Abstract
Objective
Whether there are age-related changes in slow wave activity (SWA) rise time, a marker of homeostatic sleep drive, is unknown. Additionally, although sleep medication use is highest among older adults, the quantitative electroencephalographic (EEG) profile of the most commonly prescribed sleep medication, zolpidem, in older adults is also unknown. We therefore quantified age-related and regional brain differences in sleep EEG with and without zolpidem.
Methods
Thirteen healthy young adults aged 21.9 ± 2.2 years and 12 healthy older adults aged 67.4 ± 4.2 years participated in a randomized, double-blind, within-subject study that compared placebo to 5 mg zolpidem.
Results
Older adults showed a smaller rise in SWA and zolpidem increased age-related differences in SWA rise time such that age differences were observed earlier after latency to persistent sleep. Age-related differences in EEG power differed by brain region. Older, but not young, adults showed zolpidem-dependent reductions in theta and alpha frequencies. Zolpidem decreased stage 1 in older adults and did not alter other age-related sleep architecture parameters.
Conclusions
SWA findings provide additional support for reduced homeostatic sleep drive or reduced ability to respond to sleep drive with age. Consequences of reduced power in theta and alpha frequencies in older adults remain to be elucidated.
Keywords: Hypnotic, Geriatric, Power spectrum, QEEG, Sleep medication, Sleep architecture
1. Introduction
Aging, even in healthy individuals without sleep complaints, is associated with changes in sleep electroencephalographic (EEG) parameters. Changes to sleep architecture during healthy aging include reductions in slow wave sleep (SWS) and rapid eye movement (REM) sleep, sleep efficiency (SE), and total sleep time (TST), and increases in sleep onset latency (SOL), stages 1 and 2 sleep, and wakefulness after sleep onset (WASO) [1–3]. Findings from quantitative EEG (QEEG) studies have revealed an age-related attenuation of power in EEG broad-frequency bands and decreased density of EEG waveforms thought to represent sleep-promoting processes, eg, decreases in delta/slow wave activity (SWA) [4–8], K-complexes [9–11], theta [5,6], and spindles/sigma [5,6,10–12]. Conversely, age-related increases of power in frequency bands indicating brain arousal have been reported during NREM sleep stages, eg, increases in beta and gamma activities [13,14]. All together, these age-related architectural and QEEG changes indicate a generalized “lightening” of sleep, perhaps related to a reduced homeostatic sleep drive [15] or reduced ability to respond to sleep drive [16] and a disrupted sleep phenotype which becomes the norm in older adulthood [17]. The rise in SWA in the first ~30 min of the sleep episode has been shown to be responsive to prior homeostatic sleep drive [18], yet there are no QEEG studies in which the rise in SWA has been compared for healthy young and older adults without sleep complaints. Therefore, a primary aim of the current analysis was to assess age-related differences in SWA rise time as well as regional brain differences [19].
The prevalence of nearly all sleep disorders and sleep complaints increases with advancing age, with only about half of adults remaining free of sleep complaints into older adulthood [17,20,21]. Therefore, it is not surprising that aged populations are also the most common users of sleep medications [22], including the most prescribed sleep medication zolpidem [23]. Zolpidem is a non-benzodiazepine GABA-A receptor agonist used to help treat insomnia symptoms. Recommended clinical doses for young adult males is 5–10 mg; however, it is 5 mg for women and the elderly due to clinical efficacy, slower clearance, and risk of side effects, is 5 mg for women and the elderly [24,25]. Peak plasma concentrations occur ~0.7–2.2 h post ingestion for a 5- or 10-mg dose of zolpidem [24,26,27]. Findings from placebo-controlled studies using polysomnographic (PSG) recordings for healthy young adults have shown both no changes [28–32] and improvements in sleep continuity measures (ie, increases in SE and TST, with reductions in SOL, WASO, and the number of awakenings per night) [33,34] under zolpidem with varying durations of use and doses. Reported sleep stage changes with zolpidem have been mixed for healthy young adults, but typically included increases in SWS [31,34] and decreases in REM [29,34]. Conversely, others have found a reduction in stage 2 sleep but only with a large 30-mg zolpidem dose [31]. In one previous study, effects of zolpidem on the sleep EEG of healthy older adults were examined [35]. Comparing placebo to two nights of 5, 10, 15, or 20 mg of zolpidem, this study’s findings included improvements in SOL and SE at all doses; however, no dose was found to reduce the number of nighttime awakenings. The few changes to sleep stages were an increase of percent stage 2 with 20 mg and reductions in percent REM with 10 and 20 mg.
Findings from studies examining the QEEG profile of zolpidem compared to placebo have shown mixed results. A single administration of 10 mg zolpidem in healthy young or middle-aged adults has shown decreases in SWA, theta, and low alpha powers, and increases in sigma power [29,36] or no changes to any of these QEEG bands [28,30]. Doses of 5 and 20 mg have also been shown to significantly reduce alpha but increase delta in the first 3–4 h of the night, while beta also increased but only within the first hour of sleep [37]. To date, there have been no studies on the effects of zolpidem on sleep QEEG measures in older adults. Therefore, a secondary aim of the current study was to compare effects of a single 5-mg dose of immediate-release zolpidem between healthy young and healthy older adults for changes to sleep architecture and QEEG measures of NREM sleep in the first ~2 h of a nighttime sleep episode, a time when plasma zolpidem levels are high.
2. Methods
2.1. Participants
Thirteen healthy young adults (6 females) aged 21.9 ± 2.2 years (mean ± standard deviation (SD)) and 12 healthy older adults (8 females) aged 67.4 ± 4.2 years participated. Detailed screening, demographic, and sample size information has been published [38]. Prior to study, the participants were deemed healthy based on psychological, medical, and sleep disorder screenings. Exclusion criteria included use of nicotine or illicit drugs, abnormal blood chemistries, body mass index <18.5 or >30.0, night or rotating shift work in the previous year, and travel >1 time zone in the 3 weeks prior to in-laboratory study. Screenings were conducted at the Clinical and Translational Research Center (CTRC) and the Sleep and Chronobiology Laboratory at the University of Colorado Boulder. All participants provided written informed consent, and study procedures were in accordance with the Declaration of Helsinki and approved by the University of Colorado Boulder Institutional Review Board and the Colorado Clinical & Translational Sciences Institute Scientific Advisory and Review Committee.
2.2. Pre-study controls
One week prior to each in-laboratory study visit, participants maintained habitual sleep–wakefulness schedules, which were verified with sleep diaries, call-ins at bed and wake times to a time-stamped voicemail recorder, and wrist actigraphy (Actiwatch-L, Mini Mitter Respironics, Bend, OR, USA). Participants refrained from use of caffeine, alcohol, and nonprescribed drugs for 3 days prior to each study visit. Compliance was verified with self-report, breath alcohol testing (Lifeloc Technologies Model FC10, Wheat Ridge, CO, USA), and urine toxicology for illicit drugs at the beginning of each study visit.
2.3. Study design and in-laboratory protocol
A randomized, crossover, double-blind, placebo-controlled, and within-subject study design was used in which each participant had three overnight in-laboratory study visits scheduled ~1 week apart that tested their sleep, cognition, and walking stability. The current study utilizes data collected on two of these experimental nights as the third night was a wakefulness-control condition [38]. For the current study, PSG data were examined from the two experimental visits wherein participants were administered either 5 mg zolpidem or placebo. Pill allocation and randomization sequence was performed by the CTRC pharmacist who provided pills identical in appearance containing either 5 mg immediate-release zolpidem or rice powder-filled placebo. The allocation sequence was concealed until after all participants completed the study. Pills were administered 10 min prior to lights out, which was scheduled at each participant’s habitual bedtime and they were given a 110-min sleep opportunity, after which time they were awakened for performance testing.
2.4. Polysomnography (PSG) and EEG power spectral analysis
Nighttime PSG recordings were obtained using digital sleep recorders (Siesta, Compumedics Inc., Charlotte, NC, USA) with EEG recording at brain sites F3-A2, C3-A2, C4-A1, and O1-A2, left and right electrooculograms (EOG), and left and right mentalis electromyograms (EMG). Impedances were <5 kohms. PSG data were stored and sampled at 256 Hz with a 12-bit A/D board. Sleep stages were manually scored according to standard criteria [39] from brain site C3-A2 in 30-s epochs. Sleep onset was determined in two ways, either as 1) SOL, the first epoch of three consecutive epochs (1.5 min) of any sleep stage, or as 2) latency to persistent sleep (LPS), the first epoch of 20 consecutive epochs (10 min) of any sleep stage. Sleep architecture is reported for the 110-min sleep opportunity. Epochs scored as either stages 3 or 4 were combined into SWS.
Power spectral analysis was performed on EEG data from brain sites F3-A2, C3-A2, and O1-A2 (hereafter referred to as brain regions F3, C3, and O1, respectively). Fast Fourier transform (FFT) was applied to the EEG data using a custom Matlab (MathWorks, Inc., version R2012b, Natick, MA, USA) program to calculate power using a 2-s Hanning window with no overlap, and then averaging 2-s windows within each 30-s epoch to produce estimates of power with a frequency resolution of 0.5 Hz; high- and low-pass filters of 0.75 Hz and 45.25 Hz were utilized. EEG artifacts were visually scored in 2-s epochs and removed from the EEG data prior to FFT analysis. Power spectra data are presented as the average power in each 0.5-Hz spectral frequency bin for all NREM epochs after SOL. Conditional differences in NREM EEG power spectra were calculated for each 0.5-Hz spectral frequency bin with each participant’s zolpidem power spectra expressed as a percent of their placebo power spectra. Rise time from sleep onset of EEG power in the SWA frequency range [18], defined here as 0.75–4.25 Hz, was calculated by summing the power among the 0.5-Hz bins comprising the SWA range for each epoch starting 2 min prior and ending 30 min after EEG-defined sleep onset (ie, SOL primary and LPS secondary). SWA for these epochs were then averaged across every 4 epochs to produce estimates of SWA in 2-min bins. Among the 12 young and 11 older adults included in the SWA rise time analysis from SOL, five young and 10 older adults had at least one epoch of wakefulness in the first 30 min after SOL in either or both of their placebo or zolpidem nights. Thus, as a secondary analysis, we assessed SWA rise time during continuous sleep by examining data from participants who had no wakefulness epochs in the first 30 min after LPS in both their placebo and zolpidem nights (n = 11 young and n = 6 older adults).
2.5. Data analysis
Sleep architecture data were analyzed using mixed-model analyses of variance (ANOVAs) with age group (young or older) and condition (placebo or zolpidem) as fixed factors and subject as a random factor. Repeated measures ANOVAs were used to analyze SWA rise time from sleep onset and changes in EEG power spectra. Planned comparisons were tested with independent t-tests for age group and with dependent t-tests for condition and brain region differences. Single-sample t-tests were utilized to test for zolpidem effects expressed as a percentage of placebo. Modified Bonferroni corrections were utilized to account for multiple comparisons in SWA rise time analyses [40]. Data in tables and figures are expressed as mean ± standard error of the mean (SEM). Statistics were performed with Statistica (StatSoft, Inc., version 10.0, Tulsa, OK, USA).
Placebo data from one older female were missing from sleep architecture analyses because she did not fall asleep in the 110-min sleep opportunity. Her zolpidem condition data were also excluded from QEEG analyses as she lacked the necessary placebo condition data to perform repeated measures ANOVAs. Zolpidem data from one young female were excluded from sleep architecture measures except sleep latencies because her PSG recording spontaneously terminated ~80 min into the sleep opportunity. One other young female was excluded from the QEEG analyses due to slow-frequency sweat artifact throughout her placebo night recording. Therefore, participants included in non-latency sleep architecture measures for placebo were 13 young and 11 older adults, and for zolpidem 12 young and 12 older adults, while the number of participants included in QEEG analyses was 12 young and 11 older adults.
3. Results
3.1. Sleep architecture
Sleep architecture data are presented in Table 1. Regardless of zolpidem or placebo condition, older adults showed less min and percent SWS, longer LPS, lower SE, and greater percent wakefulness, min of WASO after SOL and LPS, and number of awakenings after SOL compared to the young adults. In addition, on placebo nights, older adults showed more min and percent stage 1, longer SOL, and a greater number of awakenings after LPS compared to young adults, whereas on zolpidem nights older adults showed more min and percent stage 2 and greater durations of awakenings after SOL and LPS. Minutes and percent REM did not differ between age groups or conditions. No significant differences in sleep architecture measures were observed between placebo and zolpidem conditions for the young adult age group (all p > 0.10), whereas the min and percent of stage 1 was reduced by zolpidem compared to placebo in the older adult age group. Older adults also showed nonsignificant trends for reduced number of awakenings after SOL (p = 0.09) and LPS (p = 0.07) during zolpidem versus placebo.
Table 1.
Sleep Architecture in First 110 min of the Sleep Episode.
| Parameter | Young adults
|
Older adults
|
||
|---|---|---|---|---|
| Placebo (n = 13) | Zolpidem (n = 12) | Placebo (n = 11) | Zolpidem (n = 12) | |
| Minutes of recording time | ||||
| Stage 1 | 4.8 ± 0.5 | 5.5 ± 1.1 | 8.5 ± 1.2* | 6.5 ± 1.1† |
| Stage 2 | 48.8 ± 3.0 | 42.7 ± 2.5 | 53.3 ± 5.9 | 59.4 ± 6.1* |
| SWS | 42.2 ± 3.8 | 49.0 ± 4.8 | 18.5 ± 5.7* | 17.3 ± 6.3* |
| REM | 7.9 ± 2.3 | 5.9 ± 1.5 | 3.3 ± 1.4 | 4.0 ± 1.4 |
| Sleep Onset Latency (SOL) | 3.4 ± 0.7 | 5.5 ± 2.7 | 9.3 ± 1.2* | 10.5 ± 1.4 |
| Latency to Persistent Sleep (LPS) | 4.0 ± 0.8 | 6.4 ± 2.8 | 19.0 ± 3.9* | 13.5 ± 1.7* |
| WASO, from SOL | 3.1 ± 1.3 | 1.3 ± 0.4 | 17.5 ± 4.2* | 12.3 ± 3.9* |
| WASO, from LPS | 3.0 ± 1.3 | 1.2 ± 0.3 | 12.2 ± 3.6* | 11.4 ± 4.0* |
| Number of awakenings, from SOL | 2.5 ± 0.7 | 2.1 ± 0.6 | 6.1 ± 1.0* | 4.4 ± 0.7* |
| Duration of awakenings, from SOL (min) | 1.9 ± 1.3 | 0.6 ± 0.1 | 3.0 ± 0.6 | 2.2 ± 0.6* |
| Number of awakenings, from LPS | 2.3 ± 0.6 | 1.9 ± 0.5 | 4.6 ± 0.9* | 3.4 ± 0.6 |
| Duration of awakenings, from LPS (min) | 1.9 ± 1.3 | 0.6 ± 0.1 | 2.4 ± 0.6 | 2.8 ± 0.8* |
| Percent of recording time (%) | ||||
| Stage 1 | 4.4 ± 0.5 | 5.0 ± 1.0 | 7.7 ± 1.1* | 5.9 ± 1.0† |
| Stage 2 | 44.3 ± 2.7 | 38.8 ± 2.3 | 48.4 ± 5.4 | 54.1 ± 5.5* |
| SWS | 38.4 ± 3.5 | 44.6 ± 4.4 | 16.9 ± 5.2* | 15.8 ± 5.8* |
| REM | 7.2 ± 2.0 | 5.3 ± 1.4 | 3.0 ± 1.3 | 3.6 ± 1.3 |
| Wakefulness | 5.7 ± 1.1 | 6.3 ± 2.7 | 24.0 ± 4.3* | 20.6 ± 4.2* |
| Sleep efficiency | 94.3 ± 1.1 | 93.7 ± 2.7 | 76.0 ± 4.3* | 79.4 ± 4.2* |
Sleep architecture for young and older adults in placebo and zolpidem conditions for the 110-min sleep opportunity. Data are mean ± standard error of the mean (SEM). N = 13 for SOL and LPS parameters for young adult zolpidem condition (see text for missing data).
p < 0.05 for differences between condition within age group.
p < 0.05 for differences between age groups within condition.
3.2. Age, brain region, and drug condition-related differences in SWA rise time
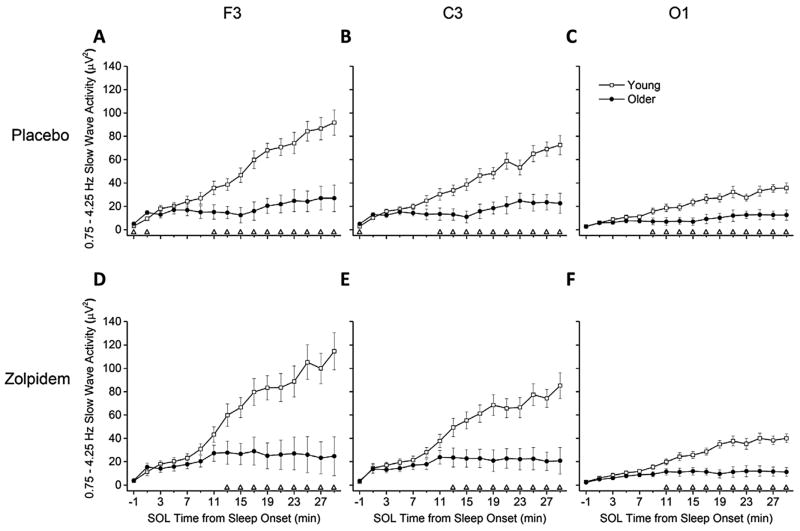
The rise in SWA after SOL is shown in Fig. 1. Both age groups showed significant increases in SWA for all brain regions in both conditions (all p < 0.05, main effects of time) with higher SWA in young adults. Compared to SWA of older adults, individual time bins for young adults that exhibited sustained higher levels of SWA were observed beginning at 9 min for O1 and 11 min for F3 and C3 after SOL under placebo and beginning at 11 min for O1 and 13 min for F3 and C3 after SOL under zolpidem. The rise time for SWA was greater in F3 and C3 compared to O1 for all age group–condition combinations for almost all time bins examined (Supplementary Fig. S1 in the online version at doi:10.1016/j.sleep.2014.05.007). SWA was greater in F3 than C3 for a few time bins mostly in the second half of the 30-min analysis episode. In addition, under the placebo condition, older adults showed more SWA in the time bin prior to SOL in F3 and C3 and immediately after SOL in F3.
Fig. 1.
SWA Rise Time from SOL between Age Groups – Absolute SWA Power. Data presented are absolute slow wave activity (SWA) power in 2-min time bins, lasting from the 2 min preceding until the 30 min after sleep onset latency (SOL). Values are expressed as mean ± standard error of the mean (SEM) for young and older age groups, and presented separately for placebo and zolpidem condition and F3, C3, and O1 brain region combinations. Data are plotted at the centers of the corresponding 2-min time bins (e.g., data for time bin 12–14 min after SOL are plotted at 13 min). Open triangles (△) above abscissa denote significant differences between young and older age groups at corresponding time bins, calculated with independent t-tests and using the modified Bonferroni correction (p < 0.04688). Young n = 12, older n = 11. Condition-brain region combinations shown are (A) Placebo-F3, (B) Placebo-C3, (C) Placebo-O1, (D) Zolpidem-F3, (E) Zolpidem-C3, and (F) Zolpidem-O1.
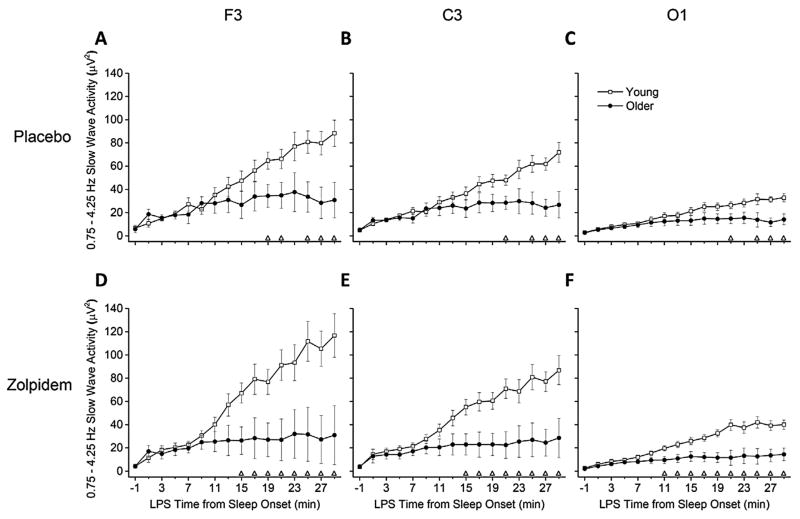
Figure 2 shows SWA rise time for participants with continuous sleep in the first 30 min after LPS. Similar to Fig. 1, both age groups showed significant increases in SWA for all brain regions in both conditions (all p < 0.05, main effects of time). However, individual time bins that showed significant differences between age groups occurred later in the sleep episode under placebo. Specifically, compared to older adults, SWA levels under placebo were higher for young adults beginning at 19 min for F3 and 21 min for C3 and O1 after LPS, whereas under zolpidem, young adults showed higher SWA levels beginning at least 4 min earlier (11 min for O1 and 15 min for F3 and C3, after LPS) compared to older adults. Rise time brain region differences from LPS (Supplementary Fig. S2 in the online version at doi:10.1016/j.sleep.2014.05.007) yielded similar results for both conditions as seen in the SOL analysis (Supplementary Fig. S1 in the online version at doi:10.1016/j.sleep.2014.05.007) for young adults; however, fewer brain region differences were observed for older adults in both conditions in the LPS analysis. Few differences in SWA rise time after SOL or LPS between zolpidem and placebo conditions were observed within age groups (Supplementary Figs S3 and S4 in the online version at doi:10.1016/j.sleep.2014.05.007).
Fig. 2.
SWA Rise Time from LPS between Age Groups – Absolute SWA Power. Data presented are absolute slow wave activity (SWA) power in 2-min time bins, lasting from the 2 min preceding until the 30 min after latency to persistent sleep (LPS). Plot details are same as Fig. 1. Young n = 11, older n = 6. Condition-brain region combinations shown are (A) Placebo-F3, (B) Placebo-C3, (C) Placebo-O1, (D) Zolpidem-F3, (E) Zolpidem-C3, and (F) Zolpidem-O1.
3.3. Age, brain region, and drug condition-related differences in NREM sleep QEEG power spectra during the first 110 min of the sleep episode
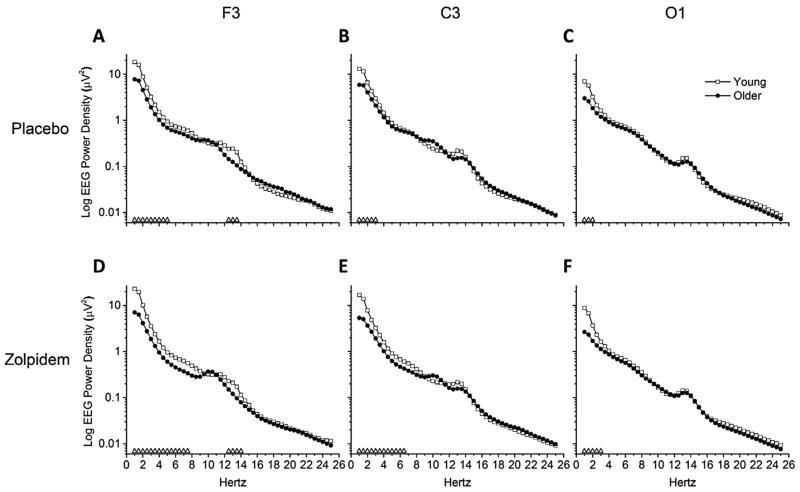
Figure 3 shows NREM sleep EEG power spectra for individual half-Hertz bins between 1 and 25 Hz for age group, drug condition, and brain region. Regardless of drug condition, young adults showed significantly higher power in the delta frequency ranges in all brain regions compared to older adults. Young adults also showed higher power in the theta and sigma frequency range in brain region F3 compared to older adults under placebo. In the zolpidem condition, young adults showed higher power in the theta frequency range than older adults for F3 and C3 brain regions. No age group differences were seen for EEG power in the alpha or beta frequency ranges.
Fig. 3.
NREM EEG Power Spectra between Age Groups – Absolute Power. Data presented are absolute EEG power spectra for average of NREM sleep epochs occurring after sleep onset latency (SOL) in the 110-min sleep opportunity. Power spectra were calculated in half-Hertz frequency bins between 0.75 and 25.25 Hz and are plotted at the centers of the corresponding half-Hertz bins. Mean values are expressed for young and older age groups, and presented on log scales separately for placebo and zolpidem conditions and F3, C3, and O1 brain region combinations. Open triangles (△) above abscissa denote significant differences between young and older age groups at corresponding frequency bins, calculated with independent t-tests and alpha level of p < 0.05. Young n = 12, older n = 11. Condition-brain region combinations shown are (A) Placebo-F3, (B) Placebo-C3, (C) Placebo-O1, (D) Zolpidem-F3, (E) Zolpidem-C3, and (F) Zolpidem-O1.
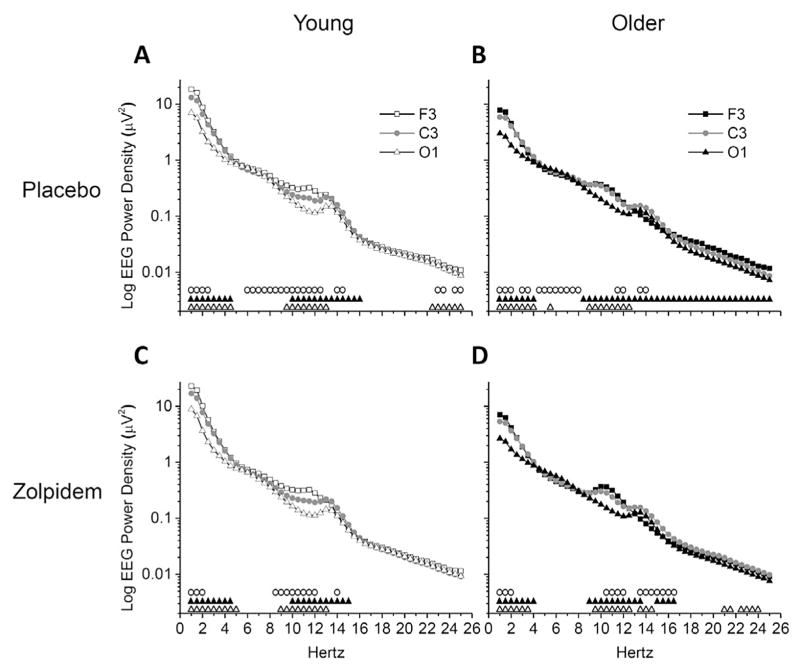
Regional differences in NREM sleep EEG power spectra are shown in Fig. 4. For young adults, observed differences between brain regions were of the order F3 > C3 > O1. With placebo (Fig. 4A), young adults showed greater power in F3 versus C3 for frequency bins 1.0–2.5, 6.0–12.5, 14.0–14.5, 23.0–23.5, and 24.5–25.0 Hz; greater power in C3 versus O1 for frequency bins 1.0–4.5 and 10.0–16.0 Hz; and greater power in F3 versus O1 for frequency bins 1.0–4.5, 9.5–13.0, and 22.5–25.0 Hz. With zolpidem (Fig. 4C), young adults showed greater power in F3 versus C3 for frequency bins 1.0–2.0, 8.5–12.0, and 14.0 Hz; greater power in C3 versus O1 for frequency bins 1.0–4.5 and 10.0–15.0 Hz; and greater power in F3 versus O1 for frequency bins 1.0–5.0 and 9.0–13.0 Hz. However, unlike young adults, brain region differences for older adults were not always of the order F3 > C3 > O1. With placebo (Fig. 4B), older adults showed greater power in F3 versus C3 for frequency bins 1.0–2.0 and 11.5–12.0 Hz, but greater power in C3 versus F3 for frequency bins 3.0–3.5, 4.5–8.0, and 13.5–14.0 Hz. Differences between C3 and O1 were all C3 > O1 and were observed for frequency bins 1.0–4.0 and 8.5–25.0 Hz. Greater power was observed in F3 versus O1 for frequency bins 1.0–4.0 and 9.0–12.5, while O1 was greater than F3 for 5.5 Hz only. Under zolpidem (Fig. 4D), older adults showed greater power in F3 versus C3 for frequency bins 1.0–2.0 and 10.5–12.0 Hz, but greater power in C3 versus F3 for frequency bins 13.5–16.5 Hz. Like placebo, differences between C3 and O1 with zolpidem were all C3 > O1 but were observed for frequency bins 1.0–4.0, 9.0–13.5, and 15.0–16.5 Hz. In addition, like placebo, almost all differences observed between F3 and O1 with zolpidem were F3 > O1 (frequency bins 1.0–3.5, 9.5–12.5, 21.0–21.5, and 22.5–24.0 Hz), while few frequency bins were O1 > F3 (13.5–14.5 Hz).
Fig. 4.
NREM EEG Power Spectra between Brain Regions – Absolute Power. Data presented are absolute EEG power spectra for average of NREM sleep epochs occurring after sleep onset latency (SOL) in the 110-min sleep opportunity. Power spectra were calculated in half-Hertz frequency bins between 0.75 and 25.25 Hz and are plotted at the centers of the corresponding half-Hertz bins. Mean values are expressed for F3, C3, and O1 brain regions, and presented on log scales separately for placebo and zolpidem condition and young and older age group combinations. Symbols above abscissa denote significant differences comparing between brain region pairs (open circles (○) = F3 vs. C3, black triangles (▲) = C3 vs. O1, open triangles (△) = F3 vs. O1) at corresponding frequency bins, calculated with dependent t-tests and alpha level of p < 0.05. Young n = 12, older n = 11. Condition-age group combinations shown are (A) Placebo-Young, (B) Placebo-Older, (C) Zolpidem-Young, and (D) Zolpidem-Older.
NREM sleep power spectra between conditions expressed as a percent of each participant’s placebo power spectra are shown in Fig. 5. No zolpidem versus placebo differences were observed in any spectral frequencies for any brain region in young adults, whereas zolpidem reduced power in theta and alpha frequencies for all brain regions in older adults.
Fig. 5.
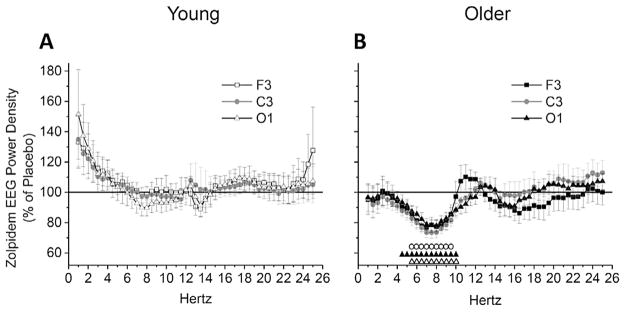
NREM EEG Power Spectra between Conditions – Percent of Placebo. Data presented are zolpidem condition NREM EEG power spectra expressed as a percent of placebo condition for average of NREM sleep epochs occurring after sleep onset latency (SOL) in the 110-min sleep opportunity. Power spectra were calculated in half-Hertz frequency bins between 0.75 and 25.25 Hz and are plotted at the centers of the corresponding half-Hertz bins. Values are expressed as mean ± standard error of the mean (SEM) for F3, C3, and O1 brain regions, and presented separately for young (A) and older (B) adult age groups. Symbols above abscissa denote significant differences between placebo (horizontal 100% line) and zolpidem conditions for individual brain regions (open circles (○) = F3, black triangles (▲) = C3, open triangles (△) = O1) at corresponding frequency bins, calculated with single-sample t-tests and alpha level of p < 0.05. Young n = 12, older n = 11.
4. Discussion
The rise of SWA after sleep onset was smaller in older than in younger adults, and administration of 5 mg zolpidem increased age-related differences in SWA rise time such that age differences were observed earlier after the latency to persistent sleep. Age-related differences in EEG power spectra also differed by brain region within condition, with young adults showing a general frontal predominance in power including frequencies across the range examined, followed by intermediate power in the central and lowest power in the occipital region, whereas older adults showed the highest power in the frontal or central region. Under placebo, young adults exhibited higher power than older adults in delta frequencies for all brain regions and higher sigma frequencies for the frontal brain region, whereas under 5 mg zolpidem, young adults also showed higher power in theta frequencies for frontal and central regions. Older, but not young, adults showed zolpidem-dependent power reductions in theta and alpha frequencies for all brain regions examined. Most sleep architecture parameters showed age-related differences for both conditions. Zolpidem did not alter sleep architecture parameters for young adults and only decreased stage 1 in older adults during the first 110 min of the sleep episode, when concentrations of zolpidem are high.
Sleep architecture in the first 110 min after lights out was not significantly altered by 5 mg zolpidem, except for a decrease in stage 1 for the older adults only. Although findings from two studies in healthy young adults indicated improvements in sleep initiation and continuity measures [33,34], the current study’s findings are consistent with findings from most other placebo-controlled studies in healthy adults that showed no improvements in sleep initiation and continuity measures with administration of zolpidem [28–32], likely reflective of a ceiling effect. Also consistent with prior findings is the lack of sleep stage changes with zolpidem, with the exception of decreased stage 1 for older adults in the current study. In older adults, SE in the 110-min recording was ~76%; thus, there was room for improvement. Consistent with age group norms for healthy sleepers [1–3], we observed age differences for sleep architecture, except minutes and percent REM for one or both conditions. These findings demonstrate that differences between the sleep of healthy young versus healthy older adults persist even after one night intervention with 5 mg zolpidem.
The rise in EEG SWA levels after sleep onset is a marker of neural synchrony that occurs due to the slowed rate of cortical firing as sleep deepens [41]. This has been previously demonstrated by the higher levels and quicker accumulation of SWA immediately following sleep onset after sleep deprivation [18]. Thus, the SWA rise time analysis in this study served as a useful tool for detecting effects of zolpidem and aging on neural synchrony. When timed from SOL and including all participants regardless of sleep continuity, SWA levels were greater for young versus older adults for time bins beginning 9–13 min after sleep onset for the three brain regions examined under both placebo and zolpidem conditions. When beginning the analysis at LPS and removing participants who awakened during the first 30 min, we found that the overall pattern of SWA rise time to be similar to that for SOL, but that the time at which age differences were first observed occurred later in the sleep episode, especially under placebo conditions. These findings suggest that neural synchrony at sleep onset declines with age and that zolpidem had a small influence on sleep EEG in the healthy young and older adults studied. Further research is needed to determine the effects of sleep medications on SWA rise time in young and older patients with insomnia.
Under placebo, NREM EEG power spectra exhibited the typical age-related power reductions in sleep EEG frequencies (ie, delta, theta, and sigma) [4–8]. Lower absolute power in the delta frequency range was ubiquitous across brain regions and conditions for older versus young adults, and EEG power in the sigma frequency range was lower under both conditions, but only in the frontal brain region. Under zolpidem, EEG power levels in the theta frequency range showed age-dependent declines in the frontal and central brain regions. These regional difference findings are consistent with findings from others who have shown age-related changes in slow-frequency EEG activity in anterior versus posterior brain regions [19,42,43]. However, unlike prior findings [13,14], we did not observe age-related differences in fast EEG frequencies during NREM sleep.
When NREM EEG power spectra were expressed as a percent of placebo, 5 mg zolpidem significantly reduced power in theta and alpha frequencies for older, but not young, adults in the brain regions examined. Though findings have been mixed, in patients with in-somnia [44], under sleep deprivation conditions [45], and in healthy young and middle-aged adults [29,36], the dominant observed effects of zolpidem on the sleep QEEG are reductions in theta and alpha powers and increases in sigma power. In the current study, findings indicate that young adults did not show any of these common QEEG changes with zolpidem, while older adults did show the power reductions in theta and alpha frequencies but not the increased power in sigma frequencies.
A number of factors may have contributed to our findings. Unlike previous studies, we administered a smaller dose of zolpidem (5 mg) that is recommended for older adults and young women. This dose may not have been sufficient in the young group composed of men and women to produce the common QEEG profile seen with doses 10 mg or higher. In addition, theta and alpha powers are dominant during lighter NREM sleep stages and quiet wakefulness; states that are increased during the night in older individuals and showed age-related increases in the current study. Although we did measure sleep when zolpidem levels are highest, we cannot rule out a delayed effect in young adults nor can we rule out an effect on REM sleep, as an analysis of REM sleep EEG was not possible given the small number of subjects with REM sleep at the time of sleep episode examined. A possible mechanism underlying age-related differences in the effects of zolpidem on NREM sleep EEG could be age-related anatomical changes in GABA-A receptors. Specifically, aged animals are reported to show increased number of GABA-A receptor α1 subunits [46]. Benzodiazepine and non-benzodiazepine medications act by binding to the GABA-A receptor’s α1 subunits which, when activated, help to open the receptor’s chloride channel, leading to hyperpolarization. Additionally, it is known that liver metabolism and renal excretion of drugs is reduced by aging [47,48], and therefore age-related differences in zolpidem effects on sleep EEG could be related to the slower drug clearance and increased plasma concentrations after administration of the same 5-mg dose used in the current study [24]. As designed, we only examined effects of zolpidem on NREM sleep EEG when plasma levels are reported to be high. Effects of zolpidem on the QEEG in healthy older adults later in the night require follow-up studies. Frey et al. (2011) [38] found that participants in the current study had walking instability and impaired cognition upon awakening at 2 h after pill administration following zolpidem compared to placebo and a wakefulness-control condition. The current findings demonstrate that zolpidem also had significant effects on brain activity during NREM sleep in these healthy older adults prior to performance testing. We examined the effects of zolpidem in healthy young and older adults without sleep complaint. Although healthy adults do not routinely take sleep medications, sleep medication use in healthy adults does occur (eg, during stress and jet travel).
Findings from the current study may have been limited by a number of other factors. We only gave one night of the drug; thus, chronic effects cannot be determined. The cross-sectional nature of the design did not test for age-related changes within individuals. Though 5 mg is the recommended zolpidem dose for young females and all older adults, 10 mg remains a recommended dose for young males; therefore, follow-up studies could test dose-dependent age-related QEEG effects of zolpidem. Newer preparations of zolpidem include extended-release versions that could also be tested for similar EEG effects.
Taken together, our findings add to the knowledge about age-related sleep EEG changes, including age-related reductions in the rise of SWA with and without zolpidem, regional brain differences in SWA rise in young versus older adults, and the QEEG profile of zolpidem administration in healthy older adults. Findings provide further evidence for reduced homeostatic sleep pressure [5,6,15] or the ability to respond to such pressure [16] in healthy older adults, with reductions in SWA across all brain regions examined. A recommended dose of zolpidem administered 10 min prior to bedtime does not appear to substantially alter EEG SWA immediately following sleep onset or sleep architecture for healthy young or older adults, but does lead to age-dependent differences in the spectral profile of NREM EEG in the first ~2 h of the night. Future research is needed to determine if such reductions in QEEG theta and alpha powers in older adults are persistent and, if so, to elucidate the possible implications of such changes.
Supplementary Material
Acknowledgments
Research was supported by the following grants: NIH R03 AG024621, NIH T32 AG000279, NIH T32 AG15332, NIH R01 HL109706, and Colorado Clinical and Translational Sciences Institute Grant UL1 TR000154 from NIH/National Center for Advancing Translational Sciences.
Appendix: Supplementary material
Supplementary data to this article can be found online at doi:10.1016/j.sleep.2014.05.007.
Footnotes
Conflict of interest
The ICMJE Uniform Disclosure Form for Potential Conflicts of Interest associated with this article can be viewed by clicking on the following link: http://dx.doi.org/10.1016/j.sleep.2014.05.007.
References
- 1.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 2.Redline S, Kirchner HL, Quan SF, Gottlieb DJ, Kapur V, Newman A. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch Intern Med. 2004;164:406–18. doi: 10.1001/archinte.164.4.406. [DOI] [PubMed] [Google Scholar]
- 3.Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA. 2000;284:861–8. doi: 10.1001/jama.284.7.861. [DOI] [PubMed] [Google Scholar]
- 4.Carrier J, Viens I, Poirier G, et al. Sleep slow wave changes during the middle years of life. Eur J Neurosci. 2011;33:758–66. doi: 10.1111/j.1460-9568.2010.07543.x. [DOI] [PubMed] [Google Scholar]
- 5.Dijk DJ, Beersma DG, van den Hoofdakker RH. All night spectral analysis of EEG sleep in young adult and middle-aged male subjects. Neurobiol Aging. 1989;10:677–82. doi: 10.1016/0197-4580(89)90004-3. [DOI] [PubMed] [Google Scholar]
- 6.Landolt HP, Dijk DJ, Achermann P, Borbely AA. Effect of age on the sleep EEG: slow-wave activity and spindle frequency activity in young and middle-aged men. Brain Res. 1996;738:205–12. doi: 10.1016/s0006-8993(96)00770-6. [DOI] [PubMed] [Google Scholar]
- 7.Mander BA, Rao V, Lu B, et al. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat Neurosci. 2013;16:357–64. doi: 10.1038/nn.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mourtazaev MS, Kemp B, Zwinderman AH, Kamphuisen HA. Age and gender affect different characteristics of slow waves in the sleep EEG. Sleep. 1995;18:557–64. doi: 10.1093/sleep/18.7.557. [DOI] [PubMed] [Google Scholar]
- 9.Colrain IM, Crowley KE, Nicholas CL, et al. Sleep evoked delta frequency responses show a linear decline in amplitude across the adult lifespan. Neurobiol Aging. 2010;31:874–83. doi: 10.1016/j.neurobiolaging.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crowley K, Trinder J, Kim Y, Carrington M, Colrain IM. The effects of normal aging on sleep spindle and K-complex production. Clin Neurophysiol. 2002;113:1615–22. doi: 10.1016/s1388-2457(02)00237-7. [DOI] [PubMed] [Google Scholar]
- 11.Wauquier A. Aging and changes in phasic events during sleep. Physiol Behav. 1993;54:803–6. doi: 10.1016/0031-9384(93)90095-w. [DOI] [PubMed] [Google Scholar]
- 12.Wei HG, Riel E, Czeisler CA, Dijk DJ. Attenuated amplitude of circadian and sleep-dependent modulation of electroencephalographic sleep spindle characteristics in elderly human subjects. Neurosci Lett. 1999;260:29–32. doi: 10.1016/s0304-3940(98)00851-9. [DOI] [PubMed] [Google Scholar]
- 13.Carrier J, Land S, Buysse DJ, Kupfer DJ, Monk TH. The effects of age and gender on sleep EEG power spectral density in the middle years of life (ages 20–60 years old) Psychophysiology. 2001;38:232–42. [PubMed] [Google Scholar]
- 14.Larsen LH, Moe KE, Vitiello MV, Prinz PN. Age trends in the sleep EEG of healthy older men and women. J Sleep Res. 1995;4:160–72. doi: 10.1111/j.1365-2869.1995.tb00165.x. [DOI] [PubMed] [Google Scholar]
- 15.Dijk DJ, Duffy JF, Riel E, Shanahan TL, Czeisler CA. Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. J Physiol. 1999;516:611–27. doi: 10.1111/j.1469-7793.1999.0611v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wright KP, Jr, Frey DJ. Age related changes in sleep and circadian physiology: from brain mechanisms to sleep behavior. In: Avidan AY, Alessi CA, editors. Geriatric sleep medicine. New York: Informa HealthCare USA, Inc; 2008. pp. 1–18. [Google Scholar]
- 17.Vitiello MV. Sleep in normal aging. Sleep Med Clin. 2006;1:171–6. doi: 10.1016/j.jsmc.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dijk DJ, Brunner DP, Borbely AA. Time course of EEG power density during long sleep in humans. Am J Physiol. 1990;258:R650–61. doi: 10.1152/ajpregu.1990.258.3.R650. [DOI] [PubMed] [Google Scholar]
- 19.Munch M, Knoblauch V, Blatter K, et al. The frontal predominance in human EEG delta activity after sleep loss decreases with age. Eur J Neurosci. 2004;20:1402–10. doi: 10.1111/j.1460-9568.2004.03580.x. [DOI] [PubMed] [Google Scholar]
- 20.Ancoli-Israel S. Sleep and its disorders in aging populations. Sleep Med. 2009;10(Suppl 1):S7–11. doi: 10.1016/j.sleep.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18:425–32. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 22.Chong Y, Fryar CD, Gu Q. NCHS data brief, no 127. Hyattsville, MD: National Center for Health Statistics; 2013. Prescription sleep aid use among adults: United States, 2005–2010. [PubMed] [Google Scholar]
- 23.Gershell L. Insomnia market. Nat Rev Drug Discov. 2006;5:15–16. doi: 10.1038/nrd1932. [DOI] [PubMed] [Google Scholar]
- 24.Olubodun JO, Ochs HR, von Moltke LL, et al. Pharmacokinetic properties of zolpidem in elderly and young adults: possible modulation by testosterone in men. Br J Clin Pharmacol. 2003;56:297–304. doi: 10.1046/j.0306-5251.2003.01852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.U.S. Food and Drug Administration (FDA) Risk of next-morning impairment after use of insomnia drugs; FDA requires lower recommended doses for certain drugs containing zolpidem (Ambien, Ambien CR, Edluar, and Zolpimist) [Internet] Silver Spring, MD: U.S. FDA; 2013. [accessed 13.11.02]. < http://www.fda.gov/downloads/Drugs/DrugSafety/UCM335007.pdf>. [Google Scholar]
- 26.Monti JM, Monti D. Overview of currently available benzodiazepine and nonbenzodiazepine hypnotics. In: Pandi-Perumal SR, Monti JM, editors. Clinical pharmacology of sleep. Basel: Birkhauser; 2006. pp. 207–23. [Google Scholar]
- 27.Salva P, Costa J. Clinical pharmacokinetics and pharmacodynamics of zolpidem: therapeutic implications. Clin Pharmacokinet. 1995;29:142–53. doi: 10.2165/00003088-199529030-00002. [DOI] [PubMed] [Google Scholar]
- 28.Blois R, Gaillard JM, Attali P, Coquelin JP. Effect of zolpidem on sleep in healthy subjects: a placebo-controlled trial with polysomnographic recordings. Clin Ther. 1993;15:797–809. [PubMed] [Google Scholar]
- 29.Brunner DP, Dijk DJ, Munch M, Borbely AA. Effect of zolpidem on sleep and sleep EEG spectra in healthy young men. Psychopharmacology (Berl) 1991;104:1–5. doi: 10.1007/BF02244546. [DOI] [PubMed] [Google Scholar]
- 30.Feige B, Voderholzer U, Riemann D, Hohagen F, Berger M. Independent sleep EEG slow-wave and spindle band dynamics associated with 4 weeks of continuous application of short-half-life hypnotics in healthy subjects. Clin Neurophysiol. 1999;110:1965–74. doi: 10.1016/s1388-2457(99)00147-9. [DOI] [PubMed] [Google Scholar]
- 31.Nicholson AN, Pascoe PA. Hypnotic activity of an imidazo-pyridine (zolpidem) Br J Clin Pharmacol. 1986;21:205–11. doi: 10.1111/j.1365-2125.1986.tb05176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Voderholzer U, Riemann D, Hornyak M, et al. A double-blind, randomized and placebo-controlled study on the polysomnographic withdrawal effects of zopiclone, zolpidem and triazolam in healthy subjects. Eur Arch Psychiatry Clin Neurosci. 2001;251:117–23. doi: 10.1007/s004060170045. [DOI] [PubMed] [Google Scholar]
- 33.Feinberg I, Maloney T, Campbell IG. Effects of hypnotics on the sleep EEG of healthy young adults: new data and psychopharmacologic implications. J Psychiatr Res. 2000;34:423–38. doi: 10.1016/s0022-3956(00)00038-8. [DOI] [PubMed] [Google Scholar]
- 34.Roth T, Roehrs T, Vogel G. Zolpidem in the treatment of transient insomnia: a double-blind, randomized comparison with placebo. Sleep. 1995;18:246–51. doi: 10.1093/sleep/18.4.246. [DOI] [PubMed] [Google Scholar]
- 35.Scharf MB, Mayleben DW, Kaffeman M, Krall R, Ochs R. Dose response effects of zolpidem in normal geriatric subjects. J Clin Psychiatry. 1991;52:77–83. [PubMed] [Google Scholar]
- 36.Walsh JK, Deacon S, Dijk DJ, Lundahl J. The selective extrasynaptic GABAA agonist, gaboxadol, improves traditional hypnotic efficacy measures and enhances slow wave activity in a model of transient insomnia. Sleep. 2007;30:593–602. doi: 10.1093/sleep/30.5.593. [DOI] [PubMed] [Google Scholar]
- 37.Patat A, Trocherie S, Thebault JJ, et al. EEG profile of intravenous zolpidem in healthy volunteers. Psychopharmacology (Berl) 1994;114:138–46. doi: 10.1007/BF02245455. [DOI] [PubMed] [Google Scholar]
- 38.Frey DJ, Ortega JD, Wiseman C, Farley CT, Wright KP., Jr Influence of zolpidem and sleep inertia on balance and cognition during nighttime awakening: a randomized placebo-controlled trial. J Am Geriatr Soc. 2011;59:73–81. doi: 10.1111/j.1532-5415.2010.03229.x. [DOI] [PubMed] [Google Scholar]
- 39.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Washington, DC: U.S. Government Printing Office; 1968. [DOI] [PubMed] [Google Scholar]
- 40.Keppel G. Design and analysis: a researcher’s handbook. Englewood Cliffs, NJ: Prentice-Hall, Inc; 1991. [Google Scholar]
- 41.Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol. 2001;85:1969–85. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- 42.Landolt HP, Borbely AA. Age-dependent changes in sleep EEG topography. Clin Neurophysiol. 2001;112:369–77. doi: 10.1016/s1388-2457(00)00542-3. [DOI] [PubMed] [Google Scholar]
- 43.Robillard R, Massicotte-Marquez J, Kawinska A, Paquet J, Frenette S, Carrier J. Topography of homeostatic sleep pressure dissipation across the night in young and middle-aged men and women. J Sleep Res. 2010;19:455–65. doi: 10.1111/j.1365-2869.2010.00820.x. [DOI] [PubMed] [Google Scholar]
- 44.Lundahl J, Deacon S, Maurice D, Staner L. EEG spectral power density profiles during NREM sleep for gaboxadol and zolpidem in patients with primary insomnia. J Psychopharmacol. 2012;26:1081–7. doi: 10.1177/0269881111424457. [DOI] [PubMed] [Google Scholar]
- 45.Landolt HP, Finelli LA, Roth C, Buck A, Achermann P, Borbely AA. Zolpidem and sleep deprivation: different effect on EEG power spectra. J Sleep Res. 2000;9:175–83. doi: 10.1046/j.1365-2869.2000.00192.x. [DOI] [PubMed] [Google Scholar]
- 46.Rissman RA, Mobley WC. Implications for treatment: GABAA receptors in aging, Down syndrome and Alzheimer’s disease. J Neurochem. 2011;117:613–22. doi: 10.1111/j.1471-4159.2011.07237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klotz U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev. 2009;41:67–76. doi: 10.1080/03602530902722679. [DOI] [PubMed] [Google Scholar]
- 48.Greenblatt DJ, Roth T. Zolpidem for insomnia. Expert Opin Pharmacother. 2012;13:879–93. doi: 10.1517/14656566.2012.667074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.