Abstract
Alterations in the gut microbiota have been implicated to play a role in potentiating inflammatory bowel diseases in both humans and mice. Mice lacking the flagellin receptor, toll-like receptor 5 (TLR5), are prone to develop spontaneous gut inflammation, but are significantly protected when treated with antibiotics or maintained in germ-free conditions. However, given that the incidence of spontaneous inflammation in TLR5KO mice is quite variable in conventional conditions (typically ∼10% show clear colitis), this result is far from definitive and does not rule out that TLR5KO mice might be prone to develop inflammation even in the absence of a microbiota. Herein, we demonstrate that neutralization of IL10 signaling induces colitis in 100% of TLR5KO mice which provide a more rigorous approach to evaluate the role of microbiota in gut inflammation. Mice treated with antibiotics or maintained in germ-free condition are substantially protected against IL-10R neutralization-induced colitis, underscoring that gut inflammation in TLR5KO mice is dependent upon the presence of a gut microbiota.
Keywords: Germ-free mice, Gut bacteria, Interleukin-10, Intestinal inflammation, Toll-like receptor
Abbreviations
- Abx
antibiotics
- GF
germ-free
- IL-10
interleukin 10
- MPO
myeloperoxidase
Introduction
Inflammatory bowel diseases (IBD), encompassing Crohn's disease (CD) and Ulcerative Colitis (UC) are driven by a seemingly aberrant immune response to the commensal microbiota, thus causing intestinal pathology characterized by immune cell infiltration. IBD are tightly linked to the host innate immunity as defects in innate immune signaling predispose gut inflammation.1 Previously, we have demonstrated the development of colitis in a fraction (typically 10%) of mice lacking toll-like receptor 5 (TLR5), a receptor for bacterial flagellin.1 Colitic TLR5KO mice display altered microbiota with significant enrichment in Proteobacteria when compared to non-colitic TLR5KO, suggesting that microbiota dysbiosis is strongly associated with disease. Accordingly, we found that germ-free (GF) conditions abrogate the disease in TLR5KO mice. The interpretation that GF conditions protect TLR5KO mice from colitis, however, is not definitive considering that about 90% of TLR5KO mice normally do not develop overt colitis. Moreover, some models such as the SAMP1/YitFc mice still exhibit disease in GF conditions, albeit to a lesser degree compared to mice maintained on specific pathogen-free conditions.2 Hence, additional studies are required to further confirm the role of microbiota in driving colitis in TLR5KO mice.
Interleukin 10 (IL-10) is a pleiotropic anti-inflammatory cytokine that exerts inhibitory effect on the pro-inflammatory functions of TH1 cells, NK cells and macrophages.3 It is required to maintain immune tolerance and suppress unregulated/aberrant inflammatory response, thus reducing tissue damage during inflammation.3 On the flip side, deficiencies in IL-10 signaling predispose mice to spontaneous colitis that is dependent on MyD88 signaling.4 In our previous study, we demonstrate that neutralization of IL-10 signaling via administration of anti-IL-10 receptor monoclonal antibody (α-IL-10R mAb; an IL-10R antagonist that block IL-10 signaling) uniformly induces colitis in all treated TLR5KO mice. In contrast to the 100% induction of colitis in TLR5KO mice, α-IL-10R mAb induced only modest increase in inflammatory markers with no obvious phenotype at the gross colon or histologic level 5 in WT mice.
While the incomplete penetrance of colitis in TLR5KO mice makes the GF approach not definitive in ruling out the role of microbiota, the 100% induction of disease due to the loss of IL-10 signaling, achieved via genetics or neutralizing antibody, would allow a much more rigorous test on whether elimination of microbiota eliminates colitis in TLR5KO mice. Hence, the current study more rigorously investigated the contribution of intestinal microbiota in the development of colitis induced by inhibition of IL-10 signaling in microbiota-ablated and GF-TLR5KO mice. We observed that α-IL-10R mAb administration failed to induce colitis in either microbiota-ablated or GF-TLR5KO mice when compared to conventionally raised TLR5KO mice, underscoring the role of microbiota in intestinal inflammation.
Results
α-IL-10R failed to induce colitis in microbiota-ablated TLR5KO mice
Gut microbiota have been implicated in the development of spontaneous colitis in susceptible genetically-modified mice. However, whether the microbiota may or may not be indispensible in the development of colitis is heavily dependent on the strains of mice being used in the study. For instance, the microbiota is necessary for colitis development in IL-10KO mice as GF-IL-10KO mice are free from colitis.6 Yet other strains such as SAMP/YitFc mice display colitic phenotype even when maintained on GF conditions. Hence, the role of microbiota needs to be carefully studied for each model. In this study, we asked the extent to which the microbiota plays a role in the colitis induced upon inhibition of IL-10 signaling in TLR5KO mice. Groups of TLR5KO mice with or without broad-spectrum antibiotics in drinking water were administered α-IL-10R mAb for 4 weeks. The antibiotics ablated 90% of the intestinal bacteria as measured by fecal 16S rRNA via qRT-PCR (data not shown).
Antibiotic given TLR5KO mice exhibited enlarged ceca that weighed fold4- higher than the ceca from conventionally raised TLR5KO mice (Fig. 1A). TLR5KO mice administered α-IL-10R mAb without antibiotics lost 10% of body weight when compared to with antibiotics (data not shown). Similar to our earlier observation,5 TLR5KO mice receiving α-IL-10R mAb without antibiotics exhibited hallmark features of colitis including shrunken ceca, colomegaly, and splenomegaly (Figs. 1A–D). Colitic TLR5KO mice also displayed substantially elevated colonic tissue myeloperoxidase (MPO) and serum inflammatory marker, Lcn2 (Figs. 1E and 1F), when compared to TLR5KO receiving isotype control antibody. All the above parameters in microbiota-ablated and α-IL-10R mAb given TLR5KO mice were comparable to isotype control antibody treated TLR5KO mice (Figs. 1A-F).
Figure 1.
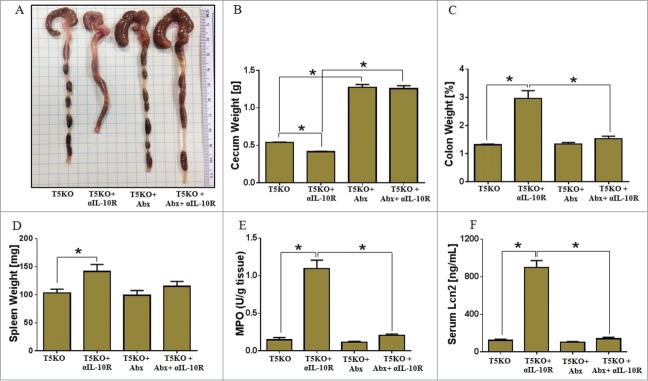
Gut sterilization prevents development of colitis in TLR5KO mice receiving α-IL-10R mAb. Age matched male TLR5KO mice (n = 5 ) with or without α-IL-10R mAb were treated with broad antibiotics (ampicillin and neomycin) in drinking water for 4 weeks and then analyzed for development of colitis. (A) Image displays gross pictures of colon. Bar graphs represent: Weights of (B) Ceca, (C) Colon, (D) Spleen, (E) MPO in colonic tissue, and (F) Serum Lcn2. Results are expressed, as mean ± SEM. Image is representative of 2 independent experiments. *p <0.05.
GF-TLR5KO mice are resistant to IL-10 receptor neutralization-induced colitis
The use of GF mice has been regarded as the gold standard to study the interaction between gut microbiota and host mucosal immunity. To further confirm our results from the microbiota-ablated mice, we took advantage of GF-WT and GF-TLR5KO mice. Groups of GF-WT and GF-TLR5KO mice were given α-IL-10R mAb for 4 weeks. As observed in microbiota-ablated TLR5KO mice, GF-TLR5KO mice did not show any symptoms of colitis at the gross level (Fig. 2A). Further, α-IL-10R mAb administration failed to induce cecal shrinkage, colomegaly and splenomegaly (Figs. 2B–D) either in GF-WT or GF-TLR5KO mice. Similarly, IL-10R neutralization in GF-TLR5KO mice failed to induce colonic MPO or upregulate systemic inflammatory marker Lcn2 (Figs. 2E and 2F). Collectively, our results demonstrate that colitis development in IL-10R neutralized TLR5KO mice is microbiota dependent.
Figure 2.
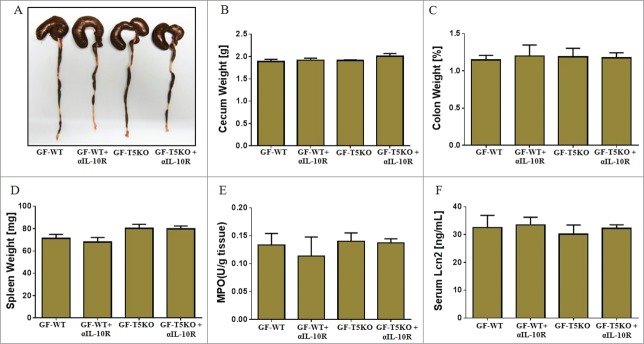
IL-10R neutralization failed to induce colitis in GF-TLR5KO mice. Age matched male GF-TLR5KO mice (n = 4−5) received α-IL-10R mAb (1.0 mg/mouse, i.p. weekly) up to 4 weeks and then analyzed for development of colitis. (A) Image displays gross picture of colon. Bar graphs represent: Weights of (B) Ceca, (C) Colon, (D) Spleen, (E) MPO in colonic tissue, and (F) Serum Lcn2. Results are expressed, as mean ± SEM.
Discussion
The substantial differences in the susceptibility to IBD in genetically identical twins underscore the importance of gut microbiota as an environmental factor in driving this disease.7 Similar to vivaria-specific colitis susceptibility in IL-10KO mice, significant variability in the colitis onset and robustness in TLR5KO mice (originally generated by Dr. Akira, Japan) can be attributable to presence or absence of specific microbial communities in the gut. For instance, TLR5KO mice from Jackson Laboratories (originally generated by Dr. Flavell, Yale University) do not show any signs of colonic inflammation, presumably due to differences in microbiota composition. Similarly, colitis susceptible TLR5KO mice do not show signs of inflammation in other facilities.8 Such incomplete disease penetrance in TLR5KO mice, as well as in other model of spontaneous colitis, presents logistical challenges in experimental design due to the inability to control the onset and uniformity of disease incidence. Hence, alternate strategies are required to better define the role of microbiota in the pathogenesis of colitis-susceptible TLR5KO mice. Accordingly, we found that IL-10 neutralization via α-IL-10R mAb can induce chronic colitis with 100% penetrance in TLR5KO mice generated by 2 independent facilities.5 The colitis development in TLR5KO mice is MyD88 dependent and TLR4 independent.5 However, the extent to which microbiota is driving colonic inflammation in this model was not known.
In this study, we demonstrate that indeed, microbiota is required for colitis development in IL-10R neutralized TLR5KO mice. Administration of α-IL-10R mAb consistently induces gut inflammation in conventionally raised TLR5KO mice, but not in microbiota-ablated and GF-TLR5KO mice. These results recapitulate our previous observation that gut inflammation in TLR5KO mice is microbiota-dependent and further suggests that all aspects of colitic phenotype in TLR5KO mice are due to their innate inability to manage the microbiota. Further, our results parallel the study that demonstrated the indispensability of the microbiota for colitis development in IL-10KO mice as GF-IL-10KO mice are free from colitis.6 While not surprising, this was not a foregone conclusion as some genetically-modified mice still develop colitis even in GF conditions, particularly SAMP/YitFc mice.2 Intriguingly for SAMP/YiFc mice, the initiation of ileal inflammation is independent of microbiota, but is primarily mediated by 1). inherent defects in gut permeability due to overexpression of claudin-2 and reduced expression of occludin in ileal epithelial cells, and 2) overexpression of pro-inflammatory mediators STAT3, IL-33, and RELMβ in the ileum.9-12 Nevertheless, the microbiota still plays a role in modulating the severity of ileitis in SAMP/YitFc mice despite being dispensable in initiation of ileitis.2 Therefore, although we demonstrate that colitis in TLR5KO mice is microbiota-dependent, caution should be exercised when extrapolating our findings to other mouse models of colitis, as each model need to be independently studied for the role of microbiota in colitis development.
The microbota-dependent colitis development in TLR5KO mice is tightly associated with dysregulated immune responses, presumably due to aberrant response to the microbiota. We have previously demonstrated that TLR5KO mice harbor highly volatile microbiota that undergo significant compositional shift at the phyla level over time from weaning to 11 weeks of age.13 This difference is observed in mice lacking only intestinal epithelial cell TLR5, even when such mice are co-housed with WT control mice.14 Although both non-colitic and colitic TLR5KO mice display variability in their microbiota composition, the family enterobacteria (phyla proteobacteria) was found to be significantly enriched in the colitic TLR5KO mice via 16S rRNA sequencing.13 Fluorescent in situ hybridization (FISH) analysis on colons from colitic TLR5KO mice not only confirmed the increased abundance of enterobacteria, but also revealed that these bacteria penetrated the mucosa and direct contact with the gut epithelium.13 To further investigate the role of enterobacteria in potentiating colitis, TLR5KO mice were infected adherent-invasive E. coli strain LF82 (a member of the proteobacteria family that is associated with Crohn's disease). TLR5KO mice infected with flagellated LF82 displayed impaired clearance of bacteria and exhibited chronic gut inflammation. However, the colitic phenotype was absent in TLR5KO mice infected with an aflagellate isogenic LF82 mutant, suggesting that TLR5 response to flagellin is protective. In the absence of TLR5, bacterial flagellin will instead induce the activation of cytosolic flagellin receptor NLRC4, whose activation triggers an inflammasome-mediated cascade resulting in the production of IL-1β, a pro-inflammatory cytokine that strongly correlates with IBD.5,15-17 Accordingly, the colitis that occurred in IL-10R-neuralized TLR5KO mice is dependent on IL-1β as mice deficient in both TLR5 and IL-1 receptor are protected from α-IL-10R mAb-induced colitis,5 suggesting that both the microbiota and immune dysregulation are tightly associated with IBD pathogenesis.
We envisage that the use of IL-10R neutralization will be helpful to mechanistically study the role of microbiota and immune dysfunction not only in TLR5KO mice, but in other model of spontaneous colitis as well. There are several advantages for using α-IL-10R mAb induced chronic colitis: (a) slow and steady induction of colitis, (b) requires relatively short duration (c) lack mortality during 4 weeks of antibody treatment, unlike those observed in other chronic colitis models (e.g. DSS or piroxicam-aggravated colitis in IL-10KO mice) (d) development of uniform colitis (unlike IL-10 spontaneous colitis which is vivaria-specific), (f) represent typical features of human IBD reminiscent of Crohn's disease. Further our model is suitable to test susceptibility of mice with genetic modification, altered microbiota or any perturbations in mucosal physiology.
Altogether, our study demonstrates that the pathogenesis of colitis in TLR5KO mice is microbiota dependent. TLR5KO mice are susceptible to develop spontaneous colitis which is further augmented upon treatment with IL-10R neutralizing mAb. However, both microbiota-ablated and germ-free TLR5KO mice are significantly protected against colitis, underscoring the requirement of microbiota in the development of gut inflammation in TLR5KO mice.
Materials and Methods
Rat anti-mouse IL-10R mAb was purchased from BioXcell, (West Lebanon, NH, USA). Hexadecyltrimethylammonium bromide, O-dianisidine and human neutrophil myeloperoxidase were purchased from Sigma, (St. Louis, MO). Mouse Lipocalin 2 duoset ELISA kit was procured from R&D Systems (Minneapolis, MN).
Mice
TLR5 knock out (TLR5KO) mice and their WT littermates on BL6 background were bred and maintained at The Pennsylvania State University, PA. Germ-free TLR5KO mice and their WT littermates were generated in Taconic facility and maintained at University of North Carolina, Chapel Hill, NC. All animal experimental procedures were approved by the institutional animal care and use committee (IACUC).
Administration of α-IL-10R to Mice
IL-10 receptor neutralization-induced colitis was induced in mice as described in.5 Briefly, 8 weeks old male GF-TLR5KO mice and their WT littermates (n = 4−5) were administered commercially available anti-mouse IL-10R (IgG1) mAb (α-IL-10R mAb, 1.0 mg/mouse, intraperitoneally) weekly. Mice were euthanized one week after the last (fourth) injection, and analyzed for standard colitic parameters. Similarly, conventionally raised 8 weeks old male TLR5KO (n = 5) were maintained on ampicillin (1.0 g/L) and neomycin (0.5 g/L) in drinking water for 4 weeks and received α-IL-10R mAb weekly as described above. Control mice were given isotype control (rat anti-PD-1) antibody.5
Blood Collection and Euthanasia
Blood was collected at the time of euthanasia in BD microtainer® (Becton, Dickinson, Franklin Lakes, NJ), via retro-orbital plexus. Hemolysis-free sera was obtained after centrifugation and stored at −800C until further use.
Colonic Myeloperoxidase (MPO) assay
MPO assay was done according to the previously described method.18 Briefly, frozen or fresh colon tissue (∼50 mg) was homogenized in 0.5% hexadecyltrimethylammonium bromide in 50 mM Potassium Phosphate buffer, (pH 6.0), freeze-thawed 3x, sonicated and centrifuged at 10000 g, 40C, and the clear supernatants were collected. O-dianisidine dihydrochloride (1.0 mg/ml) and 5 × 10−4 % H2O2 was used for MPO assay and the change in optical density was measured at 450 nm over a period of 30 min. Human neutrophil MPO was used as standard and the amount of MPO that degraded 1.0 μmol of peroxide/min at 25°C was considered as one Unit.
Serum lipocalin 2 estimation
Serum lipocalin 2 (Lcn2) was quantitated according to the manufacturer's instructions using Lcn2 ELISA assay (R&D Systems, Minneapolis, MN). Minimum detection limit for Lcn2 is 15.6 pg/ml.
Statistical Analysis
Statistical analysis for significance between 2 groups was determined using student's t-test (unpaired, 2-tailed) with p < 0.05 (*) considered as significant. GraphPad Prism 6.0 software was used to calculate statistical significance.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work is supported by NIH grants DK083275 (MV-K), DK094864 (MV-K), DK097865 (MV-K), DK083890 (ATG), DK099071 (ATG) and National Gnotobiotic Rodent Resource Center, University of North Carolina which is supported by NIH grant P40OD010995.
References
- 1. Vijay-Kumar M, Sanders CJ, Taylor RT, Kumar A, Aitken JD, Sitaraman SV, Neish AS, Uematsu S, Akira S, Williams IR, et al. Deletion of TLR5 results in spontaneous colitis in mice. J Clin Investig 2007; 117:3909-21; PMID:18008007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bamias G, Okazawa A, Rivera-Nieves J, Arseneau KO, De La Rue SA, Pizarro TT, Cominelli F. Commensal bacteria exacerbate intestinal inflammation but are not essential for the development of murine ileitis. J Immunol 2007; 178:1809-18; PMID:17237431; http://dx.doi.org/ 10.4049/jimmunol.178.3.1809 [DOI] [PubMed] [Google Scholar]
- 3. Chaudhry A, Samstein RM, Treuting P, Liang Y, Pils MC, Heinrich JM, Jack RS, Wunderlich FT, Brüning JC, Müller W, et al. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity 2011; 34:566-78; PMID:21511185; http://dx.doi.org/ 10.1016/j.immuni.2011.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoshi N, Schenten D, Nish SA, Walther Z, Gagliani N, Flavell RA, Reizis B, Shen Z, Fox JG, Iwasaki A, et al. MyD88 signalling in colonic mononuclear phagocytes drives colitis in IL-10-deficient mice. Nat Commun 2012; 3:1120; PMID:23047678; http://dx.doi.org/ 10.1038/ncomms2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carvalho FA, Nalbantoglu I, Ortega-Fernandez S, Aitken JD, Su Y, Koren O, Walters WA, Knight R, Ley RE, Vijay-Kumar M, et al. Interleukin-1beta (IL-1beta) promotes susceptibility of Toll-like receptor 5 (TLR5) deficient mice to colitis. Gut 2012; 61:373-84; PMID:21646247; http://dx.doi.org/ 10.1136/gut.2011.240556 [DOI] [PubMed] [Google Scholar]
- 6. Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, Rennick DM, Sartor RB. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun 1998; 66:5224-31; PMID:9784526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dicksved J, Halfvarson J, Rosenquist M, Jarnerot G, Tysk C, Apajalahti J, Engstrand L, Jansson JK. Molecular analysis of the gut microbiota of identical twins with Crohn's disease. ISME J 2008; 2:716-27; PMID:18401439; http://dx.doi.org/ 10.1038/ismej.2008.37 [DOI] [PubMed] [Google Scholar]
- 8. Letran SE, Lee SJ, Atif SM, Flores-Langarica A, Uematsu S, Akira S, Cunningham AF, McSorley SJ. TLR5-deficient mice lack basal inflammatory and metabolic defects but exhibit impaired CD4 T cell responses to a flagellated pathogen. J Immunol 2011; 186:5406-12; PMID:21451112; http://dx.doi.org/ 10.4049/jimmunol.1003576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Olson TS, Reuter BK, Scott KG, Morris MA, Wang XM, Hancock LN, Burcin TL, Cohn SM, Ernst PB, Cominelli F, et al. The primary defect in experimental ileitis originates from a nonhematopoietic source. J Exp Med 2006; 203:541-52; PMID:16505137; http://dx.doi.org/ 10.1084/jem.20050407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mitsuyama K, Matsumoto S, Rose-John S, Suzuki A, Hara T, Tomiyasu N, Handa K, Tsuruta O, Funabashi H, Scheller J, et al. STAT3 activation via interleukin 6 trans-signalling contributes to ileitis in SAMP1/Yit mice. Gut 2006; 55:1263-9; PMID:16682432; http://dx.doi.org/ 10.1136/gut.2005.079343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pastorelli L, Garg RR, Hoang SB, Spina L, Mattioli B, Scarpa M, Fiocchi C, Vecchi M, Pizarro TT. Epithelial-derived IL-33 and its receptor ST2 are dysregulated in ulcerative colitis and in experimental Th1/Th2 driven enteritis. Proc Natl Acad Sci U S A 2010; 107:8017-22; PMID:20385815; http://dx.doi.org/ 10.1073/pnas.0912678107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barnes SL, Vidrich A, Wang ML, Wu GD, Cominelli F, Rivera-Nieves J, Bamias G, Cohn SM. Resistin-like molecule beta (RELMbeta/FIZZ2) is highly expressed in the ileum of SAMP1/YitFc mice and is associated with initiation of ileitis. J Immunol 2007; 179:7012-20; PMID:17982092; http://dx.doi.org/ 10.4049/jimmunol.179.10.7012 [DOI] [PubMed] [Google Scholar]
- 13. Carvalho FA, Koren O, Goodrich JK, Johansson ME, Nalbantoglu I, Aitken JD, Su Y, Chassaing B, Walters WA, González A, et al. Transient Inability to Manage Proteobacteria Promotes Chronic Gut Inflammation in TLR5-Deficient Mice. Cell host & microbe 2012; 12:139-52; PMID:22863420; http://dx.doi.org/ 10.1016/j.chom.2012.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chassaing B, Ley RE, Gewirtz AT. Intestinal epithelial cell toll-like receptor 5 regulates the intestinal microbiota to prevent low-grade inflammation and metabolic syndrome in mice. Gastroenterology 2014; 147:1363-77 e17; PMID:25172014; http://dx.doi.org/ 10.1053/j.gastro.2014.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang J, Fu S, Sun S, Li Z, Guo B. Inflammasome activation has an important role in the development of spontaneous colitis. Mucosal Immunol 2014; 7:1139-50; PMID:24472848; http://dx.doi.org/ 10.1038/mi.2014.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coccia M, Harrison OJ, Schiering C, Asquith MJ, Becher B, Powrie F, Maloy KJ. IL-1beta mediates chronic intestinal inflammation by promoting the accumulation of IL-17A secreting innate lymphoid cells and CD4(+) Th17 cells. J Exp Med 2012; 209:1595-609; PMID:22891275; http://dx.doi.org/ 10.1084/jem.20111453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ligumsky M, Simon PL, Karmeli F, Rachmilewitz D. Role of interleukin 1 in inflammatory bowel disease–enhanced production during active disease. Gut 1990; 31:686-9; PMID:2379873; http://dx.doi.org/ 10.1136/gut.31.6.686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chassaing B, Srinivasan G, Delgado MA, Young AN, Gewirtz AT, Vijay-Kumar M. Fecal lipocalin 2, a sensitive and broadly dynamic non-invasive biomarker for intestinal inflammation. PloS one 2012; 7:e44328; PMID:22957064; http://dx.doi.org/ 10.1371/journal.pone.0044328 [DOI] [PMC free article] [PubMed] [Google Scholar]


