Abstract
Retinal transduction by intravitreally administered adeno-associated viral (AAV) vector is previously known to be extremely limited to the neural retina except AAV2 capsid type. Recently, we showed that prior laser photocoagulation enhances retinal transduction of intravitreally administered AAV vectors, including the outer retina and retinal pigment epithelium (RPE). Here, by performing short-pulse laser pretreatment on the mouse retina, we demonstrate RPE cells transduced by three different capsid types of AAV vectors, AAV2, AAV5, and AAV8, using RPE wholemounts. For all capsid types, laser pretreatment effectively induced the transduction of RPE cells in and around the laser site.
Adeno-associated virus (AAV) is one of the most promising viral vectors in the field of gene therapy for retinal diseases. Two kinds of vector administration methods, subretinal and intravitreal, have been introduced, but both methods possess some limitations. Subretinal injection is an invasive technique requiring a surgical procedure, while intravitreal injection is relatively safe but does not effectively transduce cells in the outer retina and retinal pigment epithelium (RPE) cells. In the previous study, using intravitreal administration route, we demonstrated enhanced focal transduction of AAV vectors after laser pretreatment on mouse retina in three different capsid types, AAV2, 5, and 8, and identified that efficiency of transduction and types of cells transduced were different depending on the vector capsid types.1 However, we did not manage to show the specific transduction pattern of RPE cells, which lies in the most outer layer of the retina.
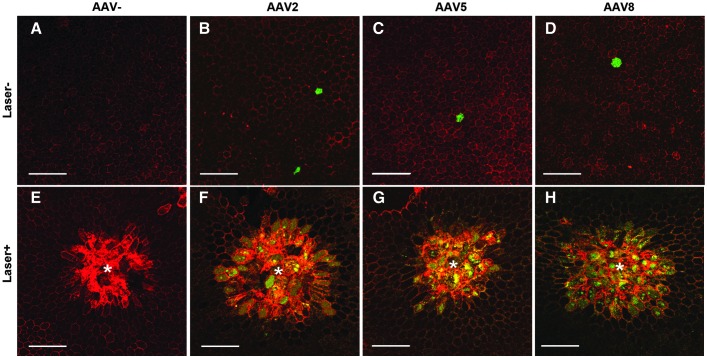
The images described here show RPE cells transduced with three different self-complementary AAV (scAAV) vectors, scAAV2, scAAV5, and scAAV8, expressing enhanced green fluorescent protein (EGFP) after laser pretreatment (Fig. 1). Forty-eight hours before intravitreal vector administration, laser photocoagulation was performed on the mouse retina of the right eye using laser parameter of 200 μm spot size, 0.02 sec duration, and 100 mW laser power. Ten laser spots were distributed in a concentric pattern 1 mm apart from the optic nerve head. Fifteen mice (5 mice for each capsid type) received 1 μl bilateral, intravitreal injections of scAAV2-CMV-EGFP (6.32 × 1011 viral genomes [vg]/ml), scAAV5-CMV-EGFP (3.38 × 1010 vg/ml), or scAAV8-CMV-EGFP (3.65 × 1010 vg/ml). Five mice did not receive vector administration to serve as controls. At 28 days of injection, eyeballs were enucleated and were dissected into posterior eye cups with neural retina removed for RPE wholemount immunohistochemistry. The wholemounts were examined with confocal microscopy (LSM510 meta; Carl Zeiss Inc., Oberkochen, Germany), and the images were captured using image-capture software (LSM image browser; Carl Zeiss Inc.) with 200× magnification.
Figure 1.
Short-pulse laser pretreatment induced transduction of adeno-associated viral (AAV) vector into retinal pigment epithelial (RPE) cells. Shown are representative confocal microscopic RPE wholemount images (200×) of the control (laser-untreated/vector-uninjected, laser-treated/vector-uninjected, and laser-untreated/vector-injected; A–E) and scAAV type 2-, 5-, and 8-treated eyes after laser pretreatment (F–H). Morphological changes of RPE cells were also observed at the lasered region. AAV5 vector (G) transduced fewer RPE cells compared with AAV2 (F) and AAV8 (H). Several RPE cells adjacent to the laser site were also transduced by AAV vectors. Laser-untreated/vector-uninjected and laser-treated/vector-uninjected control eyes did not show any EGFP expression (A and E), while laser-untreated/vector-injected eyes demonstrated few of sporadic EGFP expression in the RPE cells. The wholemount was processed with β-catenin (red) immunostaining to show the intact cell-to-cell junction. Some of the RPE cells showed cytoplasmic accumulation of β-catenin, indicating the activation of canonical Wnt signaling pathway. *Center of laser-treated region. The scale bars represent 100 μm.
Using laser pulse duration of 0.04 sec, our previous studies demonstrated that the photothermal damage was confirmed to the photoreceptors and the RPE cells.1,2 In this experiment, we used a shorter duration (0.02 sec) than previously performed, and were able to minimize the photothermal damage of photoreceptor and the RPE cells. After 28 days of injection, immunostaining with β-catenin, a well-known protein involved in both cell–cell adhesion and canonical Wnt signaling,3 clearly showed that short-pulse laser resulted in robust EGFP expression of not only RPE cells in the laser-treated site but also cells nearby the lesion for all capsid types (Fig. 1F–H). Lesser extent of EGFP expression in RPE cells was observed in scAAV5-treated eyes (Fig. 1G) compared with the results from scAAV2- and scAAV8-treated eyes. On the other hand, there were no signs of transduction in laser-treated/vector-uninjected eyes, while few of sporadic EGFP-positive RPE cells were observed in laser-untreated/vector-injected eyes (Fig. 1A–E). For the control eyes without laser treatment, the images were taken from the regions corresponding to the approximately same distance (1 mm) from the optic nerve head at which the laser photocoagulation was performed.
To quantify the transduction efficiency of each capsid type, we manually counted EGFP-positive RPE cells using ImageJ (National Institutes of Health, Bethesda, MD), and compared the differences among the types using the one-way ANOVA test with Tukey's post hoc analysis from SPSS software version 20.0 for Windows (SPSS Inc., Chicago, IL). The mean values for the number of EGFP-positive cells for each capsid type were as follows: AAV2, 46.53 ± 5.93; AAV5, 33.93 ± 4.67; and AAV8, 47.26 ± 5.97. There were statistically significant differences in the number of EGFP-positive RPE cells between scAAV2- and scAAV5-treated eyes (p < 0.001), and scAAV8- and scAAV5-treated eyes (p < 0.001), while no significant difference was observed between scAAV2- and scAAV8-treated eyes (p = 0.806). The RPE cells at laser-treated regions did not lose their gross integrity, but mild morphological changes, such as elongation and irregularity of size and shape of the cells, were observed. Moreover, cytoplasmic accumulation of β-catenin was evident in some RPE cells, indicating the possible activation of canonical Wnt signaling pathway or initiation of other functional role of the protein.
The mechanism for RPE cell transduction of AAV vector after laser pretreatment is unclear. When treated with laser parameters used here and in our previous study,2 the damage was limited to the photoreceptors and RPE cells. Hence, it seems obvious that the RPE cell transduction is not because of direct thermal disruption of vitreoretinal junction, a major barrier for AAV vector transduction.4 Previously proposed hypothesis of cell stress response and upregulation of capsid receptors induced by laser photocoagulation rather fit well with the situation.1 Still, the specific mechanism involved in the enhanced transduction process by laser treatment needs to be further elucidated. Moreover, considering the morphological changes of RPE cells observed in the laser-treated sites, we cannot rule out the fact that the function of the vector-transduced RPE cells may have been changed by laser pretreatment, even though we used short-pulse duration to minimize the damage of RPE cells. The functional aspect of the transduced RPE cells in laser-treated sites should be further evaluated in the future studies.
Notwithstanding these limitations, these results, together with the previous ones we reported,1 clearly demonstrate the laser-enhanced transduction of AAV vectors, regardless of capsid types. Additionally, short-pulse laser pretreatment induced effective and selective transduction of RPE cells by intravitreally administered AAV vectors.
Acknowledgments
This work was supported by Basic Science Research Program through the National Research Foundation of Korea funded by the ministry of Education, Science, and Technology (Grant Number 2013R1A1A2009899). This work was supported partially by the Soonchunhyang University Research Fund.
Author Disclosure
No competing financial interests exist.
References
- 1.Lee SH, Colosi P, Lee H, et al. Laser photocoagulation enhances adeno-associated viral vector transduction of mouse retina. Hum Gene Ther Methods 2014;25:83–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee SH, Kim HD, Park YJ, et al. Time-dependent changes of cell proliferation after laser photocoagulation in mouse chorioretinal tissue. Invest Ophthalmol Vis Sci 2015;56:2696–2708 [DOI] [PubMed] [Google Scholar]
- 3.McCrea PD, Gu D. The catenin family at a glance. J Cell Sci 2010;123:637–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalkara D, Kolstad KD, Caporale N, et al. Inner limiting membrane barriers to AAV-mediated retinal transduction from the vitreous. Mol Ther 2009;17:2096–2102 [DOI] [PMC free article] [PubMed] [Google Scholar]