Abstract
Human embryonic stem (ES) cells have most commonly been cultured in the presence of basic FGF (FGF2) either on fibroblast feeder layers or in fibroblast-conditioned medium. Recently, it has been reported that elevated concentrations of FGF2 permit the culture of human ES cells in the absence of fibroblasts or fibroblast-conditioned medium. Here we compare the ability of unconditioned medium (UM) supplemented with 4, 24, 40, 80, 100 and 250 ng/ml FGF2 to sustain low-density human ES cell cultures through multiple passages. In these stringent culture conditions, 4, 24, and 40 ng/ml FGF2 failed to sustain human ES cells through three passages, but 100 ng/ml sustained human ES cells with an effectiveness comparable to conditioned medium (CM). Two human ES cell lines (H1 and H9) were maintained for up to 164 population doublings (7 and 4 months) in UM supplemented with 100 ng/ml FGF2. After prolonged culture the cells formed teratomas when injected into SCID-beige mice, and expressed markers characteristic of undifferentiated human ES cells. We also demonstrate that FGF2 is degraded more rapidly in UM than in CM, partly explaining the need for higher concentrations of FGF2 in UM. These results further facilitate the large-scale, routine culture of human ES cells, and suggest that fibroblasts and fibroblast-conditioned medium sustain human ES cells in part by stabilizing FGF signaling above a critical threshold.
Keywords: human embryonic stem cell, fibroblast growth factor
Introduction
Embryonic stem (ES) cells can be expanded indefinitely while maintaining the potential to form any cell type of the body [1–4]. Both human and mouse ES cells were initially isolated on fibroblast feeder layers in medium containing serum; however, the growth factors that maintain human and mouse ES cells are distinct. In the presence of serum and leukemia inhibitory factor (LIF), the resulting activation of the JAK/STAT3 pathway supports feeder-independent growth of mouse ES cells [5, 6]. In comparable culture conditions, LIF does not maintain human ES cells, and the JAK/STAT3 pathway does not appear to become activated in conditions that maintain human ES cells [4]. In serum-free conditions, combined BMP4 and LIF activities are sufficient to support the clonal growth of mouse ES cells [7]. However, when BMP4 is added to human ES cells in culture conditions that would otherwise support undifferentiated proliferation, rapid differentiation occurs [8].
In contrast to mouse ES cells, FGF signaling appears to be of central importance to human ES cells self-renewal. FGF2 (4 ng/ml) and a commercially available serum substitute support human ES cell growth on fibroblasts [9] or in fibroblast-conditioned medium [10]. Recently it has been shown that elevated levels of FGF2 can sustain human ES cells through long-term cultures in the absence of fibroblasts [11–14]. Although 40 ng/ml FGF2 can sustain high-density human ES cell cultures through multiple passages in the absence of fibroblasts, there is significantly more background differentiation compared to control cells cultured in conditioned medium (CM: [11] and (Fig. 1C vs. A)).
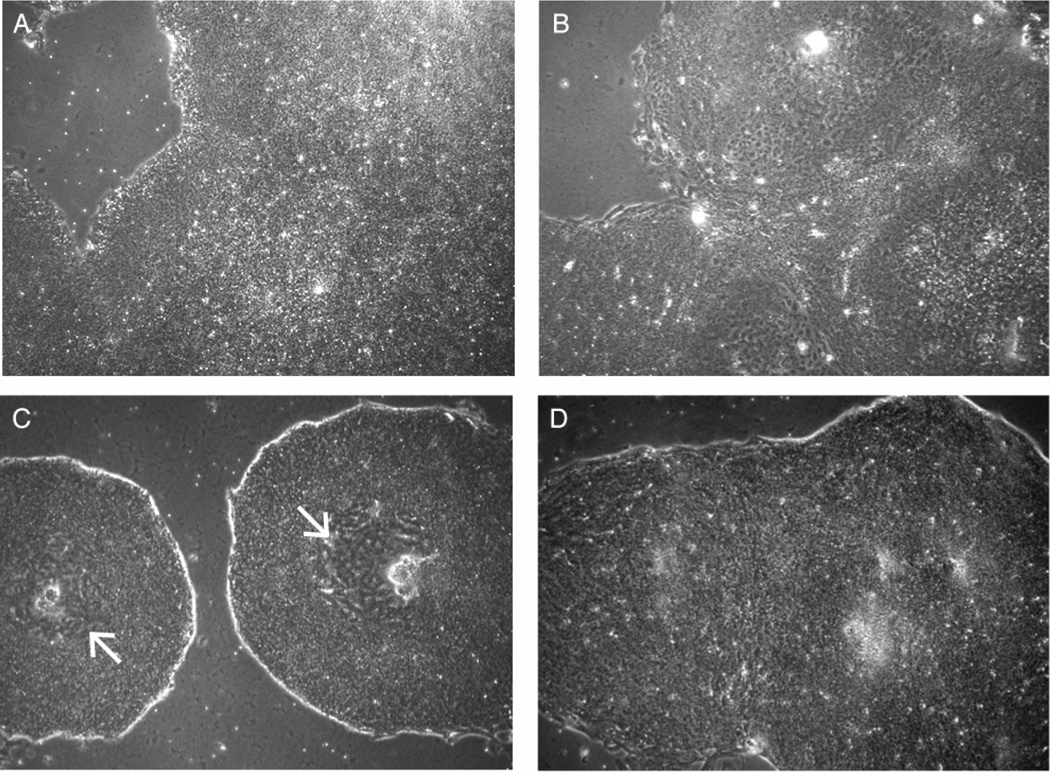
Figure 1.
Morphology of human ES cells grown in high FGF2 concentrations. 5× phase contrast images show representative morphology of H14 cells maintained for 5 days in A) CM, B) UM, C) UM 40, or D) UM 100. Arrows identify centralized regions of differentiation within UM 40 colonies. Abbreviations: CM, conditioned medium plus 4 ng/ml FGF2; UM, unconditioned medium plus 4 ng/ml FGF2; UM 40, unconditioned medium plus 40 ng/ml FGF2; UM 100, unconditioned medium plus 100 ng/ml FGF2.
FGF proteins play a role in diverse developmental pathways, and antagonism between FGF and BMP signaling is a repeated theme [15, 16]. We have previously shown that the level of BMP activity in unconditioned medium (UM) is sufficient to cause human ES cell differentiation, and that conditioning the medium reduces or blocks BMP activity [11]. UM supplemented with 40 ng/ml FGF2 and the BMP antagonist noggin sustained feeder-free, undifferentiated human ES cell proliferation through prolonged culture, with background differentiation levels comparable to those observed in CM [11]. Furthermore, elevating FGF2 levels to 100 ng/ml in UM suppressed BMP signaling to a level comparable to that achieved either by the addition of noggin or by fibroblast-conditioning the medium. Indeed, at 100 ng/ml FGF2, background differentiation was minimal and supplementation with Noggin conferred no additional benefit in short-term human ES cell cultures (data not shown).
Here we compare growth curve analyses of low-density human ES cell cultures through multiple passages in UM supplemented with 4, 24, 40, 80, 100, and 250 ng/ml FGF2. We find that culture in UM plus 100 ng/ml FGF2 closely parallels the proliferation and low background differentiation rate obtained using CM, and supports long-term, undifferentiated, feeder-independent culture of human ES cells. We further demonstrate that FGF2 is more rapidly degraded in UM than in CM, so that ES cells are exposed to dramatically different levels of FGF2 in UM at the beginning and end of the 24 hours between routine media changes. This suggests that the maintenance of FGF2 levels above a critical threshold throughout the culture period is essential, and that one of the effects of culturing on fibroblasts or in CM is stabilization of the FGF signal.
Materials and Methods
Cell Lines and Cell Culture
The human ES cell lines H1, H7, H9, and H14 were cultured on Matrigel (Becton Dickinson, Franklin Lakes, NJ) in CM as previously described [10]. UM contained 80% DMEM/F12 and 20% KNOCKOUT serum replacement supplemented with 1 mM L-glutamine, 1% Nonessential Amino Acids (all from Invitrogen, Carlsbad, CA), 0.1 mM β-mercaptoethanol (Sigma, St. Louis, MO), and 4 ng/ml human FGF2 (Invitrogen). UM 24, 40, 80, 100, and 250 contained UM supplemented with 24, 40, 80, 100, and 250 ng/ml FGF2 respectively. UM 4/RA contained UM supplemented with 4 ng/ml human FGF2 and 10 µM retinoic acid (Sigma). Cultures of human ES cells were routinely passaged onto Matrigel-coated plates in clumps at approximately weekly intervals by exposure to dispase (1 mg/ml: Gibco/Invitrogen). Cells were transitioned into test media either by direct passage from CM or by directly thawing from frozen stock.
Immunofluorescence
H1 cells growing in CM (22 passages) and UM 100 (24 passages) and H9 cells growing in UM 100 (10 passages) were fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) for 15 minutes at room temperature. Cells were then washed 3 times for 5 minutes in KPBS (150 mM NaCl, 40 mM K2HPO4, 10 mM KH2P04). After blocking in 5% milk, cells were probed with either an anti-human OCT4 mouse monoclonal or an isotype control antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Cells were washed and then probed with an alexaflour anti-mouse secondary antibody (Invitrogen) for one hour in the dark at room temperature. Finally, fluorescent images were captured with a DM IRB microscope (Leica, Frankfurt, Germany) and analyzed with IP Lab software (Scanalytics Incorporated, Fairfax, VA).
Western Blotting
H1 cells grown in CM or UM 100 (passage 24 or 26 respectively) were individualized for 10 minutes at 37° C with Trypsin/EDTA (Invitrogen), counted with a hemocytometer, and resuspended in 8 M Urea/100 mM Tris (pH 7.2). Lysates from 1.5 × 106 cells were run on 4–20% gradient polyacrylamide gels along with biotinylated standards and transferred to nitrocellulose (all from BioRad, Hercules, CA). Filters were blocked for 1 hour at room temperature in 1× Blocking Buffer (Sigma) in PBS. Anti-human OCT4 or NANOG primary antibodies (Santa Cruz Biotechnology, Inc. and R & D Systems, Minneapolis, MN) were diluted 1:500 or 1:100 in 1× Blocking Buffer and probed for 1 hour at room temperature. Filters were washed 2 × 5 minutes in TBS-T (10 mM Tris-HCl pH 7.9, 150 mM NaCl, 0.05% Tween-20). 1:2000 dilutions of appropriate HRP-conjugated secondary antibodies (Santa Cruz Biotechnology, Inc.) along with 1:5000 dilutions of avidin-HRP (BioRad) in 1× Blocking Buffer were added and probed for 1 hour at room temperature. Filters were washed 2 × 5 minutes in TBS-T and signals were detected by chemiluminescence with ECL reagents (GE Healthcare, Piscataway, NJ).
RT-PCR and FACS Analysis
For RT-PCR, total RNA was extracted from H1 cells cultured in CM or UM 100 (24 or 26 passages respectively) using an RNeasy Mini Kit (Qiagen, Valencia, CA) following manufacturer’s recommendations. Transcripts were assayed from one microgram of total RNA with 35 rounds of PCR amplification using an OneStep RT-PCR Kit (Qiagen) with previously described gene-specific primers [11] and visualized on ethidium bromide stained agarose gels.
For FACS analysis, cells were removed from the culture dish with Trypsin/EDTA (Invitrogen) containing 2% chick serum (ICN, Costa Mesa, CA) for 10 minutes at 37° C and resuspended in FACS Buffer (PBS + 2% FBS + 0.1% sodium azide). The cells (probed for internal markers) were fixed with 0.1% paraformaldehyde for 10 min. at 37° C then permeablized with 90% methanol (Fisher Scientific, Rochester, NY) for 30 min. on ice. 1 – 5 × 105 treated (OCT4) or non-treated (SSEA4 or Tra1–60) cells were then probed for 2 hours at room temperature with a 1:100 dilution of the specific monoclonal antibody or an appropriate isotype control antibody (Santa Cruz Biotechnology, Inc.) in FACS Buffer (+ 0.1% Triton-X100 for OCT4). Cells were then washed and probed in FACS Buffer (+ 0.1% Triton-X100 for OCT4) with 1:1000 dilution of an alexaflour anti-mouse secondary antibody (Invitrogen) for one hour in the dark at room temperature. The Triton-X100 was washed away, and the cells were sorted using a FACSCalibur flow cytometer (Becton Dickinson). Data was analyzed with CellQuest software (Becton Dickinson).
FGF2 Stability Assays
CM and UM samples were prepared and supplemented with 4, 24, 40, 80, 100 or 250 ng/ml FGF2. 2 ml of each sample were placed at 37°C in a single well of Day 3 (H1) ES cells cultured in CM + 4 ng/ml FGF2 in a 6-well tissue culture dish (Nunc, Rochester, NY) and the remainder was stored at 4°C. After overnight incubation FGF2 concentrations were determined in 96-well plates (Nunc) using a Human FGF-basic ELISA Development Kit following the manufacturer’s recommendations (PeproTech, Rocky Hill, NJ). Measurements were performed on a Tecan GENios Pro plate reader (Tecan US, Research Triangle Park, NC) and analyzed with Magellan5 software (Tecan US). FGF2 concentrations following incubation at 4° C or 37° C were compared using student’s t-test.
Results
High Concentrations of FGF2 Support Undifferentiated Human ES Cells Growth
H14 cells (passage 36) grown in CM (4 ng/ml FGF2) displayed the standard morphology of pluripotent human ES cells (small cell sizes, large nuclear/cytoplasmic ratio, defined colony borders: Fig. 1A). In contrast, cells grown in UM (4 ng/ml FGF2) showed significantly differentiated morphologies (flattened, “cobblestone-like” cells: Fig. 1B). Confirming previous results [11, 13], we found that UM supplemented with 40 ng/ml FGF2 (UM 40) supported human ES cells through multiple passages, but a central region of differentiation consistently formed within ES cell colonies (Fig. 1C - arrowheads). However, UM supplemented with 100 ng/ml FGF2 (UM 100) prevented this central differentiation (Fig. 1D, 3A – i, ii).
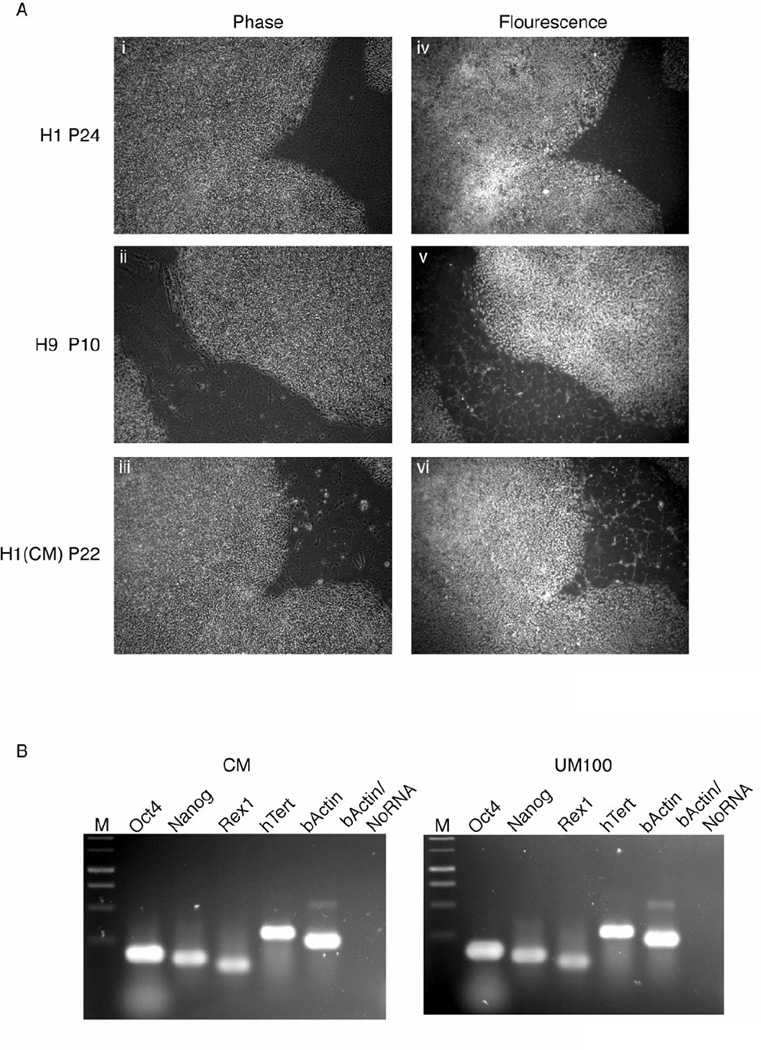
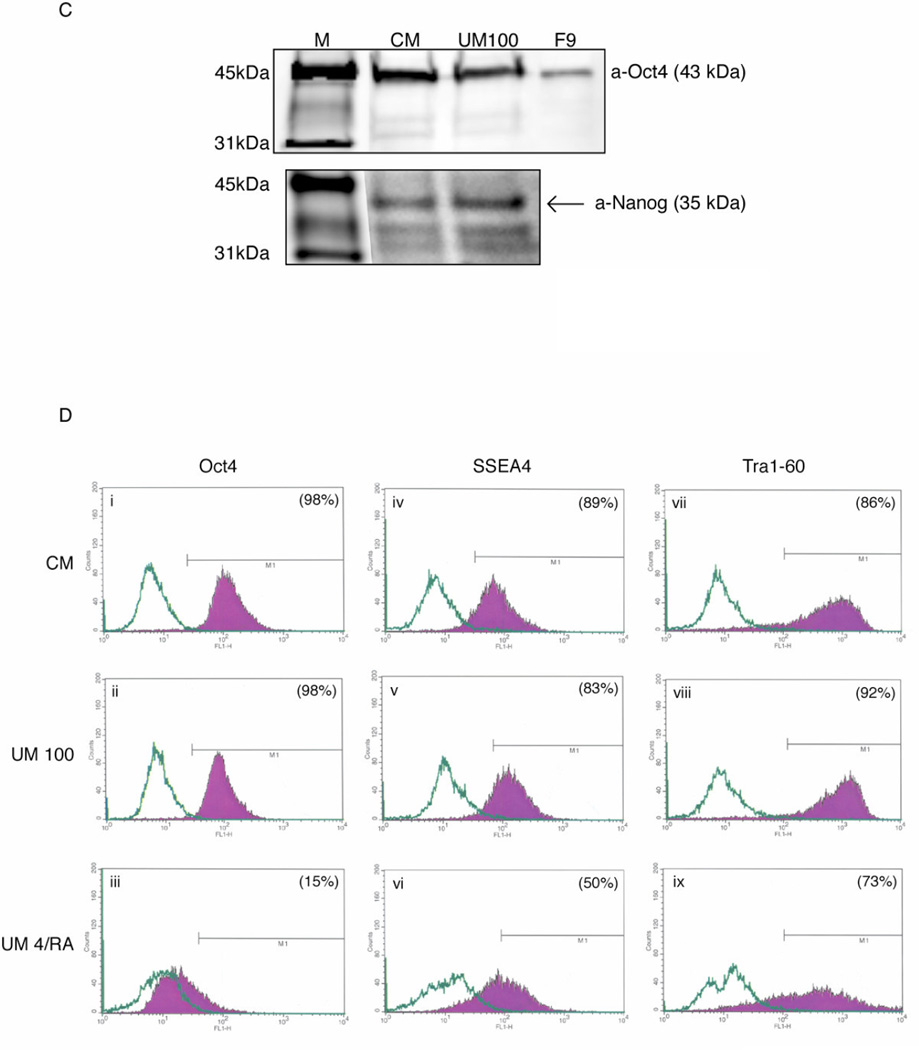
Figure 3.
Human ES cell marker expression in UM100 cultured cells. A. 5× Phase contrast and immunofluorescence for OCT4 on H1 cells (i, iv: 24 passages in UM100), H9 cells (ii, v: 10 passages in UM100), and H1 CM-cultured controls (iii, vi: 23 passages). B. RT-PCR analysis for molecular markers expressed in H1 cells cultured in CM (17 passages) or UM100 (19 passages). C. Western blot analysis for OCT4 and NANOG of H1 cells cultured in CM (24 passages) or UM100 (26 passages). F9 cell lysate (Santa Cruz Biotechnology, Inc.) served as an OCT4-positive control. D. FACS analysis of Oct4 (i – iii), SSEA4 (iv – vi), and Tra1–60 (vii – ix) expression by H1 cells (passage 30) cultured 7 days in CM (i, iv, vii), UM 100 (ii, v, viii), or UM 4/RA (iii, vi, ix). Abbreviations: CM, conditioned medium plus 4 ng/ml FGF2; UM 100, unconditioned medium plus 100 ng/ml FGF2; M, markers; OCT4, octamer-binding transcription factor-3/4; Rex1, Reduced expression-1; hTERT, human telomerase reverse transcriptase; F9, mouse embryonal carcinoma cells; SSEA4, stage-specific embryonic antigen −4; Tra1–60, tumor rejection antigen 1–60; UM 4/RA, unconditioned media plus 4 ng/ml FGF2 and 10µM retinoic acid.
Dose Response for FGF2 Support of Human ES Cells
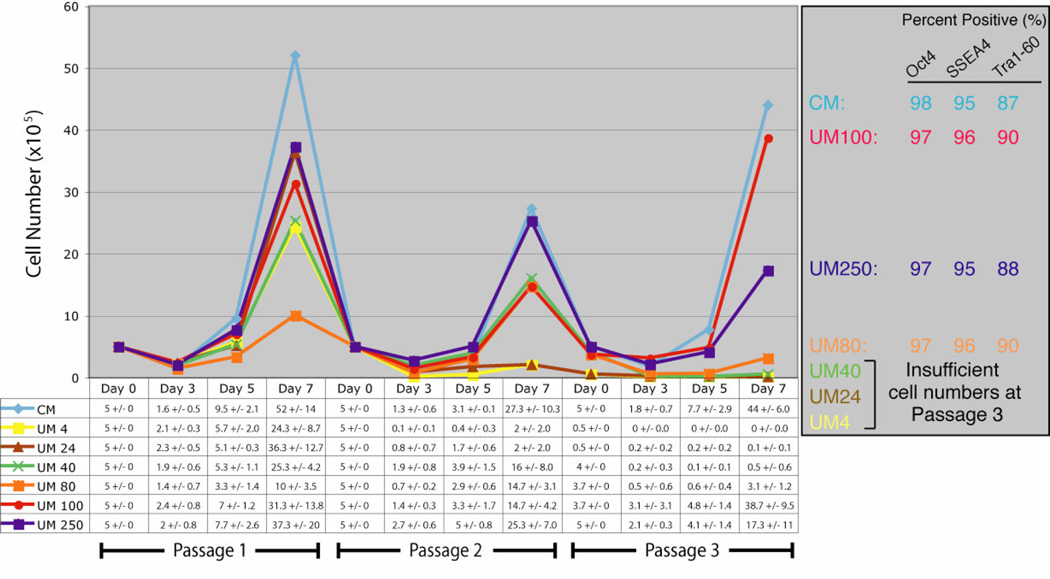
To examine the attachment efficiency and proliferation rates of human ES cells cultured in FGF2-supplemented UM, H9 cells (Passage 50/10 in CM) were grown in CM, UM 4, UM 24, UM 40, UM 80, UM 100, or UM 250. 5 × 105 cells were plated in triplicate culture wells and cell numbers were counted on Days 3, 5, and 7 after plating. On Day 7 cells were analyzed by FACS for OCT4, SSEA4, and Tra1–60 expression. After 7-day periods, if sufficient cells proliferated in a particular condition, 5 × 105 cells were re-plated for a total of three passages. UM 4, UM 24, and UM 40 all failed to support H9 culture through these three passages of low-density culture. CM, UM 100, UM 250, and with somewhat less effectiveness, UM 80, were all capable of sustaining undifferentiated human ES cell proliferation through three passages (Fig. 2). Both the percentage of cells that expressed markers of pluripotency and the total number of cells after three passages in UM 100 were comparable to cells cultured in CM (Fig. 2). In total, comparable FGF2 dose response results were compiled using three separate ES cell lines as well as alternate lots of both Knockout-SR and Matrigel highlighting the central role of FGF2 signaling in human ES cell self-renewal.
Figure 2.
FGF2 dose response for human ES cell self-renewal. Growth curve analysis of H9 cells cultured in CM, UM 4, UM 24, UM 40, UM 80, UM 100, and UM 250 for three passages. 5 × 105 cells from CM-cultured H9 cells were plated on Day 0 of passage 1. Cell numbers were counted from triplicate wells on Days 3, 5, and 7 of each passage. Initial plating density and sampling times were repeated, when possible, for 3 passages. Average cell numbers (×105 cells) with standard deviations are listed below the graph for each condition and time point. For conditions that allowed cell survival through three passages, cells were analyzed on Day 7 of passage 3 by FACS for multiple human embryonic stem cell markers (right hand box). Abbreviations: CM, conditioned medium plus 4 ng/ml FGF2; UM 4, unconditioned medium plus 4 ng/ml FGF2; UM 24, unconditioned medium plus 24 ng/ml FGF2; UM 40, unconditioned medium plus 40 ng/ml FGF2; UM 80, unconditioned medium plus 80 ng/ml FGF2; UM 100, unconditioned medium plus 100 ng/ml FGF2; UM 250, unconditioned medium plus 250 ng/ml FGF2; OCT4, octamer-binding transcription factor-3/4; SSEA4, stage-specific embryonic antigen −4; Tra1–60, tumor rejection antigen 1–60.
Long Term Culture of Low Passage Human ES Cells in 100 ng/ml FGF2
Independent human ES cell lines H9 and H1 were maintained in UM 100 for 16 and 33 passages, respectively, or for a period of 4–7 months. H1 cells (passage 41) had previously been cultured in CM for 2 passages while H9 cells (passage 22) were thawed directly into UM 100 from frozen stocks previously grown only on fibroblasts. The karyotypes of the H1 cells were examined after 10 passages in UM 100. The karyotypes of H9 cells were examined after 17 passages in UM 100. Both were confirmed normal (data not shown).
Human ES cell lines H1 (24 passages in UM 100) and H9 (10 passages in UM 100) were analyzed for OCT4 expression by immunocytochemistry, and both demonstrated staining for OCT4 that was comparable to CM-cultured human ES cells (Fig. 3A – iv and v vs. vi). Reverse transcription-PCR was performed on H1 cells (24 passages in CM or 26 passages in UM 100) confirming the continued expression of human ES cell markers OCT4, NANOG, ZFP42 (hRex1), and TERT (Fig. 3B). Whole cell protein extracts from the same H1 cells were also analyzed for both OCT4 and NANOG protein expression by Western Blot. Again, UM 100 supported expression levels comparable to CM controls (Fig. 3C). Finally, H1 (passage 30) cells were FACS analyzed for internal (Oct4) and cell surface (SSEA-4, TRA1–60) markers after 7 days of culture in CM, UM 100, or UM 4/retinoic acid. Both CM and UM 100 cultured ES cells highly expressed pluripotency markers while ES cells cultured in suboptimal growth conditions down regulated these genes (Fig. 3D – i – vi vs. vii – ix).
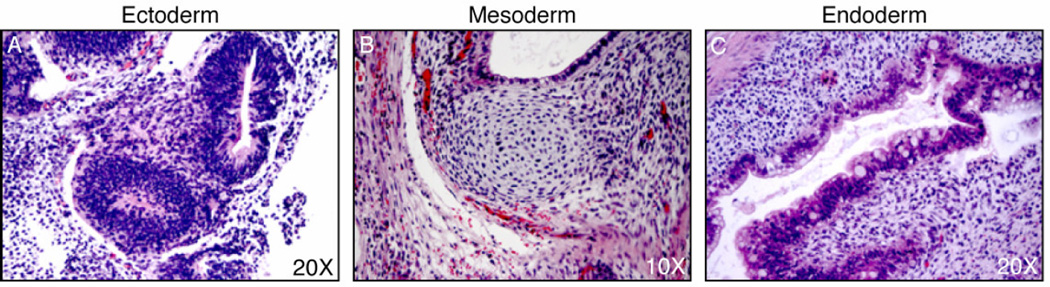
To test whether UM 100-cultured human ES cells maintain developmental potential, three SCID-beige mice were each injected with 8 × 106 H1 cells (12 passages in UM 100). Each animal formed a teratoma exhibiting advanced differentiation of multiple lineages (Fig 4.). Therefore we conclude that UM 100 supports long-term culture of human ES cells with an effectiveness comparable to CM.
Figure 4.
Teratoma Formation. UM 100-cultured H1 cells form teratomas generating structures representative of all developmental lineages. A. Neural rosette. B. Cartilage. C. Epithelium.
FGF2 Stability
100 ng/ml (5.8 nM) FGF2 is substantially above both the published high affinity kd values for FGF receptors (typically in the range of 20 pM – 200 pM) [17] as well as the 4 ng/ml FGF2 (230pM) used to support human ES cells in CM. However, FGF proteins that are not bound to heparin/heparan sulfates are known to be sensitive to proteolysis and thermal denaturation [18]. If FGF2 is unstable in our current culture conditions, then the requirement for this high level of initial FGF2 could be explained by degradation over the 24 hours between medium changes to a level below an important signaling threshold.
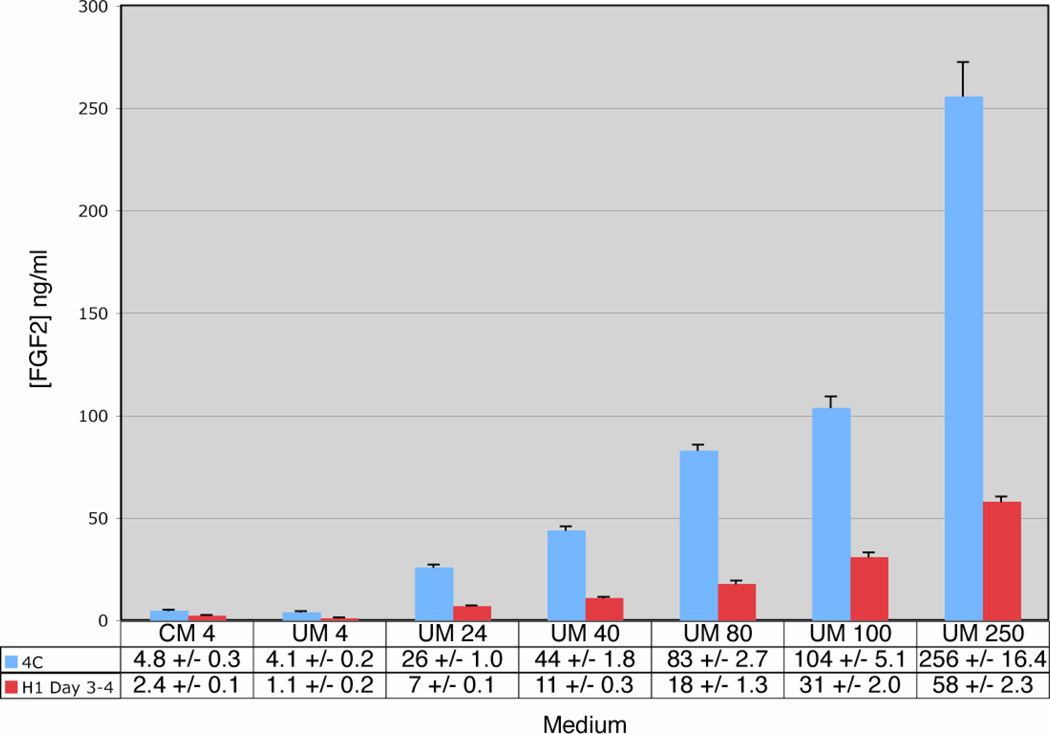
We performed ELISA-based assays to examine the concentrations of FGF2 after routine culture. No substantial loss of FGF2 was detected in either CM or UM-based media after overnight incubation at 4° C (Fig. 4). However, upon overnight incubation at 37° C we found a significant reduction of FGF2 levels regardless of the initial concentration (4, 24, 40, 80, 100 or 250 ng/ml: P < 0.01 for all concentrations). Additionally, consistently lower FGF2 levels were detected in UM cultures than were detected in CM culture following incubation (Fig. 4). Thus, it appears that one function of the conditioning process is FGF2 stabilization and that high initial concentrations of FGF2 can compensate for its instability in UM. However, there were considerable levels of FGF2 remaining in UM 40 after 24 hours of culture on H1 cells, so differences in FGF2 stability alone are likely not sufficient to explain the differences in culture performance observed between CM, UM 40, and UM 100.
Large-scale Human ES Cell Culture
Eliminating the need for fibroblast production greatly reduces the labor required for large-scale expansion of human ES cells. To demonstrate the effectiveness of large-scale culture with UM 100, we began with a single 6-well tissue culture dish of H1 cells and subsequently expanded to 40 × 100 mm culture dishes over 3 passages (26 days total). This expansion resulted in 1.8 × 109 ES cells, 92% of which were OCT4 positive (data not shown).
Discussion
Fibroblasts secrete multiple growth factors, including FGFs, TGFβ, Activin, WNTs, and antagonists of BMP signaling. Media incorporating different combinations of these factors have been reported to sustain human ES cells in the absence of fibroblasts [11–14, 19 – 22]. In each of these feeder-free media formulations, an FGF family member has been present. Because FGF2 stability is enhanced in CM (Fig 4), fibroblasts likely secrete either protease inhibitors or binding proteins that modulate FGF2 stability. Heparin and heparan sulfate proteoglycans modulate the stability and activity of FGFs by forming high affinity binding complexes [23]. However, at high concentrations FGF proteins can signal through direct interaction with FGF receptors, and the requirement for these complexes might be circumvented [24]. Thus, the addition of appropriate heparan sulfate proteoglycans to human ES cell medium may further improve the culture of human ES cells and reduce the required FGF2 concentrations. To date, we have been unsuccessful in using either exogenous heparin or heparan sulfate proteoglycans to reduce the required concentrations of FGF2 (data not shown). Because a large variety of heparin and heparan sulfates exist in multiple modified states it will be important to identify which, if any, are secreted by fibroblast feeder cells.
FGF signaling is dose dependent. It has been reported that, in some instances, thresholds must be maintained for a minimum of 12 hours for appropriate signaling [25]. While high affinity binding constants for FGF2 are in the pM range, nM levels are reported for low affinity binding [17]. We find that overnight incubation at 37° C results in a significant reduction of FGF2 regardless of the initial concentration (Fig. 5). However, our data shows that in UM 100 FGF2 concentrations remain in the nM range (1.8 nM) throughout the culture period while FGF2 levels in media with lower starting concentrations do not (Fig. 5). Interestingly, our data shows that FGF2 levels in UM 80 straddle the nM benchmark (970 pM – 1.1 nM) perhaps explaining the capacity of this media formulation to sustain ES cells for three passages in our low-density growth curve assay, albeit less effectively than UM 100 (Fig. 2 and Fig. 5).
Figure 5.
FGF2 Stability. Different starting concentrations of FGF2 added to conditioned or unconditioned medium were analyzed for remaining FGF2 concentrations after overnight incubation at 4° C (Blue Bars) or on H1 cells at 37° C (Red Bars). Samples were subjected to ELISA-based assays for final FGF2 concentrations. Values with standard deviations are listed below bars in ng/ml. Abbreviations: CM 4, conditioned medium plus 4 ng/ml FGF2; UM 4, unconditioned medium plus 4 ng/ml FGF2; UM 24, unconditioned medium plus 24 ng/ml FGF2; UM 40, unconditioned medium plus 40 ng/ml FGF2; UM 80, unconditioned medium plus 80 ng/ml FGF2; UM 100, unconditioned medium plus 100 ng/ml FGF2; UM 250, unconditioned medium plus 250 ng/ml FGF2.
Limiting spontaneous differentiation is essential for maintaining long-term human ES cell culture. BMP signaling increases differentiation in human ES cells and BMP antagonists such as noggin have a positive effect on human ES cell culture [11]. Our recent study showed that FGF2 itself suppresses BMP signaling in human ES cells [11]. However, our finding that FGF2 levels in UM degrade significantly during a 24 hour culture period suggests that cells cultured in UM with FGF2 concentrations below 80 ng/ml can be exposed to significant periods of suboptimal FGF signaling, allowing them to respond to differentiation-inducing signals including BMPs. At higher initial FGF2 concentrations (e.g. 100 ng/ml), where adequate FGF concentrations are maintained throughout the culture period, additional antagonism of the BMP pathway no longer appears beneficial. It is currently unclear whether other factors observed to have beneficial effects at lower FGF2 concentrations (such as TGFβ, activin, or Wnts) will further improve culture at these higher FGF2 concentrations, but UM 100 is already an effective medium for propagating human ES cells.
UM 100 addresses several broad goals for human ES cell media improvement. The first is to simplify human ES cell culture so that laboratories with little or no previous ES cell experience can reliably produce cells in numbers useful in a research setting. UM 100 greatly simplifies the propagation of human ES cells by removing variation and technical hurdles associated with fibroblast feeder cells. As a result, cell numbers greater then 109 can be readily cultured within three passages. Second, more defined culture conditions will allow a careful dissection of the signaling pathways essential for human ES cell self-renewal. UM 100 clearly highlights the importance of FGF signaling. However, UM 100 is not yet a defined culture system, as both the cell matrix (Matrigel, Becton Dickinson) and the Serum Replacement (Gibco/Invitrogen) contain complex, poorly defined mixtures of proteins and other molecules that influence self-renewal and differentiation. And finally, improved culture conditions will facilitate the clinical use of human ES cell-based therapies. Clearly, challenges remain for this goal, as the matrix and Serum Replacement contain animal products, and provide a possible route for introduction of pathogens to the culture system. Nonetheless, UM 100 is a simple, efficient, feeder-independent culture system that sheds additional light on the mechanism of FGF2 support of human ES cell proliferation.
Acknowledgements
The authors thank Dr. Travis Berggren, Deborah Faupel, and Lynn Schmidt for critical reading of the manuscript. We are grateful to Ruthann Peck, Marian Piekarczyk, and Dr. Amy Usborne for technical assistance. This work was supported by NIH exploratory center grant number P20 GM06998 (to M.L. and J.T.).
References
- 1.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 2.Martin GR. Isolation of a Pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomson JA, Kalishman J, Golos TG, et al. Isolation of a primate embryonic stem cell line. Proc Natl Acad Sci U S A. 1995;92:7844–7848. doi: 10.1073/pnas.92.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 5.Williams RL, Hilton DJ, Pease S, et al. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988;336:684–687. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- 6.Smith AG, Heath JK, Donaldson DD, et al. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988;336:688–690. doi: 10.1038/336688a0. [DOI] [PubMed] [Google Scholar]
- 7.Ying QL, Nichols J, Chambers I, et al. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- 8.Xu RH, Chen X, Li DS, et al. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nature Biotechnology. 2002;20:1261–1264. doi: 10.1038/nbt761. [DOI] [PubMed] [Google Scholar]
- 9.Amit M, Carpenter MK, Inokuma MS, et al. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Developmental Biology. 2000;227:271–278. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- 10.Xu C, Inokuma MS, Denham J, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nature Biotechnology. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 11.Xu RH, Peck RM, Li DS, et al. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nature Methods. 2005;2:185–190. doi: 10.1038/nmeth744. [DOI] [PubMed] [Google Scholar]
- 12.Wang L, Li L, Menendez P, et al. Human embryonic stem cells maintained in the absence of mouse embryonic fibroblasts or conditioned media are capable of hematopoietic development. Blood. 2005 doi: 10.1182/blood-2004-10-4065. (Epub). [DOI] [PubMed] [Google Scholar]
- 13.Xu C, Rosler E, Jiang J, et al. Basic fibroblast growth factor supports undifferentiated human embryonic stem cell growth without conditioned medium. Stem Cells. 2005;23:315–323. doi: 10.1634/stemcells.2004-0211. [DOI] [PubMed] [Google Scholar]
- 14.Klimanskaya I, Chung Y, Meisner L, et al. Human embryonic stem cells derived without feeder cells. Lancet. 2005;365:1636–1641. doi: 10.1016/S0140-6736(05)66473-2. [DOI] [PubMed] [Google Scholar]
- 15.Pera EM, Ikeda A, Eivers E, et al. Integration of IGF, FGF, and anti-BMP signals via Smad1 phosphorylation in neural induction. Genes & Development. 2003;17:3023–3028. doi: 10.1101/gad.1153603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delaune E, Lemaire P, Kodjabachian L. Neural induction in Xenopus requires early FGF signalling in addition to BMP inhibition. Development. 2005;132:299–310. doi: 10.1242/dev.01582. [DOI] [PubMed] [Google Scholar]
- 17.Naski MC, Ornitz DM. FGF signaling in skeletal development. Frontiers in Bioscience. 1998;3:781–794. doi: 10.2741/a321. [DOI] [PubMed] [Google Scholar]
- 18.Ornitz DM. FGFs, heparan sulfates and FGFRs: complex interactions essential for development. BioEssays. 2000;22:108–112. doi: 10.1002/(SICI)1521-1878(200002)22:2<108::AID-BIES2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 19.Amit M, Shariki C, Margulets V, et al. Feeder layer- and serum-free culture of human embryonic stem cells. Biology of Reproduction. 2004;70:837–845. doi: 10.1095/biolreprod.103.021147. [DOI] [PubMed] [Google Scholar]
- 20.Sato N, Meijer L, Skaltsounis L, et al. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nature Medicine. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 21.Kim SJ, Cheon SH, Yoo SJ, et al. Contribution of the PI3K/AksPKB signal pathway to maintenance of self-renewal in human embryonic stem cells. FEBS Letters. 2005;579:534–540. doi: 10.1016/j.febslet.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 22.Beattie GM, Lopez AD, Bucay N, et al. Activin A maintains pluripotency of human embryonic stem cells in the absence of feeder layers. Stem Cells. 2005;23:489–495. doi: 10.1634/stemcells.2004-0279. [DOI] [PubMed] [Google Scholar]
- 23.Pantoliano MW, Horlick RA, Springer BA, et al. Multivalent ligand-receptor binding interactions in the fibroblast growth factor system produce a cooperative growth factor and heparin mechanism for receptor dimerization. Biochemistry. 1994;33:10229–10248. doi: 10.1021/bi00200a003. [DOI] [PubMed] [Google Scholar]
- 24.Roghani M, Mansukhani A, Dell’Era P, et al. Heparin increases the affinity of basic fibroblast growth factor for its receptor but is not required for binding. Journal of Biological Chemistry. 1994;269:3976–3984. [PubMed] [Google Scholar]
- 25.Zhan X, Hu X, Friesel R, et al. Long term growth factor exposure and differential tyrosine phosphorylation are required for DNA synthesis in BALB/c 3T3 cells. The Journal of Biochemistry. 1993;268:9611–9620. [PubMed] [Google Scholar]