Abstract
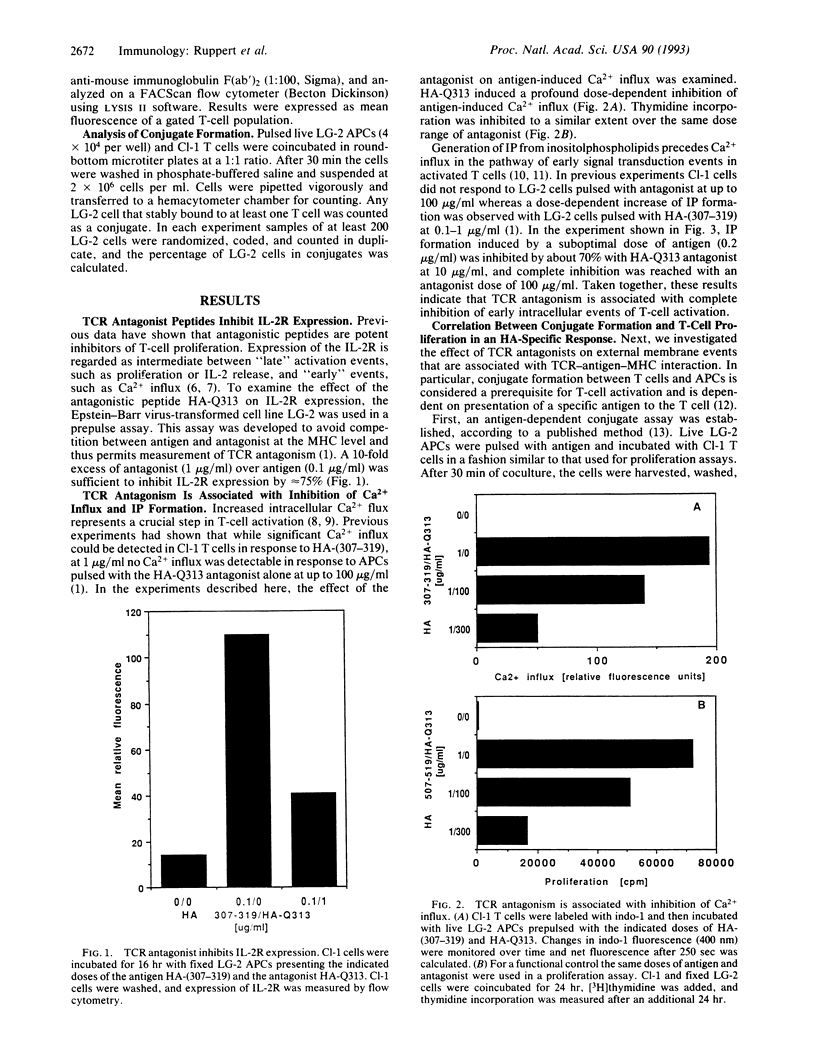
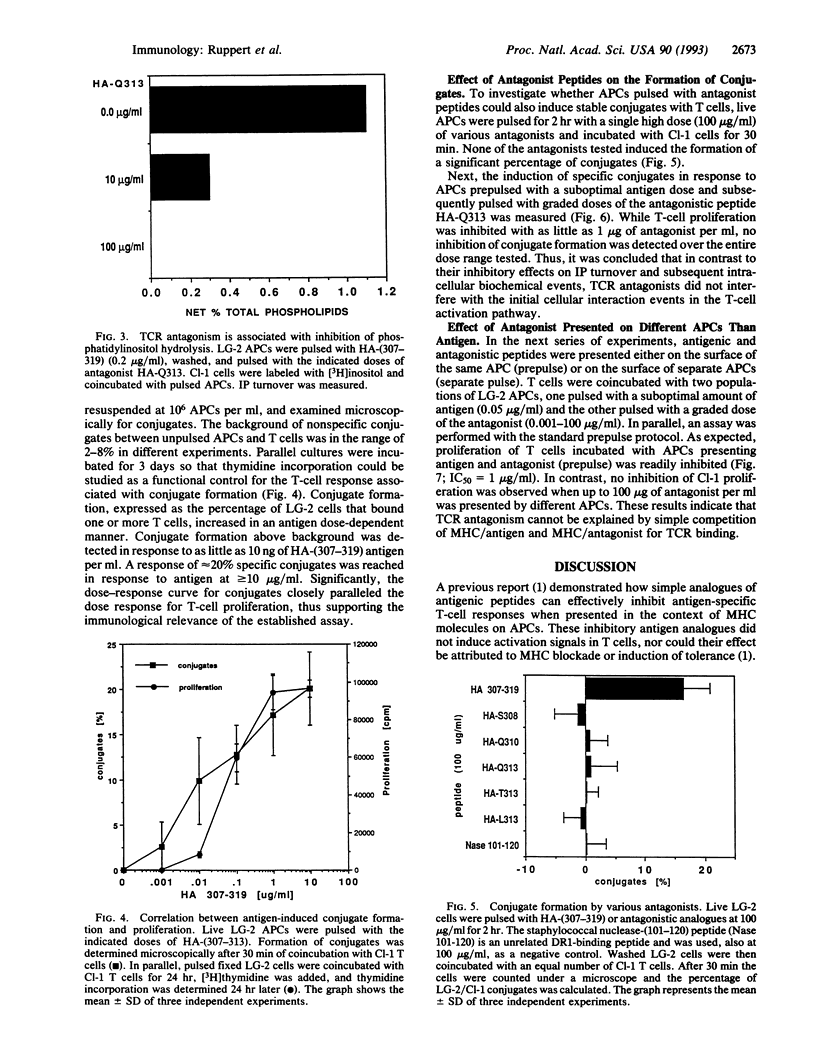
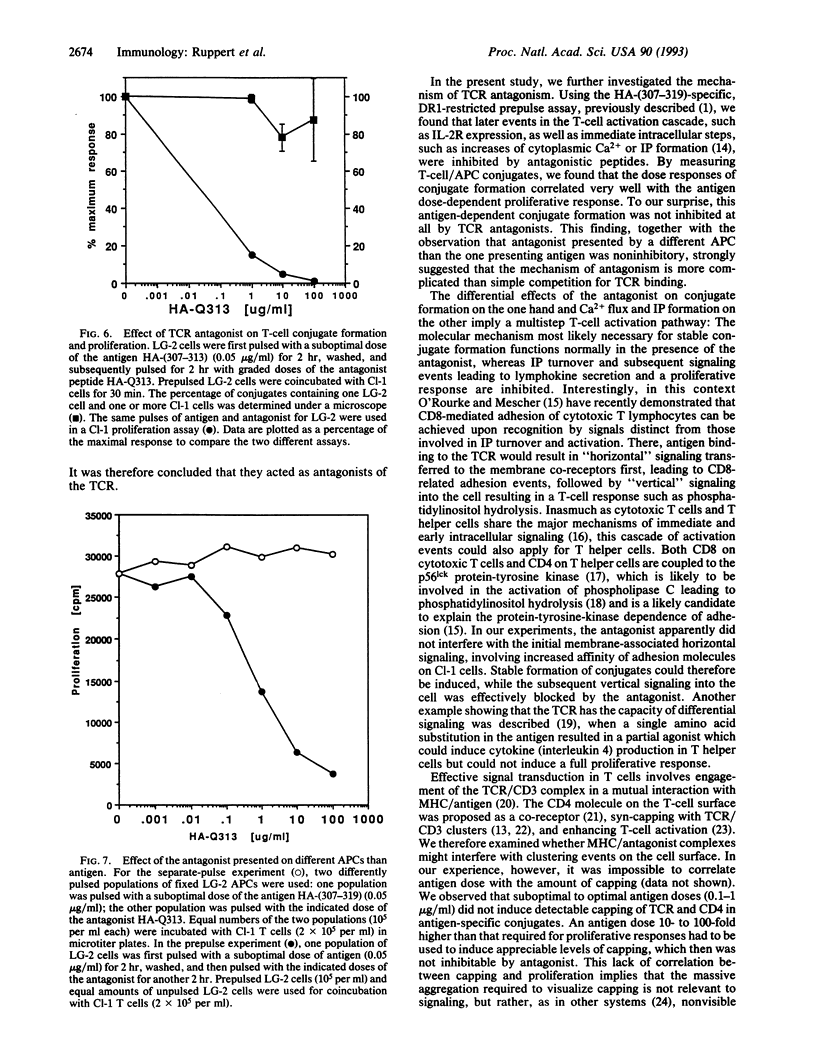
T-cell receptor (TCR) antagonism induced by complexes of antigen analogue with major histocompatibility complex (MHC) molecules results in efficient inhibition of antigen-dependent T-cell responses. We have investigated some of the possible mechanisms by which TCR antagonists bound to the MHC molecules of antigen-presenting cells (APCs) can inhibit T-cell activation. Using a nonstimulatory analogue of the antigenic peptide influenza hemagglutinin-(307-319), we showed that MHC/antagonist complexes completely inhibit very early intracellular events of antigen-dependent T-cell activation, such as inositol phosphate turnover and Ca2+ influx. In a parallel series of experiments, the effect of TCR antagonist peptide on membrane-related activation events was also investigated. It was found that MHC/antagonist complexes on the surface of APCs did not induce stable conjugates with T cells and, most interestingly, did not inhibit antigen-induced conjugate formation. Thus, our data suggest that antagonistic peptides do not interfere with the cellular events that are required for stable T-cell/APC conjugate formation but do inhibit early biochemical events required for T-cell proliferation. The data are discussed with respect to the role of surface receptor clustering in TCR antagonism.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cotner T., Williams J. M., Christenson L., Shapiro H. M., Strom T. B., Strominger J. Simultaneous flow cytometric analysis of human T cell activation antigen expression and DNA content. J Exp Med. 1983 Feb 1;157(2):461–472. doi: 10.1084/jem.157.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Magistris M. T., Alexander J., Coggeshall M., Altman A., Gaeta F. C., Grey H. M., Sette A. Antigen analog-major histocompatibility complexes act as antagonists of the T cell receptor. Cell. 1992 Feb 21;68(4):625–634. doi: 10.1016/0092-8674(92)90139-4. [DOI] [PubMed] [Google Scholar]
- Evavold B. D., Allen P. M. Separation of IL-4 production from Th cell proliferation by an altered T cell receptor ligand. Science. 1991 May 31;252(5010):1308–1310. doi: 10.1126/science.1833816. [DOI] [PubMed] [Google Scholar]
- Geppert T. D., Davis L. S., Gur H., Wacholtz M. C., Lipsky P. E. Accessory cell signals involved in T-cell activation. Immunol Rev. 1990 Oct;117:5–66. doi: 10.1111/j.1600-065x.1990.tb00566.x. [DOI] [PubMed] [Google Scholar]
- Imboden J. B., Stobo J. D. Transmembrane signalling by the T cell antigen receptor. Perturbation of the T3-antigen receptor complex generates inositol phosphates and releases calcium ions from intracellular stores. J Exp Med. 1985 Mar 1;161(3):446–456. doi: 10.1084/jem.161.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imboden J. B., Weiss A., Stobo J. D. The antigen receptor on a human T cell line initiates activation by increasing cytoplasmic free calcium. J Immunol. 1985 Feb;134(2):663–665. [PubMed] [Google Scholar]
- Janeway C. A., Jr, Dianzani U., Portoles P., Rath S., Reich E. P., Rojo J., Yagi J., Murphy D. B. Cross-linking and conformational change in T-cell receptors: role in activation and in repertoire selection. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 2):657–666. doi: 10.1101/sqb.1989.054.01.077. [DOI] [PubMed] [Google Scholar]
- Janeway C. A., Jr The role of CD4 in T-cell activation: accessory molecule or co-receptor? Immunol Today. 1989 Jul;10(7):234–238. doi: 10.1016/0167-5699(89)90260-0. [DOI] [PubMed] [Google Scholar]
- June C. H., Fletcher M. C., Ledbetter J. A., Samelson L. E. Increases in tyrosine phosphorylation are detectable before phospholipase C activation after T cell receptor stimulation. J Immunol. 1990 Mar 1;144(5):1591–1599. [PubMed] [Google Scholar]
- Krieger J. I., Karr R. W., Grey H. M., Yu W. Y., O'Sullivan D., Batovsky L., Zheng Z. L., Colón S. M., Gaeta F. C., Sidney J. Single amino acid changes in DR and antigen define residues critical for peptide-MHC binding and T cell recognition. J Immunol. 1991 Apr 1;146(7):2331–2340. [PubMed] [Google Scholar]
- Kupfer A., Singer S. J., Janeway C. A., Jr, Swain S. L. Coclustering of CD4 (L3T4) molecule with the T-cell receptor is induced by specific direct interaction of helper T cells and antigen-presenting cells. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5888–5892. doi: 10.1073/pnas.84.16.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer A., Singer S. J. Molecular dynamics in the membranes of helper T cells. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8216–8220. doi: 10.1073/pnas.85.21.8216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer A., Swain S. L., Janeway C. A., Jr, Singer S. J. The specific direct interaction of helper T cells and antigen-presenting B cells. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6080–6083. doi: 10.1073/pnas.83.16.6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D. P. Cancer. p53, guardian of the genome. Nature. 1992 Jul 2;358(6381):15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- Merćep M., Weissman A. M., Frank S. J., Klausner R. D., Ashwell J. D. Activation-driven programmed cell death and T cell receptor zeta eta expression. Science. 1989 Dec 1;246(4934):1162–1165. doi: 10.1126/science.2531464. [DOI] [PubMed] [Google Scholar]
- O'Flynn K., Zanders E. D., Lamb J. R., Beverley P. C., Wallace D. L., Tatham P. E., Tax W. J., Linch D. C. Investigation of early T cell activation: analysis of the effect of specific antigen, interleukin 2 and monoclonal antibodies on intracellular free calcium concentration. Eur J Immunol. 1985 Jan;15(1):7–11. doi: 10.1002/eji.1830150103. [DOI] [PubMed] [Google Scholar]
- O'Rourke A. M., Mescher M. F. Cytotoxic T-lymphocyte activation involves a cascade of signalling and adhesion events. Nature. 1992 Jul 16;358(6383):253–255. doi: 10.1038/358253a0. [DOI] [PubMed] [Google Scholar]
- O'Sullivan D., Sidney J., Appella E., Walker L., Phillips L., Colón S. M., Miles C., Chesnut R. W., Sette A. Characterization of the specificity of peptide binding to four DR haplotypes. J Immunol. 1990 Sep 15;145(6):1799–1808. [PubMed] [Google Scholar]
- Owens T., Fazekas de St Groth B., Miller J. F. Coaggregation of the T-cell receptor with CD4 and other T-cell surface molecules enhances T-cell activation. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9209–9213. doi: 10.1073/pnas.84.24.9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo J. M., Saizawa K., Janeway C. A., Jr Physical association of CD4 and the T-cell receptor can be induced by anti-T-cell receptor antibodies. Proc Natl Acad Sci U S A. 1989 May;86(9):3311–3315. doi: 10.1073/pnas.86.9.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samelson L. E. Lymphocyte activation. Curr Opin Immunol. 1989 Dec;2(2):210–214. doi: 10.1016/0952-7915(89)90190-8. [DOI] [PubMed] [Google Scholar]
- Segal D. M., Taurog J. D., Metzger H. Dimeric immunoglobulin E serves as a unit signal for mast cell degranulation. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2993–2997. doi: 10.1073/pnas.74.7.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. J. Intercellular communication and cell-cell adhesion. Science. 1992 Mar 27;255(5052):1671–1677. doi: 10.1126/science.1313187. [DOI] [PubMed] [Google Scholar]
- Smith K. A. Interleukin-2: inception, impact, and implications. Science. 1988 May 27;240(4856):1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- Veillette A., Bookman M. A., Horak E. M., Bolen J. B. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988 Oct 21;55(2):301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- Weiss A., Imboden J., Hardy K., Manger B., Terhorst C., Stobo J. The role of the T3/antigen receptor complex in T-cell activation. Annu Rev Immunol. 1986;4:593–619. doi: 10.1146/annurev.iy.04.040186.003113. [DOI] [PubMed] [Google Scholar]
- el-Deiry W. S., Kern S. E., Pietenpol J. A., Kinzler K. W., Vogelstein B. Definition of a consensus binding site for p53. Nat Genet. 1992 Apr;1(1):45–49. doi: 10.1038/ng0492-45. [DOI] [PubMed] [Google Scholar]



