Abstract
Urinary hydroxy polycyclic aromatic hydrocarbons (OH-PAHs) are commonly used in biomonitoring to assess exposure to polycyclic aromatic hydrocarbons (PAHs). Similar to other biologically non-persistent chemicals, OH-PAHs have relatively short biological half-lives (4.4–35 hours). Little information is available on their variability in urinary concentrations over time in non- occupationally exposed subjects. This study was designed to (i) study the variability of 9 urinary OH-PAH metabolite concentrations over time and (ii) calculate sample size requirements for future epidemiological studies based on spot urine, first morning void and 24-hour void sampling. Individual urine samples (n = 427) were collected during one week from 8 non-occupationally exposed adults. We recorded the time and volume of each urine excretion, dietary details, and the driving activities of the participants. Within subjects, the coefficients of variation (CV) for the wet-weight concentration of OH-PAHs in all samples ranged from 45% to 297%; creatinine adjustment reduced the CV to 19–288% (p < 0.001; paired t-test). The simulated 24-hour void concentrations were the least variable measure, with CVs ranging 13–182% for the 9 OH-PAHs. Within-day variability contributed on average 84%, and between-day variability accounted for 16% of the total variance of 1-hydroxypyrene (1-PYR). Intraclass correlation coefficients (ICC) of 1-PYR levels were 0.55 for spot urine samples, 0.60 for first-morning voids, and 0.76 for 24-hour voids, indicating a high degree of correlation between urine measurements collected from the same subject over time. Sample size calculations were performed to estimate the number of subjects needed for detecting differences in geometric mean at a statistical power of 80% for spot urine, first-morning, and 24-hour void sampling. These data will aid in the design of future studies of PAHs and possibly other biologically non-persistent chemicals and the interpretation of their analytical results.
Keywords: PAH, variability, first morning void, 24-hour void, spot sample, sample size
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are a group of pollutants formed during incomplete combustion and are ubiquitously distributed in air, soil, water, food, and many occupational settings (IARC 1983). Human exposure to PAHs can occur through inhalation of polluted air or cigarette smoke, ingestion of food containing PAHs, and dermal absorption from soil or other PAH-containing materials such as coal tar. For the general population, ingestion and inhalation are the two dominant routes of exposure (ATSDR 1995; Bostrom et al. 2002). For certain occupations, such as coke oven workers, dermal absorption can become a major exposure route (ATSDR 1995). PAHs such as benzo(a)pyrene have been classified as probable human and animal carcinogens (ATSDR 1995). PAHs have also been associated with reproductive, developmental, hemato-, cardio-, neuro-, and immuno-toxicities (ATSDR 1995).
Urinary PAH metabolites, specifically the mono-hydroxy PAH metabolites (OH-PAHs), have been used as biomarkers for assessing human exposure to PAHs, with 1-hydroxypyrene (1-PYR) as the most commonly used biomarker (Jacob and Seidel 2002). A number of studies have reported the levels of OH-PAHs in occupational groups, including coke oven workers and road pavers (Grimmer et al. 1993; Jongeneelen et al. 1990; Levin et al. 1995), as well as in the general population from various parts of the world (Hansen et al. 2005; Mucha et al. 2006). In the United States, OH-PAHs are included in the National Health and Nutrition Examination Survey (NHANES) conducted by the Centers for Disease Control and Prevention (CDC), an on-going survey that reports the concentrations of biomarkers in a statistically representative sample of the U.S. population. In that survey, OH-PAHs are measured in approximately 3,000 samples every two years (Li et al. 2008).
The half-life for urinary 1-PYR in humans has been reported to be 9.8 hours (Brzeznicki et al. 1997) and 6–35 hours (Jongeneelen et al. 1990) after inhalation exposure, 4.4 hours (Buckley and Lioy 1992) and 12 hours (Viau et al. 1995) after ingestion exposure, 11.5–15 hours after dermal absorption (Viau and Vyskocil 1995), and 3.9–26.7 hours (average 10.4 hours) after inhalation and dermal absorption (Boogaard and van Sittert, 1994). Information on half-lives of the other OH-PAHs is scarce; however, since these are the same group of metabolites formed under similar biological pathways, we can reasonably deduce that other OH-PAHs measured in this study would have similar half-lives. Because of the short half-lives of PAHs, the information that urinary biomonitoring provides is limited to recent exposure. Further, the half-life information should be considered in study design, and appropriate samples need to be collected within the window during which PAHs are excreted in the urine following exposure.
Theoretically, a 24-hour void specimen represents the total and average daily metabolite excretion more reliably than a spot urine sample. Thus, 24-hour urine collection was recommended by the U.S. EPA for evaluating exposure to pesticides and other toxic substances that are primarily eliminated in the urine (U.S.EPA 1996). However, this sampling method is burdensome on the study subjects, and non-compliance can lead to compromised or a biased sampling. A common alternative is to collect the first-morning urine, as has been done in many occupational and environmental exposure investigations, because the first-morning urine is often correlated with the 24-hour void (Han et al. 2008; Scher et al. 2007; Kissel et al. 2005). In epidemiological studies such as NHANES involving thousands of participants, first-morning voids are often difficult to obtain; hence, spot urine samples have been used as a more practical solution. However, non-standardized sampling increases the variance, especially for short-lived compounds like OH-PAHs, and changes in water consumption throughout the day increase the variance even further. Therefore, concentration adjustments such as creatinine correction have often been used to adjust for urine excretion rate and to minimize variability of the urinary biomarker concentrations caused by different hydration status of participants (Barr et al. 2005).
Epidemiological study design should include a good understanding of the variability within a normal, non-exposed population or group of interest (IPCS 1993). Furthermore, because most potential health effects are likely associated with exposure over time, understanding the temporal variability of urinary biomarker levels from subjects with no occupational exposure is essential for data interpretation and study design. At present, little is known about the variability of urinary OH-PAH levels. Siwinska et al. (1998) found that the concentration of 1-PYR in first-morning urine samples collected on 6 consecutive days in 30 children had a within-person coefficient of variation (CV) range of 14–109%, whereas inter-person variation was 69–109%. Grimmer et al. (1993) reported that the intra-person variation in 24-hour voids during 4 consecutive days from 4 coke workers had a range of 14–41% for 1-PYR and 16–94% for mono-hydroxy phenanthrene metabolites. Ovrebo et al (1995) found that the intra-individual CVs of 1-PYR in post-shift urine samples were less than 0.50 for half of the workers during a 2.5-year study. Han et al. (2007) reported a significant (40–62%) intra-person difference between first-morning and 24-hour voids in 100 adults. Although these studies provided some information on the variability of first-morning and 24-h void samples, they could not address the variability of spot urine samples excreted at any time of the day, nor did they address temporal variation of urinary OH-PAHs. Further, they could not provide a comparison between the three sampling approaches, that is, spot, first-morning and 24-hour void.
The objectives of the present study were to evaluate the variability of 9 commonly detectable OH-PAHs, metabolites of naphthalene, fluorene, phenanthrene, and pyrene, in adults with no occupational exposure over 7 days. Our design allowed us to determine the between- and within-subject variability and the between- and within-day variability, as well as apportion the variance to subject and day effects for spot, first-morning and 24-hour void samples. In addition, we calculated sample size requirements for the three sampling approaches. The findings of this investigation are applicable to the study design of future exposure assessments or epidemiological studies involving exposures to PAHs and potentially other non-persistent chemicals which have short biological half lives and are eliminated in urine (Needham and Sexton, 2000).
Methods
Study design
The 8 subjects in this study were healthy volunteers between 26 and 58 years of age with no known occupational PAH exposure. They were non-smokers who were not subjected to second-hand smoking. The participants (4 males and 4 females) lived in the metropolitan Atlanta area and drove to and from work in the morning and afternoon during the weekdays (Monday–Friday), with median driving time of 30 minutes (one-way) and a median distance of 21 km (one-way). The participants did not work during the weekend (Saturday–Sunday), when they engaged in only sporadic driving.
During the one-week study period, participants collected all urine excretions in a graduated beaker, recorded the volume and time of each excretion, and transferred a portion (~50 mL) to a pre-labeled sterile urine cup, which was then stored in an ice cooler. The urine samples were retrieved from each participant daily (or after the weekend), brought back to the laboratory, and frozen at −70°C until analysis. Participants also recorded detailed information on dietary intake, driving, and other outdoor activities.
Laboratory methods
The method used to measure the urinary OH-PAH metabolites has been described previously (Li et al. 2006). In brief, urine samples were spiked with 13C-labeled internal standards and sodium acetate buffer containing β-glucuronidase enzyme, hydrolyzed overnight at 37°C, and then extracted by n-pentane through semi-automated liquid-liquid extraction. The extracts were evaporated, derivatized, and analyzed on a 6890 gas chromatograph (Agilent Technology, Palo Alto, CA) coupled with a MAT 95XL high resolution mass spectrometer (Thermo Fisher Scientific Inc., Waltham, MA). All analyses were subjected to a series of quality control and quality assurance checks as described elsewhere (Li et al. 2006). Urinary creatinine was measured on a Roche Hitachi 912 Chemistry Analyzer (Hitachi Inc., Pleasanton, CA) by use of the Creatinine Plus Assay, as described in Roche’s Creatinine Plus Product Application # 03631761003.
Statistical analysis
All statistical analyses were performed through SAS 9.1.3 (SAS Institute, Cary, NC) and Statistica 7.1 (StatSoft Inc., Tulsa, OK). Concentrations below the limit of detection (LOD) were replaced with the LOD divided by the square root of 2 prior to data analysis. The urinary metabolite concentrations followed a log-normal distribution; therefore, all data were log-transformed before statistical analysis.
A total of 427 samples were available from 8 subjects over one week. We defined the first-morning void sample as the first sample collected from each subject after 5 AM each day. The simulated 24-hour void concentration was calculated as the volume-weighted average of all urine specimens collected by an individual during a 24-hour period starting at midnight. Subject 8 (S8) had a high dietary intake of PAHs on the fourth day of the study period, and concentrations of all OH-PAHs for this subject increased up to 104-fold within 12 hours. Hence, data from this subject for the two days affected by the high dietary intake were excluded from further variability and sample size calculations.
To assess the between- and within-subject as well as the between- and within-day variance, we calculated the contributions of each effect toward the total variance for four metabolites: 1-naphthol (1-NAP), 3-hydroxyfluorene (3-FLUO), 3-hydroxyphenanthrene (3-PHEN), and 1-PYR. We performed this calculation by using a random effects model fitted through the PROC NESTED, PROC MIXED, and PROC VARCOMP procedures in SAS. Since all models produced similar results, we chose to present only the results from the PROC NESTED model. We calculated intraclass correlation coefficients (ICC), defined as the ratio of between-subject variance to the total variance, as an indicator of reproducibility of repeated measurements over time (McGraw and Wong 1996). A high ICC value indicates high correlation and high reproducibility between repeated samples from the same subject. Using the findings from this study, we performed sample size calculations, including 1 to 4 repeated samples from each subject. Separate calculations were performed for spot sample, first-morning void, and 24-hour void. The percent differences in geometric means (GM) used for the sample size calculation between the two groups (e.g., control vs. case) were 10%, 25%, 50%, and 100%. All of our analyses were performed on log-transformed data; hence, we converted percent differences in the GM to a difference (d) on the mean of log-transformed concentrations for the power calculation (Supplementary Information, Appendix 1). The number of subjects (m) needed per group to achieve a desired type I error (α = 0.05) and power (P = 0.8) was calculated by use of the following formula (Diggle et al. 1994):
where: Δ = d/SD is the smallest meaningful difference between groups in standard deviation (SD) units; n = number of repeated samples per subject; Zy = yth percentile of a standard Gaussian distribution; β = type II error = 1-P.
Results
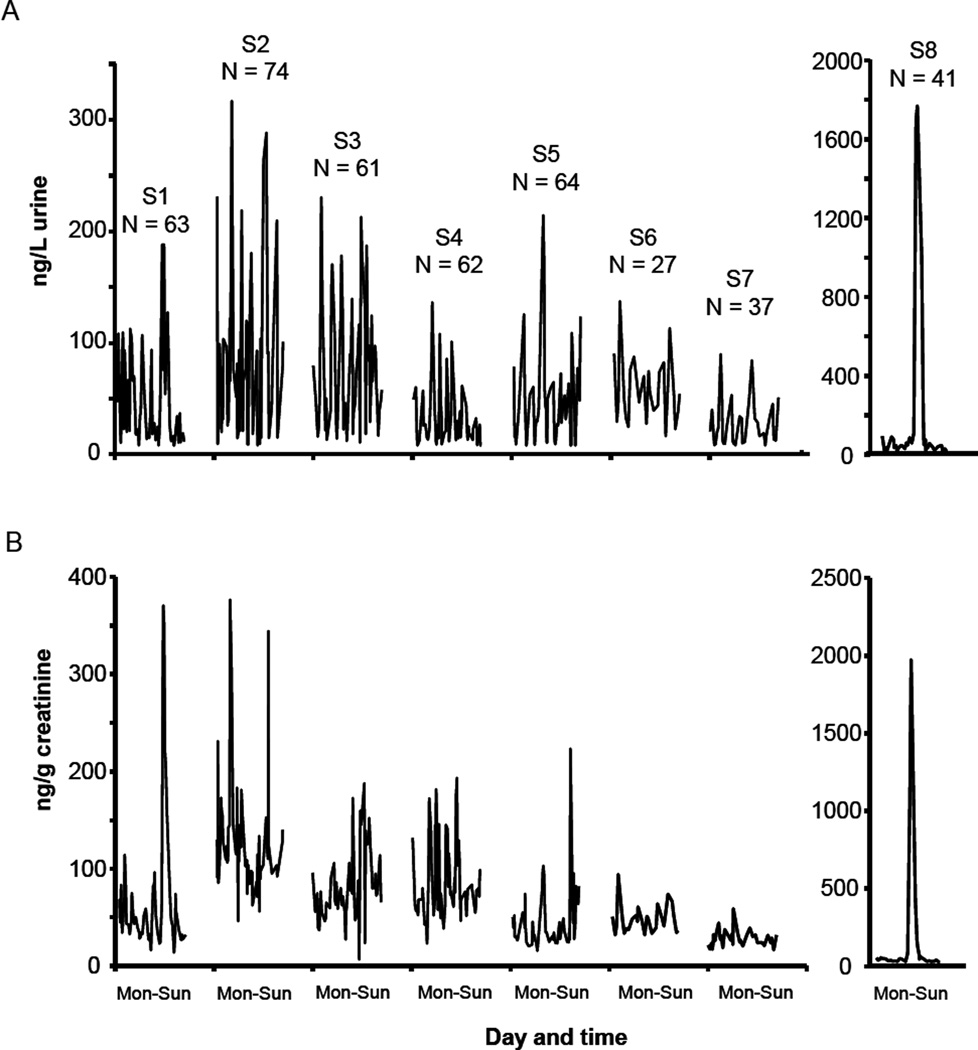
We quantified 9 OH-PAH metabolites in 427 samples collected from 8 subjects during 1 week. The GM, median, and quartile concentrations from all samples, first-morning voids, and simulated 24-hour voids are shown in Table 1. The detection rates were above 95% for all reported analytes except for 2-hydroxyphenanthrene (2-PHEN, 82%) and 3-hydroxyfluorene (3-FLUO, 87%). During the 7-day study period, each of the 8 subjects collected an average of 53 urine samples (27–74 samples/subject, Figure 1). Urinary OH-PAH concentrations within subjects varied 2 to 3 orders of magnitude (Supplementary Information, Table S-1). For example, the concentration of 2-naphthol (2-NAP) in subject S7 ranged from 86 ng/L to 17069 ng/L during the 7-day study period. Within subjects, the CVs of the 9 OH-PAHs among all samples, first-morning voids, and 24-hour voids were 45–297%, 26–189%, and 13–182%, respectively. After creatinine adjustment, the CVs were 19–288%, 9–176%, and 9–154%, respectively (Supplementary Information, Table S-2). Levels of urinary PAH biomarkers in each subject varied throughout the day, as exemplified in Figure 2. The 1-PYR concentration over time, stratified by subject, is provided in Figure 1. One of the subjects (S8) ate barbecued chicken for lunch on Thursday; as a result, concentrations of the 9 metabolites increased by 22–104 folds following that exposure (Figures 1 and 3). Twenty-four hours after the dietary exposure, the concentration of the urinary metabolites monitored returned to pre-exposure levels. The Thursday and Friday data from subject S8 were excluded from further variability analysis and sample size calculations.
Table 1.
Concentrations of urinary PAH metabolites for all samples, first-morning voids, and reconstructed 24-hour voids. Also given are reference concentrations from the U.S. adult population.
| Analyte | Abbr | all samples (n = 427) |
1st morning voids (n=56) |
24 hour voids (n = 56) |
U.S. adult b |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GMa | Median | Quartiles | GM | Median | Quartiles | GM | Median | Quartiles | |||
| Wet weight concentration (ng/L) | |||||||||||
| 1-naphthol | 1-NAP | 664 | 693 | 324–1287 | 1355 | 1268 | 789–1941 | 948 | 866 | 511–1381 | 2190 |
| 2-naphthol | 2-NAP | 1041 | 906 | 507–1968 | 1705 | 1449 | 886–3413 | 1271 | 1016 | 673–2138 | 2620 |
| 9-hydroxyfluorene | 9-FLUO | 116 | 110 | 60–242 | 189 | 226 | 105–313 | 143 | 143 | 96–210 | 230 |
| 3-hydroxyfluorene | 3-FLUO | 33 | 35 | 17–56 | 52 | 50 | 38–68 | 37 | 35 | 30–47 | 138 |
| 2-hydroxyfluorene | 2-FLUO | 91 | 99 | 50–164 | 150 | 137 | 107–191 | 107 | 109 | 84–133 | 333 |
| 3-hydroxyphenanthrene | 3-PHEN | 52 | 51 | 27–99 | 85 | 79 | 53–125 | 63 | 60 | 42–80 | 105 |
| 1-hydroxyphenanthrene | 1-PHEN | 70 | 69 | 38–130 | 116 | 118 | 76–179 | 81 | 81 | 58–101 | 145 |
| 2-hydroxyphenanthrene | 2-PHEN | 29 | 25 | 14–53 | 43 | 40 | 26–70 | 33 | 29 | 23–47 | 57 |
| 1-hydroxypyrene | 1-PYR | 35 | 33 | 17–67 | 59 | 51 | 40–87 | 41 | 39 | 28–57 | 47 |
| Creatinine corrected concentration (ng/g creatinine) | |||||||||||
| 1-naphthol | 1-NAPc | 1126 | 1050 | 638–1641 | 1166 | 1002 | 680–1741 | 1329 | 1168 | 739–1927 | 2070 |
| 2-naphthol | 2-NAPc | 1766 | 1657 | 910–3112 | 1468 | 1093 | 764–2437 | 1781 | 1356 | 925–3158 | 2480 |
| 9-hydroxyfluorene | 9-FLUOc | 196 | 203 | 125–318 | 163 | 175 | 117–238 | 201 | 210 | 158–275 | 217 |
| 3-hydroxyfluorene | 3-FLUOc | 55 | 53 | 37–77 | 45 | 45 | 32–58 | 52 | 52 | 37–72 | 131 |
| 2-hydroxyfluorene | 2-FLUOc | 155 | 154 | 113–200 | 129 | 126 | 108–166 | 150 | 147 | 116–194 | 314 |
| 3-hydroxyphenanthrene | 3-PHENc | 89 | 87 | 63–122 | 73 | 73 | 55–101 | 88 | 86 | 64–114 | 99 |
| 1-hydroxyphenanthrene | 1-PHENc | 119 | 122 | 82–173 | 100 | 96 | 71–143 | 114 | 116 | 74–165 | 137 |
| 2-hydroxyphenanthrene | 2-PHENc | 49 | 45 | 33–67 | 37 | 36 | 27–46 | 47 | 47 | 33–58 | 54 |
| 1-hydroxypyrene | 1-PYRc | 59 | 58 | 33–98 | 50 | 49 | 32–72 | 58 | 57 | 34–88 | 45 |
GM—geometric mean
Geometric mean concentrations in the general U.S. adult population (aged 20 years and older) are quoted from Li et al, 2008
Figure 1.
Wet weight (A) and creatinine-adjusted (B) concentrations of 1-hydroxypyrene in all individual urine samples from 8 subjects (S1–S8) during 7 days.
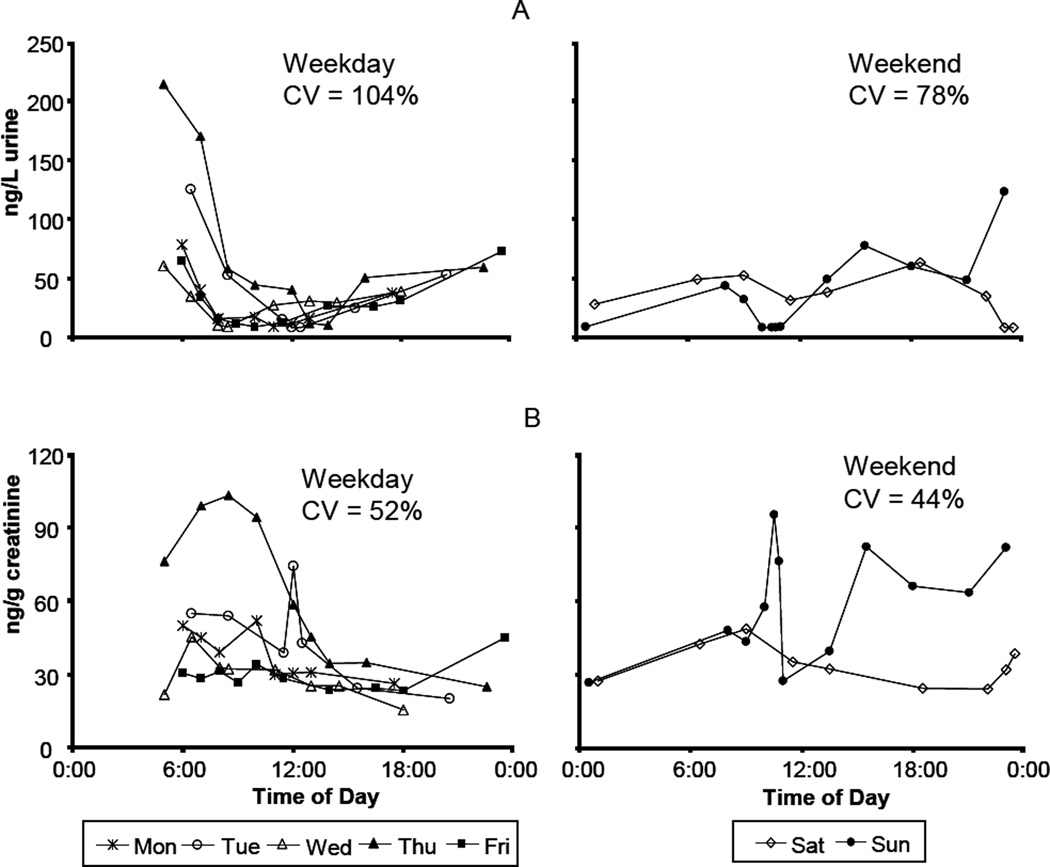
Figure 2.
Levels of 1-hydroxypyrene in Subject 5 during one week for wet weight (A) and creatinine-adjusted concentrations (B).
Figure 3.
Normalized creatinine-adjusted concentrations of 4 OH-PAH metabolites for Subject 8 during 7 days. The subject consumed barbecue chicken for lunch on Thursday.
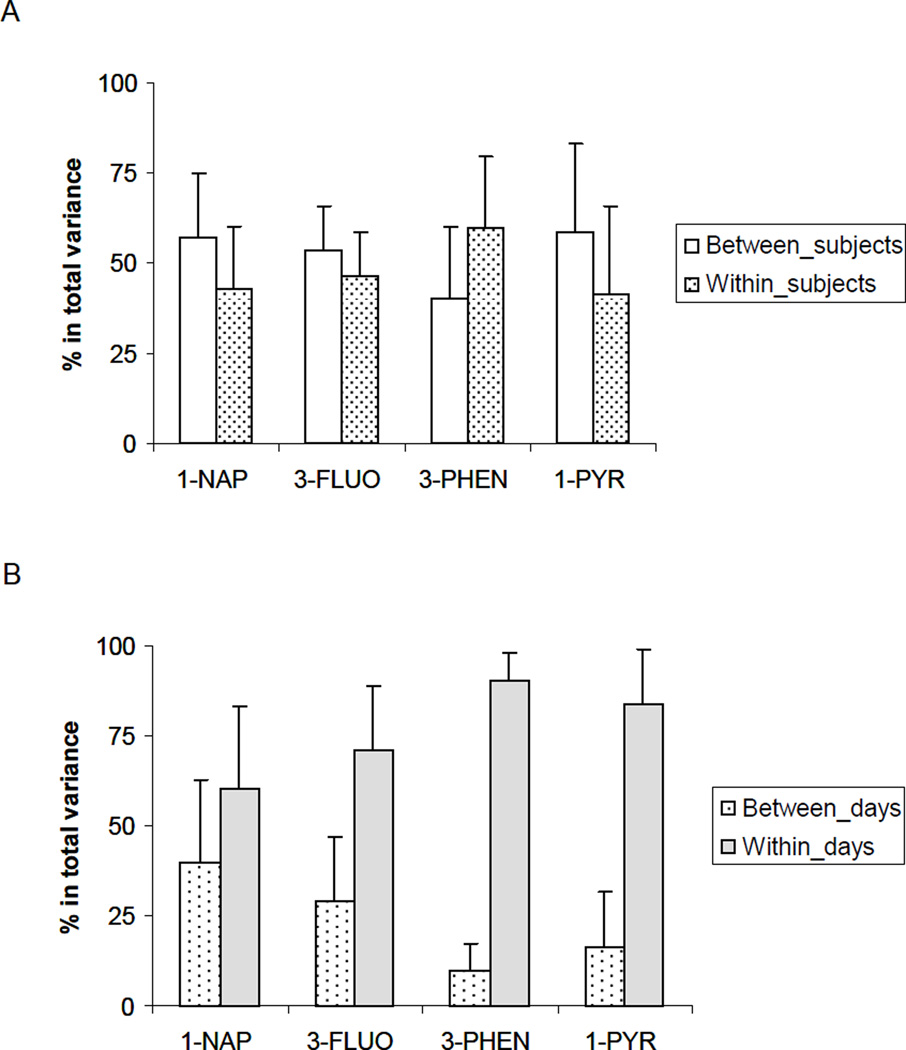
Between-subject variance contributed 49–59% to the total variance of 1-NAP, 3-FLUO, 3-PHEN, and 1-PYR (creatinine-adjusted, unless specified otherwise), and within-subject variance explained on average 41–60% for the 7 days of sampling (Figure 4A). For the 8 subjects, within-day variance for 1-NAP, 3-FLUO, 3-PHEN, and 1-PYR contributed to 60%, 71%, 90%, and 84% (average of 8 subjects) of the total variance, respectively (Figure 4B). The contributions of subject, day, and hour effect to the total variance of all samples, first-morning voids, and 24-hour voids are shown in Table 2.
Figure 4.
Contribution of between- and within-subject (A), between- and within-day (B) effects to total variance for 4 urinary OH-PAH metabolites.
Table 2.
Variance apportionment for creatinine-adjusted concentrations of 4 PAH metabolites in urine samples from 8 subjects over a period of 7 days.
| Spot samples |
1st morning voids |
24 hour voids |
||||
|---|---|---|---|---|---|---|
| Variance component |
Percent of total variance |
Variance component |
Percent of total variance |
Variance component |
Percent of total variance |
|
| 1-naphthol | ||||||
| Subject | 0.20 | 30%* | 0.27 | 33% | 0.33 | 44% |
| Day | 0.19 | 29% | 0.55 | 67% | 0.42 | 56% |
| Hour | 0.27 | 41% | - | - | - | - |
| 3-hydroxyfluorene | ||||||
| Subject | 0.14 | 44% | 0.10 | 50% | 0.14 | 67% |
| Day | 0.04 | 13% | 0.10 | 50% | 0.07 | 33% |
| Hour | 0.14 | 43% | - | - | - | - |
| 3- hydroxyphenanrene | ||||||
| Subject | 0.11 | 40% | 0.21 | 65% | 0.25 | 77% |
| Day | 0.01 | 4% | 0.12 | 35% | 0.07 | 23% |
| Hour | 0.16 | 56% | - | - | - | - |
| 1-hydroxypyrene | ||||||
| Subject | 0.26 | 55% | 0.13 | 60% | 0.13 | 76% |
| Day | 0.03 | 7% | 0.07 | 40% | 0.04 | 24% |
| Hour | 0.18 | 38% | - | - | - | - |
Numbers in italic are intraclass correlation coefficients (ICC), defined as the ratio of between-subject variance to total variance.
The number of subjects needed per group to detect a difference of 10%, 25%, 50% and 100% in urinary 1-PYR concentration is given in Table 3. Sample size calculations were performed to reflect three sampling methods: (i) spot sample collected anytime during the day; (ii) first-morning void; and (iii) 24-hour void. Separate estimates of sample size are given for one through four repeated samples from each subject.
Table 3.
Estimated number of samples needed to detect a difference of 10, 25, 50, and 100% in the 1-hydroxypyrene geometric mean concentration with a statistical power of 80% (p<0.05), for single and repeated sampling for spot, first-morning, and 24-hour void sampling.
| Target % difference on GM |
Number of repeated samples |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spot samples |
First morning voids |
24-Hour voids |
||||||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| Wet weight concentration (ng/L urine) | ||||||||||||
| 10% | 1069 | 624 | 475 | 401 | 618 | 420 | 353 | 320 | 296 | 230 | 208 | 197 |
| 25% | 195 | 114 | 87 | 73 | 113 | 77 | 64 | 58 | 54 | 42 | 38 | 36 |
| 50% | 59 | 34 | 26 | 22 | 34 | 23 | 20 | 18 | 16 | 13 | 11 | 11 |
| 100% | 20 | 12 | 9 | 8 | 12 | 8 | 7 | 6 | 6 | 4 | 4 | 4 |
| Creatinine-adjusted concentration (ng/g creatinine) | ||||||||||||
| 10% | 606 | 470 | 424 | 401 | 415 | 332 | 305 | 291 | 399 | 351 | 335 | 327 |
| 25% | 111 | 86 | 77 | 73 | 76 | 61 | 56 | 53 | 73 | 64 | 61 | 60 |
| 50% | 33 | 26 | 23 | 22 | 23 | 18 | 17 | 16 | 22 | 19 | 19 | 18 |
| 100% | 11 | 9 | 8 | 8 | 8 | 6 | 6 | 5 | 8 | 7 | 6 | 6 |
Discussion
Concentrations and profiles
Overall, concentrations found in this adult population with no occupational PAH exposure were lower than the reference levels established for the U.S. general adult population (Table 1). Wet-weight concentrations in first-morning voids were significantly higher than in all samples and simulated 24-hour voids. The differences diminished after creatinine adjustment, indicating that the higher wet-weight concentrations in the first-morning voids were caused by the more concentrated urine formed overnight and that creatinine adjustment is an effective normalization method.
Within each subject, concentrations of the urinary OH-PAH metabolites varied throughout the day (Figures 1 and 2). An apparent diurnal pattern (i.e., higher concentration in the early and late hours of the day and lower concentration during the middle of the day) of wet-weight concentrations was observed in one subject during the weekdays but not during weekends (Figure 2). Creatinine normalization effectively removed or reduced this daily pattern, suggesting that urine dilution related to variation in water consumption throughout the day was the likely explanation for the higher wet-weight concentrations at the beginning and the end of each day.
In the current study, we found that certain metabolites had distinct concentration profiles over time, compared to other metabolites from the same individual. For example, 1-NAP had an elevated peak in S8 starting on Tuesday afternoon, whereas all other analytes, including 1-PYR, stayed at background levels during the same time period (Figure 3). This subject exercised at a fitness center freshly sprayed with a cleaner or pesticide, which could be the most likely source for the elevated 1-NAP concentration shortly after the workout, since 1-NAP has been reported as a metabolite of carbaryl pesticide (Maroni et al. 2000). Similar large increases of only one or two metabolites occurred in other subjects (data not shown), but they could not be explained by the information collected. The unique concentration profile specific to only one or two OH-PAHs demonstrated the individuality of each metabolite, the importance of a multi-analyte assay for exposure assessment, and the potential of using individual biomarkers as indicators of specific source-related exposure scenarios.
One subject (S8) consumed barbecued chicken during the study period. As a result, concentrations of all 9 metabolites increased by 22–104 folds within hours of the exposure. This exposure overwhelmed other potential sources during the study period, c.f. Figure 3. Therefore, data from the two days affected by the exposure were excluded from the variability analysis for this subject. Similar observation has been noted previously that dietary PAH intake can significantly affect the inter-individual variability of the baseline excretion of urinary 1-PYR in non-smokers (Van Rooij et al, 1994). We would further strongly recommend that in an exposure assessment study investigating non-dietary sources, consumption of smoked, grilled or barbecued food should be avoided or be used as an exclusion criteria to prevent biasing the data.
Variability analysis
We found that levels of the PAH biomarkers spanned three orders of magnitude during a course of one week (Table S-1). Within each of the 7 subjects (not including S8), the CVs of wet-weight 1-PYR were 48–96%, 30–99% and 21–44% in all samples, first-morning voids and 24-hour voids, respectively. The within-subject variability in the current study was comparable to results from a study on first morning voids from 30 children (21–109%, 6 samples/person) [Siwinska et al. 1998;] and another study on 24-hour voids from 4 workers (14–41%, 4 samples/person) [Grimmer et al., 1993]. Of all 9 OH-PAH concentrations, the within-subject CVs were 45–270% in all samples, 26–153% in first-morning voids, and 13–140% in 24-hour voids (Table S-2), after exclusion of S8 because of a single high dietary exposure. Creatinine adjustment reduced the variability by 35% and 29% in all samples and first-morning voids, respectively, indicating that creatinine adjustment corrects for differences in hydration status and reduces variability. Viau et al. (2004) studied creatinine adjustment in individual urine samples collected over 24-hour or longer periods from 50 subjects. They found that creatinine excretion parallels that of 1-PYR, creatinine normalization had a smoothing effect on 1-PYR excretion profiles, and was valid and necessary for biomonitoring of exposure to PAHs. Therefore, creatinine-corrected concentrations were used for all further variance and sample size calculations for these two sampling methods. Creatinine adjustment did not affect the variability of 24-hour void concentrations, which is consistent with the premise that humans excrete creatinine at a relatively consistent rate—approximately 2% of body creatine is converted to creatinine every 24 hours (Barr et al. 2005). Therefore, creatinine adjustment is not necessary for 24-hour voids, but is essential for spot samples and first-morning voids.
For individual subjects, within-day variance exceeded the between-day variance, contributing 72–89% to total variance (Figure 4B), which could be caused by changes in daily routine, diet and metabolic pattern. This finding illustrates the need to control for known exposures when analyzing the association between a particular exposure and biomarker-level. Moreover, it illustrates the difficulty in attributing small changes in biomarker-level to a single exposure. In general, between-subject variance outweighed the between-day variance for 3-FLUO, 3-PHEN, and 1-PYR, whereas for urinary 1-NAP concentrations, the day effect contributed equally or more toward the total variance than did the subject effect (Table 2). For spot samples, 38–56% of the total variances among the 4 metabolites could not be explained by either the subject or the day factor; these variances could be attributed to the time-of-day effect—a finding that re-emphasizes the understanding of effect from time of the day during epidemiological study design.
ICC value, calculated as the ratio of between-subject variance to the total variance, had a range of 0.30–0.55 for spot samples, 0.33–0.65 for first-morning voids, and 0.44–0.77 for 24-hour voids. The lower ICC values found in spot samples indicated higher within-subject variability and lower reproducibility from repeated urine collections from the same individual. On the other hand, the 24-hour void was proven to be the most reproducible sampling technique, with markedly higher ICC values up to 0.77. As a comparison, the ICC values were 0.20–0.57 in first-morning voids during an 8-consecutive-day study of urinary metabolites of phthalates, another group of non-persistent chemicals with similar half-lives (Fromme et al. 2007). In another study of urinary phthalate metabolites in spot urine samples taken over a 3-month period, the ICCs ranged from 0.28 to 0.52 (Hauser et al. 2004). The observed ICCs of urinary OH-PAH levels in our study were very similar to those reported on metabolites from other chemical classes with short half-lives, and there was a considerable degree of temporal reproducibility for these biomarkers over the study period of a week.
Sample size recommendation
In any cross-sectional or longitudinal epidemiological studies, investigators need to know in advance the approximate number of subjects required to achieve a desired statistical power. We found only one publication that reported sample size recommendations for urinary OH-PAH biomonitoring. Siwinska et al. (1998) calculated that the minimum number of subjects for assessing exposure of a child population to environmental PAHs was 164 based on variability of urinary 1-PYR concentrations in 6 consecutive first-morning voids from 30 children. The method used in that estimation, however, was based on the assumption that the data were independent, an assumption that could be problematic because urinary biomarker concentrations are correlated. The ICCs for 1-PYR in our study were 0.55, 0.60, and 0.76, for spot samples, first morning-voids, and 24-hour voids, respectively, indicating considerable agreement between repeated measurements. Using the variability and correlation findings from our study, we calculated sample size requirements for urinary 1-PYR, with one to four repeated samples, simulating the three different sampling methods. See Table 3 for more information.
As expected, the number of subjects needed per group is the highest for spot samples, followed by first-morning voids and 24-hour voids, because of the higher variability in the spot samples. Repeated sampling from the same subjects can reduce the sample size requirements, and the lower correlation and reproducibility in spot samples makes this approach benefit more from repeated sampling. For example, taking an additional sample per person reduces the sample size by 23%, 20%, and 13% for spot samples, first-morning, and 24-hour voids, respectively. The sample size requirement can be further reduced by collecting additional repeated samples from each person; however, the benefit lessens with each additional sample, especially if 3 or more samples are taken per person. As expected, the sample size requirements when using wet-weight concentrations are significantly higher than for creatinine-adjusted concentrations in spot urine samples and first-morning voids. The greater sample size requirement for wet-weight concentrations can be explained by higher variability of wet-weight concentrations in these two sampling methods. For 24-hour voids, however, the sample size requirement is lower when using wet-weight concentrations, a finding that is consistent with the previous conclusion that creatinine adjustment is not needed for 24-hour voids.
To detect a difference of 10% in geometric mean with a statistical power of 80%, a total of 606 spot urine samples (creatinine adjusted) will be needed per group to reach the desired statistical difference, while 296 24-hour void samples (unadjusted) would be sufficient to reach the same statistical power. Hence, it is of utmost importance that these considerations be taken into account when one is planning an epidemiological study, since an investigator will need to balance the additional labor of collecting 24-hour void samples with the costs of recruiting and analyzing more than twice as many samples to reach the same statistical power.
Supplementary Material
Acknowledgments
We would like to thank the study participants for their time and devotion. We also would like to thank Pam Olive and Dr. Mary Kimberly for supplying the creatinine measurements.
Abbreviations
- PAH
polycyclic aromatic hydrocarbon
- OH-PAH
mono-hydroxy polycyclic aromatic hydrocarbon
- CV
coefficient of variation
- GM
geometric mean
- ICC
intraclass correlation coefficient
- 1-PYR
1-hydroxypyrene
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- ATSDR. Toxicological Profile for Polycyclic Aromatic Hydrocarbons. Atlanta: Agency for Toxic Substances and Disease Registry; 1995. [[accessed October 1, 2008]]. Available: http://www.atsdr.cdc.gov/toxprofiles/tp69.html. [PubMed] [Google Scholar]
- Barr DB, Wilder LC, Caudill SP, Gonzales AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005;113:192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boogaard PJ, van Sittert NJ. Exposure to polycyclic aromatic hydrocarbons in petrochemical industries by measurement of urinary 1-hydroxypyrene. Occup Environ Med. 1994;51:250–258. doi: 10.1136/oem.51.4.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostrom CE, Gerde P, Hanberg A, Jernstrom B, Johansson C, Kyrklund T, et al. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ Health Perspect. 2002;110(Suppl 3):451–488. doi: 10.1289/ehp.110-1241197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzeznicki S, Jakubowski M, Czerski B. Elimination of 1-hydroxypyrene after human volunteer exposure to polycyclic aromatic hydrocarbons. Int Arch Occup Environ Health. 1997;70:257–260. doi: 10.1007/s004200050216. [DOI] [PubMed] [Google Scholar]
- Buckley TJ, Lioy PJ. An examination of the time course from human dietary exposure to polycyclic aromatic hydrocarbons to urinary elimination of 1-hydroxypyrene. Br J Ind Med. 1992;49:113–124. doi: 10.1136/oem.49.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggle PJ, Liang K, Zeger SL. Analysis of Longitudinal Data. Oxford: Oxford University Press; 1994. pp. 27–31. [Google Scholar]
- Fromme H, Bolte G, Koch HM, Angerer J, Boehmer S, Drexler H, et al. Occurrence and daily variation of phthalate metabolites in the urine of an adult population. Int J Hyg and Environ Health. 2007;210:21–33. doi: 10.1016/j.ijheh.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Grimmer G, Dettbarn G, Jacob J. Biomonitoring of polycyclic aromatic hydrocarbons in highly exposed coke plant workers by measurement of urinary phenanthrene and pyrene metabolites (phenols and dihydrodiols) Int Arch Occup Environ Health. 1993;65:189–199. doi: 10.1007/BF00381155. [DOI] [PubMed] [Google Scholar]
- Han IK, Duan X, Zhang L, Yang H, Rhoads GG, Wei F, et al. 1-Hydroxypyrene concentrations in first morning voids and 24-h composite urine: intra- and inter-individual comparisons. J Expo Sci Environ Epidemiol. 2008;18:477–485. doi: 10.1038/sj.jes.7500639. [DOI] [PubMed] [Google Scholar]
- Hansen AM, Raaschou-Nielsen O, Knudsen LE. Urinary 1-hydroxypyrene in children living in city and rural residences in Denmark. Sci Total Environ. 2005;347:98–105. doi: 10.1016/j.scitotenv.2004.12.037. [DOI] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Park S, Silva MJ, Calafat AM. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environ Health Perspect. 2004;112:1734–1740. doi: 10.1289/ehp.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC. IARC monographs on the evaluation of carcinogenic risk of chemicals to man: polycyclic aromatic compounds, part 1, chemical and environmental data, vol 32, International Agency for Research on Cancer. Lyon: IARC; 1983. [[last accessed: February 8, 2009]]. Available: http://monographs.iarc.fr/ENG/Monographs/vol32/volume32.pdf. [Google Scholar]
- IPCS World Health Organization. [[accessed October 1, 2008]];ENVIRONMENTAL HEALTH CRITERIA 155— Biomarkers and Risk Assessment: Concepts and Principles. 1993 Available: http://www.inchem.org/documents/ehc/ehc/ehc155.htm.
- Jacob J, Seidel A. Biomonitoring of polycyclic aromatic hydrocarbons in human urine. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;778:31–47. doi: 10.1016/s0378-4347(01)00467-4. [DOI] [PubMed] [Google Scholar]
- Jongeneelen FJ, Van Leeuwen FE, Oosterink S, Anzion RB, van der LF, Bos RP, et al. Ambient and biological monitoring of cokeoven workers: determinants of the internal dose of polycyclic aromatic hydrocarbons. Br J Ind Med. 1990;47:454–461. doi: 10.1136/oem.47.7.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissel JC, Curl CL, Kedan G, Lu C, Griffith W, Barr DB, Needham LL, Fenske RA. Comparison of organorphosphorus pesticide metabolite levels in single and multiple daily urine samples collected from preschool children in Washington State. J Expos Anal Environ Epidemiol. 2005;15:164–171. doi: 10.1038/sj.jea.7500384. [DOI] [PubMed] [Google Scholar]
- Levin JO, Rhen M, Sikstrom E. Occupational PAH exposure: urinary 1-hydroxypyrene levels of coke oven workers, aluminium smelter pot-room workers, road pavers, and occupationally non-exposed persons in Sweden. Sci Total Environ. 1995;163:169–177. doi: 10.1016/0048-9697(95)04488-m. [DOI] [PubMed] [Google Scholar]
- Li Z, Romanoff LC, Trinidad DA, Hussain N, Jones RS, Porter EN, et al. Measurement of urinary monohydroxy polycyclic aromatic hydrocarbons using automated liquid-liquid extraction and gas chromatography/isotope dilution high-resolution mass spectrometry. Anal Chem. 2006;78:5744–5751. doi: 10.1021/ac0606094. [DOI] [PubMed] [Google Scholar]
- Li Z, Sandau CD, Romanoff LC, Caudill SP, Sjodin A, Needham LL, et al. Concentration and profile of 22 urinary polycyclic aromatic hydrocarbon metabolites in the US population. Environ Res. 2008;107:320–331. doi: 10.1016/j.envres.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Maroni M, Colosio C, Ferioli A, Fait A. Biological monitoring of pesticide exposure: a review. Toxicology. 2000;143:5–118. doi: 10.1016/s0300-483x(99)00152-3. [DOI] [PubMed] [Google Scholar]
- McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psychol Methods. 1996;1:30–46. [Google Scholar]
- Mucha AP, Hryhorczuk D, Serdyuk A, Nakonechny J, Zvinchuk A, Erdal S, et al. Urinary 1-hydroxypyrene as a biomarker of PAH exposure in 3-year-old Ukrainian children. Environ Health Perspect. 2006;114:603–609. doi: 10.1289/ehp.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham LL, Sexton K. Assessing children’s exposure to hazardous environmental chemicals: an overview of selected research challenges and complexities. J Expos Anal Environ Epidemiol. 2000;10:611–29. doi: 10.1038/sj.jea.7500142. [DOI] [PubMed] [Google Scholar]
- Ovrebo S, Haugen A, Hemminki K, Szyfter K, Drablos PA, Skogland M. Studies of biomarkers in aluminum workers occupationally exposed to polycyclic aromatic hydrocarbons. Cancer Detect Prev. 1995;19:258–267. [PubMed] [Google Scholar]
- Scher DP, Alexander BH, Adgate JL, Eberly LE, Mandel JS, Acquavella JF, et al. Agreement of pesticide biomarkers between morning void and 24-h urine samples from farmers and their children. J Expo Sci Environ Epidemiol. 2007;17:350–357. doi: 10.1038/sj.jes.7500505. [DOI] [PubMed] [Google Scholar]
- Siwinska E, Mielzynska D, Smolik E, Bubak A, Kwapulinski J. Evaluation of intra- and interindividual variation of urinary 1-hydroxypyrene, a biomarker of exposure to polycyclic aromatic hydrocarbons. Sci Total Environ. 1998;217:175–183. doi: 10.1016/s0048-9697(98)00186-7. [DOI] [PubMed] [Google Scholar]
- U.S.EPA. [[accessed October 1, 2008]];Occupational and Residential Exposure Test Guidelines: 875.1500 Biological monitoring. 1996 Available: http://www.epa.gov/opptsfrs/publications/OPPTS_Harmonized/875_Occupational_and_Residential_Exposure_Test_Guidelines/Series/875-1500.pdf.
- Van Rooij JGM, Veeger MMS, Bodelier-Bade MM, Scheepers PTJ, Jongeneelen FJ. Smoking and dietary-intake of polycyclic aromatic-hydrocarbons as sources of interindividual variability in the base-line excretion of 1-hydroxypyrene in urine. Int Arch Occup Environ Health. 1994;66:55–65. doi: 10.1007/BF00386580. [DOI] [PubMed] [Google Scholar]
- Viau C, Carrier G, Vyskocil A, Dodd C. Urinary excretion kinetics of 1-hydroxypyrene in volunteers exposed to pyrene by the oral and dermal route. Sci Total Environ. 1995;163:179–186. doi: 10.1016/0048-9697(95)04494-l. [DOI] [PubMed] [Google Scholar]
- Viau C, Lafontaine M, Payan JP. Creatinine normalization in biological monitoring revisited: the case of 1-hydroxypyrene. Int Arch Occup Environ Health. 2004;77:177–185. doi: 10.1007/s00420-003-0495-9. [DOI] [PubMed] [Google Scholar]
- Viau C, Vyskocil A. Patterns of 1-hydroxypyrene excretion in volunteers exposed to pyrene by the dermal route. Sci Total Environ. 1995;163:187–190. doi: 10.1016/0048-9697(95)04495-m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.