Abstract
Background
The relationship between prenatal tobacco exposure and hyperactivity remains controversial. To mitigate limitations of prior studies, we used a strategy involving comparison of maternal and paternal smoking reports in a historical sample where smoking during pregnancy was common.
Method
Data were drawn from a longitudinally followed subsample of the Child Health and Development Study (n = 1752), a population-based pregnancy cohort ascertained in 1961–1963 in California. Maternal prenatal smoking was common (33.4%). Maternal and paternal smoking patterns were assessed at three time points by mother report. Hyperactivity was assessed at the mean of age of 10 years based on mother report to a personality inventory.
Results
Unadjusted, maternal smoking during pregnancy was associated with offspring hyperactivity [β = 0.22, 95% confidence interval (CI) 0.11–0.33] and, to a similar degree, when the father smoked (β = 0.18, 95% CI 0.07–0.30). After adjustment, maternal smoking remained robustly predictive of offspring hyperactivity (β = 0.25, 95% CI 0.09–0.40) but father smoking was not (β = 0.02, 95% CI −0.20 to 0.24). When examined among the pairs matched on propensity score, mother smoking was robustly related to offspring hyperactivity whether the father smoked (β = 0.26, 95% CI 0.03–0.49) or did not smoke (β = 0.30, 95% CI 0.04–0.57). By number of cigarettes, associations with hyperactivity were present for 10–19 and 20+ cigarettes per day among mothers.
Conclusions
In a pregnancy cohort recruited in a time period in which smoking during pregnancy was common, we document associations between prenatal smoking exposure and offspring hyperactivity. Novel approaches to inferring causality continue to be necessary in describing the potential adverse consequences of prenatal smoking exposure later in life.
Keywords: Attention deficit hyperactivity disorder, causal inference, hyperactivity, prenatal smoking
Introduction
Prenatal exposure to tobacco smoke is causally linked to low birth weight and other neonatal health problems (Sexton & Hebel, 1984; Kramer, 1987; DiFranza et al. 2004; Tyrrell et al. 2012). It is also associated with child hyperactivity as well as other childhood outcomes such as conduct problems and cognitive dysregulation (Knopik, 2009), but the causality of this association remains questionable. Many longitudinal cohort and case–control studies have found a robust association between maternal smoking during pregnancy and child offspring hyperactivity/inattention, whether measured by symptom scales (Denson et al. 1975; Weitzman et al. 1992; Fergusson et al. 1993; Thapar et al. 2003), attention and reaction time to cued tests (Naeye & Peters, 1984; Streissguth et al. 1984; Kristjansson et al. 1989; Fried et al. 1992), Diagnostic and Statistical Manual of Mental Disorders (DSM)-defined attention deficit hyperactivity disorder (ADHD) (McGee & Stanton, 1994; Milberger et al. 1996; Mick et al. 2002; Langley et al. 2005), or International Classification of Diseases (ICD)-defined hyperkinetic disorder (Linnet et al. 2005). Animal studies have found that chronic nicotine exposure during pregnancy produced ‘hyperactivity’ (e.g. measured by spontaneous motor activity and rearing) in offspring, via an increase in brain nicotinic receptors that regulate anxiety responses (Johns et al. 1982; van de Kamp & Collins, 1994; DiPietro et al. 1996; Eriksson et al. 2000).
However, other studies have documented no association (Weissman et al. 1999; Hill et al. 2000; Cornelius et al. 2001; Knopik et al. 2006, Agrawal et al. 2010; Ball et al. 2010), and studies designed to minimize or assess uncontrolled confounding by genetic and shared familial factors have suggested that the association between prenatal smoking and child hyperactivity is not causal (D’Onofrio et al. 2008; Thapar et al. 2009; Lindblad & Hjern, 2010; Obel et al. 2011; Langley et al. 2012). In particular, in studies that compared siblings discordant for prenatal tobacco exposure, no strong associations were evident (D’Onofrio et al. 2008; Lindblad & Hjern, 2010; Obel et al. 2011). Sibling designs add strengths for causal inference but also have their own limitations (Susser et al. 2010; Donovan & Susser, 2011; Frisell et al. 2012). For example, these studies only control for stable features of the family context. Changes in the family context that relate to changes in smoking thus remain a threat to inferential validity because family factors are no longer stable (Susser et al. 2010; Donovan & Susser, 2011). In addition, recent methodological work has suggested that sibling designs may sometimes be more biased than non-sibling designs by factors that are non-shared between siblings and related to the outcome (Frisell et al. 2012; Keyes et al. 2013a).
Smoking during pregnancy remains an important modifiable risk factor and diagnoses of hyperactivity in children continue to increase (Olfson et al. 2003). Additional studies using an array of techniques to control confounding are needed in order to build consensus as to whether the association between prenatal smoking and child hyperactivity reflects a causal relationship. The approach of the present study combines three novel features. First, longitudinal data were collected over more than 15 years on both maternal and paternal smoking patterns in a prospective pregnancy cohort in Oakland, California. This allows for a comparison of the effect of maternal smoking with the effect of paternal smoking, which has been used previously to uncover potentially causal relationships between intrauterine exposures and offspring health (Leary et al. 2006a,b; Brion et al. 2007; Davey Smith, 2008, 2012). An intrauterine causal relationship should be detectable by a stronger association of prenatal maternal than paternal smoking with child hyperactivity. Second, several studies of pregnant women in the early to mid-1960s have reported relatively modest associations with socio-economic indicators (Graham et al. 2006; Gilman et al. 2008; Dow & Rehkopf, 2010; Power & Jefferis, 2002), certainly less of an association with socio-economic indicators than is typically seen in the association between smoking in pregnancy and socio-economic status in samples collected more recently (Keyes et al. 2013b). In the present sample, 34% of the women reported smoking during pregnancy, indicating a relatively common exposure, and all pregnancies in this sample occurred during 1961–1963, prior to the landmark Surgeon General Report (United States Department of Health, 1964). Thus, the use of these historical data may offer a more robust approach to exploring the potential influence of the myriad difficult-to-measure factors that differ between women who smoke in pregnancy and those who do not. Third, offspring were prospectively followed from birth to adolescence. The follow-up at the mean age of 10 years of the child included an assessment of hyperactivity by report of the mother, and queries on maternal and paternal smoking.
Method
Study population and design
Data were drawn from the Child Health and Development Study (CHDS; van den Berg et al. 1988), the first large epidemiologic sample of women in pregnancy assembled and studied at a single site. The CHDS included pregnant women participating in the Kaiser Permanente Health Plan and residing in the East Bay Area of California. Almost 100% of women receiving prenatal care from late 1959 to the fall of 1966 participated in the study (n = 20754).
The present study focuses on those women and children who participated in a baseline survey during pregnancy and two follow-up studies into the child’s adolescence (CHDS-A; n = 1752). Demographic differences between mothers who did versus did not participate in the adolescent interview (and had complete demographic information at baseline) are given in Table 1. Compared with those who did not participate, the CHDS-A included a greater proportion of subjects who were white, completed fewer years of education, were married at original intake, and were older. We used these demographic variables to create an inverse probability weight of selection into the adolescent sample. Demographic distributions of the weighted CHDS-A sample are also reported in Table 1. Henceforth, all analyses reported in the present study incorporated this inverse probability weight. Not shown, maternal smoking at baseline was unrelated to participation in the adolescent interview (χ2 = 4.1, degrees of freedom = 2, p = 0.13).
Table 1.
Characteristics of children and their parents in the Child Health and Development Study, comparing those who participated in the adolescent follow-up (CHDS-A) versus all others
| CHDS-A (n = 1752), % |
All others (n = 12041), % |
Test statistic, df, p | Re-weighted CHDS-A sample, % |
|
|---|---|---|---|---|
| Mother self-reported race | ||||
| White | 73.1 | 67.0 | χ2 = 27.2, df = 2, p<0.01 | 66.3 |
| Black | 19.1 | 22.8 | 23.3 | |
| Other | 7.8 | 10.2 | 10.4 | |
| Mother’s highest level of education | ||||
| Less than high school | 55.0 | 50.3 | χ2 = 49.9, df = 2, p<0.01 | 50.8 |
| High school | 33.4 | 31.9 | 32.2 | |
| More than high school | 11.4 | 17.8 | 16.9 | |
| Father’s highest level of education | ||||
| Less than high school | 57.5 | 55.1 | χ2 = 12.3, df = 2, p<0.01 | 49.1 |
| High school | 28.1 | 27.1 | 38.0 | |
| More than high school | 14.4 | 17.9 | 12.9 | |
| Mother’s marital status | ||||
| Not married | 0.9 | 2.4 | χ2 = 18.6, df = 1, p<0.01 | 2.2 |
| Married | 99.2 | 97.6 | 97.8 | |
| Age, years | ||||
| 14–19 | 4.0 | 6.4 | χ2 = 111.77, df = 3, p<0.01 | 6.1 |
| 20–29 | 53.0 | 63.0 | 61.7 | |
| 30–39 | 39.0 | 27.3 | 28.7 | |
| 40–50 | 4.1 | 3.4 | 3.5 | |
| Child’s gender | ||||
| Male | 50.3 | 50.9 | χ2 = 0.04, df = 1, p = 0.84 | 50.8 |
| Female | 49.7 | 49.1 | 49.2 |
df, Degrees of freedom.
Measures
Smoking
Women were queried regarding their own and their husbands’ current and former smoking patterns (including daily amount) at the pregnancy interview and at the offspring mean age 10-years assessment (range of ages was 9–11 years; we refer to this as the ‘age 10’ assessment hereafter). There was a positive association between maternal and paternal smoking. Substantial evidence indicates that self-reported smoking status in pregnancy is highly correlated with serum cotinine concentration (Klebanoff et al. 1998; Pickett et al. 2005), including analyses of the women in this sample (Eskenazi et al. 1995), indicating the validity of self-reports for smoking during pregnancy.
There is likely to be more measurement error in father smoking than in mother smoking, because women were reporting on their husbands’ smoking status. Therefore we also conducted sensitivity analyses of potential misclassification of father smoking. Assuming that most misclassification would be women reporting their husbands do not smoke when they do, we first changed a random 5–25% of ‘nonsmoking’ husbands to be ‘smokers’. We then considered that women who smoke may be more accurate in reporting their husbands’ smoking, and in a second sensitivity analysis, reclassified 5% of ‘non-smoking’ husbands to be ‘smokers’ if the woman was a smoker, and then reclassified 10, 15, 20, and 25%, in separate analyses, of ‘non-smoking’ husbands to be ‘smokers’ if the woman was a non-smoker. Results of maternal smoking and hyperactivity are shown in Supplementary Fig. S1; results of paternal smoking and hyperactivity are shown in Supplementary Fig. S2. In none of these scenarios would the conclusions presented here change: there remains an unadjusted association between paternal smoking and offspring hyperactivity that is no longer apparent when controlled for relevant confounders.
Hyperactivity
Hyperactivity at the age 10 assessment was culled from a 100-item battery of child characteristics, administered to the mother. Details on the origin and development of these questions can be found elsewhere (Tuddenham et al. 1974). Items assessing hyperactivity were assessed using factor analysis: hates to sit still, restless; butterfingers – spills things, drops things without meaning to; clumsy, falls over things; shows off, likes attention; is a dare-devil, wants to do things that are dangerous; gabby – talks, talks, talks; and two reverse coded items: takes good care of his/her possessions; tries to keep things neat. Items exhibited unidimensionality in an exploratory analytic framework [eigenvalue = 4.78, comparative fit index (CFI) = 0.95, Tucker-Lewis index (TLI) = 0.94, root mean square error of approximation (RMSEA) = 0.07, standardized root mean square residual (SRMR) = 0.05] and high internal consistency (α = 0.71). The latent dimension factor score was extracted and standardized to a z-score (sample mean = 0.0, sample s.d. = 1.0). These items are classic indicators of attention deficit and hyperactivity, and the majority of these items correspond to ICD-9 hyperkinesis disorder.
Potential confounders
Potential confounding factors included maternal cognitive ability measured via the Peabody Picture Vocabulary Test at the child’s age 10 assessment, post-birth smoking patterns in mother and father, socioeconomic position (maternal and paternal education, income, and father’s job status as manual or non-manual), as well as child age, race/ethnicity and gender. We also controlled for maternal self-reported alcohol and caffeine use (number of cups of coffee consumed in an average day) during pregnancy. Questions on alcohol and caffeine use were added to the interview after the start of data collection; 26.8% of respondents are missing data on caffeine use and 27.2% on alcohol use. Due to the high level of missingness, we controlled for these variables among the subset with data as an auxiliary to the main analysis.
Birth weight
We also examined the relationship of prenatal maternal smoking to hospital recorded offspring birth weight to test the validity of our approach given the well-documented relationship between maternal smoking and offspring birth weight.
Statistical analysis
We first report the unadjusted associations of the covariates described above with mother and father prenatal smoking using odds ratios (ORs), as well as with offspring hyperactivity using linear regression.
For our main analysis, we examined the effect of maternal and paternal smoking on offspring hyperactivity using linear regression models; normality assumptions were met. We then conducted three additional analyses. First, we divided the sample into four groups: (1) during the pregnancy period both mother and father smoked; (2) during the pregnancy period only father smoked; (3) during the pregnancy period only mother smoked; and (4) during the pregnancy period neither mother nor father smoked. We then examined relationships with hyperactivity. Second, we examined the relationship between dose of smoking (1–9, 10–19, and 20+ cigarettes per day versus 0 cigarettes) during the pregnancy period among mothers and fathers. Third, we disaggregated non-smokers into lifetime abstainers and former smokers. All analyses used linear regression and controlled for aforementioned covariates.
We also created a propensity score using a binary variable of any maternal smoking during pregnancy versus none as the outcome. Covariates used to create the propensity score included aforementioned covariates as well as the inverse probability weight of selection into the sample (Little & Vartivarian, 2003). We then conducted a nearest-neighbor match based on propensity score (total matched pairs = 480). We then analysed the four-level parental dyad of smoking (both parents smoked, only one parent smoked, or neither parent smoked) with respondents matched on propensity score. The Stata psmatch2 program (StataCorp LP, USA) was used for propensity score matching. All other analyses were conducted using SAS 9.2 (SAS Institute Inc., USA).
Results
Association between maternal and paternal smoking during mother’s pregnancy and potential confounders
The unadjusted associations of the covariates with father and mother smoking are shown in Supplementary Table S1. Maternal age, caffeine use, and mother’s highest education level were related to both mother and father smoking. Several covariates were associated with mother but not father smoking, including child non-white race [OR 0.55, 95% confidence interval (CI) 0.43–0.75] and male offspring (OR 0.78, 95% CI 0.62–0.97). Smoking was more common among fathers in a manual occupation (OR 1.76, 95% CI 1.40–2.22) and odds of father smoking decreased as maternal cognitive ability increased.
Association between potential confounders and hyperactivity
The unadjusted associations of the covariates with offspring hyperactivity are shown in Supplementary Table S2. Factors that were related to hyperactivity include child being male (β = 0.20, 95% CI 0.05–0.10) and child being non-white (β = 0.15, 95% CI 0.06–0.27). Further, mean hyperactivity was higher among children in low socio-economic positions as measured by father’s education, mother’s education, father’s job, with the strongest association for father’s less than high school compared with more than high school (β = 0.27, 95% CI 0.07–0.13). Finally, mean hyperactivity was increased among women who reported consuming >2 drinks per day on average during pregnancy compared with those reporting no drinks (β = 0.16, 95% CI 0.04–0.27).
Maternal versus paternal smoking during mother’s pregnancy and offspring birth weight
In a replication of previous literature, we first demonstrated that birth weight is more strongly related to maternal smoking. In unadjusted analysis, mean birth weight was approximately 186 g lower in the offspring of women who smoked during pregnancy compared with non-smokers (b = −185.6, 95% CI −242.6 to −128.6) and adjusting for covariates increased the strength of the association (b = −228.3, 95% CI −295.3 to −161.3). In contrast, the association with paternal smoking was of substantially smaller magnitude, and was attenuated to a small degree when controlled for measured covariates (unadjusted: b = −62.7, 95% CI −122.0 to −3.3) and was slightly attenuated when controlled for measured covariates (adjusted: b = −54.2, 95% CI −150.6 to 42.3). When maternal alcohol and caffeine use were added as covariates and analysed among the subset with data, maternal smoking remained a robust predictor of low birth weight (maternal smoking: b = −177.9, 95% CI −272.9 to −82.91). To specifically compare the maternal effect with the paternal effect, we separated the mothers and fathers into dyads of smoking (both smoked, mother only, father only, neither). We observed strong associations for mother smoking on offspring birth weight both when the father smoked (b = −139.9, 95% CI −26.1 to −253.45) and did not smoke (b = −129.2, 95% CI −20.1 to −278.5) directly compared with the effect for father-only smoking. Birth weight was unrelated to offspring hyperactivity (b = 0.0007, 95% CI −0.003 to 0.002) and thus did not mediate or confound associations between prenatal smoking and offspring hyperactivity.
Main effects of maternal and paternal smoking during mother’s pregnancy on hyperactivity
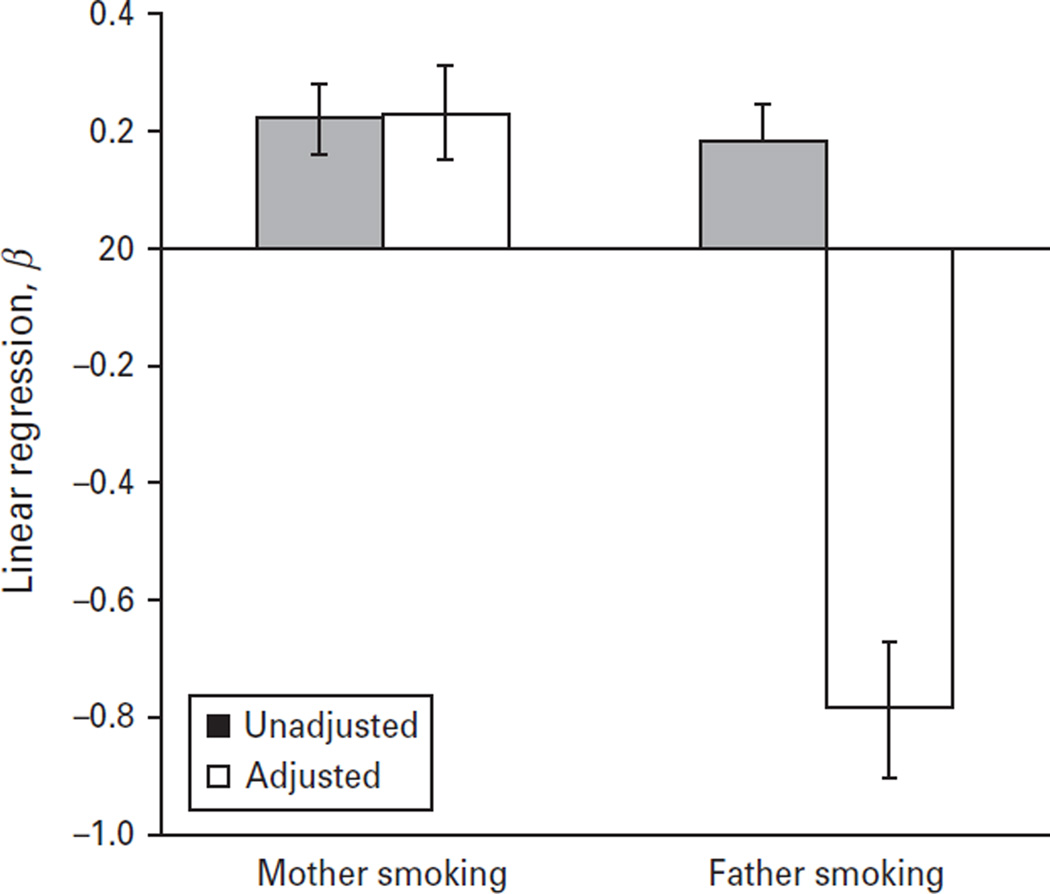
In unadjusted analysis, the mean hyperactivity score was approximately 0.22 standard deviations higher among offspring of mothers who smoked during pregnancy compared with offspring of mothers who did not (β = 0.22, 95% CI 0.11–0.33). The unadjusted regression coefficient for the effect of father smoking on offspring hyperactivity was similar (β = 0.18, 95% CI 0.07–0.30). When estimates were simultaneously adjusted for partner smoking patterns, socio-economic position and other demographics, we observed a stronger effect of maternal smoking on offspring hyperactivity than paternal smoking (Fig. 1). In contrast, we observed little effect of paternal smoking (Fig. 1). Supplementary Table S3 shows the β estimate from regression models in which each of the items used to measure a hyperactivity dimension is considered as an outcome separately; the strongest results were observed for ‘butterfingers…’ and ‘shows off, likes attention’. We performed additional sensitivity analyses with alternative control variables and results did not change; for example, controlling for maternal alcohol and caffeine use among the subset with data, there remained an effect of maternal smoking (β = 0.25, 95% CI 0.09–0.40) but not paternal smoking (β = 0.02, 95% CI −0.20 to 0.24).
Fig. 1.
Effect of maternal and paternal smoking during the pregnancy period on offspring hyperactivity at the mean age of 10 years. β Estimates were derived from linear regressions comparing each category with no smoking in either mother or father; adjusted estimates are also shown controlling for partner smoking during pregnancy period, father’s education, manual job, maternal smoking at child’s age 10 assessment, paternal smoking at child’s age 10 assessment, offspring race/ethnicity, child gender, maternal cognition, maternal age and child age.
Dose–response relationship between cigarette use and offspring hyperactivity
Fig. 2 shows the relationship between number of cigarettes smoked by mothers and fathers at the time of pregnancy and offspring hyperactivity. Each estimate among mothers is compared with non-smoking mothers; each estimate among fathers is compared with non-smoking fathers. Mother’s amount of smoking is controlled for father’s amount of smoking and vice versa. In the adjusted model, the offspring of mothers who smoked 10–19 cigarettes and those who smoked 20+ cigarettes during the pregnancy period had similarly elevated hyperactivity scores (β = 0.33, 95% CI 0.10–0.55 and β = 0.30, 95% CI 0.13–0.47, respectively); no increase was observable according to father smoking. When controlled for maternal alcohol and caffeine use among the subset with data, an elevation in hyperactivity symptoms remained observable among mothers (compared with 0 cigarettes per day: 20+ cigarettes per day: β = 0.41, 95% CI 0.22–0.61; 1–19 cigarettes per day: β = 0.40, 95% CI 0.15–0.65) and remained unobserved among fathers. Hyperactivity symptoms showed no consistent association or dose–response relationship with paternal smoking.
Fig. 2.
Effect of maternal and paternal level of smoking during the pregnancy period on offspring hyperactivity at the mean age of 10 years. Each estimate was derived comparing with no smoking in the parent group (e.g. effect of mother smoking 1–9 cigarettes compared with mothers who did not smoke at all). β Estimates were derived from linear regressions comparing each category with no smoking in either mother or father; adjusted estimates are also shown controlling for partner smoking during pregnancy period, father’s education, manual job, maternal smoking at child’s age 10 assessment, paternal smoking at child’s age 10 assessment, offspring race/ethnicity, child gender, maternal cognition, maternal age and child age.
Joint associations between maternal and paternal smoking during the pregnancy period (Table 2)
Table 2.
Four levels of maternal and paternal smoking: associations with offspring hyperactivity at the mean age of 10 years
| At pregnancy | n | Mean hyperactivity factor score at age 10 (S.D.) |
Unadjusted β (95% CI) |
Adjusted β (95% CI)a |
Propensity score matched (n = 960): β (95% CI)b |
|---|---|---|---|---|---|
| Maternal current smoking/Paternal current smoking | 289 | 0.07 (1.01) | 0.28 (0.13 to 0.43) | 0.15 (−0.10 to 0.41) | 0.26 (0.03 to 0.49) |
| Maternal smoking abstention/Paternal current smoking | 225 | −0.05 (0.98) | 0.13 (−0.03 to 0.29) | −0.04 (−0.30 to 0.21) | 0.16 (−0.06 to 0.39) |
| Maternal current smoking/Paternal smoking abstention | 101 | 0.11 (0.99) | 0.28 (0.06 to 0.50) | 0.29 (0.04 to 0.53) | 0.30 (0.04 to 0.57) |
| Maternal smoking abstention/Paternal smoking abstention | 341 | −0.18 (0.96) | Ref. | Ref. | Ref. |
s.d., Standard deviation; CI, confidence interval; Ref., reference.
β Estimates were derived from linear regressions comparing each category with no smoking in either mother or father, controlling for partner smoking during pregnancy period, father’s education, manual job, maternal smoking at child’s age 10 assessment, paternal smoking at child’s age 10 assessment, offspring race/ethnicity, child gender, maternal cognition, maternal age and child age.
Propensity score analysis was based on 480 matched pairs.
When the mother smoked during pregnancy and the father did not smoke during the pregnancy period, we see an effect of smoking on hyperactivity (unadjusted: β = 0.28, 95% CI 0.06–0.50; adjusted: β = 0.29, 95% CI 0.04–0.53). When the mother and the father both smoked, we see an effect of mother smoking on hyperactivity in an unadjusted model (β = 0.28, 95% CI 0.13–0.43); in the adjusted model the estimate is in the direction of a detrimental effect but the CI is wide (β = 0.15, 95% CI −0.10 to 0.41). When the father smoked but the mother did not, we see no effect on offspring hyperactivity in any model (see Table 2). These results held in magnitude when additionally controlled for maternal alcohol and caffeine use among the subset with data (compared with neither parent smoking, both parent smoked: β = 0.28, 95% CI −0.02 to 0.59; mother only smoked: β = 0.22, 95% CI −0.07 to 0.51; father only smoked: β = −0.04, 95% CI −0.34 to 0.27), but were underpowered to detect robust effects. When examined among the pairs matched on propensity score, mother smoking was robustly related to offspring hyperactivity, and to a similar degree whether the father smoked (β = 0.26, 95% CI 0.03–0.49) or did not smoke (β = 0.30, 95% CI 0.04–0.57).
Former smoking and its relationship with offspring hyperactivity
Finally, we also disaggregated lifetime abstainers from former smokers to examine whether there was heterogeneity in the effect. For maternal smoking, there was an effect of both current smoking versus lifetime abstention (adjusted: β = 0.35, 95% CI 0.09–0.61) and former smoking versus lifetime abstention (adjusted: β = 0.25, 95% CI 0.03–0.48). There was no effect of current or former smoking versus lifetime abstention among fathers. We then evaluated time since quitting among mothers, comparing those who quit just before pregnancy, 1–2 years before pregnancy, and 3 or more years before pregnancy with those who never smoked. Quitting just prior to pregnancy was associated with offspring hyperactivity (β = 0.32, 95% CI 0.01–0.63), whereas quitting 3 or more years before the pregnancy was less associated (β = 0.12, 95% CI −0.18 to 0.42). These results held when additionally controlled for maternal alcohol and caffeine use among the subset with data. Associations between time since quitting and study covariates are provided in Supplementary Table S4.
Discussion
These data provide some support for the hypothesis that maternal prenatal smoking exposure exerts a causal effect on symptoms of hyperactivity during childhood, especially smoking at high levels. First, we demonstrate that maternal smoking during the gestational period is associated with offspring hyperactivity, after controls for subsequent maternal and paternal smoking during the childhood period, maternal cognitive ability, maternal alcohol and caffeine use in pregnancy, and other important confounders. Second, among women matched on a propensity score predicting smoking during pregnancy, there remained a robust association between maternal smoking and offspring hyperactivity. Third, using paternal smoking during the gestational period as a marker of background confounding due to the shared family context, we demonstrate a stronger association for maternal smoking than for paternal smoking, as would be consistent with an intrauterine pathway for the effect of smoking on offspring hyperactivity (Davey Smith, 2008). However, this difference is only evident after adjustment for covariates. Fourth, we observed an increase in the relationship between maternal prenatal smoking and offspring hyperactivity among those smoking 10–19 and 20+ cigarettes per day, but observed no such relationship for paternal smoking during the pregnancy period at any level of cigarette use. These data offer unique strengths for this research question, including comprehensive prospectively collected information on mothers, fathers and their children beginning at birth, and assessments during a historical time period in a sample in which smoking was common.
As noted in a recent review of the literature on offspring effects of maternal smoking by Knopik (2009) and emphasized by others (Davey Smith, 2008), associations between maternal smoking in pregnancy have been documented with a range of outcomes including cognitive dysfunction, conduct and other behavioral problems, obesity, and other health outcomes. Constraints on causal inference are common across all of these studies; thus innovative approaches that mitigate sources of confounding are critical to advancing our understanding of child health. One increasingly common strategy is to compare outcomes among siblings that were discordant for maternal smoking during pregnancy, which mitigates sources of confounding that are shared within families. With regarding to hyperactivity, our results differ from sibling match studies, which have generally suggested that the link between prenatal smoking and offspring hyperactivity largely reflects shared familial confounding (D’Onofrio et al. 2008; Lindblad & Hjern, 2010; Obel et al. 2011). Sibling matched studies provide a within-family estimate whereas the parameters estimated in the present study combine within- and across-family effects. However, if there is a causal relationship between intrauterine exposure to tobacco smoke and offspring hyperactivity, we would expect consistent results. While sibling match studies are a powerful approach to use observational data for better causal inference, there are limitations to the design and unique potential biases (Susser et al. 2010; Donovan & Susser, 2011; Frisell et al. 2012). Examinations across multiple study designs of the effects of prenatal smoking exposure on offspring health are beneficial in developing consensus regarding the strength and validity of these associations (Keyes et al. 2013a).
Several other studies have provided evidence suggesting that the association between maternal smoking and hyperactivity is the result of confounding (Thapar et al. 2009; Langley et al. 2012), and recent maternal/paternal comparisons have been inconsistent (Nomura et al. 2010; Langley et al. 2012). Studies exhibit differences in time period of data collection, which could yield different results due to secular trends in rearing practices across time, or from differences in outcome measurement. Compared with studies initiated in the past several decades, the present study was conducted in a time period in which mothers may have had a greater role in child rearing, given that women are increasingly pursuing careers outside of the home. This may introduce additional confounding in the present study, though the present study has the benefit of being conducted in a time period in which smoking during pregnancy was less socially sanctioned. With regard to outcome measurement, we also note that additional analyses in our data examining each item as a singular outcome produced similar results to our main analyses (i.e. an effect of maternal smoking that was greater in magnitude than the effect of paternal smoking; see Supplementary Table S3).
Three additional findings in these data warrant discussion as they suggest that other pathways may also be operable in the effect of maternal smoking on offspring health. First, women who reported quitting smoking just prior to pregnancy also had offspring with increased hyperactivity symptoms compared with never smokers. Preconception smoking could have effects on the developing fetus by altering metabolite pathways in the mother in ways that persist during at least the early part of pregnancy (Foley et al. 2009). However, this association could also be indicative of residual confounding or underreporting of cigarette use. The hypothesis that preconception smoking could affect offspring outcomes should be further investigated before strong conclusions are attached. Second, when controlled for relevant confounders, only the association between maternal smoking in the absence of paternal smoking was strongly related to hyperactivity in offspring. It is possible that these women had a greater accumulation of risk factors that influence the risk for offspring hyperactivity. This raises the possibility that non-intrauterine pathways might also contribute to the difference in child hyperactivity risk for maternal versus paternal smoking during pregnancy. Third, our data suggest somewhat different patterns of confounding for maternal smoking than for paternal smoking. For example, some socio-economic indicators as well as offspring race were associated with paternal smoking but not maternal smoking. The validity of the approach comparing the strengths of the estimates of maternal versus paternal smoking relies on paternal smoking as a marker for shared environmental confounding; to the extent that these indicators have unique confounding structures, the approach will yield less valid inference.
These data and the validity of the present analysis should be viewed with several important limitations. First, the adolescent subsample of the CHDS represents approximately 8% of the original cohort, and sociodemographic factors were associated with participation in the adolescent interview. Thus, the subsample is not fully representative of the source sample, limiting generalizability. However, our main exposure, maternal smoking, was unrelated to participation in the subsample, and we incorporated an inverse probability weight based on baseline sociodemographic factors that increase the representativeness of the adolescent subsample. Second, the validity of the maternal/paternal comparison rests on the assumption that paternal smoking is an adequate marker for confounding via familial context, and that there are not factors that are unshared between mothers and fathers than may influence offspring hyperactivity (Davey Smith, 2008). While this threat cannot be evaluated systematically, we note that in these and other data (Davey Smith, 2008), the approach consistently shows maternal but not paternal smoking effects on outcomes such as birth weight, where we know there is an intrauterine effect. However, as noted earlier, our data suggest that some correlates of maternal and paternal smoking were not shared, suggesting the potential for residual confounding. Further, the present results rely on mother reporting about the hyperactivity in her offspring; it is possible that women who smoke have a different reporting style than women who do not smoke. Third, we did not have a diagnostic measure of ADHD or hyperkinetic disorder. However, our scale was made up of items that are classically representative of hyperkinetic disorders, and an itemlevel analysis indicated that the relationships presented here were approximately equal across indicators used in our scale (Supplementary Table S3), such that the results are not dependent on the any one of the scaled items. Finally, we did not have information on parental history of hyperactivity or any information on other parental psychopathology, which could confound the relationship between parental smoking and offspring hyperactivity (Griesler et al. 2008; Agrawal et al. 2010). This information is important to collect in future studies examining the impact of prenatal exposures with offspring mental health.
Much remains to be understood about the potential mechanisms through which maternal smoking may potentially cause health consequences such as hyperactivity and ADHD in offspring. While animal models can suggest plausible biological pathways (Johns et al. 1982; van de Kamp & Collins, 1994; DiPietro et al. 1996; Eriksson et al. 2000), observational data are inconsistent in demonstrating an effect of prenatal smoking within human populations. We believe that the case for a potentially intrauterine effect of maternal smoking on offspring hyperactivity should continue to be investigated, and that further research using novel study designs (including Mendelian randomization; Davey Smith & Ebrahim, 2003) providing insight into this issue is warranted.
Supplementary Material
Acknowledgements
We acknowledge the following individuals for their contributions to this work: Roberta Christianson, M.A., and Barbara Cohn, Ph.D. We also thank the National Institute for Child Health and Development, and the Public Health Institute, Berkeley, CA.
Funding for this study was provided by the Department of Epidemiology of Columbia University and the New York State Psychiatric Institute (to K.M.K.) and by the National Institutes of Health (P01-AG023028-01) (to E.S.). G.D.S.'s work is supported by the European Research Council DEVHEALTH grant (269874).
Footnotes
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0033291713000986.
Declaration of Interest
None.
References
- Agrawal A, Scherrer JF, Grant JD, Sartor CE, Pergadia ML, Duncan AE, Madden PA, Haber JR, Jacob T, Bucholz KK, Xian H. The effects of maternal smoking during pregnancy on offspring outcomes. Preventative Medicine. 2010;50:13–18. doi: 10.1016/j.ypmed.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball SW, Gilman SE, Mick E, Fitzmaurice G, Ganz ML, Seidman LJ, Buka SL. Revisiting the association between maternal smoking during pregnancy and ADHD. Journal of Psychiatric Research. 2010;44:1058–1062. doi: 10.1016/j.jpsychires.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Brion MJ, Leary SD, Davey Smith G, Ness AR. Similar associations of parental prenatal smoking suggest child blood pressure is not influenced by intrauterine effects. Hypertension. 2007;49:1422–1428. doi: 10.1161/HYPERTENSIONAHA.106.085316. [DOI] [PubMed] [Google Scholar]
- Cornelius MD, Ryan CM, Day NL, Goldschmidt L, Willford JA. Prenatal tobacco effects on neuropsychological outcomes among preadolescents. Journal of Developmental and Behavioral Pediatrics. 2001;22:217–225. doi: 10.1097/00004703-200108000-00002. [DOI] [PubMed] [Google Scholar]
- D’Onofrio BM, Van Hulle CA, Waldman ID, Rodgers JL, Harden KP, Rathouz PJ, Lahey BB. Smoking during pregnancy and offspring externalizing problems: an exploration of genetic and environmental confounds. Development and Psychopathology. 2008;20:139–164. doi: 10.1017/S0954579408000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey Smith G. Assessing intrauterine influences on offspring health outcomes: can epidemiological studies yield robust findings? Basic and Clinical Pharmacology and Toxicology. 2008;102:245–256. doi: 10.1111/j.1742-7843.2007.00191.x. [DOI] [PubMed] [Google Scholar]
- Davey Smith G. Negative control exposures in epidemiologic studies. Epidemiology. 2012;23:350–351. doi: 10.1097/EDE.0b013e318245912c. [DOI] [PubMed] [Google Scholar]
- Davey Smith G, Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? International Journal of Epidemiology. 2003;32:1–22. doi: 10.1093/ije/dyg070. [DOI] [PubMed] [Google Scholar]
- Denson R, Nanson JL, McWatters MA. Hyperkinesis and maternal smoking. Canadian Psychiatry Association Journal. 1975;20:183–187. doi: 10.1177/070674377502000302. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Aligne CA, Weitzman M. Prenatal and postnatal environmental tobacco smoke exposure and children’s health. Pediatrics. 2004;113:1007–1015. [PubMed] [Google Scholar]
- DiPietro JA, Hodgson DM, Costigan KA, Hilton SC, Johnson TR. Fetal neurobehavioral development. Child Development. 1996;67:2553–2567. [PubMed] [Google Scholar]
- Donovan SJ, Susser E. Commentary: advent of sibling designs. International Journal of Epidemiology. 2011;40:345–349. doi: 10.1093/ije/dyr057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow WH, Rehkopf DH. Socioeconomic gradients in health in international and historical context. Annals of the New York Academy of Sciences. 2010;1186:24–36. doi: 10.1111/j.1749-6632.2009.05384.x. [DOI] [PubMed] [Google Scholar]
- Eriksson P, Ankarberg E, Fredriksson A. Exposure to nicotine during a defined period in neonatal life induces permanent changes in brain nicotinic receptors and in behaviour of adult mice. Brain Research. 2000;853:41–48. doi: 10.1016/s0006-8993(99)02231-3. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Prehn AW, Christianson RE. Passive and active maternal smoking as measured by serum cotinine: the effect on birthweight. American Journal of Public Health. 1995;85:395–398. doi: 10.2105/ajph.85.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Lynskey MT. Maternal smoking before and after pregnancy: effects on behavioral outcomes in middle childhood. Pediatrics. 1993;92:815–822. [PubMed] [Google Scholar]
- Foley DL, Craig JM, Morley R, Olsson CA, Dwyer T, Smith K, Saffery R. Prospects for epigenetic epidemiology. American Journal of Epidemiology. 2009;169:389–400. doi: 10.1093/aje/kwn380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried PA, Watkinson B, Gray R. A follow-up study of attentional behavior in 6-year-old children exposed prenatally to marihuana, cigarettes, and alcohol. Neurotoxicology and Teratology. 1992;14:299–311. doi: 10.1016/0892-0362(92)90036-a. [DOI] [PubMed] [Google Scholar]
- Frisell T, Oberg S, Kuja-Halkola R, Sjolander A. Sibling comparison designs: bias from non-shared confounders and measurement error. Epidemiology. 2012;23:713–720. doi: 10.1097/EDE.0b013e31825fa230. [DOI] [PubMed] [Google Scholar]
- Gilman SE, Gardener H, Buka SL. Maternal smoking during pregnancy and children’s cognitive and physical development: a causal risk factor? American Journal of Epidemiology. 2008;168:522–531. doi: 10.1093/aje/kwn175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham H, Inskip HM, Francis B, Harman J. Pathways of disadvantage and smoking careers: evidence and policy implications. Journal of Epidemiology and Community Health. 2006;60(Suppl. 2):7–12. doi: 10.1136/jech.2005.045583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesler PC, Hu MC, Schaffran C, Kandel DB. Comorbidity of psychiatric disorders and nicotine dependence among adolescents: findings from a prospective, longitudinal study. Journal of the Ameircan Academy of Child Adolescent Psychiatry. 2008;47:1340–1350. doi: 10.1097/CHI.0b013e318185d2ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Lowers L, Locke-Wellman J, Shen SA. Maternal smoking and drinking during pregnancy and the risk for child and adolescent psychiatric disorders. Journal of Studies on Alcohol. 2000;61:661–668. doi: 10.15288/jsa.2000.61.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns JM, Louis TM, Becker RF, Means LW. Behavioral effects of prenatal exposure to nicotine in guinea pigs. Neurobehavioral Toxicology and Teratology. 1982;4:365–369. [PubMed] [Google Scholar]
- Keyes KM, Davey Smith G, Susser E. On sibling designs. Epidemiology. 2013a;24:473–474. doi: 10.1097/EDE.0b013e31828c7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, March D, Link BG, Chilcoat H, Susser E. Do socio-economic gradients in smoking emerge differently across time by gender? Implications for the tobacco epidemic from a pregnancy cohort in California, USA. Social Science and Medicine. 2013b;76:101–106. doi: 10.1016/j.socscimed.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff MA, Levine RJ, Clemens JD, DerSimonian R, Wilkins DG. Serum cotinine concentration and self-reported smoking during pregnancy. American Journal of Epidemiology. 1998;148:259–262. doi: 10.1093/oxfordjournals.aje.a009633. [DOI] [PubMed] [Google Scholar]
- Knopik VS. Maternal smoking during pregnancy and child outcomes: real or spurious effect? Developmental Neuropsychology. 2009;34:1–36. doi: 10.1080/87565640802564366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopik VS, Heath AC, Jacob T, Slutske WS, Bucholz KK, Madden PA, Waldron M, Martin NG. Maternal alcohol use disorder and offspring ADHD: disentangling genetic and environmental effects using a children-of-twins design. Psychological Medicine. 2006;36:1461–1471. doi: 10.1017/S0033291706007884. [DOI] [PubMed] [Google Scholar]
- Kramer MS. Intrauterine growth and gestational duration determinants. Pediatrics. 1987;80:502–511. [PubMed] [Google Scholar]
- Kristjansson EA, Fried PA, Watkinson B. Maternal smoking during pregnancy affects children’s vigilance performance. Drug and Alcohol Dependence. 1989;24:11–19. doi: 10.1016/0376-8716(89)90003-3. [DOI] [PubMed] [Google Scholar]
- Langley K, Heron J, Davey Smith G, Thapar A. Maternal and paternal smoking during pregnancy and risk of ADHD symptoms in offspring: testing for intrauterine effects. American Journal of Epidemiology. 2012;176:261–268. doi: 10.1093/aje/kwr510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley K, Rice F, van den Bree MB, Thapar A. Maternal smoking during pregnancy as an environmental risk factor for attention deficit hyperactivity disorder behaviour. A review. Minerva Pediatrica. 2005;57:359–371. [PubMed] [Google Scholar]
- Leary S, Davey Smith G, Ness A. Smoking during pregnancy and components of stature in offspring. American Journal of Human Biology. 2006a;18:502–512. doi: 10.1002/ajhb.20518. [DOI] [PubMed] [Google Scholar]
- Leary SD, Davey Smith G, Rogers IS, Reilly JJ, Wells JC, Ness AR. Smoking during pregnancy and offspring fat and lean mass in childhood. Obesity (Silver Spring) 2006b;14:2284–2293. doi: 10.1038/oby.2006.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblad F, Hjern A. ADHD after fetal exposure to maternal smoking. Nicotine and Tobacco Research. 2010;12:408–415. doi: 10.1093/ntr/ntq017. [DOI] [PubMed] [Google Scholar]
- Linnet KM, Wisborg K, Obel C, Secher NJ, Thomsen PH, Agerbo E, Henriksen TB. Smoking during pregnancy and the risk for hyperkinetic disorder in offspring. Pediatrics. 2005;116:462–467. doi: 10.1542/peds.2004-2054. [DOI] [PubMed] [Google Scholar]
- Little RJ, Vartivarian S. On weighting the rates in non-response weights. Statistics in Medicine. 2003;22:1589–1599. doi: 10.1002/sim.1513. [DOI] [PubMed] [Google Scholar]
- McGee R, Stanton WR. Smoking in pregnancy and child development to age 9 years. Journal of Paediatrics and Child Health. 1994;30:263–268. doi: 10.1111/j.1440-1754.1994.tb00631.x. [DOI] [PubMed] [Google Scholar]
- Mick E, Biederman J, Faraone SV, Sayer J, Kleinman S. Case–control study of attention-deficit hyperactivity disorder and maternal smoking, alcohol use, drug use during pregnancy. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:378–385. doi: 10.1097/00004583-200204000-00009. [DOI] [PubMed] [Google Scholar]
- Milberger S, Biederman J, Faraone SV, Chen L, Jones J. Is maternal smoking during pregnancy a risk factor for attention deficit hyperactivity disorder in children? American Journal of Psychiatry. 1996;153:1138–1142. doi: 10.1176/ajp.153.9.1138. [DOI] [PubMed] [Google Scholar]
- Naeye RL, Peters EC. Mental development of children whose mothers smoked during pregnancy. Obstetrics and Gynecology. 1984;64:601–607. [PubMed] [Google Scholar]
- Nomura Y, Marks DJ, Halperin JM. Prenatal exposure to maternal and paternal smoking on attention deficit hyperactivity disorders symptoms and diagnosis in offspring. Journal of Nervous and Mental Disease. 2010;198:672–678. doi: 10.1097/NMD.0b013e3181ef3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obel C, Olsen J, Henriksen TB, Rodriguez A, Jarvelin MR, Moilanen I, Parner E, Linnet KM, Taanila A, Ebeling H, Heiervang E, Gissler M. Is maternal smoking during pregnancy a risk factor for hyperkinetic disorder?–Findings from a sibling design. International Journal of Epidemiology. 2011;40:338–345. doi: 10.1093/ije/dyq185. [DOI] [PubMed] [Google Scholar]
- Olfson M, Gameroff MJ, Marcus SC, Jensen PS. National trends in the treatment of attention deficit hyperactivity disorder. American Journal of Psychiatry. 2003;160:1071–1077. doi: 10.1176/appi.ajp.160.6.1071. [DOI] [PubMed] [Google Scholar]
- Pickett KE, Rathouz PJ, Kasza K, Wakschlag LS, Wright R. Self-reported smoking, cotinine levels, and patterns of smoking in pregnancy. Paediatric and Perinatal Epidemiology. 2005;19:368–376. doi: 10.1111/j.1365-3016.2005.00660.x. [DOI] [PubMed] [Google Scholar]
- Power C, Jefferis BJ. Fetal environment and subsequent obesity: a study of maternal smoking. International Journal of Epidemiology. 2002;31:413–419. [PubMed] [Google Scholar]
- Sexton M, Hebel JR. A clinical trial of change in maternal smoking and its effect on birth weight. Journal of the American Medical Association. 1984;251:911–915. [PubMed] [Google Scholar]
- Streissguth AP, Martin DC, Barr HM, Sandman B, Kirchner GL, Darby B. Intrauterine alcohol and nicotine exposure: attention and reaction time in 4-year-old children. Developmental Psychology. 1984;20:533–541. [Google Scholar]
- Susser E, Eide MG, Begg M. Invited commentary: The use of sibship studies to detect familial confounding. American Journal of Epidemiology. 2010;172:537–539. doi: 10.1093/aje/kwq196. [DOI] [PubMed] [Google Scholar]
- Thapar A, Fowler T, Rice F, Scourfield J, van den Bree M, Thomas H, Harold G, Hay D. Maternal smoking during pregnancy and attention deficit hyperactivity disorder symptoms in offspring. American Journal of Psychiatry. 2003;160:1985–1989. doi: 10.1176/appi.ajp.160.11.1985. [DOI] [PubMed] [Google Scholar]
- Thapar A, Rice F, Hay D, Boivin J, Langley K, van den Bree M, Rutter M, Harold G. Prenatal smoking might not cause attention-deficit/hyperactivity disorder: evidence from a novel design. Biological Psychiatry. 2009;66:722–727. doi: 10.1016/j.biopsych.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuddenham RD, Brooks J, Milkovich L. Mothers’ reports of behavior of ten-year-olds: relationships with sex, ethnicity, and mother’s education. Developmental Psychology. 1974;10:959–995. [Google Scholar]
- Tyrrell J, Huikari V, Christie JT, Cavadino A, Bakker R, Brion MJ, Geller F, Paternoster L, Myhre R, Potter C, Hohnson PC, Ebrahim S, Feenstra B, Harikainen AL, Hattersley AT, Hofman A, Kaakinen M, Lowe LP, Magnus P, McConnachie A, Melbye M, Ng JW, Nohr EA, Power C, Ring SM, Sebert SP, Sengpiel V, Taal HR, Watt GC, Sattar N, Relton CL, Jacobsson B, Frayling TM, Sorensen TI, Murray JC, Lawlor DA, Pennell CE, Jaddoe VW, Hypponen E, Lowe WL, Jarvelin MR, Davey Smith G, Freathy RM Early Growth Genetics Consortium. Genetic variation in the 15q25 nicotinic acetylcholine receptor gene cluster (CHRNA5-CHRNA3-CHRNB4) interacts with maternal self-reported smoking status during pregnancy to influence birth weight. Human Molecular Genetics. 2012;21:5344–5358. doi: 10.1093/hmg/dds372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Department of Health, Education, and Welfare. Smoking and Health: Report of the Advisory Committee to the Surgeon General of the Public Health Service. Washington, DC: Public Health Service Office of the Surgeon General; 1964. Public Health Service Publication no. 1103. [Google Scholar]
- van de Kamp JL, Collins AC. Prenatal nicotine alters nicotinic receptor development in the mouse brain. Pharmacology, Biochemist, and Behavior. 1994;47:889–900. doi: 10.1016/0091-3057(94)90293-3. [DOI] [PubMed] [Google Scholar]
- van den Berg BJ, Christianson RE, Oechsli FW. The California Child Health and Development Studies of the School of Public Health University of California at Berkeley. Paediatric and Perinatal Epidemiology. 1988;2:265–282. doi: 10.1111/j.1365-3016.1988.tb00218.x. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Warner V, Wickramaratne PJ, Kandel DB. Maternal smoking during pregnancy and psychopathology in offspring followed to adulthood. Journal of the American Academy of Child Adolescent Psychiatry. 1999;38:892–899. doi: 10.1097/00004583-199907000-00020. [DOI] [PubMed] [Google Scholar]
- Weitzman M, Gortmaker S, Sobol A. Maternal smoking and behavior problems of children. Pediatrics. 1992;90:342–349. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.