Abstract
Cardiovascular disease (CVD) is a major cause of death worldwide. Of the many etiological factors, microorganisms constitute one. From the local impact of the gut microbiota on energy metabolism and obesity, to the distal association of periodontal disease with coronary heart disease, microbes have a significant impact on cardiovascular health. In terms of the ability to modulate or influence the microbes, probiotic applications have been considered. These are live microorganisms which when administered in adequate amounts confer a benefit on the host. While a number of reports have established the beneficial abilities of certain probiotic bacterial strains to reduce cholesterol and hypertension, recent research suggests that their use could be more widely applied. This review presents an up-to-date summary of the known associations of the microbiome with CVD, and potential applications of probiotic therapy.
Keywords: cardiovascular disease, cardioprotection, dysbiosis, microbiome, probiotics, periodontal disease
Abbreviations
- ACE
Angiotensin converting enzyme
- ASD
Autism Spectrum Disorder
- BSH
Bile salt hydrolase
- CLA
Conjugate linoleic acid
- CVD
Cardiovascular disease
- CRP
C-reactive protein
- HSP
Heat shock protein
- I/R
Ischemia/reperfusion
- LDL-C
Low density lipoprotein cholesterol
- PD
Periodontal disease
- TLR
Toll-like receptor
- TMA
Trimethylamine
- TMAO
Trimethylamine-N-oxide
Introduction
Much has been written in recent years about the dynamic microbial communities that have an increasingly recognized impact on human health, including the cardiovascular system. The gut, skin, vagina, urinary tract and oral cavity are among several colonisation sites in which microbial communities exist in a specific equilibrium that is required for proper function and health.1 As a whole, these communities in terms of their genomes, activity, size and compositions, and surrounding ecosystems, represent the human microbiome.1-3 Using high-throughput next-generation DNA sequencing of the 16 S rRNA genome, the analysis of over 4000 specimens collected from 242 adults revealed that each site has a characteristic microbiome with constituents adapted to specific niches.1,2,4 The colon, for example, in North American adults appears to be dominated by 2 phyla: Firmicutes and Bacteriodetes, while other phyla, including Proteobacteria and Actinobacteria, exist in smaller proportions.5,6 These bacteria perform a multitude of endogenous functions from the transformation of bile acids, breakdown of insoluble fibers, to the production of specific vitamins and cofactors,7 (Figure 1, Table 1) .
Figure 1.
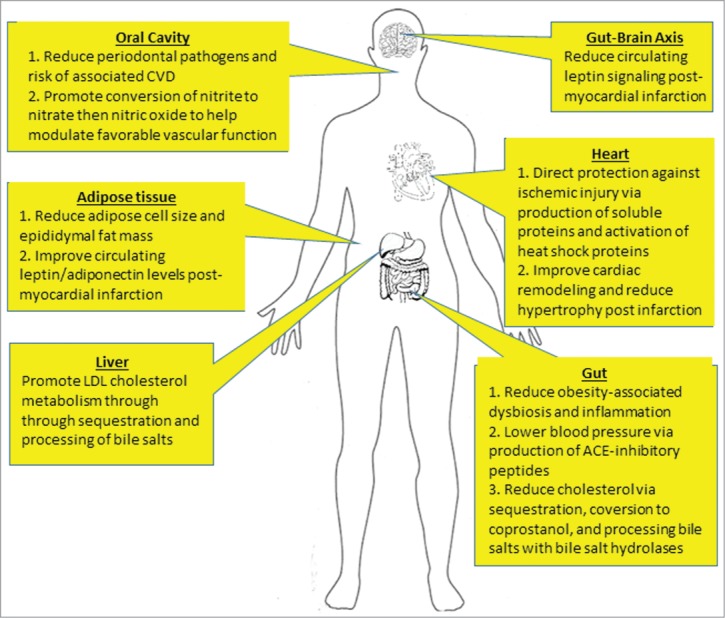
The potential sites of action of probiotics in preventing and treating CVD. Schematic diagram indicates sites throughout the body in which probiotic bacteria may confer a cardiovascular benefit on the host. Of note, many of these activities remain to be proven in humans.
Table 1.
Probiotic microorganisms shown to benefit cardiovascular health
| Species | Strain | Model | Benefit |
|---|---|---|---|
| Lactobacillus rhamnosus | GG | Mouse | Weight reduction103, prevent ischemia in I/R injury1124 |
| GR-1 | Rat | Attenuate heart failure, cardiac hypertrophy127 | |
| PL60 | Mouse | Reduce adipose tissue mass107 | |
| Lactobacillus sakei | NR28 | Mouse | Weight reduction103 |
| Lactobacillus/Bifidobacterium spp | Multi-strain | Human | Reduce BMI in obese adults, reduce serum cholesterol105 |
| Multi-strain | In vitro culture | Produce ACE-inhibitory peptides121,122 | |
| Lactobacillus plantarum | PL62 299 v | Mouse Rat | Reduce adipose tissue mass108 Reduce severity of ischemia in I/R injury125 |
| Lactobacillus reuteri | NCIMB 30242 | Human | Lower serum cholesterol118 |
The effects of the microbiome on sites distant to the gut are the subject of intense investigations, particularly related to the brain, reproductive tract, mammary glands and vasculature. Only very recently has the concept of gut organisms affecting the heart been considered. The focus of the enclosed review is to examine the role of the microbiome and probiotic interventions in preventing and treating cardiovascular disease (CVD), a group of disorders of the heart and the blood vessels that supply the heart, brain, and extremities.8
The role of the microbiome in cardiovascular health
The prevalence of CVD is increasing in magnitude across the globe8 and presents an immense burden to health care systems worldwide. Innovative approaches to effective prevention, intervention, and management for treating CVD are key to mitigating some of this burden. Although CVD represents a myriad of symptoms and dysfunctions, from hypertension to cardiomyopathy, there is a link between microbes and cardiovascular health.
The oral microbiota
The teeth, tongue, cheek, attached gingiva are all surfaces among others within the oral cavity which are colonised by distinct and complex microbial communities.9 Over 500 oral bacterial species have been identified, with the healthy “core microbiome” consisting predominantly of taxa belonging to Firmicutes, Proteobacteria, Actinobacteria, Bacteroidetes, and Fusobacteria.10 These commensals preserve homeostasis in the oral cavity by helping to produce nutrients, maintain pH, modulate saliva production, and generate inhibitory substances, all of which act to prevent colonisation and growth of exogenous or pathogenic species.11
Members of the oral microbiota have a bearing upon cardiovascular health- Streptococcus pyogenes being the classic example. Cross reactive antibodies for the organism affect the heart valves and other parts of vasculature12 and it is routinely carried in the oropharynx of up to 20% of children.13 The ability of dental infections and other oral microbial disturbances to contribute to CVD has been closely examined over the years. In particular, periodontal disease (PD), driven by an overgrowth of anaerobic bacteria in subgingival plaque communities, has been associated with CVD through a variety of epidemiological studies and meta-analyses.14-16 While these diseases share a number of risk factors (age, smoking status, oral hygiene practices) the finding of a definitive causative link between PD and specific CVD events has proven elusive.17 Nevertheless consistent evidence that the presence of PD results in an increased risk of CVD.18 During PD, an overt, consistent host immune response directed against pathogenic bacteria results in chronic gingival inflammation and destruction of tissues and bones that surround and support the teeth.19 Different mechanisms have been proposed to explain how PD may influence the development of CVD, with an emphasis placed on the infectious and inflammatory aspects of this oral disease. The increase in gingival bleeding during PD offers oral bacteria access to the bloodstream, where they can circulate and interact with atheromatous plaque deposits. Numerous studies have detected oral bacterial DNA in these atherosclerotic lesions.20-22 These bacteria may be capable of invading and activating endothelial cells, increasing Toll-like receptor (TLR) interactions, or inducing the expression of metalloproteinases, all of which contribute to the development of CVD. In addition, host antibodies generated against Porphyromonas gingivalis, a periodontal pathogen, demonstrate cross-reactivity with human heat shock proteins (HSPs), including HSP60, commonly expressed by endothelial cells in atherosclerotic lesions.23 As higher levels of anti-HSP60 antibodies correlate with morbidity and mortality from atherosclerosis,24 it is possible that “auto-immunity” developed against P. gingivalis during PD may lead to detrimental cardiovascular outcomes.
The ability of PD to affect systemic inflammation has also garnered significant attention, given the role inflammation plays in the pathogenesis of CVD. Plasma levels of the inflammatory marker C-reactive protein (CRP) have been correlated with PD status,25,26 as well as the pro-inflammatory cytokine IL-6.27 These immune modulators may be either produced locally in the oral environment and subsequently dumped into systemic circulation, or arise as a result of low grade, short-lived bacteremia.28 Given the high prevalence of PD in the adult population, management or prevention of this disease, in conjunction with other healthcare related interventions, may offer a way to reduce the incidence of CVD.
The intestinal microbiota and diet
In the so-called ‘Western world’, the prevalence of heart disease coincides with other chronic diseases such as obesity, type II diabetes, and inflammation. Metabolic syndrome is largely diet-dependent and is a key preventable risk factor for CVD. It has been well documented that individuals with obesity have a gut microbiome profile distinct from those of lean individuals.5,29,30,31 In diet-induced obesity, over-nutrition can alter composition of the gut microbiome, with dietary nutrients influencing the growth of certain species. Diets rich in cholesterol, saturated fats, and simple carbohydrates are associated with a gut microbiota rich in particular organisms belonging to the Firmicutes phylum.6,32 In obese individuals, the decreased proportion of constituents from the Bacteriodetes phylum in comparison to Firmicutes can be normalized with a low-calorie diet-associated weight loss.6 Conceivably, these obesity-associated microbiome profiles feature organisms in theory that are more adept at processing the energy-rich diets. This theory is supported by metagenomic and biochemical analyses showing that the core gut microbiome of obese individuals has an increased capacity for energy harvesting, compared to lean individuals.33 Furthermore, when the gut microbes from normal mice are transplanted into germ-free recipients, there is an increase in weight and adiposity without any added food consumption.6 This showed that the increased energy harvesting capacity is transmissible, although most of the microbes implicated in this outcome are based in the colon, which is distal to the small intestine where most absorption of lipids takes place. The modern Western diet, high in lipids and fructose and lacking of complex fermentable fibers , is seemingly mismatched to the capacity of our “ancestral” microbiota. This consequently results in less diversity and a shifted microbiome profile.32,34 It has been suggested that a return to unprocessed food diets, consisting mainly of plant-based complex fibers, seasonally fresh raw food, fermented foods and low in red meat, can help promote the proliferation of the beneficial microbes that are considered indigenous to our gut.32
Epidemiological studies indicate that vegetarians and vegans have lower blood cholesterol and lower risk for CVD compared to omnivores.35,36 The elimination of red meat from the diet is beneficial for cardiovascular health, as its consumption has long been associated with increased risk for CVD.37,38 Dietary carnitine and phosphotidyl choline, predominantly from red meat, are converted to trimethylamine (TMA) by colonic microbes.39 TMA is then converted to the proatherogenic metabolite trimethylamine-N-oxide (TMAO), which accelerates atherosclerosis in mice. The conversion of dietary carnitine to TMAO is microbe dependent, as indicated by individuals receiving oral antibiotics for a week prior to consuming red meat experienced a complete suppression of endogenous TMAO production. The same study also reports that vegetarians and vegans had significantly lower fasting baseline TMAO levels, compared to omnivores. Correspondingly, vegetarian and vegans had significantly higher abundance of Bacteriodes species and lower abundance of Prevotella species in the gut microbiome than omnivores, and a decreased risk for coronary heart disease and the traditional risk factors for CVD such as hypertension, atherosclerosis, peripheral artery disease, and stenosis. As vegetarian diet typically consists of very high portions of fermentable substrates low in carnitine, and all these components are metabolized by gut microbes, this provides strong evidence for a role of the microbiota in CVD.
In terms of prevention and treatment strategies, an understanding of the role of diet in cardiovascular health has proven extraordinarily valuable. The increased adiposity, angiogenesis, blood flow, and cardiac output associated with over-nutrition and obesity is a major risk factor for hypertension and hyperlipidemia, atherosclerosis, myocardial infarction and coronary heart disease, all of which predispose to congestive heart failure.40 If modulation of the gut microbiome can interrupt this progression at any point, there is a potential to improve an individual's cardiovascular health.
Modulation of the human microbiome
As our understanding of the elaborate symbiotic relationship between the human microbiome and the host expands, strategies for modulating the latter have evolved. The primary modulator is antibiotics. Modern medicine has relied heavily on the prescription of antibiotics in efforts to eradicate infectious microbial pathogens. Gut infections such as Clostridum difficile, Escherichia coli, Salmonella spp., and Helicobacter pylori can be controlled with the use of antibiotics,41 however not without significant detriment to the host. Antibiotics are relatively non-discriminating drugs, unable to distinguish between pathogenic and non-pathogenic bacteria at any site they encounter, thus leading to side effects such as diarrhea. The widespread use of broad spectrum antibiotics is something that should be discouraged, and alternative options are being explored.
Application of probiotics
The consumption of fermented food products from various grains, vegetables, beans, fish, and dairy products dates back to Neolithic times. The theorization by Metchnikoff in the early 1900s that the longevity of certain ethnic cultures is the result of consuming fermented foods is credited with reinvigorating the probiotic concept.42 Today, probiotics are defined as live microorganisms which when administered in adequate amounts confer a health benefit on the host.43 There is strong clinical evidence supporting probiotic treatment for relieving symptoms of bacterial vaginosis,44 diarrhea,45,46 and irritable bowel syndrome,47-50 as well as preventing Clostridium difficile-associated diarrhea.51 In most cases, the effects are strain specific and the precise mechanisms not well elucidated.
Probiotics can interact with the existing microbial community dynamic through competition with pathogens.52,53 Various mechanisms have been studied, including production of bacteriocins, biosurfactants and simple competitive exclusion.54-56 The net effect of suppressing pathogenic activity can be restoration of a ‘normal’ microbial community. Probiotic strains also participate in epithelial cross talk with the host immune system.57 The gut epithelium is a major defense barrier against foreign pathogens and antigens. Probiotics not only improve the integrity of epithelial barriers and function of tight junctions,55,58 but also can interact with toll-like receptors and transcription factors in the gut that regulate inflammatory responses.59,60 Probiotic strains produce many metabolites, enzymes, co-factors, and vitamins that become active in modulating our health. For example, the fermentation of carbohydrates by the gut microbiota and probiotics results in the production of short chain fatty acids such as acetate or propionate which are used as energy in the host.7 Studies also show that certain probiotic strains produce vitamin K, B2, B12, and folate,61-64 all of which are utilized by the host.
There are numerous examples showing the potential for probiotics to have a positive impact on the oral cavity, such as preventing dental caries,65,66 decreasing halitosis,67,68 and reducing episodes of streptococcal pharyngeal infections,69 Teughels et al.70 recently showed that the daily usage of lozenges containing Lactobacillus reuteri by patients suffering from chronic periodontitis following standard dental scaling and root planing, resulted in significantly more pocket depth reduction and attachment gain in deep periodontal pockets, as well as a decrease in P. gingivalis levels. A similar study using Lactobacillus salivarius WB21-containing tablets demonstrated the ability of this probiotic to reduce the plaque index and periodontal pocket depth in subjects at high risk of PD.71 It seems reasonable to now examine the potential of oral probiotics to reduce CVD risk via affecting PD.
Another area to explore is the ability of oral bacteria to convert dietary-derived inorganic nitrate (NO3-) to nitrite (NO2-) and other subsequent toxic compounds.72 Importantly, the generation of nitric oxide (NO) through the “nitrate-nitrite-nitric oxide” pathway, of which oral bacteria play a crucial role, has been shown to increase vasodilatation, improve vascular endothelial function, and decrease blood pressure. In this process, ingested inorganic nitrate (found in high levels in leafy vegetables) is absorbed in the small intestine, where it enters the bloodstream and can be taken up and concentrated in the salivary glands. In the oral cavity, facultative anaerobic bacteria reduce some of this nitrate secreted in saliva to nitrite, which is swallowed and can eventually be further processed to NO in the stomach, blood, or tissues.73
Human studies have shown that consumption of nitrate in the diet results in an increase in plasma nitrite levels, with corresponding decreases in systolic and diastolic blood pressures.74-76 The importance of the oral microbiome in this process has been shown through the use of antibacterial mouthwashes, as the cardioprotective effects of dietary nitrate consumption are lost when oral bacterial populations are reduced.77,78 Studies have shown that bacterial nitrate reduction mostly occurs at the dorsal surface of the tongue,79 with several bacterial taxa identified as key players, including Veillonella and Actinomyces.80 In vitro experiments have demonstrated the high capacity of Actinomyces odontolyticus to reduce nitrate without subsequent nitrite reduction.80 This species could be explored as a novel probiotic designed to be taken immediately prior to, or in conjunction with a meal, in order to maximize nitrate utilization from the diet, increase NO generation, and help lower the risk of CVD.
Probiotic influence on obesity and adiposity
Obesity is one of the primary risk factors for CVD, as the progression from overweight to obese introduces a slew of comorbidities that are detrimental to cardiovascular health.81 The excessive energy intake and fat accumulation in obesity presents major risk for type II diabetes, chronic inflammation, and hyperlipidemia, all of which predispose to coronary heart disease, cardiac arrest, and heart failure. The use of probiotics to re-set the dysbiotic obese gut microbiome is one proposed approach to improving outcomes. A study performed on healthy weight mice found that there were significant changes in the gut microbiome as well as significant weight reduction in mice receiving either Lactobacillus rhamnosus GG or Lactobacillus sakei NR28 daily by oral gavage for 3 weeks.82 There was no significant difference in food consumption between control and probiotic treatment groups, indicating that the significant reduction in epididymal fat mass was not due to a reduction of energy consumption. Results from this study indicate that the modulation of the gut microbiome with probiotic administration produces an anti-obesity effect that directly reduces epididymal fat mass. However, efforts to significantly alter the human gut microbiota using probiotics have shown more subtle effects83 and it is expecting too much to think that a single probiotic will resolve the obesity epidemic. Nevertheless, understanding the mechanisms by which probiotics affect adipose tissue, cholesterol, satiety and other factors associated with cardiovascular health, will contribute to identifying new probiotic interventions.
The twice daily administration of a multi-strain probiotic capsule (Streptococcus thermophilus, Lactobacillus plantarum, Lactobacillus acidophilus, Lactobacillus rhamnosus, Bifidobacterium lactis, Bifidobacterium longum, and Bifidobacterium breve) to overweight individuals with a body mass index greater than 25 resulted in a significant reduction in weight, waist circumference, and serum cholesterol after 8 weeks.84 These metabolic changes correlated to a significant increase in Lactobacillus plantarum populations and the overall ratio of gram negative bacteria, presumably representing the Bacteriodetes phylum. Conjugated linoleic acid (CLA) produced by certain Lactobacillus species have shown to reduce obesity and arteriosclerosis in mice.30,85 Studies investigating CLA-producing probiotic strains have demonstrated that Lactobacillus rhamnosus PL60 and Lactobacillus plantarum PL62 reduce body weight and adipose tissue mass in mice on a high fat diet in a CLA-dependent manner, without any changes in food intake.86,87 Of note, while the trans-10,cis-12 isomer of CLA have been reported to reduce adiposity and increase lean mass in mice and other animals when included at <or = 1% of the diet, there remain concerns about possible deleterious effects of trans-10,cis-12 CLA on lipid profile, glucose metabolism and insulin sensitivity.88 Although many studies show the potential benefits of CLS in a variety of conditions, as much dietary CLA in humans comes from dairy product consumption, debate continues over aspects of dairy foods on human health. Probiotics have also shown to directly reduce adipocyte cell size in high fat diet mice,89,90 which can improve oxidative stress and the subsequent chronic inflammation that is associated with inadequate blood supply to enlarged adipocytes in obesity. Several of these results are strain-specific, highlighting the variability of mechanisms of action across the multitude of probiotic strains and species.
The lowering of cholesterol and hypertension by probiotics
One of the most popular and thoroughly investigated applications for probiotic therapy for CVD is the reduction of serum cholesterol. Elevated low-density lipoprotein cholesterol (LDL-C) is a major risk factor for CVD.91 The accumulation of LDL-C in the blood is a precursor to hypertension, hyperlipidaemia, and causes the formation and build-up of atherosclerotic plaque in the arteries. Meta-analyses of randomized controlled clinical trials have been performed to evaluate the effect of probiotic consumption on serum LDL-C and total cholesterol levels. Pooled data from a total 485 total participants with ‘high,’ ‘borderline high,’ and ‘normal’ serum cholesterol levels found that probiotic consumption significantly lowered LDL-C and total cholesterol levels among all categories, compared to the control.92 The cholesterol-lowering properties of probiotics are strain and species specific with several postulated mechanisms of action. Their ingestion can sequester cholesterol from the gut by incorporation into the cellular membrane.93,94 They also covert cholesterol to coprostanol94 which is subsequently excreted in feces, potentially reducing cholesterol absorption in the gut.
Free cholesterol, of course, is an important component for cardiomyocyte function. A study of membrane incorporation of arachidonic acid (C20:4 ω6, AA) or docosahexaenoic acid (C22:6 ω3, DHA) as ω6 or ω3 polyunsaturated fatty acids on cholesterol homeostasis, showed a 2.7-fold lower cholesterol biosynthesis in AA cells than the DHA cells.95 The results demonstrated that AA incorporation into cardiomyocyte membranes decreased the free cholesterol turnover by markedly decreasing the endogenous cholesterol synthesis and by decreasing the ABCA1- and ABCG1-cholesterol efflux pathways, whereas DHA had the opposite effects. Yogurt lactic acid bacteria which produce high amounts of exopolysaccharide can remove free cholesterol from solution 96. It is important to select the probiotic strain(s) that can lower LDL cholesterol and not adversely affect cardiomyocyte function or increase fat deposition. This can be specific for probiotic strains, as shown in a study where administration of B. breve NCIMB 702258 to mice increased visceral fat mass and weight gain whereas administration of B. breve DPC 6330 did not.97 On the other hand, B. breve DPC 6330 had a greater influence on the fatty acid composition of epididymal adipose tissue, with higher palmitic acid, palmitoleic acid, and DHA, while B. breve NCIMB 702258 had a greater effect on the fatty acid composition of the brain. These effects may be direct via the probiotic, or through modulating the gut Clostridiaceae, Eubacteriaceae or other constituents. Since AA and DHA play important roles in neurogenesis, neurotransmission, and protection against oxidative stress, and their concentrations in the brain influence cognitive processes, including learning and memory,97-99 the selection and application of probiotics must be carefully conceived.
Perhaps most accepted mechanism is the processing of bile acids in the gut by Gram-positive organisms including Lactobacillus and Bifidobacterium.100,101 Metabolism of cholesterol, a precursor of bile acids, is mediated through gut microbes expressing the enzyme bile salt hydrolase (BSH). Probiotics with high BSH activity promote the deconjugation of bile acids in the gut to secondary amino acid conjugates. When these secondary conjugates are excreted, cholesterol is broken down to replace the processed bile salts. Overall, this process promotes the catabolism of cholesterol, leading to reduced serum levels. There is variability in BSH phenotypes among probiotic species, indicating that the genes which encode it are likely to be horizontally acquired.102 Many probiotic strains express more than one BSH homolog,103 potentially helping them survive in the gut when exposed to different types of bile salts.
An important aspect of the bile acid effect is the pool size, metabolite composition and compartment concentrations which relate to the composition of the gut microbiota and how it metabolizes bile acids and impacts host metabolic processes and adiposity. 104 The organisms alter expression of genes controlled by the farnesoid X receptor (FXR) through bile acids, leading to differential activation by the acids and their metabolites [chenodeoxycholic acid (CDCA) > deoxycholic acid (DCA) > lithocholic acid (LCA) >> cholic acid (CA)].105 Intake of 8 strain probiotic VSL#3 enhanced bile acid deconjugation and fecal excretion, albeit in mice. The mechanism was believed to be due to changes in ileal bile acid absorption, repression of the enterohepatic FXR-fibroblast growth factor 15, and increased hepatic bile acid neosynthesis.106,107 Induction of such physiological effects are quite dramatic for a probiotic, and warrant further study to determine if it is due to modulation of the indigenous microbiota, or specific metabolic effects of the ingested probiotic strains.
To date, Health Canada has approved only one probiotic product with cardiovascular health claims. This product, Cardioviva™, also available in the USA and Europe, contains 2 billion encapsulated Lactobacillus reuteri NCIMB 30242, clinically proven to lower LDL-cholesterol levels by 11.6% in hypercholesterolemic adults.108
Hypertension, closely tied to hypercholesterolemia, is a major risk factor for CVD.109 About 30% of Americans are hypertensive, doubling their risk for developing CVD. Reducing hypertension alone, using diuretics, angiotensin converting enzyme (ACE)-inhibitors and β-blockers is about 30% less effective than reducing hypertension by treating hypercholesterolemia in hypertensive patients. If probiotic therapy improves lipid blood chemistry, they could potentially improve hypertension and outcomes for CVD patients. A meta-analysis of 14 randomized placebo-controlled clinical trials with 702 participants show that probiotic fermented milk significantly reduced both systolic and diastolic blood pressure in pre-hypertensive and hypertensive patients.110 Certain probiotic strains produce peptides with ACE-inhibitory activity through the proteolysis and fermentation of milk proteins.111,112 When growth of Lactobacillus and Bifidobacterium strains was enhanced using fermentation substrates, or prebiotics (inulin, pectin, fructooligosaccharides, and mannitol), proteolytic activity and ACE inhibition was proportionally increased.112
By reducing cholesterol and hypertension, the risk for developing coronary heart disease, atherosclerosis, heart attack, and stroke is reduced by nearly half. The strong clinical evidence for the attenuation of hypercholesterolemia and hypertension with probiotic consumption provides support for use of these organisms in the treatment of CVD.
The protective and therapeutic effect of probiotics against myocardial infarction and heart failure
Until recently, probiotic applications for cardiovascular health were limited to metabolic and diet-associated processes. The aforementioned evidence for probiotic therapy for CVD pertains mostly to symptoms of CVD that are precursory to direct heart damage incurred by coronary artery disease, myocarditis, myocardial infarction, and heart failure. Now, there is emerging evidence that probiotics can provide a direct, cardioprotective effect to the heart that results in reduced ischemic injury and improved cardiac function, post-infarction. The protective role of probiotics against apoptotic injury was first investigated in intestinal cells. While exploring the mechanisms of action against inflammatory bowel disease, it was found the L. rhamnosus GG prevents TNF, IL-1α, and IFN-γ-induced apoptosis in mouse colon cells.113 This was achieved through both activation of the anti-apoptotic Akt pathway, and inhibition of the pro-apoptotic p38 mitogen-activated protein kinase pathway. The purification of L. rhamnosus GG supernatant identified a novel protein, p75, to be responsible.58 The effect of this protein on ischemia/reperfusion (I/R) induced heart cell injury was evaluated using a rat model. The pre-treatment of rats with the purified p75 protein isolated from L. rhamnosus GG 30 minutes prior to I/R surgery significantly attenuated heart tissue infarction in a dose-dependent manner. This phenotype was reportedly generated by enhanced expression of HSPs with p75 pretreatment114 suggesting that proteins produced by Lactobacillus probiotics have a direct cardioprotective effect against ischemic injury. Further mechanistic research is required, as the isolated p75 protein, delivered in a bolus, bypassed the gastrointestinal system. Studies examining the production and kinetics of p75 from L. rhamnosus GG within the gut can contribute to an understanding of the role of the microbiome in this phenotype.
A recent myocardial infarction study in rats demonstrated that the oral consumption of probiotics could be cardioprotective. Rats administered the probiotic drink marketed as “GoodBelly,” containing Lactobacillus plantarum 299 v, in their drinking water for up to 14 days before I/R heart surgery saw a 29% reduction in ischemia and a 23% recovery of post-ischemic mechanical, as measured by left ventricular diastolic pressure.115 This cardioprotection seems to be gut microbiome-dependent, as similar administration of vancomycin generated the same phenotype. It was found that the attenuated ischemia was independent of cytokine mediation, but dependent on serum leptin reduction. There was a significant increase in serum leptin post-I/R that was attenuated with pretreatment using GoodBelly and vancomycin. Pre-administration of exogenous leptin abolished the cardioprotection. Leptin is a hormone mainly produced by adipocytes but also by cardiomyocytes, and is typically upregulated and deleterious in CVD patients. This novel finding linked communication between the gut and the heart through hormone signaling and demonstrates an ability of orally administered probiotics to protect against myocardial infarction-associated ischemic injury.
While the application of probiotic to prevent and protect against CVD and direct damage to the heart is gaining interest, the use of probiotics as a therapy for CVD after a major cardiac event may have more practical applications for 2 reasons: Firstly, many patients do not realize they are at risk of infarction and therefore would be unlikely consuming probiotics as prophylaxis; and secondly while the mortality of myocardial infarction has improved by 40% over the past decade, the prevalence of heart failure is stagnant116 due to the irreversible damage to heart tissue caused by ischemia and infarction. Heart failure is a complex syndrome and in many cases is considered the final outcome of several manifestations of CVD. Myocardial infarction, coronary heart disease, hypertension, and chronic inflammation are all examples of confounding factors that instigate and perpetuate heart failure.
Heart failure is a progressive disease that is difficult to reverse. As a result, 50% of patients will die within the first 5 years of diagnosis. Novel strategies are desired for treating heart failure, to complement the current use of ACE-inhibitors and β-blockers. A recent animal study investigating the outcome of oral probiotic administration in rats with moderate heart failure has produced encouraging results. Following a coronary artery ligation surgery without reperfusion, rats were provided Lactobacillus rhamnosus GR-1 in their drinking water for ad libitum consumption. After 6 weeks of daily probiotic administration and sustained coronary artery ligation there was a significant attenuation of several indices of heart failure, including cardiac hypertrophy and ventricular remodeling.117 Cardiac mechanical function was also maintained at normal levels whereas mechanical function significantly deteriorated in animals on placebo treatment over the course of 6 weeks. Cecum digesta microbial analysis using 16 S rRNA next-generation sequencing indicated no significant community-level changes in the microbiota. This suggests that the attenuation of heart failure by L. rhamnosus GR-1 may be independent of the gut microbiome and instead due to a direct interaction of the probiotic with the heart. Studies in efforts to elucidate such mechanisms are ongoing. This seminal research is the first to report that probiotics might be an effective therapy for heart failure and other comorbidities that occur after a major cardiovascular event.
Conclusions
Probiotics represent one of the fastest growing consumer items on the functional food and nutraceutical market today118 meaning they are an affordable and accessible health product. They also have few side effects unlike chronic use of CVD medications such as β-blockers and ACE-inhibitors, or any pharmacological agent, which are accompanied by many adverse side effects, including cough, dizziness, hypotension, and bradycardia.119
There is a great potential to reduce the cost of CVD medication by implementing combination therapy. Patients diagnosed with CVD spend around $340 US per month on ACE-inhibitors or β-blockers alone, and around $300 US on medication for other non-cardiovascular comorbidities such as diabetes.120 For patients without access to health insurance and lacking personal financial resources, these costs stand as a strong deterrent for medication compliance. Lack of sufficient medication can exacerbate the progression of HF and eventually result in hospitalization and further economic burden. Based on the suggested retail price of commercially available probiotic products such as GoodBelly, the cost of daily consumption would be less than $40 per month. While, significantly more research is needed to understand the role of the microbiome and probiotics in CVD, the potential exists that such studies can lead to new approaches to preventing and managing this disease.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Human Microbiome Project Consortium . Structure, function and diversity of the healthy human microbiome. Nature 2012; 486:207-214; PMID:22699609; http://dx.doi.org/ 10.1038/nature11234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cho I, Blaser MJ. The human microbiome: At the interface of health and disease. Nat Rev Genet 2012; 13:260-270; PMID:22411464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature 2007; 449:804-810; PMID:17943116; http://dx.doi.org/ 10.1038/nature06244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hamady M, Knight R. Microbial community profiling for human microbiome projects: Tools, techniques, and challenges. Genome Res 2009; 19:1141-1152; PMID:19383763; http://dx.doi.org/ 10.1101/gr.085464.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science 2005; 308:1635-1638; PMID:15831718; http://dx.doi.org/ 10.1126/science.1110591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: Human gut microbes associated with obesity. Nature 2006; 444:1022-1023; PMID:17183309; http://dx.doi.org/ 10.1038/4441022a [DOI] [PubMed] [Google Scholar]
- 7. Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature 2012; 489:242-249; PMID:22972297; http://dx.doi.org/ 10.1038/nature11552 [DOI] [PubMed] [Google Scholar]
- 8. Mendis S, Puska P, Norrving B. Global atlas on cardiovascular disease prevention and control. World Health Organization, World Heart Federation, World Stroke Organization 2011; ISBN 978-92-4-156437-3 [Google Scholar]
- 9. Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, Lakshmanan A, Wade WG. The human oral microbiome. J Bacteriol 2010; 192:5002-5017; PMID:20656903; http://dx.doi.org/ 10.1128/JB.00542-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zaura E, Keijser BJ, Huse SM, Crielaard W. Defining the healthy “core microbiome” of oral microbial communities. BMC Microbiol 2009; 9:259-2180-9-259; PMID:19144191; http://dx.doi.org/ 10.1186/1471-2180-9-259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marsh P. Role of the oral microflora in health. Microb Ecol Health Dis 2000; 12:130-137; http://dx.doi.org/ 10.1080/089106000750051800 [DOI] [Google Scholar]
- 12. Marijon E, Mirabel M, Celermajer DS, Jouven X. Rheumatic heart disease. Lancet 2012; 379:953-964; PMID:22405798; http://dx.doi.org/ 10.1016/S0140-6736(11)61171-9 [DOI] [PubMed] [Google Scholar]
- 13. Bessen DE. Population biology of the human restricted pathogen, Streptococcus pyogenes. Infect Genet Evol 2009; 9:581-593; PMID:19460325; http://dx.doi.org/ 10.1016/j.meegid.2009.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Desvarieux M, Demmer RT, Jacobs DR, Papapanou PN, Sacco RL, Rundek T. Changes in clinical and microbiological periodontal profiles relate to progression of carotid intima-media thickness: The oral infections and vascular disease epidemiology study. J Am Heart Assoc 2013; 2:e000254; PMID:24166489; http://dx.doi.org/ 10.1161/JAHA.113.000254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mustapha IZ, Debrey S, Oladubu M, Ugarte R. Markers of systemic bacterial exposure in periodontal disease and cardiovascular disease risk: A systematic review and meta-analysis. J Periodontol 2007; 78:2289-2302; PMID:18052701; http://dx.doi.org/ 10.1902/jop.2007.070140 [DOI] [PubMed] [Google Scholar]
- 16. Dietrich T, Sharma P, Walter C, Weston P, Beck J. The epidemiological evidence behind the association between periodontitis and incident atherosclerotic cardiovascular disease. J Clin Periodontol 2013; 40 Suppl 14:S70-84 [DOI] [PubMed] [Google Scholar]
- 17. Lockhart PB, Bolger AF, Papapanou PN, Osinbowale O, Trevisan M, Levison ME, Taubert KA, Newburger JW, Gornik HL, Gewitz MH, et al. American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young, Council on Epidemiology and Prevention, Council on Peripheral Vascular Disease, and Council on Clinical Cardiology. Periodontal disease and atherosclerotic vascular disease: Does the evidence support an independent association? A scientific statement from the American heart association. Circulation 2012; 125:2520-2544; PMID:22514251; http://dx.doi.org/ 10.1161/CIR.0b013e31825719f3 [DOI] [PubMed] [Google Scholar]
- 18. Teles R, Wang CY. Mechanisms involved in the association between periodontal diseases and cardiovascular disease. Oral Dis 2011; 17:450-461; PMID:21223455; http://dx.doi.org/ 10.1111/j.1601-0825.2010.01784.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Darveau RP. Periodontitis: A polymicrobial disruption of host homeostasis. Nat Rev Microbiol 2010; 8:481-490; PMID:20514045; http://dx.doi.org/ 10.1038/nrmicro2337 [DOI] [PubMed] [Google Scholar]
- 20. Haraszthy VI, Zambon JJ, Trevisan M, Zeid M, Genco RJ. Identification of periodontal pathogens in atheromatous plaques. J Periodontol 2000; 71:1554-1560; PMID:11063387; http://dx.doi.org/ 10.1902/jop.2000.71.10.1554 [DOI] [PubMed] [Google Scholar]
- 21. Ott SJ, El Mokhtari NE, Musfeldt M, Hellmig S, Freitag S, Rehman A, Kühbacher T, Nikolaus S, Namsolleck P, Blaut M, et al. Detection of diverse bacterial signatures in atherosclerotic lesions of patients with coronary heart disease. Circulation 2006; 113:929-937; PMID:16490835; http://dx.doi.org/ 10.1161/CIRCULATIONAHA.105.579979 [DOI] [PubMed] [Google Scholar]
- 22. Koren O, Spor A, Felin J, Fåk F, Stombaugh J, Tremaroli V, Behre CJ, Knight R, Fagerberg B, Ley RE, et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci U S A 2011; 108 Suppl 1:4592-4598; PMID:20937873; http://dx.doi.org/ 10.1073/pnas.1011383107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ford PJ, Gemmell E, Hamlet SM, Hasan A, Waler PJ, West MJ, Cullinan MP, Seymour GJ. Cross-reactivity of GroEL antibodies with human heat shock protein 60 and quantification of pathogens in atherosclerosis. Oral Microbiol Immunol 2005; 20:296-302; PMID:16101965; http://dx.doi.org/ 10.1111/j.1399-302X.2005.00230.x [DOI] [PubMed] [Google Scholar]
- 24. Metzler B, Schett G, Kleindienst R, van der Zee R, Ottenhoff T, Hajeer A, Bernstein R, Xu Q, Wick G. Epitope specificity of anti-heat shock protein 65/60 serum antibodies in atherosclerosis. Arterioscler Thromb Vasc Biol 1997; 17:536-541; PMID:9102173; http://dx.doi.org/ 10.1161/01.ATV.17.3.536 [DOI] [PubMed] [Google Scholar]
- 25. Noack B, Genco RJ, Trevisan M, Grossi S, Zambon JJ, De Nardin E. Periodontal infections contribute to elevated systemic C-reactive protein level. J Periodontol 2001; 72: 1221-1227; PMID:11577954; http://dx.doi.org/ 10.1902/jop.2000.72.9.1221 [DOI] [PubMed] [Google Scholar]
- 26. Yoshii S, Tsuboi S, Morita I, Takami Y, Adachi K, Inukai J, Inagaki K, Mizuno K, Nakagaki H. Temporal association of elevated C-reactive protein and periodontal disease in men. J Periodontol 2009; 80:734-739; PMID:19405826; http://dx.doi.org/ 10.1902/jop.2009.080537 [DOI] [PubMed] [Google Scholar]
- 27. Loos BG, Craandijk J, Hoek FJ, Wertheim-van Dillen PM, van der Velden U. Elevation of systemic markers related to cardiovascular diseases in the peripheral blood of periodontitis patients. J Periodontol 2000; 71:1528-1534; PMID:11063384; http://dx.doi.org/ 10.1902/jop.2000.71.10.1528 [DOI] [PubMed] [Google Scholar]
- 28. Loos BG. Systemic markers of inflammation in periodontitis. J Periodontol 2005; 76:2106-2115; PMID:16277583; http://dx.doi.org/ 10.1902/jop.2005.76.11-S.2106 [DOI] [PubMed] [Google Scholar]
- 29. Abdallah Ismail N, Ragab SH, Abd Elbaky A, Shoeib AR, Alhosary Y, Fekry D. Frequency of Firmicutes and Bacteroidetes in gut microbiota in obese and normal weight Egyptian children and adults. Arch Med Sci 2011; 7:501-507; PMID:22295035; http://dx.doi.org/ 10.5114/aoms.2011.23418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fukuda S, Ohno H. Gut microbiome and metabolic diseases. Semin Immunopathol 2014; 36:103-114; PMID:24196453; http://dx.doi.org/ 10.1007/s00281-013-0399-z [DOI] [PubMed] [Google Scholar]
- 31. Gargano LM, Hughes JM. Microbial origins of chronic diseases. Annu Rev Public Health 2014; 35:65-82; PMID:24365095; http://dx.doi.org/ 10.1146/annurev-publhealth-032013-182426 [DOI] [PubMed] [Google Scholar]
- 32. Wong JM, Esfahani A, Singh N, Villa CR, Mirrahimi A, Jenkins DJ, Kendall CW. Gut microbiota, diet, and heart disease. J AOAC Int 2012; 95:24-30; PMID:22468338; http://dx.doi.org/ 10.5740/jaoacint.SGE_Wong [DOI] [PubMed] [Google Scholar]
- 33. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006; 444:1027-1031; PMID:17183312; http://dx.doi.org/ 10.1038/nature05414 [DOI] [PubMed] [Google Scholar]
- 34. Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, et al. A core gut microbiome in obese and lean twins. Nature 2009; 457:480-484; PMID:19043404; http://dx.doi.org/ 10.1038/nature07540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sacks FM, Ornish D, Rosner B, McLanahan S, Castelli WP, Kass EH. Plasma lipoprotein levels in vegetarians: the effect of ingestion of fats from dairy products. JAMA 1985; 254:1337-1341; PMID:4021011; http://dx.doi.org/ 10.1001/jama.1985.03360100087019 [DOI] [PubMed] [Google Scholar]
- 36. Jenkins DJ, Kendall CW, Marchie A, Jenkins AL, Connelly PW, Jones PJ, Vuksan V. The Garden of Eden-plant based diets, the genetic drive to conserve cholesterol and its implications for heart disease in the 21st century. Comp Biochem Physiol A Mol Integr Physiol 2003; 136:141-151; PMID:14527636; http://dx.doi.org/ 10.1016/S1095-6433(02)00345-8 [DOI] [PubMed] [Google Scholar]
- 37. Bernstein AM, Sun Q, Hu FB, Stampfer MJ, Manson JE, Willett WC. Major dietary protein sources and risk of coronary heart disease in women. Circulation 2010; 122:876-883; PMID:20713902; http://dx.doi.org/ 10.1161/CIRCULATIONAHA.109.915165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: A systematic review and meta-analysis. Circulation 2010; 121:2271-2283; PMID:20479151; http://dx.doi.org/ 10.1161/CIRCULATIONAHA.109.924977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 2013; 19:576-585; PMID:23563705; http://dx.doi.org/ 10.1038/nm.3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Re RN. Obesity-related hypertension. Ochsner J 2009; 9:133-136; PMID:21603428 [PMC free article] [PubMed] [Google Scholar]
- 41. Lesbros-Pantoflickova D, Corthesy-Theulaz I, Blum AL. Helicobacter pylori and probiotics. J Nutr 2007; 137:812S-8S; PMID:17311980 [DOI] [PubMed] [Google Scholar]
- 42. Howell TH. Metchnikoff and prolongation of life. Age Ageing 1988; 17:420-421; PMID:3071118; http://dx.doi.org/ 10.1093/ageing/17.6.420 [DOI] [PubMed] [Google Scholar]
- 43. Food and Agriculture Association on the United Nations , World Health Organization. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria 2001 [Google Scholar]
- 44. Reid G, Beuerman D, Heinemann C, Bruce AW. Probiotic Lactobacillus dose required to restore and maintain a normal vaginal flora. FEMS Immunol Med Microbiol 2001; 32:37-41; PMID:11750220; http://dx.doi.org/ 10.1111/j.1574-695X.2001.tb00531.x [DOI] [PubMed] [Google Scholar]
- 45. Ruszczynski M, Radzikowski A, Szajewska H. Clinical trial: Effectiveness of Lactobacillus rhamnosus (strains E/N, Oxy and Pen) in the prevention of antibiotic-associated diarrhoea in children. Aliment Pharmacol Ther 2008; 28:154-161; PMID:18410562; http://dx.doi.org/ 10.1111/j.1365-2036.2008.03714.x [DOI] [PubMed] [Google Scholar]
- 46. Swidsinski A, Loening-Baucke V, Kirsch S, Doerffel Y. Functional biostructure of colonic microbiota (central fermenting area, germinal stock area and separating mucus layer) in healthy subjects and patients with diarrhea treated with Saccharomyces boulardii. Gastroenterol Clin Biol 2010; 34:S79-92; PMID:20889010; http://dx.doi.org/ 10.1016/S0399-8320(10)70025-7 [DOI] [PubMed] [Google Scholar]
- 47. Guglielmetti S, Mora D, Gschwender M, Popp K. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life-a double-blind, placebo-controlled study. Aliment Pharmacol Ther 2011; 33:1123-1132; PMID:21418261; http://dx.doi.org/ 10.1111/j.1365-2036.2011.04633.x [DOI] [PubMed] [Google Scholar]
- 48. Francavilla R, Miniello V, Magista AM, De Canio A, Bucci N, Gagliardi F, Lionetti E, Castellaneta S, Polimeno L, Peccarisi L, et al. A randomized controlled trial of Lactobacillus GG in children with functional abdominal pain. Pediatrics 2010; 126:e1445-52; PMID:21078735; http://dx.doi.org/ 10.1542/peds.2010-0467 [DOI] [PubMed] [Google Scholar]
- 49. Lyra A, Krogius-Kurikka L, Nikkila J, Malinen E, Kajander K, Kurikka K, Korpela R, Palva A. Effect of a multispecies probiotics supplement on quantity of irritable bowel syndrome-related intestinal microbial phylotypes. BMC Gastroenterol 2010; 10:110-230X-10-110; PMID:20102635; http://dx.doi.org/ 10.1186/1471-230X-10-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ducrotte P, Sawant P, Jayanthi V. Clinical trial: Lactobacillus plantarum 299v (DSM 9843) improves symptoms of irritable bowel syndrome. World J Gastroenterol 2012; 18:4012-4018; PMID:22912552; http://dx.doi.org/ 10.3748/wjg.v18.i30.4012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. McFarland LV, Surawicz CM, Greenberg RN, Fekety R, Elmer GW, Moyer KA, Melcher SA, Bowen KE, Cox JL, Noorani Z, et al. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for clostridium difficile disease. JAMA 1994; 271:1913-1918; PMID:8201735; http://dx.doi.org/ 10.1001/jama.1994.03510480037031 [DOI] [PubMed] [Google Scholar]
- 52. Dylag K, Hubalewska-Mazgaj M, Surmiak M, Szmyd J, Brzozowski T. Probiotics in the mechanism of protection against gut inflammation and therapy of gastrointestinal disorders. Curr Pharm Des 2014; 20:1149-55; PMID:23755726; http://dx.doi.org/ 10.2174/13816128113199990422 [DOI] [PubMed] [Google Scholar]
- 53. Lee YK, Puong KY. Competition for adhesion between probiotics and human gastrointestinal pathogens in the presence of carbohydrate. Br J Nutr 2002; 88:S101-8; PMID:12215184; http://dx.doi.org/ 10.1079/BJN2002635 [DOI] [PubMed] [Google Scholar]
- 54. Nes IF, Diep DB, Havarstein LS, Brurberg MB, Eijsink V, Holo H. Biosynthesis of bacteriocins in lactic acid bacteria. Antonie Van Leeuwenhoek 1996; 70:113-128; PMID:8879403; http://dx.doi.org/ 10.1007/BF00395929 [DOI] [PubMed] [Google Scholar]
- 55. Reid G, Younes JA, Van der Mei HC, Gloor GB, Knight R, Busscher HJ. Microbiota restoration: Natural and supplemented recovery of human microbial communities. Nat Rev Microbiol 2011; 9:27-38; PMID:21113182; http://dx.doi.org/ 10.1038/nrmicro2473 [DOI] [PubMed] [Google Scholar]
- 56. Reid G, Cook RL, Bruce AW. Examination of strains of lactobacilli for properties that may influence bacterial interference in the urinary tract. J Urol 1987; 138:330-335; PMID:3599250 [DOI] [PubMed] [Google Scholar]
- 57. Walker WA. Mechanisms of action of probiotics. Clin Infect Dis 2008; 46:S87-91; PMID:18181730; http://dx.doi.org/ 10.1086/523335 [DOI] [PubMed] [Google Scholar]
- 58. Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology 2007; 132:562-575; PMID:17258729; http://dx.doi.org/ 10.1053/j.gastro.2006.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Corthesy B, Gaskins HR, Mercenier A. Cross-talk between probiotic bacteria and the host immune system. J Nutr 2007; 137:781S-90S; PMID:17311975 [DOI] [PubMed] [Google Scholar]
- 60. Isolauri E, Sutas Y, Kankaanpaa P, Arvilommi H, Salminen S. Probiotics: Effects on immunity. Am J Clin Nutr 2001; 73:444S-450S; PMID:11157355 [DOI] [PubMed] [Google Scholar]
- 61. Ebel B, Lemetais G, Beney L, Cachon R, Sokol H, Langella P, Gervais P. Impact of probiotics on risk factors for cardiovascular diseases. A review. Crit Rev Food Sci Nutr 2014; 54:175-189; PMID:24188267; http://dx.doi.org/ 10.1080/10408398.2011.579361 [DOI] [PubMed] [Google Scholar]
- 62. Crittenden RG, Martinez NR, Playne MJ. Synthesis and utilisation of folate by yoghurt starter cultures and probiotic bacteria. Int J Food Microbiol 2003; 80:217-222; PMID:12423923; http://dx.doi.org/ 10.1016/S0168-1605(02)00170-8 [DOI] [PubMed] [Google Scholar]
- 63. Morishita T, Tamura N, Makino T, Kudo S. Production of menaquinones by lactic acid bacteria. J Dairy Sci 1999; 82:1897-1903; PMID:10509247; http://dx.doi.org/ 10.3168/jds.S0022-0302(99)75424-X [DOI] [PubMed] [Google Scholar]
- 64. Pompei A, Cordisco L, Amaretti A, Zanoni S, Matteuzzi D, Rossi M. Folate production by Bifidobacteria as a potential probiotic property. Appl Environ Microbiol 2007; 73:179-185; PMID:17071792; http://dx.doi.org/ 10.1128/AEM.01763-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ahola AJ, Yli-Knuuttila H, Suomalainen T, Poussa T, Ahlstrom A, Meurman JH, Korpela R. Short-term consumption of probiotic-containing cheese and its effect on dental caries risk factors. Arch Oral Biol 2002; 47:799-804; PMID:12446187; http://dx.doi.org/ 10.1016/S0003-9969(02)00112-7 [DOI] [PubMed] [Google Scholar]
- 66. Caglar E, Cildir SK, Ergeneli S, Sandalli N, Twetman S. Salivary mutans streptococci and lactobacilli levels after ingestion of the probiotic bacterium Lactobacillus reuteri ATCC 55730 by straws or tablets. Acta Odontol Scand 2006; 64:314-318; PMID:16945898; http://dx.doi.org/ 10.1080/00016350600801709 [DOI] [PubMed] [Google Scholar]
- 67. Burton JP, Chilcott CN, Moore CJ, Speiser G, Tagg JR. A preliminary study of the effect of probiotic Streptococcus salivarius K12 on oral malodour parameters. J Appl Microbiol 2006; 100:754-764; PMID:16553730; http://dx.doi.org/ 10.1111/j.1365-2672.2006.02837.x [DOI] [PubMed] [Google Scholar]
- 68. Kang MS, Kim BG, Chung J, Lee HC, Oh JS. Inhibitory effect of Weissella cibaria isolates on the production of volatile sulphur compounds. J Clin Periodontol 2006; 33:226-232; PMID:16489950; http://dx.doi.org/ 10.1111/j.1600-051X.2006.00893.x [DOI] [PubMed] [Google Scholar]
- 69. Di Pierro F, Donato G, Fomia F, Adami T, Careddu D, Cassandro C, Albera R. Preliminary pediatric clinical evaluation of the oral probiotic Streptococcus salivarius K12 in preventing recurrent pharyngitis and/or tonsillitis caused by Streptococcus pyogenes and recurrent acute otitis media. Int J Gen Med 2012; 5:991-997; PMID:23233809; http://dx.doi.org/ 10.2147/IJGM.S38859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Teughels W, Durukan A, Ozcelik O, Pauwels M, Quirynen M, Haytac MC. Clinical and microbiological effects of Lactobacillus reuteri probiotics in the treatment of chronic periodontitis: A randomized placebo-controlled study. J Clin Periodontol 2013; 40:1025-1035; PMID:24164569; http://dx.doi.org/ 10.1111/jcpe.12155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Shimauchi H, Mayanagi G, Nakaya S, Minamibuchi M, Ito Y, Tamaki K, Hirata H. Improvement of periodontal condition by probiotics with Lactobacillus salivarius WB21: A randomized, double-blind, placebo-controlled study. J Clin Periodontol 2008; 35:897-905; PMID:18727656; http://dx.doi.org/ 10.1111/j.1600-051X.2008.01306.x [DOI] [PubMed] [Google Scholar]
- 72. Hezel M, Weitzberg E. The oral microbiome and nitric oxide homoeostasis. Oral Dis 2013; PMID:23837897 [DOI] [PubMed] [Google Scholar]
- 73. Weitzberg E, Lundberg JO. Novel aspects of dietary nitrate and human health. Annu Rev Nutr 2013; 33:129-159; PMID:23642194; http://dx.doi.org/ 10.1146/annurev-nutr-071812-161159 [DOI] [PubMed] [Google Scholar]
- 74. Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, Rashid R, Miall P, Deanfield J, Benjamin N, et al. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension 2008; 51:784-790; PMID:18250365; http://dx.doi.org/ 10.1161/HYPERTENSIONAHA.107.103523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kapil V, Milsom AB, Okorie M, Maleki-Toyserkani S, Akram F, Rehman F, Arghandawi S, Pearl V, Benjamin N, Loukogeorgakis S, et al. Inorganic nitrate supplementation lowers blood pressure in humans: Role for nitrite-derived NO. Hypertension 2010; 56:274-281; PMID:20585108; http://dx.doi.org/ 10.1161/HYPERTENSIONAHA.110.153536 [DOI] [PubMed] [Google Scholar]
- 76. Ghosh SM, Kapil V, Fuentes-Calvo I, Bubb KJ, Pearl V, Milsom AB, Khambata R, Maleki-Toyserkani S, Yousuf M, Benjamin N, et al. Enhanced vasodilator activity of nitrite in hypertension: Critical role for erythrocytic xanthine oxidoreductase and translational potential. Hypertension 2013; 61:1091-1102; PMID:23589565; http://dx.doi.org/ 10.1161/HYPERTENSIONAHA.111.00933 [DOI] [PubMed] [Google Scholar]
- 77. Kapil V, Haydar SM, Pearl V, Lundberg JO, Weitzberg E, Ahluwalia A. Physiological role for nitrate-reducing oral bacteria in blood pressure control. Free Radic Biol Med 2013; 55:93-100; PMID:23183324; http://dx.doi.org/ 10.1016/j.freeradbiomed.2012.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Petersson J, Carlström M, Schreiber O, Phillipson M, Christoffersson G, Jägare A, Roos S, Jansson EA, Persson AE, Lundberg JO, et al. Gastroprotective and blood pressure lowering effects of dietary nitrate are abolished by an antiseptic mouthwash. Free Radic Biol Med 2009; 46:1068-1075; PMID:19439233; http://dx.doi.org/ 10.1016/j.freeradbiomed.2009.01.011 [DOI] [PubMed] [Google Scholar]
- 79. Doel JJ, Benjamin N, Hector MP, Rogers M, Allaker RP. Evaluation of bacterial nitrate reduction in the human oral cavity. Eur J Oral Sci 2005; 113:14-19; PMID:15693824; http://dx.doi.org/ 10.1111/j.1600-0722.2004.00184.x [DOI] [PubMed] [Google Scholar]
- 80. Hyde ER, Andrade F, Vaksman Z, Parthasarathy K, Jiang H, Parthasarathy DK, Torregrossa AC, Tribble G, Kaplan HB, Petrosino JF, et al. Metagenomic analysis of nitrate-reducing bacteria in the oral cavity: Implications for nitric oxide homeostasis. PLoS One 2014; 9:e88645; PMID:24670812; http://dx.doi.org/ 10.1371/journal.pone.0088645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: An update of the 1997 American Heart Association scientific statement on obesity and heart disease from the obesity committee of the council on nutrition, physical activity, and metabolism. Circulation 2006; 113:898-918; PMID:16380542; http://dx.doi.org/ 10.1161/CIRCULATIONAHA.106.171016 [DOI] [PubMed] [Google Scholar]
- 82. Ji YS, Kim HN, Park HJ, Lee JE, Yeo SY, Yang JS, Park SY, Yoon HS, Cho GS, Franz CM, et al. Modulation of the murine microbiome with a concomitant anti-obesity effect by Lactobacillus rhamnosus GG and Lactobacillus sakei NR28. Benef Microbes 2012; 3:13-22; PMID:22348905; http://dx.doi.org/ 10.3920/BM2011.0046 [DOI] [PubMed] [Google Scholar]
- 83. McNulty NP, Yatsunenko T, Hsiao A, Faith JJ, Muegge BD, Goodman AL, Henrissat B, Oozeer R, Cools-Portier S, Gobert G, et al. The impact of a consortium of fermented milk strains on the gut microbiome of gnotobiotic mice and monozygotic twins. Sci Transl Med 2011; 3:106; http://dx.doi.org/ 10.1126/scitranslmed.3002701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lee SJ, Bose S, Seo JG, Chung WS, Lim CY, Kim H. The effects of co-administration of probiotics with herbal medicine on obesity, metabolic endotoxemia and dysbiosis: A randomized double-blind controlled clinical trial. Clin Nutr 2013; S0261-5614:00329-4 [DOI] [PubMed] [Google Scholar]
- 85. West DB, Delany JP, Camet PM, Blohm F, Truett AA, Scimeca J. Effects of conjugated linoleic acid on body fat and energy metabolism in the mouse. Am J Physiol 1998; 275:R667-72; PMID:9728060 [DOI] [PubMed] [Google Scholar]
- 86. Lee HY, Park JH, Seok SH, Baek MW, Kim DJ, Lee KE, Paek KS, Lee Y, Park JH. Human originated bacteria, Lactobacillus rhamnosus PL60, produce conjugated linoleic acid and show anti-obesity effects in diet-induced obese mice. Biochim Biophys Acta 2006; 1761:736-744; PMID:16807088; http://dx.doi.org/ 10.1016/j.bbalip.2006.05.007 [DOI] [PubMed] [Google Scholar]
- 87. Lee K, Paek K, Lee HY, Park JH, Lee Y. Antiobesity effect of trans-10,cis-12-conjugated linoleic acid-producing Lactobacillus plantarum PL62 on diet-induced obese mice. J Appl Microbiol 2007; 103:1140-1146; PMID:17897219; http://dx.doi.org/ 10.1111/j.1365-2672.2007.03336.x [DOI] [PubMed] [Google Scholar]
- 88. Silveira MB, Carraro R, Monereo S, Tébar J. Conjugated linoleic acid (CLA) and obesity. Public Health Nutr 2007. Oct; 10(10A):1181-6; PMID:17903328; http://dx.doi.org/ 10.1017/S1368980007000687 [DOI] [PubMed] [Google Scholar]
- 89. Hamad EM, Sato M, Uzu K, Yoshida T, Higashi S, Kawakami H, Kadooka Y, Matsuyama H, Abd El-Gawad IA, Imaizumi K. Milk fermented by Lactobacillus gasseri SBT2055 influences adipocyte size via inhibition of dietary fat absorption in Zucker rats. Br J Nutr 2009; 101:716-724; PMID:18684338; http://dx.doi.org/ 10.1017/S0007114508043808 [DOI] [PubMed] [Google Scholar]
- 90. Takemura N, Okubo T, Sonoyama K. Lactobacillus plantarum strain no. 14 reduces adipocyte size in mice fed high-fat diet. Exp Biol Med (Maywood) 2010; 235:849-856; PMID:20558839; http://dx.doi.org/ 10.1258/ebm.2010.009377 [DOI] [PubMed] [Google Scholar]
- 91. Grundy SM. Promise of low-density lipoprotein-lowering therapy for primary and secondary prevention. Circulation 2008; 117:569-73; PMID:18227397; http://dx.doi.org/ 10.1161/CIRCULATIONAHA.107.720300 [DOI] [PubMed] [Google Scholar]
- 92. Guo Z, Liu XM, Zhang QX, Liu XM, Zhang QX, Shen Z, Tian FW, Zhang H, Sun ZH, Zhang HP, et al. Influence of consumption of probiotics on the plasma lipid profile: A meta-analysis of randomised controlled trials. Nutr Metab Cardiovasc Dis 2011; 21:844-850; PMID:21930366; http://dx.doi.org/ 10.1016/j.numecd.2011.04.008 [DOI] [PubMed] [Google Scholar]
- 93. Kimoto H, Ohmomo S, Okamoto T. Cholesterol removal from media by lactococci. J Dairy Sci 2002; 85:3182-3188; PMID:12512591; http://dx.doi.org/ 10.3168/jds.S0022-0302(02)74406-8 [DOI] [PubMed] [Google Scholar]
- 94. Lye HS, Rahmat-Ali GR, Liong MT. Mechanisms of cholesterol removal by lactobacilli under conditions that mimic the human gastrointestinal tract. Int Dairy J 2010; 20:169-175; http://dx.doi.org/ 10.1016/j.idairyj.2009.10.003 [DOI] [Google Scholar]
- 95. Doublet A, Robert V, Vedie B, Rousseau-Ralliard D, Reboulleau A, Grynberg A, Paul JL, Fournier N. Contrasting effects of arachidonic acid and docosahexaenoic acid membrane incorporation into cardiomyocytes on free cholesterol turnover. Biochim Biophys Acta 2014. Oct; 1842(10):1413-21 [DOI] [PubMed] [Google Scholar]
- 96. Tok E, Aslim B. Cholesterol removal by some lactic acid bacteria that can be used as probiotic. Microbiol Immunol 2010. May; 54(5):257-64 [DOI] [PubMed] [Google Scholar]
- 97. Wall R, Marques TM, O’Sullivan O, Ross RP, Shanahan F, Quigley EM, Dinan TG, Kiely B, Fitzgerald GF, Cotter PD, et al. Contrasting effects of Bifidobacterium breve NCIMB 702258 and Bifidobacterium breve DPC 6330 on the composition of murine brain fatty acids and gut microbiota. Am J Clin Nutr 2012. May; 95(5):1278-87; PMID:22492373; http://dx.doi.org/ 10.3945/ajcn.111.026435 [DOI] [PubMed] [Google Scholar]
- 98. Maekawa M, Takashima N, Matsumata M, Ikegami S, Kontani M, Hara Y, Kawashima H, Owada Y, Kiso Y, Yoshikawa T, et al. Arachidonic acid drives postnatal neurogenesis and elicits a beneficial effect on prepulse inhibition, a biological trait of psychiatric illnesses. PLoS One 2009; 4(4):e5085; PMID:19352438; http://dx.doi.org/ 10.1371/journal.pone.0005085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Yurko-Mauro K, McCarthy D, Rom D, Nelson EB, Ryan AS, Blackwell A, Salem N, Jr, Stedman M. MIDAS Investigators. Beneficial effects of docosahexaenoic acid on cognition in age-related cognitive decline. Alzheimers Dement 2010. Nov; 6(6):456-64; http://dx.doi.org/ 10.1016/j.jalz.2010.01.013 [DOI] [PubMed] [Google Scholar]
- 100. Pavlovic N, Stankov K, Mikov M. Probiotics-interactions with bile acids and impact on cholesterol metabolism. Appl Biochem Biotechnol 2012; 168:1880-1895; PMID:23054820; http://dx.doi.org/ 10.1007/s12010-012-9904-4 [DOI] [PubMed] [Google Scholar]
- 101. Elkins CA, Moser SA, Savage DC. Genes encoding bile salt hydrolases and conjugated bile salt transporters in Lactobacillus johnsonii 100-100 and other Lactobacillus species. Microbiology 2001; 147:3403-3412; PMID:11739773 [DOI] [PubMed] [Google Scholar]
- 102. Kumar M, Nagpal R, Kumar R, Hemalatha R, Verma V, Kumar A, Chakraborty C, Singh B, Marotta F, Jain S, et al. Cholesterol-lowering probiotics as potential biotherapeutics for metabolic diseases. Exp Diabetes Res 2012; 2012:902917; PMID:22611376; http://dx.doi.org/ 10.1155/2012/902917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Begley M, Hill C, Gahan CG. Bile salt hydrolase activity in probiotics. Appl Environ Microbiol 2006. Mar; 72(3):1729-38; PMID:16517616; http://dx.doi.org/ 10.1128/AEM.72.3.1729-1738.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Joyce SA, MacSharry J, Casey PG, Kinsella M, Murphy EF, Shanahan F, Hill C, Gahan CG. Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut. Proc Natl Acad Sci USA 2014. May 20;111(20):7421-6; PMID:24799697; http://dx.doi.org/ 10.1073/pnas.1323599111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall HU, Bamberg K, Angelin B, Hyötyläinen T, Orešič M, Bäckhed F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-β-muricholic acid, a naturally occurring FXR antagonist. Cell Metab 2013. Feb 5; 17(2):225-35; http://dx.doi.org/ 10.1016/j.cmet.2013.01.003 [DOI] [PubMed] [Google Scholar]
- 106. Jones ML, Tomaro-Duchesneau C, Prakash S. The gut microbiome, probiotics, bile acids axis, and human health. Trends Microbiol 2014. Jun; 22(6):306-8; PMID:24836108; http://dx.doi.org/ 10.1016/j.tim.2014.04.010 [DOI] [PubMed] [Google Scholar]
- 107. Degirolamo C, Rainaldi S, Bovenga F, Murzilli S, Moschetta A. Microbiota modification with probiotics induces hepatic bile acid synthesis via downregulation of the Fxr-Fgf15 axis in mice. Cell Rep 2014. Apr 10; 7(1):12-8; PMID:24656817; http://dx.doi.org/ 10.1016/j.celrep.2014.02.032 [DOI] [PubMed] [Google Scholar]
- 108. Jones ML, Martoni CJ, Prakash S. Cholesterol lowering and inhibition of sterol absorption by Lactobacillus reuteri NCIMB 30242: A randomized controlled trial. Eur J Clin Nutr 2012; 66:1234-1241; PMID:22990854; http://dx.doi.org/ 10.1038/ejcn.2012.126 [DOI] [PubMed] [Google Scholar]
- 109. Egan BM, Li J, Qanungo S, Wolfman TE. Blood pressure and cholesterol control in hypertensive hypercholesterolemic patients: National health and nutrition examination surveys 1988-2010. Circulation 2013; 128:29-41; PMID:23817481; http://dx.doi.org/ 10.1161/CIRCULATIONAHA.112.000500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Dong JY, Szeto IM, Makinen K, Gao Q, Wang J, Qin LQ, Zhao Y. Effect of probiotic fermented milk on blood pressure: A meta-analysis of randomised controlled trials. Br J Nutr 2013; 110:1188-1194; PMID:23823502; http://dx.doi.org/ 10.1017/S0007114513001712 [DOI] [PubMed] [Google Scholar]
- 111. Gonzalez-Gonzalez C, Gibson T, Jauregi P. Novel probiotic-fermented milk with angiotensin I-converting enzyme inhibitory peptides produced by Bifidobacterium bifidum MF 20/5. Int J Food Microbiol 2013; 167:131-137; PMID:24135669; http://dx.doi.org/ 10.1016/j.ijfoodmicro.2013.09.002 [DOI] [PubMed] [Google Scholar]
- 112. Yeo SK, Liong MT. Angiotensin I-converting enzyme inhibitory activity and bioconversion of isoflavones by probiotics in soymilk supplemented with prebiotics. Int J Food Sci Nutr 2010; 61:161-181; PMID:20085504; http://dx.doi.org/ 10.3109/09637480903348122 [DOI] [PubMed] [Google Scholar]
- 113. Yan F, Polk DB. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J Biol Chem 2002; 277:50959-50965; PMID:12393915; http://dx.doi.org/ 10.1074/jbc.M207050200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Zhao B, Sun G, Feng G, Duan W, Zhu X, Chen S, Hou L, Jin Z, Yi D. Carboxy terminus of heat shock protein (HSP) 70-interacting protein (CHIP) inhibits HSP70 in the heart. J Physiol Biochem 2012; 68:485-491; PMID:22456997; http://dx.doi.org/ 10.1007/s13105-012-0161-3 [DOI] [PubMed] [Google Scholar]
- 115. Lam V, Su J, Koprowski S, Hsu A, Tweddell JS, Rafiee P, Gross GJ, Salzman NH, Baker JE. Intestinal microbiota determine severity of myocardial infarction in rats. FASEB J 2012; 26:1727-1735; PMID:22247331; http://dx.doi.org/ 10.1096/fj.11-197921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2014 update: A report from the American Heart Association. Circulation 2014; 129:e28-e292; PMID:24352519; http://dx.doi.org/ 10.1161/01.cir.0000441139.02102.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Gan XT, Ettinger G, Huang CX, Burton JP, Haist JV, Rajapurohitam V, Sidaway JE, Martin G, Gloor GB, Swann JR, et al. Probiotic administration attenuates myocardial hypertrophy and heart failure following myocardial infarction in the rat. Circ Heart Fail 2014; 7:491-9; PMID:24625365; http://dx.doi.org/ 10.1161/CIRCHEARTFAILURE.113.000978 [DOI] [PubMed] [Google Scholar]
- 118. Sanders ME. Probiotics: Definition, sources, selection, and uses. Clin Infect Dis 2008; 46:S58-61; PMID:18181724; http://dx.doi.org/ 10.1086/523341 [DOI] [PubMed] [Google Scholar]
- 119. Metra M, Giubbini R, Nodari S, Boldi E, Modena MG, Dei Cas L. Differential effects of beta-blockers in patients with heart failure: A prospective, randomized, double-blind comparison of the long-term effects of metoprolol versus carvedilol. Circulation 2000; 102:546-551; PMID:10920067; http://dx.doi.org/ 10.1161/01.CIR.102.5.546 [DOI] [PubMed] [Google Scholar]
- 120. Hussey LC, Hardin S, Blanchette C. Outpatient costs of medications for patients with chronic heart failure. Am J Crit Care 2002; 11:474-478; PMID:12233973 [PubMed] [Google Scholar]


