006) and impaired emotion regulatory abilities (e.g., Mazefsky et al., 2013; White et al., 2014) have also been reported in ASD. It is clear that deficits in these domains are pervasive and transdiagnostic. They are also private, often not outwardly observable, processes not readily assessed with traditional behavioral approaches. As such, neurotechnologies might be especially useful for both assessment and rehabilitation of deficits in these socioemotional processes. Below, we describe some of the research in these areas, noting that the uncontrolled, correlational nature of the extant research prevents us from drawing inferences about causality or mechanisms of action. Nevertheless, such studies can offer indication as to viable courses to pursue in rigorous research to firmly establish directionality. After summarizing the relevant research, we address the potential utility of such systems for the study of problems in socioemotional functioning, specifically its relevance for better understanding of the mechanisms underlying such problems.
Assessment
There have been recent efforts to apply neurotechnology for assessment of serious mental illness. Traditional functional assessment, in a behavior analytic sense (i.e., structured observation of antecedents, behaviors, and consequences), is often conducted to understand the factors maintaining a problem. Functional assessment offers a direct, unfiltered view of the client and the behavior(s) of interest, and it has been used successfully in many clinical contexts such as understanding the causes of problematic behavior in individuals with limited speech or developmental disabilities (Jacobson & Holburn, 2004) and evaluation, and modification, of environmental factors that prompt and maintain occurrence of self-injurious behavior (Carr, 1977; Durand, 1987). However, structured functional assessment can be complicated for a variety of reasons, including ethical considerations (e.g., privacy of others in the setting), degree of stimulus control required (e.g., delivery of antecedent and consequent events needs to be precise), behavioral responsivity (e.g., the target behavior is exacerbated or squelched temporarily as a result of observation), and the impact of the clinician’s presence on test validity (e.g., client alters behavior intentionally because s/he is being observed) (e.g., e.g., Emerson, 1992; Iwata et al., 2000). Perhaps most germane to use of neurotechnology in this regard, functional assessment may not be viable for phenomena that are not readily observable (e.g., actively interpreting a perceived emotion), as these behaviors do not easily lend themselves to structured behavioral observation (Groth-Marnat, 2009; Iwata et al., 2000). In addition, use of technology may limit time burden on human assessors.
A technology-based functional assessment for socioemotional processes has the potential to overcome these limitations (Principle 2: Useful). For instance, virtual environments that closely simulate real-world situations (e.g., a classroom, a store) can be designed and used to determine what a person would do in the actual situation. Parsons and colleagues (2007) found that children with ADHD demonstrated more commission and omission errors and more superfluous body movements (hyperkinesis), relative to children without ASD, in a VR classroom. They also found group differences in behavior when distracters were introduced into the virtual environment. The investigators concluded that the virtual environment was able to effectively differentiate between participants with and without ADHD, and behavioral results from the VR classroom correlated with widely used ADHD assessment tools, including a parent clinician-administered tools and parent-completed questionnaires (Parsons, Bowerly, Buckwalter, & Rizzo, 2007). These results provide preliminary support for application of neurotechnology in the assessment of socioemotional processes involved in psychopathology (e.g., emotional reactivity), and suggest that interactive, VR technology might be applied to functional assessment. Further evaluation is warranted to determine incremental validity (Principle 2: Useful), stability of results (Principle 4: Reproducible) as well as generalizability and ecological soundness (Principle 7: Deployable).
Assessments employing VR might be especially useful in assessing for emotion regulation deficits since tools for direct measurement in clinical populations are lacking. Self-report of emotion regulation is likely insufficient on its own, as it requires insight into one’s own emotions and ability to label those emotions – skills that are often deficient in individuals struggling with effective emotional regulation. To supplement self-reports, observational approaches (e.g., frustration tolerance tasks) are often utilized. Accurate assessment of emotion regulation should include assessment of client’s attention to their emotional state, their ability to describe the emotion, and ability to differentiate the different emotions (Mazefsky & White, 2013). Neurotechnology may prove useful in allowing for these observations in a more controlled environment that targets the underlying mechanisms (Principle 5: Mechanism-driven). In summary, neurotechnology might provide a useful and hypothesis-driven approach to the assessment of emotional states, especially when the individual is not able to accurately self-report.
Intervention
With respect to rehabilitation of faulty socioemotional processes, there has been momentum in the development of computerized and psychosocial interventions to target facial identity/emotion recognition (e.g., Faja et al., 2012; Tanaka et al., 2010). These programs often assess change in emotion recognition ability alongside more traditional, ‘end-goal’ behavioral outcomes (e.g., Dadds, Cauchi, Wimalaweera, Hawes, & Brennan, 2012; Lopata et al., 2010). There are also programs that target emotion regulation development in children and adults (e.g., Goldstein et al., in press; Linehan, 1993; Webster-Stratton & Reid, 2004). More general (i.e., not focused on emotion regulation per se) neurofeedback-based approaches for treatment of psychopathy or antisocial disorder have shown good outcomes as well (e.g., van Outsem, 2011). Park and colleagues (2011) found a VR-based treatment to develop social skills in adults with schizophrenia to be promising. These programs have, by and large, yielded promising findings with respect to clinical and behavioral outcomes.
Building upon these targeted treatment approaches, the next challenge is to develop and apply client-interactive technologies to understand and remediate the socioemotional processes that underlie clinical symptoms or maladaptive behaviors (Principle 1: Verifiable and Principle 5: Mechanism-driven). Most such systems to date have been mouse-operated, rather than fully interactive directly with the user’s nervous system. For example, the user might indicate their response by clicking among a set of response options. With BCI, the user controls and interacts with the virtual environment neurally, without need for manual response, which provides several advantages. There is emerging literature on the application of real-time fMRI for neurofeedback in clinical disorders (Sulzer et al., 2013; Weiskopf, 2012). For example, neurofeedback in eating disorders may target regions identified in TMS (Broadbent et al., 2011), such as the dorsal circuits that contribute to reward processing (Bartholdy, Musiat Campbell, & Schmidt, 2013). Additional work has demonstrated that real-time EEG and fMRI neurofeedback can be helpful in the treatment of depression through harnessing power asymmetry in the EEG and activation in the amygdala when reporting emotional memories (Zotev, Phillips, Yuan, Misaki, & Bodurka, 2014).
The Potential of Neurotechnology
There are several potential benefits of applying neurotechnology to the study and treatment of socioemotional processes involved in psychopathology. First, this approach allows the user to engage in a virtual social environment in which interactions and practice with emotion expression, recognition, and self-regulation can happen spontaneously and in which feedback occurs in real-time. As previously noted, neurotechnology provides an opportunity to design assessment and intervention tools that do not depend on person’s ability to respond to stimuli verbally or physically (Principle 7: Deployable). This is important when working with young children, but also with clients who are unwilling or unable to report on their feelings and experiences (e.g., those with intellectual or verbal impairments). A bottom-up approach (Principle 5: Mechanism-driven) to development of assessment and treatment approaches, in which we directly target the underlying mechanism(s), rather than the manifest pathology, may be both parsimonious and clinically effective (e.g., Lerner, White, & McPartland, 2012). Neurotechnology has promise in helping us achieve this critical translation. By intervening at the deeper, underlying process, it may be possible to effect change at the manifest level, transdiagnostically. Neurotechnologies are promising in this regard since they allow tighter experimental control in efforts to intervene at the process (mediator) level of the problem.
Compared to many other forms of treatment (e.g., medication), it is conceivable that untoward side effects are minimal or maybe even nonexistent. From a usability perspective, neurotechnologies are promising for many populations (e.g., children) because they are portable and fun to use. In addition, the technology may be used to supplant or, more likely, augment more traditional in-person therapies, thereby reducing cost and promoting accessibility. They also allow a ‘safe’ training environment. Unlike practice in a real social situation, the client/user can be freed of fear of negative evaluation by others (e.g., rejection because of poor facial recognition). The user can be completely independent and not reliant on a therapist or ‘coach’ in the interactions. These qualities make it likely that clinically effective technologies will be highly translational and well-disseminated. Finally, though perhaps most critical, such technology permits direct connection between the interface and central nervous system (CNS) functioning. Owing to the tremendous advances made in clinical neuroscience over the last decade, we have the benefit of knowledge of several identified brain regions involved in socioemotional processes in the context of different forms of psychopathology.
Elucidating Mechanisms of Psychopathology
Neural mediators of emotion processing at different stages (e.g., detection of, response to, and interpretation of emotion) potentially include interactions between the amygdala, striatum, and the prefrontal cortex (see Monk, 2008 for review). These regions show structural and functional alterations in clinical populations with noted difficulties with emotion behaviors. For example, structural brain differences in amygdala volume are present in youth with an anxiety disorder (De Bellis, 2000; Milham et al., 2005), adults with depression (Sheline, Gado, & Price, 1998; Siegle, Konecky, Thase, & Carter, 2003), and individuals with ASD (Monk, 2008; Schuman et al., 2004). Similarly, while results from studies using different tasks demonstrate differential activation, studies often identify differences in amygdala, striatum, and prefrontal cortex activation in these populations compared to healthy controls. Several other brain structures, including subgenual prefrontal cortex and the orbitofrontal cortex, are also reduced in size in adults with depression (Öngür, Drevets, & Price, 1998). Functionally, several studies have found greater amygdala activation in individuals with anxiety and depression relative to healthy controls (Monk, 2008).
These are just a few examples of how brain systems may function atypically in clinical populations; these examples illustrate that many of the same brain regions subserve socioemotional deficits across distinct clinical disorders. Thus, it is important to investigate the mechanisms involved in socioemotional deficits across disorders and domains. This transdiagnostic approach will allow us to better understand the mechanism(s) behind socioemotional difficulties in clinical populations irrespective of a specific disorder. By having a clear understanding of how neural circuitry that subserves emotion is altered in clinical populations, interventions can be more readily available to aid these individuals in rehabilitation and perhaps recovery. Neurotechnologies may offer the potential to shape more adaptive brain functioning in terms of socio-emotional deficits in psychopathology.
Technical and Methodological Hurdles of Neurotechnology
Different types of observations are required to understand the complex nature of CNS functioning during emotion detection, interpretation, and response. Here we focus on two techniques that provide different, yet complementary, information about brain processes. These measurement techniques, EEG and fMRI, are leading neurotechnologies for understanding emotion processes in healthy and clinical populations. EEG and fMRI are certainly not the only technologies that interface with the CNS. However, these technologies are noninvasive, have low associated risk, and are more widely used than many other technologies (e.g., PET scan, transcranial magnetic stimulation). Although highly utilized technologies, they both have shortcomings.
EEG is considered by many to be one of the most efficient and relatively inexpensive methods for examining emotion processes. EEG measures the electrical potential between two electrodes on the scalp, with the signal having temporal resolution on the order of milliseconds. Thus, postsynaptic changes are immediately reflected in the EEG, making this methodology outstanding for tracking rapid shifts in brain functioning during emotion processing. The EEG signal recorded from the scalp is composed of multiple sine waves cycling at different frequencies. Fourier analysis decomposes the EEG into these different sine waves and estimates the spectral power (in mean square microvolts), which is a measure of the excitability of groups of neurons. Over the long history of this methodology, scientists have identified standard frequency bands (or rhythms) and their associated psychological processes (Bell & Cuevas, 2012).
Although EEG is one of the more favorable methods for examining brain processes associated with emotion, there is one major caveat. The EEG signal has excellent temporal resolution, but poor spatial resolution. The skull behaves like a low-pass filter and distorts the underlying brain electrical activity over a large area of the scalp. Thus, potentials recorded at the scalp are likely generated by multiple groupings of cortical and subcortical generators spread across a relatively wide area (Pizzagalli, 2007). This means that a scalp electrode is likely detecting electrical activity generated from non-local groups of neurons, which is why it is better to discuss EEG activity at a specific electrode location rather than resulting from a particular brain area. Use of dense electrode arrays (typically considered to be a minimum of 64 electrodes) may alleviate some of the concerns with spatial resolution (Reynolds & Richards, 2009).
fMRI provides the spatial resolution required to focus on specific brain areas of emotion processing, although at great monetary expense. This brain imaging technique measures changes in metabolic and hemodynamic processes in the brain, with the blood oxygenated level dependent (BOLD) signal being the most common fMRI measure. There is a change in magnetization between oxygen-rich and oxygen-poor blood as brain cells use energy. This means that fMRI is inferring underlying changes in groups of neurons, making it an indirect measure of neuronal activity (Shmuel, 2010). Because changes in blood flow occur on a timescale of hundreds of milliseconds to seconds, fMRI has poor temporal resolution relative to EEG.
Because of the disparate spatial and temporal dynamics of EEG and fMRI, it would seem logical to use simultaneous EEG–fMRI for a more informed assessment of brain structure and functioning during emotion processing. For simultaneous recordings, the EEG electrodes are worn inside the MRI scanner, with concurrent recordings using both techniques. However, it is not the simple case that simultaneous recordings yield brain measures with high temporal (due to EEG) and spatial (due to fMRI) resolution. The recordings are more complex because they retain the shortcomings of the two techniques (i.e., poor spatial resolution of EEG and poor temporal resolution of fMRI; Lemieux & Mulert, 2010). In addition, there are technical hurdles with simultaneous EEG-fMRI recordings. For example, random head movements, as well as the slight head movements associated with the beating of the heart (cardioballistic head motions), introduce artifact into both types of records.
Technological challenges extend beyond the recording of simultaneous EEG-fMRI. Once data are in hand, there is the issue of how to integrate the information to best inform about brain structure and function during emotion processing. Most EEG and fMRI integration techniques are asymmetrical, with information from one modality used to guide the analysis of the data from the other. fMRI informed EEG is best suited when examining neural generators of scalp EEG, whereas EEG informed fMRI yields information about functional networks of single trial activity (Huster, Debener, Eichele, & Herrmann, 2012). The next frontier of data analysis of simultaneous EEG-fMRI recordings goes beyond asymmetrical integration to data fusion and cross-platform analysis (Huster et al., 2012; Meyer et al., 2013; Shi et al, 2010; Villinger, Mulert, & Lemieux, 2010; Warnat et al, 2005). These fusion techniques and cross-platform analysis jointly assess information from both EEG and fMRI to discover classifications of hidden factors in the simultaneously recorded data. Although necessarily based on complex models, these machine learning algorithms may be the key to making neurotechnology fully translational.
With data fusion techniques and cross-platform analysis, neurotechnology has the potential to exploit data from simultaneous EEG-fMRI recordings and use that information to create treatments for difficulties in emotion-related processing (Zotev, et al., 2014). However, there is great advantage to thinking ahead with respect to moving away from initial EEG-fMRI recordings to the more ambulatory and inexpensive modality of EEG (Principle 7: Deployable). There are ambulatory devices (i.e., commercially available dry-fit EEG sensors), that can be used in rehabilitation, especially when paired with neurofeedback applications. The neurofeedback literature with individuals with ASD gives examples of how EEG can be used to help modify brain activity. The most stringent of these feedback studies feature a randomized control trial (RCT) study design.
For example, mu rhythm (8–13 Hz) is associated with the mirror-neuron system and neurofeedback in a RCT study resulted in increased mu suppression (Pineda et al., 2008). Mu suppression is associated with social imitation, critical for practicing emotion modulation and monitoring of others’ emotions. Theta (4–7 Hz) is associated with anterior cingulate cortex. Individuals with ASD have high amounts of theta activity. Neurofeedback research that featured RCT resulted in reducing theta and increasing social interaction and communication (Kouijzer van Schie, de Moor, Gerrits, & Buitelaar, 2010). Gamma (30–50 Hz) has not been targeted in RCT neurofeedback studies, but gamma in occipital areas is absent in individuals with ASD when viewing emotion on faces (Wright et al, 2012). This makes gamma a good target for neurofeedback RCT studies.
Using neurofeedback from EEG-fMRI informed models, neurotechnology has the potential to aid in rehabilitation of individuals who have difficulties in socio-emotional functioning. Even more intriguing is the potential for neurotechnology to help shape more adaptive brain functioning.
Summary and Conclusions
There is an inherent tension between our understanding of the brain as an ever changing and evolving organ (neuroplasticity), which can be affected through intervention, and our efforts to accurately measure and classify brain structure and activity. In our quest to understand the brain we are searching for patterns that can capture and describe brain processes and activities at different levels of complexities, both in space and time. These patterns should be widely applicable and yet take into account individual differences and contexts. Neurotechnologies provide a window into brain activity. However, the different neurotechnologies (e.g., fMRI and EEG) do not have equivalent spatial, temporal, and signal resolutions leading to measurement spaces differing in dimensionality and precision. We need a holistic and integrative approach that correlates and ‘fuses’ measurements and insights gained by individual neurotechnologies. The fused measurements should provide coordinated and multiple views into the brain that not only allow us to better identify brain patterns but also to more accurately map these patterns onto individual views (individual neurotechnology measurement spaces). For example, fusing fMRI and EEG data could help us improve brain activity pattern detection in the EEG measurement space, which could allow for reduction in the number of electrodes (lower spatial resolution) required – thus making commodity BCI devices capable of identifying more brain patterns.
The parable of the three-legged stool applies to the marriage of neurotechnology and clinical science. For a stool to function optimally, three legs of equal length are required. Historically, treatment research has been segmented into two arms – biological interventions (including surgical, pharmacological, and dietary) and psychosocial interventions (including educational, therapeutic, and other non-medical approaches). We propose that neurotechnology might provide another class of treatment, the ‘third leg’ in the stool so to speak - strengthening our ability to find what works for whom, allowing us to target specific mechanisms that underlie transdiagnostic problems, and providing stability by complementing and augmenting established, effective treatments.
In the United States, approximately one out of every five people is affected by a mental illness (Merikangas et al., 2010) and nearly 57.5 billion dollars is spent annually on mental healthcare (National Institute of Mental Health, 2014). Yet, less than half of the people who struggle with mental illness receive effective treatment (National Institute of Mental Health, 2012c). These grim figures underscore the high demand for more widely available clinically- and cost-effective treatment choices that target underlying mechanisms of pathology. Despite billions of dollars spent on drug development and pharmaceutical clinical trials, we are seeing sharp declines in the funding dedicated to new compound development and fewer clinical trials on medications for mental health problems (Cressey, 2011). Psychosocial treatments are the mainstay of clinical psychologists; indeed, there are over 750 treatment protocols for children and adolescents, many of which are empirically supported (Chorpita et al., 2011). Yet, among this dizzying array of treatment choices, it is a challenge to identify a single established treatment that has a clearly established and empirically supported neural mechanism of action. In other words, we know our ‘best treatments’ work based on manifest symptom reduction and behavioral outcomes, but for the most part these approaches have a high rate of non-optimal outcomes and are not available to most people who need them, nor do we know why or how they work when they do at the neural level. As noted by Insel and colleagues (2012), neurotechnologies may be the way forward. Neurotechnologies may well provide a key, a tool to open up the ‘black box’ of therapeutic mechanisms of action.
We assert that application of neurotechnology to the evaluation and treatment of maladaptive cognitive and socioemotional processes is a logical step as we move forward, because the technology allows us to more directly study the human emotional experience. As human and computer continue to merge, with fully thought-powered robots and virtual avatars, mental health fields (e.g., psychiatry, clinical psychology) will be affected. Clinical scientists should think about how neurotechnologies might be used to help decode some of the enigmas of internal mental processes and perhaps apply these technologies to the treatment of psychiatric illness.
As an example, with training, BCI has potential implications for cognitive restructuring, which holds obvious implications for treatment of psychological disorders (Neumann & Kubler, 2003). An early indicator of the viability of this line of research is demonstrated by Treder and Blankertz (2010), who observed improvement in performance on BCI-controlled spelling tasks, improved modulation of early covert attentional processes and later overt attentional processes (as indexed by increased ERP amplitude in the P1, N1, P2, N2, and P3), as well as increased ability to classify brain responses of both covert and overt attention, indicating that both forms of attentional processes may be measured and shaped. There is obvious utility in improved classification of the neural response for individuals who do not have other means of communication (e.g., amyotrophic lateral sclerosis). However, these findings also indicate potential utility in shaping cognitive patterns that maintain psychological disorders. For example, it might be possible to retrain automatic cognitions through feedback for positive internal statements (i.e., unspoken thoughts about personal inadequacies) while either consequating or ignoring biased, negative self-statements. Cognitive demand during BCI tasks has been shown to be comparable between individuals with disabilities and those without (Felton, Williams, Vanderheiden, & Radwin, 2012). Thus, there are minimal grounds for asserting that BCI is only relevant for use with healthy populations and may not be applicable for individuals with disabilities (Felton et al., 2012). It is important to note that many tests of BCI technology have been conducted within a laboratory setting (Moore, 2003). Thus, it will be necessary to extend these tasks into the real-world.
In clinical application of any neurotechnology, basic behavioral principles likely apply. For instance, it is important to determine the patient’s intrinsic motivation to learn the technology, to ascertain what the patient finds motivating and reinforcing, and to supply the patient with only one question at a time that requires only one specific and predetermined answer (Neumann & Kubler, 2004). These insights make intuitive sense, in that the implementation and training will be more successful if the clients themselves seek reduction in symptoms or enhancement of skills. Additionally, the sought after neural response (in the case of neurofeedback and brain-controlled interface; e.g., EEG) will be shaped more quickly if responses progressively approximating the target response are immediately reinforced.
BCI devices may hold particular import in characterizing and training individuals with deficits in emotion processing. BCI devices are capable of reading the emotional landscape of users, and thus may provide key insights into processes central to emotion regulation and recognition (Garcia-Molina, 2013). Emerging work indicates that it is possible to detect accurate recognition of emotion in humans through EEG (Murugappan et al., 2010). Through harnessing EEG correlates of emotion recognition, it is possible to train individuals with BCI devices to recognize emotions more quickly.
Team training is an emerging example of the use of BCI technology. Using BCI and other devices across multiple cognitive resources, it is possible to measure cognitive load during training. Cognitive Load Theory (CLT) can be combined with multiple resource theory to create a model of adaptive training (Coyne et al., 2009) and better understand how allocation of verbal and spatial mental working affects overall cognitive load. EEG-derived measures of team members’ engagement can be classified into patterns by self-organizing artificial neural networks and hidden Markov models. These patterns are then mapped onto team events and team members’ interactions to provide a framework for rapid monitoring of team performance (Stevens et al., 2012). These results could be expanded to more general social and group interaction issues and related challenges.
Impaired socioemotional functioning, including perception and interpretation of others’ emotions, regulation of one’s own emotions, and ability to interact with other people effectively and reciprocally, is implicated in many forms of psychopathology. Neurotechnologies may be ideally suited for targeting deficits in socioemotional processing for many reasons. There are, however, ethical considerations as we travel down this road. The potential of neurotechnology to help us understand and simplify (and perhaps unwittingly oversimplify) the biology of complex behavior is alluring. We must be cautious in not assuming complete correspondence to behavioral outcomes or over-extending potential clinical and scientific application (cf. Giordano, Kulkarni, & Farwell, 2014), even in the face of rising consumer demand for such technology (e.g., Borgelt, Buchman, Weiss, & Illes, 2014).
These cautions notwithstanding, we assert that neurotechnologies hold promise in helping clinical scientists better understand the neural processes that underlie socioemotional difficulties. From a clinical research perspective, neurotechnologies may propel our desire to move beyond outcome-based clinical trials that focus exclusively on change in the diagnosis or symptom cluster. Instead, the focus is on establishing that the target mechanism can be altered and, subsequently, that change in the mechanism translates to change at the symptom level. With respect to basic science research that will inform our understanding of clinical phenomena, as previously discussed we must work to integrate different neurotechnologies and fuse data streams in order to maximize resolution and, ultimately, deployability. Of equal importance, we encourage use of neurotechnology to first establish plasticity in targeted mechanisms. For example, with the right paradigm manipulation, it might be possible to show that we can engage regions of the amygdala and prefrontal cortex in a reliable way, to change an individual’s neural response over time. Thus, it might be possible to examine causation over time, to determine how change in the neural mechanism is linked to the problem of interest. In terms of clinical significance, our penultimate hope is that practitioners will have the tools to intervene at a deeper ‘mechanistic’ level, targeting pivotal processes that will move us beyond disorder-specific treatment paradigms to ultimately effect broader, more diffuse change behaviorally.
Acknowledgments
This project was supported by grants from NIMH (R21MH100268: PI, White; R03MH102651: PI, Richey).
References
- Albus JS, Bekey GA, Holland JH, Kanwisher NG, Krichmar JL, Mishkin M, Tononi G. A proposal for a decade of the mind initiative. Science. 2007;317:1321. doi: 10.1126/science.317.5843.1321b. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Arlington, VA: APA; 2013. [Google Scholar]
- Arns M, de Ridder S, Strehl U, Breteler M, Coenen S. Efficacy of neurofeedback treatment in ADHD: The effects on inattention, impulsivity and hyperactivity: a meta-analysis. Clinical EEG and Neuroscience. 2009;40:180–189. doi: 10.1177/15500594090400031119715181. [DOI] [PubMed] [Google Scholar]
- Babbage C. Passages from the life of a philosopher. London: Longman, Green, Longman, Roberts, & Green; 1864. [Google Scholar]
- Bartholdy S, Musiat P, Campbell IC, Schmidt U. The Potential of Neurofeedback in the Treatment of Eating Disorders: A Review of the Literature. European Eating Disorders Review. 2013;21(6):456–463. doi: 10.1002/erv.2250. [DOI] [PubMed] [Google Scholar]
- Beard C, Sawyer AT, Hofmann SG. Efficacy of attention bias modification using threat and appetitive stimuli: A meta-analytic review. Behavior Therapy. 2012;43(4):724–740. doi: 10.1016/j.beth.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MA, Cuevas K. Using EEG to study cognitive development: Issues and practices. Journal of Cognition and Development. 2012;13:281–294. doi: 10.1080/15248372.2012.691143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgelt E, Buchman DZ, Weiss M, Illes J. In search of “anything that would help”: Parent perspectives on emerging neurotechnologies. Journal of Attention Disorders. 2014;18:5–395. 401. doi: 10.1177/1087054712445781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent HJ, van den Eynde F, Guillaume S, Hanif EL, Stahl D, David AS, Schmidt U. Blinding success of rTMS applied to the dorsolateral prefrontal cortex in randomised sham-controlled trials: a systematic review. World Journal of Biological Psychiatry. 2011;12(4):240–248. doi: 10.3109/15622975.2010.541281. [DOI] [PubMed] [Google Scholar]
- Browning M, Holmes EA, Murphy SE, Goodwin GM, Harmer CJ. Lateral prefrontal cortex mediates the cognitive modification of attentional bias. Biological Psychiatry. 2010;67(10):919–925. doi: 10.1016/j.biopsych.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr EG. The motivation of self-injurious behavior: A review of some hypotheses. Psychological Bulletin. 1977;84:800–816. [PubMed] [Google Scholar]
- Chorpita BF, Daleiden EL, Ebesutani C, Young J, Becker KD, Nakamura BJ, Starace N. Evidence-based treatments for children and adolescents: An updated review of indicators of efficacy and effectiveness. Clinical Psychology: Science and Practice. 2011;18:154–172. [Google Scholar]
- Clarke PJF, Browning M, Hammond G, Notebaert L, Macleod C. The causal role of the dorsolateral prefrontal cortex in the modification of attentional bias: Evidence from tanscranial direct current stimulation. Biological Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.03.003. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Coyne JT, Baldwin C, Cole A, Sibley C, Roberts DM. Applying real time physiological measures of cognitive load to improve training. Proceedings of the 5th International on Foundations of Augmented Cognition. Neuroergonomics and Operational Neuroscience; 2009. pp. 469–478. [Google Scholar]
- Cressey D. Traditional drug-discovery model ripe for reform. Nature. 2011;471:17–18. doi: 10.1038/471017a. [DOI] [PubMed] [Google Scholar]
- Dadds MR, Cauchi AJ, Wimalaweera S, Hawes DJ, Brennan J. Outcomes, moderators, and mediators of empathic-emotion recognition training for complex conduct problems in childhood. Psychiatry Research. 2012;199:201–207. doi: 10.1016/j.psychres.2012.04.033. [DOI] [PubMed] [Google Scholar]
- Dadds MR, Masry YE, Wimalaweera S, Guastella AJ. Reduced eye gaze explains “fear blindness” in childhood psychopathic traits. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:4–455. 463. doi: 10.1097/CHI.0b013e31816407f1. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Casey BJ, Dahl RE, Birmaher B, Williamson DE, Thomas KM, Ryan ND. A pilot study of amygdala volumes in pediatric generalized anxiety disorder. Biological psychiatry. 2000;48(1):51–57. doi: 10.1016/s0006-3223(00)00835-0. [DOI] [PubMed] [Google Scholar]
- deCharms RC. Applications of real-time fMRI. Nature Reviews Neuroscience. 2008;9:720– 729. doi: 10.1038/nrn241418714327. [DOI] [PubMed] [Google Scholar]
- deCharms RC, Maeda F, Glover GH, Ludlow D, Pauly JM, Soneji D, Mackey SC. Control over brain activation and pain learned by using real-time functional MRI. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:18626–18631. doi: 10.1073/pnas.050521010216352728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirel H, Yesilbas D, Ozver I, Yuksek E, Sahin F, Aliustaoglu S, Emul M. Psychopathy and facial emotion recognition ability in patients with bipolar affective disorder with or without delinquent behaviors. Comprehensive Psychiatry. 2014;55:542–546. doi: 10.1016/j.comppsych.2013.11.022. [DOI] [PubMed] [Google Scholar]
- Donno R, Parker G, Gilmour J, Skuse DH. Social communication deficits in disruptive primary-school children. British Journal of Psychiatry. 2010;196:282–289. doi: 10.1192/bjp.bp.108.061341. [DOI] [PubMed] [Google Scholar]
- Durand VM. “Look homeward angel”: A call to return to our (functional) roots. Behavior Analyst. 1987;10:299–302. doi: 10.1007/BF03392443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, Mazefsky CA, Minshew NJ. Misinterpretation of facial expressions of emotion in verbal adults with autism spectrum disorder. Autism. 2014 doi: 10.1177/1362361314520755. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einstein A. On the Method of Theoretical Physics. Philosophy of Science. 1934;1(2):163–169. [Google Scholar]
- Eldar S, Apter A, Lotan D, Perez-Edgar K, Naim R, Fox NA, Pine DS, Bar-Haim Y. Attention bias modification treatment for pediatric anxiety disorders: A randomized controlled trial. American Journal of Psychiatry. 2012;169:213–220. doi: 10.1176/appi.ajp.2011.11060886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson E. Self-injurious behaviour: An overview of recent trends in epidemiological and behavioural research. Mental Handicap Research. 1992;5:49–81. [Google Scholar]
- Fairchild G, Van Goozen SHM, Calder AJ, Stollery SJ, Goodyer IM. Deficits in facial expression recognition in male adolescetns with early-onset or adolescent-onset conduct disorder. Journal of Child Psychology and Psychiatry. 2009;50:627–636. doi: 10.1111/j.1469-7610.2008.02020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faja S, Webb SJ, Jones E, Merkle K, Kamara D, Bavaro J, Aylward E, Dawson G. The effects of face expertise training on the behavioral performance and brain activity of adults with high functioning autism spectrum disorders. Journal of Autism and Developmental Disorders. 2012;42:278–93. doi: 10.1007/s10803-011-1243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felton EA, Williams JC, Vanderheiden GC, Radwin RG. Mental workload during brain–computer interface training. Ergonomics. 2012;55(5):526–537. doi: 10.1080/00140139.2012.662526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia–Molina G, Tsoneva T, Nijholt A. Emotional brain–computer interfaces. International Journal of Autonomous and Adaptive Communications Systems. 2013;6(1):9–25. [Google Scholar]
- Giordano J. Neurotechnology as demiurgical force: Avoiding Icarus’ folly. In: Giordano J, editor. Neurotechnology: Premises, potential, and problems. Boca Raton, FL: Taylor & Francis; 2012. pp. 1–14. [Google Scholar]
- Giordano J, Kulkarni A, Farwell J. Deliver us from evil? The temptation, realities, and neuroethico-legal issues of employing assessment neurotechnologies in public safety initiatives. Theoretical Medicine and Bioethics. 2014;35:73–89. doi: 10.1007/s11017-014-9278-4. [DOI] [PubMed] [Google Scholar]
- Goldstein TR, Fersch RK, Rivera M, Axelson DA, Merranko J, Yu H, Brent DA, Birmaher B. Dialectical behavior therapy (DBT) for adolescents with bipolar disorder: Results from a pilot randomized trial. Journal of Child and Adolescent Psychopharmacology. doi: 10.1089/cap.2013.0145. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey S, Mathews A. Effects of training on interpretation of emotional ambiguity. Quarterly Journal of Experimental Psychology: Section A. 2000;53(4):1143–1162. doi: 10.1080/02724980050156335. [DOI] [PubMed] [Google Scholar]
- Grey SJ, Mathews AM. Cognitive bias modification – priming with an ambiguous homograph is necessary to detect an interpretation training effect. Journal of Behavior Therapy and Experimental Psychiatry. 2009;40(2):338–343. doi: 10.1016/j.jbtep.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Jazaieri H. Emotion, emotion regulation, and psychopathology: An affective science perspective. Clinical Psychological Science. 2014;2:4–387. 401. [Google Scholar]
- Groth-Marnat G. Handbook of psychological assessment. 5. Hoboken, NJ: John Wiley & Sons; 2009. Behavioral assessment; pp. 95–118. [Google Scholar]
- Hakamata Y, Lissek S, Bar-Haim Y, Britton JC, Fox N, Leibenluft E, Pine DS. Attention bias modification treatment: A meta-analysis towards the establishment of novel treatment for anxiety. Biological Psychiatry. 2010;68(11):982–990. doi: 10.1016/j.biopsych.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallion LS, Ruscio AM. A meta-analysis of the effect of cognitive bias modification on anxiety and depression. Psychological Bulletin. 2011;137(6):940–958. doi: 10.1037/a0024355. [DOI] [PubMed] [Google Scholar]
- Hirshberg LM, Chiu S, Frazier JA. Emerging brain-based interventions for children and adolescents: Overview and clinical perspective. Child and Adolescent Psychiatric Clinics of North America. 2005;14:1–19. doi: 10.1016/j.chc.2004.07.01115564050. [DOI] [PubMed] [Google Scholar]
- Huster RJ, Debener S, Eichele T, Herrmann CS. Methods for simultaneous EEG-fMRI: An introductory review. The Journal of Neuroscience. 2012;32:6053–6060. doi: 10.1523/JNEUROSCI.0447-12.2012. doi:0.1523/JNEUROSCI.0447-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Wang P. Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. American Journal of Psychiatry. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Insel T, Sahakian BJ. A plan for mental illness. Nature. 2012;483:269. doi: 10.1038/483269a. [DOI] [PubMed] [Google Scholar]
- Iwata BA, Kahng S, Wallace MD, Lindberg JS. The functional analysis model of behavioral assessment. In: Austin J, Carr JE, editors. Handbook of applied behavior analysis. Reno, NV: Context Press; 2000. pp. 61–90. [Google Scholar]
- Jackson A, Fetz EE. Compact movable microwire array for long-term chronic unit recording in cerebral cortex of primates. Journal of Neurophysiology. 2007;98(5):3109–3118. doi: 10.1152/jn.00569.2007. [DOI] [PubMed] [Google Scholar]
- Jacobson JW, Holburn S. History and current status of applied behavior analysis in developmental disabilities. In: Matson JL, Laud RB, Matson ML, editors. Behavior modification for persons with developmental disabilities: Treatments and supports. Vol. 1. Kingston: NADD Press; 2004. pp. 1–32. [Google Scholar]
- Kaner C, Bach J, Pettichord B. Lessons learned in software testing: A context-driven approach. 1. New York: Wiley; 2001. [Google Scholar]
- Kondziolka D, Flickinger JC, Hudak R. Results following gamma knife radiosurgical anterior capsulotomies for obsessive compulsive disorder. Neurosurgery. 2011;68(1):28–33. doi: 10.1227/NEU.0b013e3181fc5c8b. [DOI] [PubMed] [Google Scholar]
- Kouijzer MEJ, van Schie HT, de Moor JMH, Gerrits BJI, Buitelaar JK. Neurofeedback treatment in autism: Preliminary findings in behavioral, cognitive, and neurophysiological functioning. Research in Autism Spectrum Disorders. 2010;4:386–399. [Google Scholar]
- Kristensen HA, Parker JDA, Taylor RN, Keefer KV, Kloosterman PH, Summerfeldt LJ. The relationship between trait emotional intelligence and ADHD symptoms in adolescents and young adults. Personality and Individual Differences. 2014;65:36–41. doi: 10.1016/j.paid.2014.01.031. [DOI] [Google Scholar]
- LaConte SM. Decoding fMRI brain states in real-time. NeuroImage. 2011;56:440–54. doi: 10.1016/j.neuroimage.2010.06.052. [DOI] [PubMed] [Google Scholar]
- LaConte SM, Peltier SJ, Hu SP. Real-time fMRI using brain-state classification. Human Brain Mapping. 2007;28:1033–1044. doi: 10.1002/hbm.20326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larman C. Applying UML and patterns: An introduction to object-oriented analysis and design and iterative development. 3. Upper Saddle River, NJ: Prentice Hall; 2004. [Google Scholar]
- Lee TS, Goh SJA, Quek SY, Phillips R, Guan C, Cheung YB, Krishnan KRR. A brain-computer interface based cognitive training system for healthy elderly: A randomized control pilot study for usability and preliminary efficacy. PLoS ONE. 2013;8(11):e79419. doi: 10.1371/journal.pone.0079419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux L, Mulert C. Preface. In: Mulert C, Lemieux L, editors. EEG-fMRI: Physiological basis, technique, and applications. Berlin: Springer-Verlag; 2010. pp. vi–ix. [Google Scholar]
- Lerner MD, White SW, McPartland JC. Mechanisms of change in psychosocial interventions for autism spectrum disorders. Dialogues in Clinical Neuroscience. 2012;14(3):307–318. doi: 10.31887/DCNS.2012.14.3/mlerner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim CG, Lee TS, Guan C, Fung DSS, Zhao Y, Teng SSW, Krishnan KRR. A brain-computer interface based attention training program for treating attention deficit hyperactivity disorder. PLoS ONE. 2012;7(10):e46692. doi: 10.1371/journal.pone.0046692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linehan MM. Cognitive-behavioral treatment of borderline personality disorder. New York, NY: Guilford Publications; 1993. [Google Scholar]
- Lopata C, Thomeer ML, Volker MA, Toomey JA, Nida RE, Lee GK, Rodgers JD. RCT of a manualized social treatment for high-functioning autism spectrum disorders. Journal of Autism and Developmental Disorders. 2010;40:1297–1310. doi: 10.1007/s10803-010-0989-8. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Mathews A. Selective memory effects in anxiety disorders: An overview of research findings and their implications. In: Reisberg D, Hertel P, editors. Memory and emotion. New York: Oxford University Press; 2004. pp. 155–185. [Google Scholar]
- MacLeod C, Mathews A. Cognitive bias modification approaches to anxiety. Annual Review of Clinical Psychology. 2012;8:189–217. doi: 10.1146/annurev-clinpsy-032511-143052. [DOI] [PubMed] [Google Scholar]
- MacPherson HA, Fristad MA. Evidence-based psychosocial treatments for pediatric mood and anxiety disorders. Current Treatment Options in Psychiatry. 2014;1:48–65. [Google Scholar]
- Mandy W, Skuse D, Steer C, StPurcain B, Oliver BR. Oppositionality and socioemotional competence: Interacting risk factors in the development of childhood conduct disorder symptoms. Journal of the American Academy of Child & Adolescent Psychiatry. 2013;52(7):718–727. doi: 10.1016/j.jaac.2013.04.011. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Blair RJR. Deficits in facial affect recognition among antisocial populations: A meta-analysis. Neuroscience and Biobehavioral Reviews. 2008;32:454–465. doi: 10.1016/j.neubiorev.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marton I, Wiener J, Rogers M, Moore C, Tannock R. Empathy and social perspective taking in children with attention-deficit/hyperactivity disorder. Journal of Abnormal Child Psychology. 2009;37:107–118. doi: 10.1007/s10802-008-9262-4. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.01415748841. [DOI] [PubMed] [Google Scholar]
- Mazefsky CA, White SW. Emotion regulation: Concepts & practice in autism spectrum disorder. Child and Adolescent Psychiatric Clinics of North America. 2013 doi: 10.1016/j.chc.2013.07.002. Early online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell K, Ries A. Foundations of Augmented Cognition. Springer; Berlin Heidelberg: 2013. A translational approach to neurotechnology development; pp. 353–360. [Google Scholar]
- Merikangas KR, He J, Burstein M, Swanson SA, Avenevoli S, Cui L, Swendsen J. Lifetime prevalence of mental disorders in U.S. adolescents: Results from the national comorbidity study-adolescent supplement (NCS-A) Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(10):980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer FR, Grausgruber H, Binter C, Mair GE, Guelly C, Vogl C, Steinborn R. Cross-platform microarray Meta-analysis for the mouse jejunum selects novel reference genes with highly uniform levels of expression. PLoS ONE. 2013;8(5):e63125. doi: 10.1371/journal.pone.0063125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham MP, Nugent AC, Drevets WC, Dickstein DS, Leibenluft E, Ernst M, Pine DS. Selective reduction in amygdala volume in pediatric anxiety disorders: a voxel-based morphometry investigation. Biological Psychiatry. 2005;57(9):961–966. doi: 10.1016/j.biopsych.2005.01.038. [DOI] [PubMed] [Google Scholar]
- Moghimi S, Kushki A, Guerguerian AM, Chau T. A review of EEG-based brain- computer interfaces as access pathways for individuals with severe disabilities. Assistive Technology. 2013;25:99–110. doi: 10.1080/10400435.2012.723298. [DOI] [PubMed] [Google Scholar]
- Monk CS. The development of emotion-related neural circuitry in health and psychopathology. Development and Psychopathology. 2008;20:1231–1250. doi: 10.1017/S095457940800059X. [DOI] [PubMed] [Google Scholar]
- Moore MM. Real-world applications for brain-computer interface technology. Neural Systems and Rehabilitation Engineering, IEEE Transactions. 2003;11(2):162–165. doi: 10.1109/TNSRE.2003.814433. [DOI] [PubMed] [Google Scholar]
- Murugappan M, Ramachandran N, Sazali Y. Classification of human emotion from EEG using discrete wavelet transform. Journal of Biomedical Science and Engineering. 2010;3:390–396. doi: 10.4236/jbise.2010.34054. [DOI] [Google Scholar]
- National Institutes of Health. BRAIN 2025: A scientific vision. Brain research through advancing innovative neurotechnologies (BRAIN) working group report to the advisory committee of the director, NIH. 2014 Jul; Retrieved from http://www.nih.gov/science/brain/2025/BRAIN2025.pdf.
- National Institute of Mental Health. Statistics: Any disorder among adults. 2012a Retrieved May 27, 2014 from http://www.nimh.nih.gov/statistics/1ANYDIS_ADULT.shtml.
- National Institute of Mental Health. Statistics: Any disorder among children. 2012b Retrieved May 27, 2014 from http://www.nimh.nih.gov/statistics/1ANYDIS_CHILD.shtml.
- National Institute of Mental Health. Use of mental health services and treatment among children. 2012c Retrieved May 27, 2014 from http://www.nimh.nih.gov/statistics/1NHANES.shtml.
- National Institute of Mental Health. Statistics. 2014 Jul; Retrieved from http://www.nimh.nih.gov/Statistics/index.shtml.
- Neumann N, Kubler A. Training locked-in patients: A challenge for the use of brain- computer interfaces. Neural Systems and Rehabilitation Engineering, IEEE Transactions. 2003;11(2):169–172. doi: 10.1109/TNSRE.2003.814431. [DOI] [PubMed] [Google Scholar]
- Nicolas-Alonso LF, Gomez-Gil J. Brain computer interfaces: A review. Sensors (Basel, Switzerland) 2012;12(2):1211–1279. doi: 10.3390/s120201211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuske HJ, Vivanti G, Dissanayake C. Are emotion impairments unique to, universal, or specific in autism spectrum disorder? A comprehensive review. Cognition & Emotion. 2013;27(6) doi: 10.1080/02699931.2012.762900. [DOI] [PubMed] [Google Scholar]
- Öngür D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proceedings of the National Academy of Sciences. 1998;95(22):13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papageorgiou TD, Lisinski JM, McHenry MA, White JP, LaConte SM. Brain-computer interfaces increase whole-brain signal-to-noise. Proceedings of the National Academy of Science. 2013;110:1360–1365. doi: 10.1073/pnas.1210738110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K, Ku J, Choi S, Jang H, Park J, Kim SI, Kim J. A virtual reality application in role-plays of social skills training for schizophrenia: A randomized, controlled trial. Psychiatry Research. 2011;189(2):166–172. doi: 10.1016/j.psychres.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Parsons TD, Bowerly T, Buckwalter JG, Rizzo AA. A controlled clinical comparison of attention performance in children with ADHD in a virtual reality classroom compared to standard neuropsychological methods. Child Neuropsychology. 2007;13(4):363–381. doi: 10.1080/13825580600943473. [DOI] [PubMed] [Google Scholar]
- Pineda JA, Brang D, Hecht E, et al. Positive behavioral and electrophysiological changes following neurofeedback training in children with autism. Research in Autism Spectrum Disorders. 2008;2:557–581. doi: 10.1016/j.rasd.2007.12.003. [DOI] [Google Scholar]
- Pizzagalli DA. Electroencephalography and high-density electrophysiological source localization. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. 3. Cambridge, UK: Cambridge University Press; 2007. pp. 56–84. [Google Scholar]
- Prasad A, Xue Q-S, Sankar V, Nishida T, Shaw G, Streit WJ, Sanchez JC. Comprehensive characterization and failure modes of tungsten microwire arrays in chronic neural implants. Journal of Neural Engineering. 2012;9(5):056015. doi: 10.1088/1741-2560/9/5/056015. [DOI] [PubMed] [Google Scholar]
- Reynolds GD, Richards JE. Cortical source localization of infant cognition. Developmental Neuropsychology. 2009;34:312–329. doi: 10.1080/87565640902801890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rump KM, Giovannelli JL, Minshew NJ, Strauss MS. The development of emotion recognition in individuals with autism. Child Development. 2009;80(5):1434–1447. doi: 10.1111/j.1467-8624.2009.01343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhanam G, Ryu SI, Yu BM, Afshar A, Shenoy KV. A high-performance brain–computer interface. Nature. 2006;442(7099):195–198. doi: 10.1038/nature04968. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Richey JA, Buckner JD, Timpano KR. Attention training for generalized social anxiety disorder. Journal of Abnormal Psychology. 2009;118:1–5. 14. doi: 10.1037/a0013643. [DOI] [PubMed] [Google Scholar]
- Schumann CM, Hamstra J, Goodlin-Jones BL, Lotspeich LJ, Kwon H, Buonocore MH, Amaral DG. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. The Journal of Neuroscience. 2004;24(28):6392–6401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman LD, Swedish EF, Ollendick TH. Anxiety disorders in children. In: Alfano CA, Beidel DC, editors. Comprehensive evidence-based interventions for children and adolescents. Hoboken, NJ: Wiley; 2014. pp. 93–110. [Google Scholar]
- Shalom DB, Mostofsky SH, Hazlett RL, Goldberg MC, Landa RJ, Faran Y, Hoehn-Saric R. Normal physiological emotional but differences in expression of conscious feelings in children with high-functioning autism. Journal of Autism and Developmental Disorders. 2006;36:3–395. 400. doi: 10.1007/s10803-006-0077-2. [DOI] [PubMed] [Google Scholar]
- Shechner T, Rimon-Chakir A, Britton JC, Lotan D, Apter A, Bliese PD, Pine DS, Bar-Haim Y. Attention bias modification treatment augmenting effects on cognitive behavioral therapy in children with anxiety: Randomized controlled trial. Journal of the American Academy of Child & Adolescent Psychiatry. 2014;53(1):61–71. doi: 10.1016/j.jaac.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan JP, Patterson G, Schlesinger D, Xu Z. Gamma Knife surgery anterior capsulotomy for severe and refractory obsessive-compulsive disorder: Clinical article. Journal of Neurosurgery. 2013;119(5):1112–1118. doi: 10.3171/2013.5.JNS13201. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Price JL. Amygdala core nuclei volumes are decreased in recurrent major depression. Neuroreport. 1998;9(9):2023–2028. doi: 10.1097/00001756-199806220-00021. [DOI] [PubMed] [Google Scholar]
- Shi L, Cambell G, Jones WD, Campagne F, Wen Z, Walker SJ, Wolfinger RD. The microarray quality control II study of common practices for the development and validation of microarray-based predictive models. Nature Biotechnology. 2010;28 (8):827–838. doi: 10.1038/nbt.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuel A. Locally measured neuronal correlates of functional MRI signals. In: Mulert C, Lemieux L, editors. EEG-fMRI: Physiological basis, technique, and applications. Berlin: Springer-Verlag; 2010. pp. 63–82. [Google Scholar]
- Siegle GJ, Konecky RO, Thase ME, Carter CS. Relationships between amygdala volume and activity during emotional information processing tasks in depressed and never depressed individuals. Annals of the New York Academy of Sciences. 2003;985(1):481–484. doi: 10.1111/j.1749-6632.2003.tb07105.x. [DOI] [PubMed] [Google Scholar]
- Soken NH, Pick AD. Infants’ perception of dynamic affective expressions: Do infants distinguish specific expressions? Child Development. 1999;70(6):1275–1282. doi: 10.1111/1467-8624.00093. [DOI] [PubMed] [Google Scholar]
- Stevens RH, Galloway TL, Wang P, Berka C, Ayben E. Cognitive neurophysiologic synchronies: What can they contribute to the study of teamwork? Human Factors: The Journal of the Human Factors and Ergonomics Society. 2012;54:489–502. doi: 10.1177/0018720811427296. [DOI] [PubMed] [Google Scholar]
- Stoeckel LE, Garrison KA, Ghosh S, Wighton P, Hanlon CA, Gilman JAS, Evins AE. Optimizing real time fMRI neurofeedback for therapeutic discovery and development. NeuroImage: Clinical. 2014 doi: 10.1016/j.nicl.2014.07.002. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer J, Haller S, Scharnowski F, Weiskopf N, Birbaumer N, Blefari ML, Sitaram R. Real-time fMRI neurofeedback: progress and challenges. Neuroimage. 2013;76:386–399. doi: 10.1016/j.neuroimage.2013.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka JW, Wolf JM, Klaiman C, Koenig K, Cockburn J, Herlihy L, et al. Using computerized games to teach face recognition skills to children with autism spectrum disorder: the let’s face It! program. Journal of Child Psychology and Psychiatry. 2010;51:944–52. doi: 10.1111/j.1469-7610.2010.02258.x. [DOI] [PubMed] [Google Scholar]
- Taylor GJ, Bagby MR, Parker JDA. Disorders of Affect Regulation: Alexithymia in Medical and Psychiatric Illness. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- Tell D, Davidson D, Camras LA. Recognition of emotion from facial expressions with direct or averted eye gaze and varying expression intensities in children with autism disorder and typically developing children. Autism Research and Treatment. 2014 doi: 10.1155/2014/816137. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RA. Emotion regulation: a theme in search of definition. Monographs of the Society for Research in Child Development. 1994;59:25–52. [PubMed] [Google Scholar]
- Treder MS, Blankertz B. (C) overt attention and visual speller design in an ERP-based brain-computer interface. Behavioral and Brain Functions. 2010;6(28) doi: 10.1186/1744-9081-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uljarevic M, Hamilton A. Recognition of emotions in autism: A formal meta- analysis. Journal of Autism and Developmental Disorders. 2013;43:1517–1526. doi: 10.1007/s10803-012-1695-5. [DOI] [PubMed] [Google Scholar]
- van Outsem R. The applicability of neurofeedback in forensic psychotherapy: A literature review. Journal of Forensic Psychiatry & Psychology. 2011;22:223–242. [Google Scholar]
- Villinger A, Mulert C, Lemieux L. Principles of multimodal functional imaging and data integration. In: Mulert C, Lemieux L, editors. EEG-fMRI: Physiological basis, technique, and applications. Berlin: Springer-Verlag; 2010. pp. 3–17. [Google Scholar]
- Warnat P, Eils R, Brors B. Cross-platform analysis of cancer microarray data improves gene expression based classification and phenotypes. BMC Bioinformatics. 2005;6:265. doi: 10.1186/1471-2015-6-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster-Stratton C, Reid MJ. Strengthening social and emotional competence in young children – the foundation for early school readiness and success: Incredible Years Classroom Social Skills and Problem-Solving Curriculum. Infants and Young Children. 2004;17(2):96–113. [Google Scholar]
- Weiskopf N. Real-time fMRI and its application to neurofeedback. Neuroimage. 2012;62(2):682–692. doi: 10.1016/j.neuroimage.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Weiss EM, Kohler CG, Nolan KA, Czobor P, Volavka J, Platt MM, Gur RC. The relationship between history of violent and criminal behavior and recognition of facial expression of emotions in men with schizophrenia and schizoaffective disorder. Aggressive Behavior. 2006;32:187–194. doi: 10.1002/ab.20120. [DOI] [Google Scholar]
- Weisz JR, Kuppens S, Eckshtain D, Ugueto AM, Hawley KM, Jensen-Doss A. Performance of evidence-based youth psychotherapies compared with usual clinical care: A multilevel meta-analysis. Journal of the American Medical Association. 2013;70(7):750–761. doi: 10.1001/jamapsychiatry.2013.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SW, Mazefsky CA, Dichter GS, Chiu PH, Richey JA, Ollendick TH. Social-cognitive, physiological, and neural mechanisms underlying emotion regulation impairments: Understanding anxiety in autism spectrum disorder. International Journal of Developmental Neuroscience. 2014 doi: 10.1016/j.ijdevneu.2014.05.012. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SW, Ollendick TH, Albano AM, Oswald D, Johnson C, Southam-Gerow M, Kim I, Scahill L. Randomized controlled trial: Multimodal anxiety and social skill intervention for adolescents with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2013;43(2):382–394. doi: 10.1007/s10803-012-1577-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright B, Alderson-Day B, Prendergast G, Bennett S, Jordan J, Whitton C, Green G. Gamma activation in young people with autism spectrum disorders and typically- developing controls when viewing emotions on faces. PLoS ONE. 2012;7(7):e41326. doi: 10.1371/journal.pone.0041326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young-Browne G, Rosenfeld HM, Horowitz FD. Infant discrimination of facial expressions. Child Development. 1977;48(2):555–562. [Google Scholar]
- Zotev V, Phillips R, Yuan H, Misaki M, Bodurka J. Self-regulation of human brain activity using simultaneous real-time fMRI and EEG neurofeedback. NeuroImage. 2014;85:985–995. doi: 10.1016/j.neuroimage.2013.04.126. [DOI] [PubMed] [Google Scholar]