Abstract
Objective
This two-stage phase II study assessed activity of single agent dalantercept in patients with recurrent/persistent endometrial carcinoma (EMC).
Methods
Eligible patients had persistent/recurrent EMC after 1–2 prior cytotoxic regimens, measurable disease (RECIST 1.1), and GOG performance ≤ 2. Dalantercept 1.2 mg/kg subcutaneous was administered once every 3 weeks until disease progression (PD)/development of prohibitory toxicity. Primary objectives were to estimate the proportion of patients with persistent/recurrent EMC, who survive progression-free without receiving non-protocol therapy (TPFS) for at least 6 months and to estimate the proportion having objective tumor response.
Results
All 28 enrolled patients were eligible and evaluable. Median age: 62 years. Most common histologies: 32% Grade 1/2 endometrioid and 54% serous tumors. Prior treatment: 1 or 2 regimens in 82% and 18% of patients, respectively. Eighteen patients received prior radiation therapy. Patients received 1–12 cycles of dalantercept, and 46% of patients received ≤2 cycles. The most common adverse events (AE) were fatigue, anemia, constipation and peripheral edema. Grade 3/4 AEs occurred in 39% and 4% of patients. One grade 5 gastric hemorrhage in a patient with a history of radiation fibrosis/small bowel obstruction was deemed possibly dalantercept-related. All patients are off study: 86% for PD. No ORs were observed; 57% had stable disease and 11% had TPFS ≥ 6 mos. Median progression-free and overall survival: 2.1 months (90% CI: 1.4–3.2) and 14.5 months (90% CI: 7.0–17.5), respectively.
Conclusions
Dalantercept has insufficient single agent activity in recurrent EMC to warrant further investigation at this dose level and schedule.
Keywords: Dalantercept, Recurrent endometrial cancer, ALK-1
INTRODUCTION
Endometrial adenocarcinoma (EMC) is the most common of all uterine malignancies and is the most common gynecologic malignancy in the US estimated to affect 52,630 women and to result in 8,590 deaths in 2014. (1). Several randomized trials have been performed to address optimal therapy for patients with advanced endometrial cancer. The most recent study, GOG 209, has identified paclitaxel and carboplatin as the standard initial regimen (2). Once this initial therapy has been delivered, either in the adjuvant or advanced disease setting, there are limited treatment options, with no standard options available. Trials evaluating cytotoxic agents in the second-line setting (GOG 129 series) have shown that pegylated liposomal doxorubicin (9.5%), topotecan (9%), weekly docetaxel (7.7%), ixabepilone (12%), pemetrexed (3.8%) and gemcitabine (4.3%) have minimal activity (3–8). The only active agent identified in the second-line setting in patients who did not have prior taxane-based therapy is paclitaxel (GOG 129C) with a response rate of 27.3% (9).
Nonetheless, most recurrent patients will not be cured by salvage therapy and will go on to other programs with much lower anticipated response. This represents an important unmet need in endometrial cancer care. Recently, a myriad of targeted therapies from rapalogues, Akt inhibitors, combination PI3K/mTor inhibitors, VEGF inhibitors, Tie2 receptor inhibitors and FGFR2 inhibitors have been evaluated in the recurrent disease setting in single arm phase II studies including: thalidomide (0229-B) (10), gefitinib (0229-C) (11), lapatinib (0229-D) (12), bevacizumab (0229-E) (13), aflibercept (0229-F) (14), bevacizumab plus temsirolimus (0229-G) (15), selumetinib (0229-H) (16), brivanib (0229-I) (17), cediranib (0229-J), nintedanib (229-K) (18), and trebananib (229-L).
There is evidence that angiogenesis plays a role in endometrial cancer progression and prognosis. A Phase II GOG study of thalidomide in refractory endometrial cancer demonstrated an association between elevated plasma VEGF levels and poor prognosis (10).
Several anti-vascular agents have been investigated in recurrent or persistent EMC. Single agent bevacizumab was studied by the GOG in study 229-E (13). Fifty-two patients with one (63.5%) or two (36.5%) prior regimens were treated. Bevacizumab was deemed active based on seven patients (13.5%) having clinical responses (one complete response and six partial responses, with median response duration of 6 months) and 21 patients (40.4%) surviving progression-free for at least six months. Median PFS and OS times were 4.2 and 10.5 months, respectively.
GOG 0229-G, a Phase II evaluation of combination bevacizumab and temsirolimus in the treatment of recurrent or persistent endometrial cancer revealed that 12 patients (24.5%) experienced clinical responses (one complete and 11 partial responses), and 23 patients (46.9%) survived progression-free for at least six months. Median progression-free survival (PFS) and overall survival (OS) were 5.6 and 16.9 months, respectively. The combination of temsirolimus and bevacizumab was deemed active, but also associated with significant toxicity in this pretreated group (15).
Activin receptor-like kinase 1 (ALK1) is a type I receptor that mediates signaling of BMP9 (bone morphogenetic protein) (19, 20) and BMP10 (21), proteins in the transforming growth factor-β (TGF-β) superfamily. Signaling through ALK1 results in phosphorylation of the intracellular Smad 1/5/8 cascade which activates proangiogenic transcription factors such as ID1 and ID3 (22). ALK1 is selectively and transiently expressed in proliferating, arterial endothelium, unlike VEGFR2, which is constitutively expressed. Targeting ALK1 is expected to provide a distinct anti-angiogenic safety profile, by sparing established vasculature.
ALK1 signaling is necessary for angiogenesis during embryogenesis, wound healing, and tumor growth. Homozygous null mutations of ALK1 (−/−) result in severe vascular malformations that cause embryonic lethality (20, 22). There is a wide range of ALK1 expression in endometrial cancer. Preliminary data from The Cancer Genome Atlas (TCGA) endometrial project indicates that most samples (N= 212) are diploid at the ALK1 locus and demonstrate a wide range of expression (23) suggesting that a correlation between expression and response is worthwhile.
Dalantercept (ALK1-IgG1), a homodimeric recombinant fusion protein consisting of the extracellular domain (ECD) of human ALK1 linked to the Fc (hinge, CH2, CH3 domains) portion of human immunoglobulin G1 (IgG1), is a first-in-class inhibitor of the ALK1 pathway. Dalantercept binds to BMP9 and BMP10, and prevents these ligands from signaling through ALK1 (30), which results in the inhibition of vascular endothelial cell maturation and disruption of the process of vascular development (24, 25). In contrast to other anti-angiogenic agents (e.g., bevacizumab) that block the proliferative phase of angiogenesis, dalantercept blocks the maturation phase of angiogenesis. In addition to potentially preventing resistance, this approach may also be safer because the ability of VEGF to function as a survival factor for normal progenitor cells is not inhibited by dalantercept (24, 26).
In a completed phase I study (A041–01) in 37 patients with recurrent/progressive solid tumors, dalantercept was administered at dose levels ranging 0.1–4.8 mg/kg. A maximum tolerated dose (MTD) as defined in the protocol was not determined. (30) The MTD of dalantercept monotherapy was designated at 1.6 mg/kg based upon the cumulative toxicity observed at this dose level and beyond which consisted primarily of edema, fluid retention, and anemia. Toxicities commonly associated with anti-VEGF therapies were not reported. Indications of antitumor activity were observed in fourteen of 29 evaluable patients. These included a partial response (33% reduction) by cycle nine at 0.4 mg/kg in one patient with squamous cell carcinoma of the head and neck. Thirteen patients had stable disease per RECISTv1.1. Of these, 8 patients had prolonged periods of stable disease (12 weeks) across the dose range (0.2 to 4.8 mg/kg). The dose level of 1.2 mg/kg (75% MTD) was selected as an acceptable dose level for phase II monotherapy trials. Other monotherapy phase II trials of dalantercept include ongoing single arm studies in ovarian cancer (GOG-170R, NCT01720173) and SCCHN (NCT01458392). Furthermore, based upon preclinical experience demonstrating additive anti-tumor effects with ALK1 and VEGFR inhibition, additional dalantercept combination studies are underway in advanced renal cell cancer (NCT01727336) and hepatocellular cancer (NCT02024087).
Based on results of prior antiangiogenic agent trials, as well dalantercept’s novel mechanism of action and phase I clinical experience, a phase II trial of single agent dalantercept was conducted in patients with recurrent or persistent endometrial cancer. The primary objective was to evaluate efficacy in terms of the proportion of patients with persistent or recurrent endometrial cancer who survive progression-free without receiving non-protocol therapy (TPFS) for at least 6 months, or the proportion of patients who have objective tumor response (ORR) (complete or partial) when treated with dalantercept.
PATIENTS AND METHODS
Patient selection
Histologic confirmation of the primary tumor by the GOG Pathology Committee central review process was required. To be eligible, patients must have had one prior chemotherapeutic regimen. Patients with the following histologic epithelial cell types are eligible: Endometrioid adenocarcinoma, serous adenocarcinoma, undifferentiated carcinoma, clear cell adenocarcinoma, mixed epithelial carcinoma, adenocarcinoma not otherwise specified mucinous adenocarcinoma, squamous cell carcinoma, and transitional cell carcinoma. Initial treatment may have included chemotherapy, chemotherapy and radiation therapy, and/or consolidation/maintenance therapy; biologic therapy as part of adjuvant therapy was allowed. Chemotherapy administered in conjunction with primary radiation as a radio-sensitizer was counted as a systemic chemotherapy regimen. Patients were allowed to have received one additional cytotoxic regimen for management of recurrent or persistent disease. All prior chemotherapy and biologic therapy, including bevacizumab, was to be discontinued at least three weeks prior registration. Prior hormonal therapy was allowed but was to be discontinued at least one week prior to registration. GOG performance status of 0 to 2 was required; and had to be 1 or less if patients had received two cytotoxic regimens in the past. All patients were required to have measurable disease by Response Criteria in Solid Tumors (RECIST 1.1). Patients must have adequate hematologic parameters (absolute neutrophil count ≥1500/mcl, hemoglobin ≥9 g/dl, and platelets ≥100,000/mcl), renal function (serum creatinine ≤1.5 x the institutional upper limit of normal [ULN] and sodium ≥130 mEq/L, urine protein ≤2+, and if ≥2+, 24-hour urine with protein level of < 1000 mg), hepatic function (serum bilirubin ≤1.5 ULN and AST, ALT, and alkaline phosphatase ≤ 3 x ULN), and coagulation parameters (prothrombin time such that international normalized ratio [INR] ≤ 1.5 x ULN or INR range between 2 and 3 for patients on a stable dose of therapeutic warfarin, and partial thromboplastin time ≤ 1.5 x ULN); and left ventricular ejection fraction ≥50%. A signed approved informed consent in accordance with federal, state and local requirements and an authorization permitting release of personal health information were required for all patients. Participation in this trial required protocol approval by institutional review boards.
Patients were ineligible if they met any of the following criteria: prior therapy with dalantercept or other ALK1 pathway inhibitor; prior malignancies (other than non-melanomatous skin cancer) evident within three years of prior cancer treatment; prior radiation therapy to any portion of the abdominal cavity or pelvis other than for the treatment of endometrial cancer within the last three years (prior radiation for localized breast, head and neck, or skin cancer is permitted if it was completed > 3 years prior to registration); prior chemotherapy for any abdominal or pelvic tumor other than for treatment of endometrial cancer within the last 3 years (adjuvant chemotherapy for localized breast cancer if > 3 years prior to registration is allowed); known CNS disease; non-healing wound, ulcer or bone fracture; abdominal fistula, anastomotic leak, GI perforation, or intra-abdominal abscess within 6 months of registration; coexistent active bleeding, hereditary hemorrhagic telangiectasia, platelet function abnormality, autoimmune or hereditary hemolysis, coagulopathy or tumor involving major vessels; treatment with full dose aspirin, clopidogrel or dabigatran; coexistent peripheral edema ≥ grade 1 within 4 weeks of registration; clinically significant cardiovascular disease; history of syndrome of inappropriate antidiuretic hormone secretion; therapeutic paracentesis within 4 weeks of registration; history of hepatitis B, C or HIV; patients who are pregnant or nursing; and active pulmonary edema, pulmonary hypertension, or pulmonary embolism.
Treatment
Enrolled patients were to receive dalantercept 1.2 mg/kg SC on day 1 of a 3 week cycle. The maximum starting dose was 120 mg. Patients weighing more than 100 kg started at a dose of 120 mg, and if dalantercept was tolerated for 2 cycles, the patient could be dose escalated to dosing based on actual body weight. Treatment was continued until disease progression or adverse events prohibited further therapy.
Toxicity was monitored with history, weight assessment, physical exam and laboratory assessments before each treatment cycle, with adverse events defined and graded according to Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
Dalantercept was withheld for non-hematologic toxicity for a maximum of three weeks to allow for recovery to ≤ grade 1. Dose modifications for dalantercept were made for a weight change of ≥ 5% from baseline. Dalantercept was to be discontinued for a bleeding event ≥ grade 2; a grade 3 or higher cardiopulmonary event; ≥ grade 2 fistula or perforation; more than 2 occurrences of grade 3 or higher ascites; more than 4 occurrences of grade 2 or higher ascites; ≥ grade 2 decrease in ejection fraction; and any other grade 3 or higher adverse event or lab value. Three dose level reductions were allowed and were defined as follows: 1 level reduction, 0.90 mg/kg once SC every 3 weeks; 2 level reduction 0.68 mg/kg SC once every 3 weeks; 3 dose level reduction 0.51 mg/kg. No fourth level dose reduction nor dose escalations or re-escalations were allowed with the exception of the one-time escalation in patients whose weight is greater than 100 kg when dalantercept was tolerated for the first 2 cycles. In this case the patient could be dose escalated to dosing based on actual body weight after cycle 2.
Evaluation Criteria
Activity of dalantercept was assessed according to the RECIST version 1.1 guidelines by computed tomography or magnetic resonance imaging at baseline, every two cycles (or equivalent time frame for patients off treatment prior to disease progression) for the first 6 months, and then every 3 months thereafter until disease progression is documented. Responses (CR and PR) required confirmation at greater than or equal to 4 weeks from initial documentation of response. Survival progression-free without receiving non-protocol therapy (TPFS) was defined as the time in months from study entry that a patient is alive, without evidence of progression until subsequent therapy. The minimum time to death, documentation of progression or initiation of subsequent therapy from study entry was used; if none of these occur, TPFS was censored at the date of last contact.
Statistical Methods
The primary objective of this trial was to evaluate the efficacy of the dalantercept through the frequency of patients with objective tumor response and the frequency of patients who survived progression-free without receiving non-protocol therapy (TPFS) for at least 6 months. Activity based on either measure was deemed worthy of further investigation.
The null hypothesis (Ho) defined a region of uninteresting levels of activity and was based on an analysis of an historical GOG dataset of a similar population of patients treated with study drugs believed to be inactive or modestly active. The null hypothesis jointly specified the probability of a patient experiencing a tumor response as less than or equal to 10% and the probability of a patient surviving progression-free for at least 6 months as less than or equal to 20%. A probability of response of 25% or a probability of TPFS at 6 months of 45% was of clinical interest and worthy of further investigation and was used as the basis for determining statistical power.
The null hypothesis was evaluated with a flexible method provided by Sill et al (27), which is a two-stage design used to limit patient exposure to inactive regimens. If the observed numbers of patients enrolled during the first stage of accrual with responses or TPFS at 6 months were both less than or equal to their respective critical values, then the study would close, and the regimen would be declared clinically uninteresting. Otherwise, with medical judgment indicating, the study would open to the second stage of accrual to further evaluate the regimen. If, after the second phase of accrual, either the number of patients with responses or who were TPFS at 6 months exceeded their respective critical values, then the regimen would be considered worthy of further investigation.
The targeted accrual for the first stage was 25 patients but was allowed to deviate slightly for administrative purposes. If 28 patients were accrued, the critical value for the number of patients with responses was three (3) and the critical value for the number of patients who were TPFS at 6 months was seven (7). The cumulative targeted accrual for the second stage was 49 but was also allowed to deviate slightly.
The goal of the design was to limit the expected probabilities of type I and II errors to approximately 10% under the assumed accrual ranges of 21 to 28 (stage 1) and 45 to 52 (cumulatively after stage 2). Using the method of Sill et al. (27), the expected type I error at the end of stage two was about 8 to 8.8%, depending on the level of association between response and TPFS at 6 months. The expected probability of early termination when the agent is uninteresting was likely between 48 % and 55%, depending on the level of association between the two endpoints. With this design there was between 89.5% and 95% power of detecting a clinically significant effect, depending on the level of association between the primary endpoints.
Kaplan-Meier estimates were used for estimating the OS and PFS distributions. Exact confidence intervals (CIs) were used for binary parameters. (28) Confidence intervals for median OS and PFS accounted for censoring. (29) The analyses presented include data reported as of September 19, 2014.
RESULTS
Patient characteristics
From September 4, 2012 to February 19, 2013, GOG member institutions enrolled 28 patients on to this trial. No patients were deemed ineligible. One patient withdrew consent for treatment and for all followup after completion of cycle 1 of therapy, and 27 patients were treated and have follow-up. The median age of patients at study entry was 62 years (range 47–79). The median weight prior to cycle 1 was 72 kg; three participants weighed more than 100 kg. Eighty-two percent of patients received 1 prior chemotherapy regimen and 18% received two prior chemotherapy regimens. Eighteen (64%) of patients received prior radiation therapy. (Table 1) Patients received 1–12 cycles of dalantercept treatment, and 13 patients (46%) received ≤ 2 cycles (Table 3).
Table 1.
Patient characteristics
| Characteristic | Category | Dalantercept | |
|---|---|---|---|
| n | % | ||
| Age Group | 40–49 | 1 | 3.6 |
| 50–59 | 9 | 32.1 | |
| 60–69 | 13 | 46.4 | |
| 70–79 | 5 | 17.9 | |
| Ethnicity | Hispanic or Latino | 2 | 7.1 |
| Non-Hispanic | 23 | 82.1 | |
| Unknown/Not specified | 3 | 10.7 | |
| Race | White | 24 | 85.7 |
| Black/African American | 3 | 10.7 | |
| Unspecified | 1 | 3.6 | |
| Performance Status | 0 | 20 | 71.4 |
| 1 | 8 | 28.6 | |
| Cell Type/Grade | Endometrioid, grade 1 | 4 | 14.3 |
| Endometrioid, grade 2 | 6 | 21.4 | |
| Serous | 15 | 53.6 | |
| Clear Cell | 2 | 7.1 | |
| Mixed Epithelial | 1 | 3.6 | |
| Prior Chemotherapy | 1 Prior Regimen | 23 | 82.1 |
| 2 Prior Regimens | 5 | 17.9 | |
| Prior Radiation | No | 10 | 35.7 |
| Yes | 18 | 64.3 | |
| Prior Hormonal | No | 25 | 89.3 |
| Therapy | Yes | 3 | 10.7 |
| Prior Surgery | Yes | 28 | 100.0 |
Table 3.
Response, 6-month PFS, cycles of treatment and status.
| Endpoint | Category | n | % |
|---|---|---|---|
| Off study therapy Cycles of Treatment | Yes | 28 | 100.0 |
| 1 | 1 | 3.6 | |
| 2 | 12 | 42.9 | |
| 3 | 3 | 10.7 | |
| 4 | 4 | 14.3 | |
| 5 | 1 | 3.6 | |
| 6 | 3 | 10.7 | |
| 8 | 1 | 3.6 | |
| 11 | 1 | 3.6 | |
| 12 | 2 | 7.1 | |
| Reason off therapy | Disease Progression | 24 | 85.7 |
| Patient Refused | 1 | 3.6 | |
| Toxicity | 2 | 7.1 | |
| Death | 1 | 3.6 | |
| RECIST 1.1 Response | Complete or partial response | 0 | 0.0 |
| Stable Disease | 16 | 57.1 | |
| Disease Progression | 11 | 39.3 | |
| Indeterminate | 1 | 3.6 | |
| PFS ≥ 6 months | No | 23 | 82.1 |
| Yes | 5 | 17.9 | |
| TPFS ≥ 6 months | No | 25 | 89.3 |
| Yes | 3 | 10.7 | |
| Survival status | Alive | 10 | 35.7 |
| Dead – Treatment-related | 1 | 3.6 | |
| Dead – Disease-related | 17 | 60.7 |
Adverse Events
As shown in Table 2, safety of dalantercept was analyzed descriptively. Eighteen patients have died. Of these, 17 were due to disease and 1 was due to treatment. The one treatment-related death was due to a gastric hemorrhage in patient with prior history of small bowel obstruction and radiation-induced fibrosis. The gastric hemorrhage in this patient occurred following cycle 3 of therapy, and a disease assessment just prior to cycle 3 revealed stable disease.
Table 2. Reported Adverse Events.
Frequencies of the maximum grade of acute adverse events within a system organ class or specific term, regardless of attribution, over all patients who initiated study treatment and have been evaluated for toxicity (CTCAE v4.0).
| System Organ Class/Term | Treatment/Adverse Event Grade | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |
| Blood/Lymphatics | 13 | 5 | 8 | 1 | 1 | 0 |
| Anemia | 13 | 5 | 8 | 1 | 1 | 0 |
| Gastrointestinal | 3 | 13 | 7 | 4 | 0 | 1 |
| Abdominal distension | 24 | 2 | 2 | 0 | 0 | 0 |
| Abdominal pain | 20 | 6 | 2 | 0 | 0 | 0 |
| Ascites | 24 | 0 | 2 | 2 | 0 | 0 |
| Constipation | 11 | 12 | 5 | 0 | 0 | 0 |
| Diarrhea | 22 | 5 | 1 | 0 | 0 | 0 |
| Gastric hemorrhage | 27 | 0 | 0 | 0 | 0 | 1 |
| Nausea | 16 | 11 | 1 | 0 | 0 | 0 |
| Rectal fistula | 27 | 0 | 0 | 1 | 0 | 0 |
| Rectal hemorrhage | 26 | 2 | 0 | 0 | 0 | 0 |
| Vomiting | 19 | 8 | 0 | 1 | 0 | 0 |
| General and administration site | 2 | 18 | 7 | 1 | 0 | 0 |
| Edema face | 26 | 1 | 1 | 0 | 0 | 0 |
| Edema limbs | 11 | 15 | 2 | 0 | 0 | 0 |
| Fatigue | 4 | 17 | 6 | 1 | 0 | 0 |
| Infections/infestations | 23 | 1 | 4 | 0 | 0 | 0 |
| Investigations | 15 | 6 | 6 | 1 | 0 | 0 |
| Activated partial thromboplastin time prolonged | 27 | 0 | 0 | 1 | 0 | 0 |
| Alkaline phosphatase increased | 22 | 4 | 2 | 0 | 0 | 0 |
| Creatinine increased | 19 | 5 | 4 | 0 | 0 | 0 |
| Lymphocyte count decreased | 25 | 1 | 1 | 1 | 0 | 0 |
| Weight loss | 24 | 2 | 2 | 0 | 0 | 0 |
| Metabolism/nutrition | 14 | 5 | 7 | 2 | 0 | 0 |
| Anorexia | 24 | 2 | 2 | 0 | 0 | 0 |
| Dehydration | 26 | 0 | 2 | 0 | 0 | 0 |
| Hyperglycemia | 22 | 5 | 1 | 0 | 0 | 0 |
| Hypoalbuminemia | 19 | 4 | 4 | 1 | 0 | 0 |
|
| ||||||
| Hypoglycemia | 27 | 0 | 1 | 0 | 0 | 0 |
| Hypokalemia | 24 | 1 | 2 | 1 | 0 | 0 |
| Musculoskeletal/connective tissue | 11 | 10 | 5 | 2 | 0 | 0 |
| Arthralgia | 24 | 4 | 0 | 0 | 0 | 0 |
| Back pain | 19 | 4 | 4 | 1 | 0 | 0 |
| Myalgia | 25 | 3 | 0 | 0 | 0 | 0 |
| Nervous system | 11 | 16 | 1 | 0 | 0 | 0 |
| Dizziness | 26 | 2 | 0 | 0 | 0 | 0 |
| Headache | 15 | 13 | 0 | 0 | 0 | 0 |
| Neuralgia | 27 | 0 | 1 | 0 | 0 | 0 |
| Renal/urinary | 24 | 3 | 0 | 1 | 0 | 0 |
| Urinary tract obstruction | 27 | 0 | 0 | 1 | 0 | 0 |
| Reproductive/breast | 25 | 3 | 0 | 0 | 0 | 0 |
| Respiratory/thoracic/mediastinal | 11 | 13 | 2 | 2 | 0 | 0 |
| Dyspnea | 18 | 8 | 1 | 1 | 0 | 0 |
| Epistaxis | 24 | 4 | 0 | 0 | 0 | 0 |
| Pleural effusion | 24 | 1 | 2 | 1 | 0 | 0 |
| Vascular disorders | 23 | 1 | 2 | 2 | 0 | 0 |
| Hypertension | 25 | 1 | 1 | 1 | 0 | 0 |
| Thromboembolic event | 26 | 0 | 1 | 1 | 0 | 0 |
The most commonly reported grade 1–2 adverse events included: anemia, lower extremity edema, fatigue, myalgias/arthralgias, headache, and dyspnea. A grade 3 thromboembolic event was reported in 1 patient after switching therapy and was felt unlikely to be study treatment-related. Other grade 3 serious adverse events at least possibly treatment-related included dyspnea, pleural effusion, ascites, vomiting, hypokalemia, and anemia. Other grade 3 adverse events included hypertension, urinary tract-obstruction, lower extremity pain, back pain, lymphocytopenia, and fatigue. A grade 3 rectal fistula, which was possibly treatment-related, also occurred on study. One episode of grade 4 anemia at least possibly treatment-related was reported in the same patient with the grade 5 gastric hemorrhage. Twenty-four (85.7%) patients discontinued treatment for disease progression, 2 (7.1%) for toxicity, 1 (3.6%) for patient refusal, and 1 (3.6%) for death. Three patients remained on study drug for 11 or 12 cycles; treatment was discontinued due to progression of disease in all three cases (Table 3).
Activity of Dalantercept
The activity of dalantercept was analyzed in 28 patients and is presented in Table 3. At the time of this report all patients are off study treatment. The best overall response was stable disease in 16 (57.1%) patients, disease progression in 11 (39.3%), and no patients’ tumors completely or partially responded. There were only three patients (11%) with a progression date > 6 months following study enrollment and prior to subsequent non-protocol treatment. All three with TPFS >6 months had serous carcinomas and were previously treated with a carboplatin/taxane regimen. One of these patients also received IVRT.
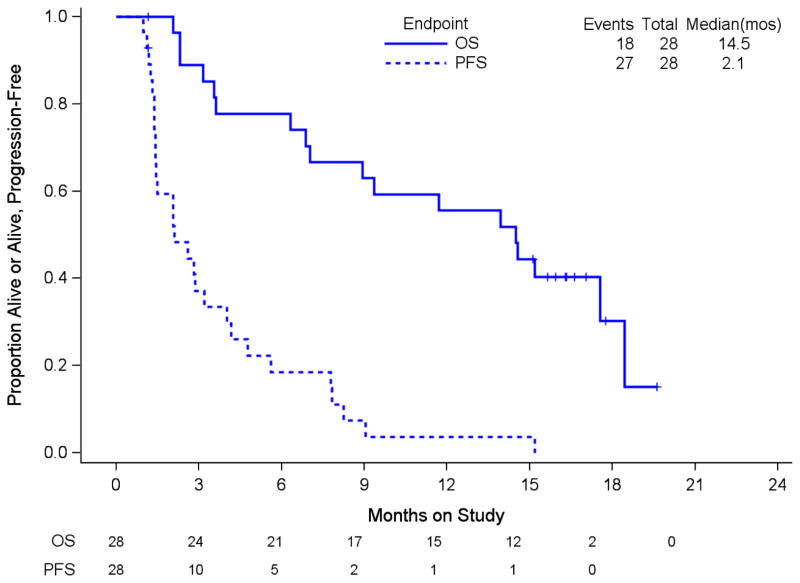
Three patients discontinued study drug prior to progression; two withdrew due to toxicity and the other withdrew consent. The first patient started on non-protocol treatment 1.4 months after discontinuing study drug and 2.2 months after entry. In addition, the response of this patient could not be evaluated due to ascites. The second patient started radiation treatment 4.6 months after enrollment and the third withdrew consent 1.2 months after study entry. This patient had stable disease at an assessment three weeks after study entry. Eleven percent of patients had TPFS lasting ≥ 6 months. The estimate of median PFS is 2.1 months (90% CI: 1.4–3.2) and the median OS 14.5 months (90% CI: 7.0–17.5), (Figure 1). Neither endpoint met its respective criteria for declaring dalantercept active (>3 responses or >7 patients with TPFS ≥ 6 months).
Figure 1.
Overall survival and progression-free survival. PFS: progression-free survival, mos: months Numbers at risk at time 0, 3, 6, 9, 12, 15, 18 and 21 months by endpoint are provided below the graph
DISCUSSION
Angiogenesis is one of the cardinal processes leading to invasion and metastasis of solid tumors (30). There is evidence that angiogenesis plays a role in endometrial cancer progression and prognosis. The angiogenic-signaling pathway may be triggered by the release of angiogenic promoters such as vascular endothelial growth factor (VEGF) from tumor cells into the local microenvironment. Vascular endothelial growth factor (VEGFR) expression has been found to be present in up to 67% of endometrial adenocarcinoma specimens, (31) and VEGF expression has been shown to be higher in endometrial adenocarcinoma than in normally cycling endometrium (32). VEGFR-2 (flk-1) and VEGFR-3 have been found to be poor prognostic factors in endometrial cancer (33, 34). VEGF-A/VEGF-1 expression is associated with decreased 5 and 10-year disease-free survival in post-menopausal patients with endometrial carcinoma (35, 36). Further study of the VEGF, VEGF-R (KDR) pathway in stage I endometrial carcinoma has demonstrated a worse prognosis for tumors bearing activated KDR (pKDR) (34–36). KDR activation was also associated with an elevation of HIF-1alpha, an up-regulator of VEGF (35, 36). These relationships point to a VEGF autocrine loop which can serve as a therapeutic target.
On the basis of the activity of bevacizumab in GOG 229-E, which was associated with an objective response rate of 13.5%, a 6 month progression-free survival of 40.4% and median overall survival of 10.5 months (13), we investigated the activity of dalantercept, a first-in-class receptor-fusion protein that inhibits the activin receptor-like kinase 1 (ALK1) signaling pathway, in advanced/recurrent endometrial cancer.
Single agent dalantercept at a dose level of 1.2 mg/kg once every 3 weeks in advanced/recurrent endometrial cancer in patients who have had 1–2 prior lines of cytotoxic therapy, failed to reach its primary endpoint, and has insufficient activity to warrant further investigation as a monotherapy in recurrent EMC. In contrast to other anti-angiogenic agents (e.g., bevacizumab) that block the proliferative phase of angiogenesis, dalantercept blocks the maturation phase of angiogenesis, and we posit that inhibition of the proliferative phase of angiogenesis via VEGF inhibition may be a more efficacious approach in the treatment of advanced endometrial carcinoma.
At the time of this report all patients are off study treatment. Overall, dalantercept was well-tolerated with 2/28 (7.1%) patients discontinuing treatment due to toxicity. However, nearly half of the patients received two or less cycles of study treatment. The majority of patients (16/28) had a best overall response of stable disease and subsequently discontinued study treatment due to disease progression. There were no patients with complete or partial responding tumors and there were only three patients with a progression date ≥ 6 months following study enrollment and prior to subsequent non-protocol treatment. While the optimal approach to angiogenesis blockade in advanced EMC is yet to be determined, it is known that key endothelial cell-selective growth factor receptors include VEGFR 1 and 2 and the Tie-2 tyrosine kinase receptor, and combination studies of such agents could prove to be active and may be worthy of further exploration in this disease.
RESEARCH HIGHLIGHTS.
Angiogenesis plays a role in endometrial cancer progression and prognosis.
Dalantercept binds BMP9/BMP10 and prevents signaling through activin receptor-like kinase, which results in inhibition of the maturation phase of angiogenesis.
Single agent dalantercept in advanced/recurrent endometrial cancer has insufficient activity to warrant further investigation as a monotherapy in this disease.
Acknowledgments
This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group Administrative Office (CA 27469), the Gynecologic Oncology Group Statistical and Data Center (CA 37517) and the NRG Oncology Grant number: U10 CA180822. The authors would like to thank Acceleron Pharmaceuticals for their support of this research.
The following NRG Oncology/Gynecologic Oncology Group (GOG) institutions participated in this study: Memorial Sloan-Kettering Cancer Center, UCSF–Mount Zion, Maine Medical Center–Bramhall Campus, Florida Hospital Cancer Institute, St. Vincent Oncology Center, University of Oklahoma Health Sciences Center, Abington Memorial Hospital, Northwest Hospital, Cleveland Clinic Foundation, and Carolinas Medical Center/Levine Cancer Institute.
Footnotes
CONFLICT OF INTEREST
Dr. Virginia Filiaci reports grants and non-financial support from National Institutes of Health, National Cancer Institute, other from Acceleron Pharma, Inc., during the conduct of the study. Additionally, Dr. Carol Aghajanian received an honorarium as a one-time ad board member in addition to travel expenses. Dr. Aghajanian also received funding for travel from Abbvie for clinical trial planning meetings. All other authors on this manuscript have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Miller D, Filiaci V, Fleming G, Mannel R, Cohn D, Matsumoto T, et al. Randomized phase III noninferiority trial of first line chemotherapy for metastatic or recurrent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2012;125:771. (abst) [Google Scholar]
- 3.Muggia FM, Blessing JA, Sorosky J, Reid GC. Phase II trial of the pegylated liposomal doxorubicin in previously treated metastatic endometrial cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2002;20:2360–64. doi: 10.1200/JCO.2002.08.171. [DOI] [PubMed] [Google Scholar]
- 4.Miller DS, Blessing JA, Lentz SS, Waggoner SE. A phase II trial of topotecan in patients with advanced, persistent, or recurrent endometrial carcinoma: a gynecologic oncology group study. Gynecol Oncol. 2002;87:247–51. doi: 10.1006/gyno.2002.6804. [DOI] [PubMed] [Google Scholar]
- 5.Garcia AA, Blessing JA, Nolte S, Mannel RS. A phase II evaluation of weekly docetaxel in the treatment of recurrent or persistent endometrial carcinoma: a study by the Gynecologic Oncology Group. Gynecol Oncol. 2008;111:22–6. doi: 10.1016/j.ygyno.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Dizon DS, Blessing JA, McMeekin DS, Sharma SK, Disilvestro P, Alvarez RD. Phase II trial of ixabepilone as second-line treatment in advanced endometrial cancer: gynecologic oncology group trial 129-P. J Clin Oncol. 2009;27:3104–08. doi: 10.1200/JCO.2008.20.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller DS, Blessing JA, Drake RD, Higgins R, McMeekin DS, Puneky LV, et al. A phase II evaluation of pemetrexed (Alimta, LY231514, IND #40061) in the treatment of recurrent or persistent endometrial carcinoma: a phase II study of the Gynecologic Oncology. Gynecol Oncol. 2009;115:443–46. doi: 10.1016/j.ygyno.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Tait DL, Blessing JA, Hoffman JS, Moore KN, Spirtos NM, Lachance JA, et al. A phase II study of gemcitabine (Gemzar, LY188011) in the treatment of recurrent or persistent endometrial carcinoma: a gynecologic oncology group study. Gynecol Oncol. 2011;121:118–21. doi: 10.1016/j.ygyno.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 9.Lincoln S, Blessing JA, Lee RB, Rocereto TF. Activity of paclitaxel as second-line chemotherapy in endometrial carcinoma: a gynecologic oncology group study. Gynecol Oncol. 2003;88:277–81. doi: 10.1016/s0090-8258(02)00068-9. [DOI] [PubMed] [Google Scholar]
- 10.McMeekin DS, Sill MW, Benbrook D, Darcy KM, Stearns-Kurosawa DJ, Eaton L, et al. A phase II trial of thalidomide in patients with refractory endometrial cancer and correlation with angiogenesis biomarkers: a Gynecologic Oncology Group study. Gynecol Oncol. 2007;105:508–16. doi: 10.1016/j.ygyno.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leslie KK, Sill MW, Fischer E, Darcy KM, Mannel RS, Tewari KS, et al. A phase II evaluation of gefitinib in the treatment of persistent or recurrent endometrial cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 2013;129:486–94. doi: 10.1016/j.ygyno.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leslie KK, Sill MW, Lankes HA, Fischer EG, Godwin AK, Gray H, et al. Lapatinib and potential prognostic value of EGFR mutations in a Gynecologic Oncology Group phase II trial of persistent or recurrent endometrial cancer. Gynecol Oncol. 2012;127:345–50. doi: 10.1016/j.ygyno.2012.07.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aghajanian C, Sill MW, Darcy K, Greer B, McMeekin DS, Rose PG, et al. A phase II evaluation of bevacizumab in the treatment of recurrent or persistent endometrial cancer: A Gynecologic Oncology Group (GOG) study. J Clin Oncol. 2011;29:2259–65. doi: 10.1200/JCO.2010.32.6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coleman RL, Sill MW, Lankes HA, Fader AN, Finkler NJ, Hoffman JS, et al. A phase II evaluation of aflibercept in the treatment of recurrent or persistent endometrial cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 2012;127:538–43. doi: 10.1016/j.ygyno.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alvarez EA, Brady WE, Walker JL, Rotmensch J, Zhou XC, Kendrick JE, et al. Phase II trial of combination bevacizumab and temsirolimus in the treatment of recurrent or persistent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2013;129:22–27. doi: 10.1016/j.ygyno.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 16.Coleman RL, Sill MW, Thaker P, Bender DP, DeGeest K, Street D, et al. A phase II evaluation of AZD6244, a selective MEK-1/2 inhibitor in the treatment of recurrent or persistent endometrial cancer: A Gynecologic Oncology Group Study. Gynecol Oncol. 2013 doi: 10.1016/j.ygyno.2015.04.005. SGO Abstract Supplement: S14; SGO#27) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Powell MA, Sill MW, Goodfellow PJ, Benbrook DM, Lankes HA, Leslie KK, et al. A phase II trial of brivanib in recurrent or persistent endometrial carcinoma : An NRG Oncology/Gynecologic Oncology Group Study. Gynecol Oncol. 2014 doi: 10.1016/j.ygyno.2014.07.083. pii: S0090–8258(14)01158–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dizon DS, Sill M, Schilder JM, McGonigle KF, Rhaman Z, Miller DS, et al. Results of 229K: A phase II trial of BIBF-1120 for women with advanced, recurrent, or metastatic endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2014;133:55. (SGO#133) [Google Scholar]
- 19.Chen H, Shi S, Acosta L, Li W, Lu J, Bao S, et al. BMP10 is essential for maintaining cardiac growth during murine cardiogenesis. Development. 2004;131:2219–31. doi: 10.1242/dev.01094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oh SP, Seki T, Goss KA, Imamura T, Yi Y, Donahoe PK, et al. Activin receptor-like kinase 1 modulates transforming growth factor-b1 signaling in the regulation of angiogenesis. Proc Natl Acad Sci. 2000;97:2626–31. doi: 10.1073/pnas.97.6.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi Y, Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 22.Cunha SI, Pietras K. ALK1 as an emerging target for antiangiogenic therapy of cancer. Blood. 2011;117:6999–7006. doi: 10.1182/blood-2011-01-330142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–4. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bendell JC, Gordon MS, Hurwitz HI, Jones SF, Mendelson DS, Blobe GC, et al. Safety, pharmacokinetics, pharmacodynamics, and antitumor activity of dalantercept, an activin receptor-like kinase-1 ligand trap, in patients with advanced cancer. Clin Cancer Res. 2014;20:480–89. doi: 10.1158/1078-0432.CCR-13-1840. [DOI] [PubMed] [Google Scholar]
- 25.Brusselmans K, Bono F, Collen D, Herbert JM, Carmeliet P, Dewerchin M, et al. A novel role for vascular endothelial growth factor as an autocrine survival factor for embryonic stem cells during hypoxia. J Biol Chem. 2005;280:3493–99. doi: 10.1074/jbc.M406613200. [DOI] [PubMed] [Google Scholar]
- 26.Furuya M, Nishiyama M, Kasuya Y, Kimura S, Ishikura H. Pathophysiology of tumor neovascularization. Vasc Health Risk Manag. 2005;1:277–90. doi: 10.2147/vhrm.2005.1.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sill MW, Rubinstein L, Litwin S, Yothers G. A method for utilizing co-primary efficacy outcome measures to screen regimens for activity in two-stage Phase II clinical trials. Clin Trials. 2012;9:385–95. doi: 10.1177/1740774512450101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–13. [Google Scholar]
- 29.Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics. 1982;38:29–41. [Google Scholar]
- 30.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Talvensaari-Mattila A, Soini Y, Santala M. VEGF and its receptors (flt-1 and KDR/flk-1) as prognostic indicators in endometrial carcinoma. Tumour Biol. 2005;26:81–7. doi: 10.1159/000085589. [DOI] [PubMed] [Google Scholar]
- 32.Saito M, Sato Y, Watanabe J, Kuramoto H, Sadayuki K, Fukuda T. Angiogenic factors in normal endometrium and endometrial adenocarcinoma. Pathol Int. 2007;57:140–47. doi: 10.1111/j.1440-1827.2006.02071.x. [DOI] [PubMed] [Google Scholar]
- 33.Yokoyama Y, Charnock-Jones DS, Licence D, Yanaihara A, Hastings JM, Holland CM, et al. Expression of vascular endothelial growth factor (VEGF)-D and its receptor, VEGF receptor 3, as a prognostic factor in endometrial carcinoma. Clin Cancer Res. 2003;9:1361–69. [PubMed] [Google Scholar]
- 34.Giatromanolaki A, Sivridis E, Brekken R, Thorpe PE, Anastasiadis P, Gatter KC, et al. The angiogenic “vascular endothelial growth factor/flk-1(KDR) receptor” pathway in patients with endometrial carcinoma: prognostic and therapeutic implications. Cancer. 2001;92:2569–77. doi: 10.1002/1097-0142(20011115)92:10<2569::aid-cncr1609>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 35.Hirai M, Nakagawara A, Oosaki T, Hayashi Y, Hirono M, Yoshihara T. Expression of vascular endothelial growth factors (VEGF-A/VEGF-1 and VEGF-C/VEGF-2) in postmenopausal uterine endometrial carcinoma. Gynecol Oncol. 2001;80:181–88. doi: 10.1006/gyno.2000.6056. [DOI] [PubMed] [Google Scholar]
- 36.Giatromanolaki A, Koukourakis MI, Turley H, Sivridis E, Harris AL, Gatter KC Tumour and Angiogenesis Research Group. Phosphorylated KDR expression in endometrial cancer cells relates to HIF1alpha/VEGF pathway and unfavorable prognosis. Mod Pathol. 2006;19:701–7. doi: 10.1038/modpathol.3800579. [DOI] [PubMed] [Google Scholar]