Abstract
Background. Secondary bacterial infections following influenza represent a major cause of mortality in the human population, which, in turn, has led to a call for stockpiling of bacterial vaccines for pandemic preparedness.
Methods. To investigate the efficacy of bacterial vaccination for protection against secondary pneumococcal infection, mice were immunized with pneumococcal capsular polysaccharide conjugate vaccine, and then sequentially coinfected 5 weeks later with PR8 influenza virus and A66.1 Streptococcus pneumoniae.
Results. In the absence of influenza virus exposure, vaccination with polysaccharide conjugate vaccine was highly effective, as indicated by 100% survival from lethal pneumococcal pneumonia and 10 000-fold greater efficiency in clearance of bacteria from the lung compared to unvaccinated mice. Enhanced clearance after vaccination was dependent upon Fc receptor (FcR) expression. However, following influenza, <40% of vaccinated mice survived bacterial coinfection and FcR-dependent clearance of antibody-opsonized bacteria reduced bacterial levels in the lungs only 5–10 fold. No differences in lung myeloid cell numbers or in FcR cell surface expression were observed following influenza.
Conclusions. The results show that induction of antibacterial humoral immunity is only partially effective in protection against secondary bacterial infections that occur following influenza, and suggest that additional therapeutic strategies to overcome defective antibacterial immunity should be explored.
Keywords: adaptive immunity, coinfection, influenza virus, innate immunity, pneumococcus, pneumonia, vaccination
Influenza virus and Streptococcus pneumoniae are the 2 pathogens that cause the majority of respiratory infections in humans. Although influenza infection alone may cause pneumonia, secondary bacterial pneumonia is a common cause of severe disease and often leads to excess morbidity and mortality during typical influenza pandemics, including the major pandemic of 1918–1919 [1, 2]. Indeed, studies by Morens and colleagues [3, 4] of published autopsy case reports found that over 90% of deaths during the 1918 influenza pandemic likely resulted from secondary pneumococcal pneumonia. Similarly, many deaths in subsequent pandemics, including the 2009 H1N1 pandemic, were due to secondary bacterial pneumonia [5–8].
The fact that secondary bacterial infections are responsible for a significant proportion of deaths during influenza pandemics has led to a recent call for stockpiling of antibiotics and pneumococcal conjugate vaccines as part of plans for influenza preparedness [9]. It is known that antibodies induced by vaccination are highly effective in preventing invasive bacterial infections. On the other hand, studies by us [10, 11] and others [12–17] have demonstrated that influenza virus infection inhibits innate antibacterial immunity. It is therefore unclear whether enhancement of adaptive antipneumococcal immunity by vaccination would be able to fully overcome the functional innate defects that occur during coinfection. Evidence in humans suggests that pneumococcal vaccination does protect against secondary bacterial infection following influenza [18]. In fact, however, the actual observed level of protection is approximately 50% or less.
The current study was designed to experimentally test in mice the ability of humoral immunity induced by pneumococcal polysaccharide (PPS) conjugate vaccine to protect against induction of pneumococcal pneumonia following influenza. The goal was to directly determine whether immunization to induce antibody-mediated bacterial clearance would compensate for defective innate immunity in the lung following influenza.
MATERIALS AND METHODS
Mice
Specific pathogen-free, 6–8-week-old C57Bl/6 wild-type (WT) mice were purchased from Taconic Laboratories (Germantown, New York) or Charles River Laboratories (Wilmington, Massachusetts). C57Bl/6 FcγR−/− mice were purchased from Taconic Laboratories. All mice were maintained at the Albany Medical College Animal Facility and all experiments followed approved Institutional Animal Care and Use Committee guidelines.
Vaccination
To induce antipneumococcal immunity, mice were immunized intramuscularly (i.m.) with 1 µg Prevnar (a 13-valent vaccine containing S. pneumoniae polysaccharide serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F conjugated to diphtheria cross-reactive material [CRM197] as the carrier protein [Pfizer]). The animals were boosted at 3 weeks, bled, and then challenged with influenza virus 2 weeks later. Serum anti-PPS3 titers were determined in all cases to be at least 1:320 by enzyme-linked immunosorbant assay using Maxisorp microtiter plates (Nalge Nunc International, Rochester, New York) coated with unconjugated PPS3 (American Type Culture Collection, Manassas, Virginia) as previously described [19].
Viral and Bacterial Infections
Influenza challenge was performed with 10 plaque-forming units (PFU) of A/PR8/34 (PR8) virus administered intranasally (i.n.) to anesthetized mice in 50 µL of sterile phosphate-buffered saline (PBS). Titers of virus were determined by plaque assays on Madin Darby canine kidney cell monolayers. After viral infection, the mice were weighed daily and bacterial coinfection was performed 7–10 days later, specifically at the particular time point when the mice began to recover from viral infection and which has been previously determined to be the window of greatest susceptibility to bacterial superinfection [10]. To induce bacterial pneumonia, anesthetized mice were inoculated i.n. with 50 µL PBS containing S. pneumoniae strain A66.1. For passive protection, mice were inoculated i.n. with bacteria and 10% immune or normal, heat-inactivated mouse serum diluted in PBS. Bacterial burdens in the lung were measured by sacrificing infected mice at 4 hours and 48 hours, and plating serial 10-fold dilutions of lung homogenates onto blood agar plates. The plates were incubated at 37°C overnight, and colony forming units (CFUs) were enumerated 24 hours later.
To denote the various experimental groups, each set of mice is first designated as vaccinated or naive, then challenged with PBS or a sublethal dose of PR8, and finally, additionally challenged with PBS or pneumococcus. For example, immunized mice coinfected with influenza and pneumococcus are referred to as “vacc FLU pneumo” mice; unimmunized mice challenged with pneumococcus only are referred to as “naive PBS pneumo” mice.
Flow Cytometry Staining
Lungs were harvested 2 days postpneumococcal infection and single-cell suspensions were obtained by incubation with 2 mg/mL collagenase D (Roche Diagnostics), 0.25 mg/mL Dnase I (Roche Diagnostics), and 10 mM MgCl2 for 1 hour at 37°C. The cells were washed with PBS containing 2% fetal calf serum (FCS) and stained with fluorescent antibodies in microtiter plates (5 × 105 cells/well). For CD64 staining, FcγRII/III was blocked by incubation with mouse anti-CD16/32 mAb (clone 2.4G2) for 20 minutes at 4°C. The cells were then stained with an antibody cocktail containing fluorescein isothiocyanate (FITC)–conjugated anti-CD11b (clone M1/70; eBioscience), phycoerythrin (PE)–conjugated anti-F4/80 (clone BM8; eBioscience), PE-Cy7 conjugated anti-Ly6G (clone 1A8; BioLegend), APC conjugated anti-CD64 (clone MAR-1; eBioscience), and APC-Cy7-conjugated CD11c (clone N418; BioLegend) mAbs. For CD16/32 staining, cells were first stained with FITC-conjugated anti-CD16/32 (clone 2.4G2; BD Pharmingen). After washing with PBS-2% FCS, the cells were incubated with unlabeled 2.4G2 blocking antibody, followed by incubation with an antibody cocktail containing PE-conjugated anti-F4/80 (clone BM8; eBioscience), PE-Cy7 conjugated anti-Ly6G (clone 1A8; BioLegend), allophycocyanin (APC)–conjugated anti-CD11c (clone HLC; BD Pharmingen), and APC-Cy7-conjugated CD11b (clone M1/70; BD Pharmingen) mAbs. The stained cells were washed twice with PBS-2% FCS, resuspended in 200 µL PBS-2% FCS, and quantitated using a FACSCanto flow cytometer.
Statistical Analyses
The data are expressed as means ± SD. Analysis of variance followed by Bonferroni correction was used for statistical analysis of bacterial clearance. Survival analyses were performed using the Kaplan–Meier log rank test. P < .05 was considered to be significant. Statistical procedures were performed using GraphPad InStat 3 software.
RESULTS
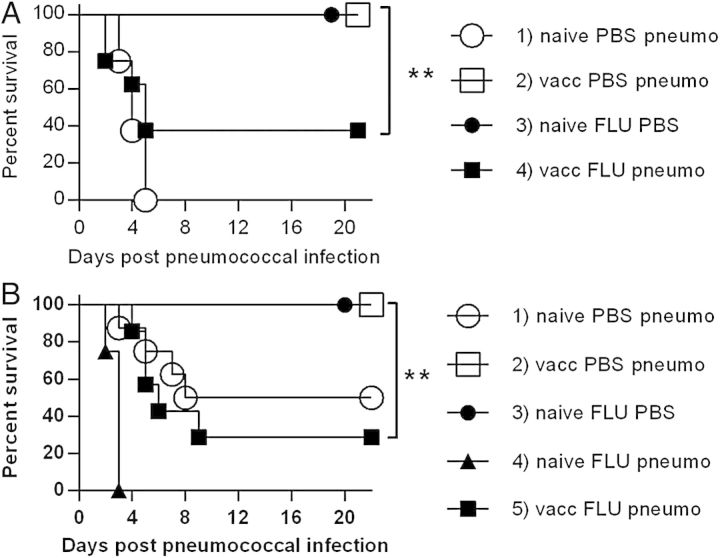
To compare the ability of PPS-conjugate vaccine to protect mice infected with a lethal dose of bacteria versus mice coinfected with both influenza and bacteria, mice were vaccinated and boosted at 3 weeks, rested for 2 weeks, and then inoculated i.n. with either PBS or a sublethal dose of influenza virus. When the animals began to regain weight, indicating recovery from the virus infection and the period of greatest susceptibility to secondary bacterial infection [10], they were challenged i.n. with a lethal dose of A66.1 (serotype 3) pneumococci (106 CFUs/mouse). As expected, 100% of naive, unvaccinated mice succumbed to bacterial infection in 3–5 days (Figure 1A, Group 1) and all immunized mice challenged only with pneumococci survived (Figure 1A, Group 2). These results confirmed the basic effectiveness of the vaccination procedure for protection against high doses of bacteria. All of the mice infected with influenza alone survived, demonstrating that the viral infection was sublethal (Figure 1A, Group 3). However, in influenza- and pneumococcus coinfected mice, protection after vaccination was only partial (survival rate of 40%) (P < .01) (Figure 1A, Group 4).
Figure 1.
Effect of vaccination on survival following coinfection with influenza virus and pneumococcus. Mice were immunized intramuscularly with 1 µg pneumococcal polysaccharide-conjugate vaccine and boosted 3 weeks later. Two weeks after that, all mice received 10 plaque-forming units of PR8 influenza virus or phosphate-buffered saline (PBS), and 10 days later, were challenged intranasally with PBS or 106 colony-forming units (CFUs)/mouse (A) or 5 × 104 CFUs/mouse of A66.1 S. pneumoniae (serotype 3) (B). Each group consisted of 8 mice. **P < .01. Abbreviations: naive PBS pneumo, unimmunized mice challenged with pneumococcus only; vacc FLU pneumo, immunized mice coinfected with influenza and pneumococcus.
Because a lethal dose of pneumococcus was administered to the mice in the above experiment, the synergistic influence of viral and bacterial coinfection in unvaccinated mice could not be assessed. Thus, a second experiment was performed using a lethal dose 50% (LD50) of bacteria (5 × 104) (Figure 1B, Group 1). In this experiment, again all pneumococcal-vaccinated mice not infected with influenza survived bacterial challenge (Figure 1B, Group 2) as did all mice infected only with influenza virus (Figure 1B, Group 3). Consistent with previous studies [10], however, all unvaccinated mice infected with influenza followed by pneumococci succumbed to infection within 2–3 days (Figure 1B, Group 4), demonstrating viral-bacterial synergy. Pneumococcal vaccination provided limited protection (30% survival) to this latter group of mice (Figure 1B, Group 5), fully consistent with the results observed above (Figure 1A, Group 4–40% survival). Thus, vaccination completely protected singly infected mice against pneumococcal pneumonia, but after influenza coinfection, vaccination efficacy was significantly diminished.
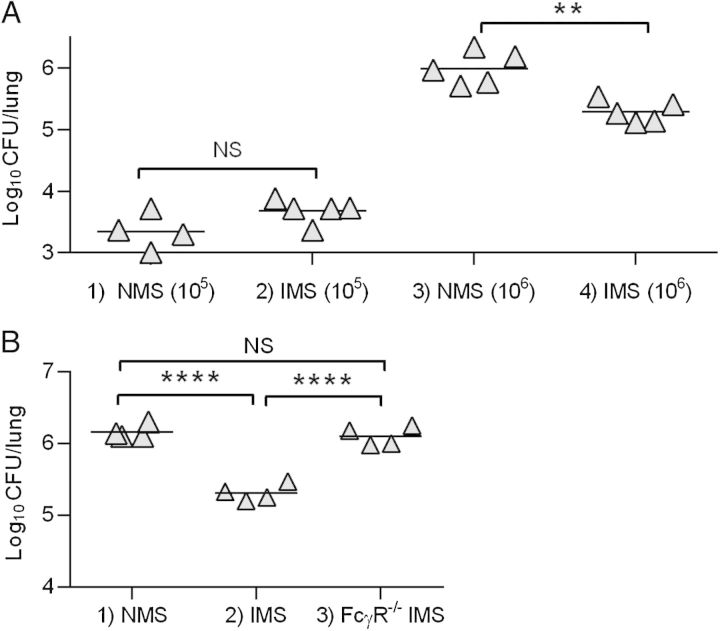
We have previously reported that innate immune mechanisms, specifically alveolar macrophage killing, quickly clear nearly all pneumococci from the airways of noninfluenza-infected mice, if low inoculation doses are used (105 or fewer bacterial CFUs) [10]. At higher doses, however, this first line of defense becomes overwhelmed, innate clearance fails, and the animal succumbs to infection. Thus, adaptive immunity is likely necessary to provide protection against high doses of pneumococci in the lungs. Indeed, after inoculation of 106 pneumococci, unvaccinated mice were not able to clear the bacteria from their lungs while all vaccinated mice cleared >95% of bacteria within 48 hours (Figure 2, Groups 1 and 2). In mice previously infected with influenza, clearance was severely compromised, with bacterial outgrowth in unvaccinated mice approaching levels of 109 CFUs (Figure 2, Group 3), which was approximately 10-fold higher than the CFU levels seen in unvaccinated mice not previously infected with influenza (Group 1). Of note, vaccination with PPS-conjugate vaccine had minimal influence on this pattern; in other words, influenza infection significantly disrupted pulmonary clearance in both unvaccinated and vaccinated mice, and resulted in considerable bacterial outgrowth (Group 2 vs Group 4).
Figure 2.
Effect of vaccination on bacterial clearance following coinfection with influenza virus and pneumococcus. Mice were immunized intramuscularly with 1 µg pneumococcal polysaccharide-conjugate vaccine and boosted 3 weeks later. Two weeks after that, all mice received 10 plaque-forming units of PR8 influenza virus or phosphate-buffered saline (PBS), and 10 days later, were challenged intranasally with 106 colony-forming units (CFUs)/mouse of A66.1 S. pneumoniae (serotype 3). Bacterial burdens in total lungs were determined 48 hours after pneumococcal infection. Each symbol represents an individual mouse (4 mice/group). The data represent results from at least 2 independent experiments. **P < .01. Abbreviations: naive PBS pneumo, unimmunized mice challenged with pneumococcus only; NS, not significant; vacc FLU pneumo, immunized mice coinfected with influenza and pneumococcus.
To directly examine the protective efficacy of antipneumococcal antibody in the lungs of mice, passive immunization experiments were performed. Mice were inoculated i.n. with bacteria together with serum from normal, unvaccinated mice (NMS) or serum from mice vaccinated with PPS-conjugate (IMS). Lung bacterial burdens were then assessed to determine macrophage-mediated clearance. It was found that at a low bacterial inoculum dose (105 CFUs), approximately 98% of the bacteria were cleared regardless of whether the animals had been given NMS or IMS (Figure 3A, Groups 1 and 2). At a higher, lethal dose 100% of bacteria, a dose that overwhelms alveolar macrophage-mediated phagocytosis, nearly all NMS-opsonized pneumococci remained in the lungs (Figure 3A, Group 3). Inoculation of IMS resulted in approximately 80% reduction in bacterial numbers, yet significant levels (>105 CFUs) still remained in the airways (Figure 3A, Group 4). The enhanced antibody-mediated clearance that occurred at this higher challenge dose appeared to be mediated by FcγR because levels of bacteria in FcγR−/− mice receiving IMS (Figure 3B, Group 3) were similar to those seen in WT animals receiving NMS (Figure 3B, Group 1). Thus, while innate immunity in naive mice was effective in almost completely clearing a low bacterial challenge dose, such clearance failed at a higher bacterial dose. FcγR-mediated antibody opsonization facilitated clearance at this high pathogen dose, but did not result in complete rescue.
Figure 3.
Bacterial clearance of pneumococci from noninfluenza-infected lungs after coinoculation of serum from normal, unvaccinated mice (NMS) or serum from mice vaccinated with PPS-conjugate (IMS). A, Bacterial colony-forming units (CFUs) were determined 4 hours after inoculation of C57Bl/6 mice with 105 (Groups 1 and 2) or 106 (Groups 3 and 4) CFUs of A66.1 S. pneumoniae and NMS or IMS. B, Numbers of bacterial CFUs 4 hours after inoculation of C57Bl/6 WT or FcγR−/− mice with 106 CFUs of A66.1 S. pneumoniae and IMS. Each symbol represents an individual mouse (4–5 mice/group). The data represent results from at least 2 independent experiments. **P < .01; ****P < .0001. Abbreviations: NS, not significant; PPS, pneumococcal polysaccharide.
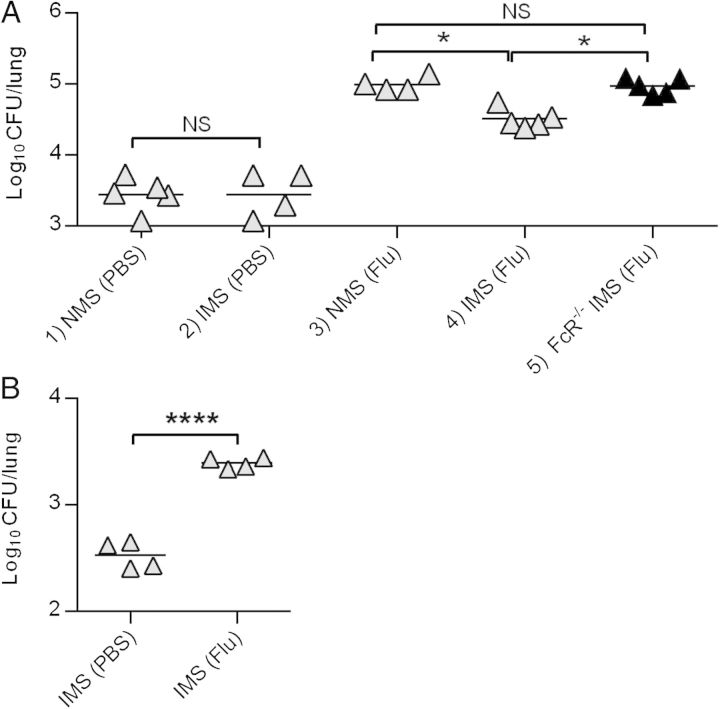
The effect of preceding influenza infection on IMS-mediated pulmonary clearance of S. pneumoniae was next determined. Mice were infected with a sublethal dose of PR8 influenza virus and were then challenged with a normally sublethal dose of A66.1 S. pneumoniae (105 CFUs/mouse). The results showed that, again, mice not infected with influenza could completely clear >90% of a low dose of S. pneumoniae (105 CFUs) from the lungs, while influenza-infected mice had defective innate immunity and were unable to effectively clear the bacteria (Figure 4A, Groups 1 and 3). Inoculation of IMS enhanced clearance in influenza-infected mice (Figure 4A, Group 4) and this occurred through an FcγR-dependent mechanism (Figure 4A, Group 5), yet significant numbers of bacteria still remained in the lung, similar to the results seen above with noninfluenza-infected mice challenged with high bacterial doses (106 CFUs). The limited bacterial clearance that was observed following influenza infection was even seen at a minimal dose of 104 CFUs/mouse (Figure 4B). We conclude that innate bacterial clearance is at least 100-fold less effective in influenza-infected mice compared to naive mice, and even in the presence of antibody, there is decreased bacterial clearance activity in the lung following influenza. This explains why pneumococcal vaccination had a marginal ability to prevent mortality of mice from secondary bacterial infection following influenza.
Figure 4.
Bacterial clearance of pneumococci from influenza-infected lungs after coinoculation of serum from normal, unvaccinated mice (NMS) or serum from mice vaccinated with PPS-conjugate (IMS). C57Bl/6 mice were treated intranasally with phosphate-buffered saline (PBS) or with 10 plaque-forming units of PR8 influenza virus. One week later, they were inoculated with 105 (A) or 104 (B) colony-forming units (CFUs) of A66.1 S. pneumoniae and NMS or IMS. Numbers of bacteria in whole lungs were determined 4 hours later. Each symbol represents an individual mouse (4–5 mice/group). The data represent results from at least 2 independent experiments. *P < .05; ****P < .0001. Abbreviations: NS, not significant; PPS, pneumococcal polysaccharide.
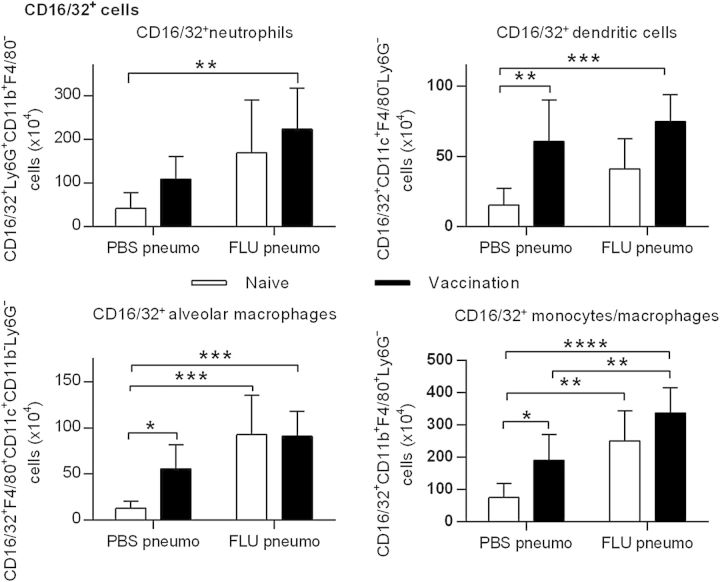
Cell populations in the lungs of mice recovering from influenza were next characterized to understand possible reasons for defective bacterial clearance. Flow cytometry analysis of alveolar macrophage, monocyte, and dendritic cell expression (see Supplementary Figures 1–3) did not show any evidence of differences among unvaccinated mice (Figure 5, open bars) or vaccinated mice (Figure 5, closed bars) that were challenged with S. pneumoniae alone (PBS/pneumo groups) or following influenza (FLU/pneumo groups). However, levels of neutrophils were increased following pneumococcal infection, which was an expected result. In addition, there were no changes among the various mouse groups in expression of CD4+ or CD8+ T cells, B cells, or natural killer cells (see Supplementary Figures 4–6). We conclude that influenza does not cause significant decreases in pulmonary cell numbers that would explain the inability of pneumococcal vaccination to provide full protection from bacterial challenge.
Figure 5.
Lung myeloid cell subsets in naive and pneumococcal polysaccharide conjugate-vaccinated mice infected with pneumococcus alone or coinfected with influenza virus and pneumococcus. The mice were inoculated intranasally (i.n.) with phosphate-buffered saline (PBS) or 10 plaque-forming units of PR8 influenza and then 1 week later, challenged i.n. with 2 × 106 colony-forming units of A66.1 S. pneumoniae. Lung cell subsets were analyzed 48 hours later by flow cytometry as described in “Material and Methods” section. The cells were defined as follows: neutrophils, Ly6G+CD11b+F4/80−; dendritic cells, CD11c+F4/80−Ly6G−; alveolar macrophages, F4/80+CD11c+CD11b−Ly6G−; monocytes/tissue macrophages, CD11b+F4/80+Ly6G−. Each group consisted of 5–7 mice. The data represent means ± SD. *P < .05, **P < .01, ***P < .001, ****P < .0001.
Opsonophagocytosis is considered to be the most reliable correlate of protection against pneumococcal infection and is typically required to assess the functional activity of vaccine-induced antibodies [20]. The results in Figures 3 and 4 indicated that IMS-mediated activity was dependent upon FcγR expression, but this pathway was unable to completely facilitate clearance in influenza-infected mice. Thus, we determined whether expression of FcγR on cells in the lung might be inhibited by influenza. The results showed that in naive mice infected with S. pneumoniae, there were relatively few cells that expressed CD16/32 (FcγRII/III) (Figure 6, open bars, PBS/pneumo groups). After PPS-conjugate vaccination, the numbers of FcγR+ cells increased, particularly among the alveolar macrophage population (closed bars, PBS/pneumo groups). Influenza infection did not decrease FcγR+ cell expression, and in some cases, tended to even increase levels of FcγR-bearing cells (FLU/pneumo groups). Similar results were obtained from analysis of cells expressing CD64 (FcγRI) (Figure 7). In no case did infection with influenza virus inhibit expression of FcγR on lung myeloid cell subsets. Analysis of median fluorescence intensity of these cells similarly showed little effect of influenza on FcγR expression levels (data not shown). We conclude that the inability of IMS to fully protect influenza-infected mice from secondary pneumococcal infection is not due to decreases in FcγR+ cell populations.
Figure 6.
Expression of CD16/32 (FcγRII/III) on lung myeloid cell subsets in naive and pneumococcal polysaccharide conjugate-vaccinated mice infected with pneumococcus alone or coinfected with influenza virus and pneumococcus. Naive and vaccinated mice were inoculated intranasally (i.n.) with phosphate-buffered saline (PBS) or 10 plaque-forming units of PR8 influenza and then 1 week later, challenged i.n. with 2 × 106 colony-forming units of A66.1 S. pneumoniae. Lung cell subsets were analyzed 48 hours later by flow cytometry as described in “Material and Methods” section. The cells were defined as follows: neutrophils, Ly6G+CD11b+F4/80−; dendritic cells, CD11c+F4/80−Ly6G−; alveolar macrophages, F4/80+CD11c+CD11b−Ly6G−; monocytes/tissue macrophages, CD11b+F4/80+Ly6G−. Each group consisted of 5–7 mice. The data represent means ± SD. *P < .05, **P < .01, ***P < .001, ****P < .0001.
Figure 7.
Expression of CD64 (FcγRI) on lung myeloid cell subsets in naive and pneumococcal polysaccharide conjugate-vaccinated mice infected with pneumococcus alone or coinfected with influenza virus and pneumococcus. Naive and vaccinated mice were inoculated intranasally (i.n.) with phosphate-buffered saline (PBS) or 10 plaque-forming units of PR8 influenza and then 1 week later, challenged i.n. with 2 × 106 colony-forming units of A66.1 S. pneumoniae. Lung cell subsets were analyzed 48 hours later by flow cytometry as described in “Material and Methods” section. The cells were defined as follows: neutrophils, Ly6G+CD11b+F4/80−; dendritic cells, CD11c+F4/80−Ly6G−; alveolar macrophages, F4/80+CD11c+CD11b−Ly6G−; monocytes/tissue macrophages, CD11b+F4/80+Ly6G−. Each group consisted of 5–7 mice. The data represent means ± SD. *P < .05, **P < .01, ***P < .001, ****P < .0001.
DISCUSSION
Our results show that influenza-infected mice had increased susceptibility to pneumococcal pneumonia even in the presence of established antibacterial humoral immunity. While PPS vaccination fully protected against lethal pulmonary bacterial infection in mice not exposed to influenza virus, vaccination of animals recovering from influenza provided only partial protection. In the absence of influenza, vaccinated mice were almost 10 000 more efficient in pulmonary clearance of bacteria compared to unvaccinated mice. However, following influenza virus infection, this clearance was severely compromised, and even in vaccinated mice, bacterial lung levels were reduced only 5–10 fold. The defective bacterial clearance in influenza-infected mice was not due to inadequate adaptive immune responses because passive transfer of immune serum resulted in similar findings (ie, significantly better bacterial clearance in nonviral-infected mice compared to influenza-infected mice). In addition, inhibition of bacterial clearance was not a result of decreased numbers of pulmonary FcγR+ cells following influenza. We [10, 11] and others [12–14] have previously shown that influenza infection causes a subsequent defect in phagocytosis of nonopsonized bacteria; our current results indicate that vaccine-induced humoral immunity can only partly overcome defects in this dominant first line of defense.
It is interesting that vaccination only protected approximately half of influenza-infected mice from secondary pneumococcal pneumonia. These results in mice agree well with the experience in Prevnar-vaccinated humans who also show only about 50% protection against secondary pneumococcal infection following influenza [18]. McCullers and colleagues have recently found that bacterial colonization of the upper-respiratory tract following influenza is likewise poorly controlled by pneumococcal vaccination [21]. Although a decrease in total numbers of alveolar macrophages following influenza has been reported [16], staining of lung cells with the PKH-226 lipophilic dye before infection has indicated no significant reduction in alveolar macrophage numbers, but rather a modified phenotype [10, 22, 23]. In the current study, we likewise saw no reduction in alveolar macrophage levels nor in numbers of other lung cell types following influenza that could result in suppressed phagocytosis of antibody-opsonized bacteria. We also observed no decreases in FcγR expression on these cells. We have previously found that interferon-γ (IFN-γ) produced during the immune response to influenza virus infection causes significantly lower expression of the macrophage receptor with collagenous structure (MARCO) scavenger receptor on alveolar macrophages [10], a receptor that is required for recognition of nonopsonized S. pneumoniae as well as survival from pneumococcal pneumonia [24]. Others [25] have similarly reported a detrimental role for IFN-γ during secondary bacterial infection. Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-mediated killing of staphylococci is also depressed following influenza [11]. It would be of interest to determine whether lung macrophages from influenza-infected mice are functionally active in mediating opsinophagocytosis of antibody-coated bacteria. However, alveolar macrophages are very difficult to manipulate in vitro because they are fragile, and also become activated and highly adherent to plastic very soon after removal from the lung. Thus, in our hands, such detailed assays have not been possible with this cell population.
It should be stressed that our results apply to pneumococcal infection caused by a serotype 3 strain; vaccination against other serotypes or bacteria that cause secondary infections following influenza, such as Staphylococcus aureus, Haemophilus influenzae, or S. pyogenes, might be more effective. In addition, we and others have found that vaccination against influenza, including the attenuated FluMist vaccine, prevents both viral infection in mice as well as accompanying secondary bacterial infections [21, 26–28]. Nevertheless, we predict that a strong adaptive immune response to viral vaccination could disable innate antibacterial defenses through mechanisms similar to viral infection itself and that this defect might not be overcome by established antibacterial adaptive immunity. Indeed, it has been recently reported that vaccination of mice with live attenuated influenza virus can prime the upper-respiratory tract for increased bacterial colonization similar to that seen following influenza virus infection [29]. Nevertheless, the same vaccine prevents invasive bacterial infection and mortality from secondary bacterial infections [27]. This ongoing issue, in addition to the crisis of growing antibiotic resistance, suggests that adding currently available bacterial vaccines or antibiotics to influenza pandemic preparedness stockpiles may be only moderately effective in preventing secondary bacterial infections. Rather, an alternative therapeutic approach involving modulation of host immunity, perhaps using an IFN-γ antagonist, could be a focus for future investigation.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Financial support. This work was supported by the National Institute of Allergy and Infectious Disease, National Institute of Health grant RO1 AI075312 to D. W. M. and an American Lung Association Senior Research Training Fellowship RT-226959-N to Y. F.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Stiver HG. The threat and prospects for control of an influenza pandemic. Expert Rev Vaccines 2004; 3:35–42. [DOI] [PubMed] [Google Scholar]
- 2.McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev 2006; 19:571–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis 2008; 198:962–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuiken T, Taubenberger JK. Pathology of human influenza revisited. Vaccine 2008; 26:D59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oswald NC, Shooter RA, Curwen MP. Pneumonia complicating Asian influenza. Br Med J 1958; 2:1305–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hers JF, Masurel N, Mulder J. Bacteriology and histopathology of the respiratory tract and lungs in fatal Asian influenza. Lancet 1958; 2:1141–3. [DOI] [PubMed] [Google Scholar]
- 7.Potter TA, Pabst LJ, Fiore AE. Bacterial coinfections in lung tissue specimens from fatal cases of 2009 pandemic influenza A (H1N1)—United States, May–August 2009. MMWR Morb Mortal Wkly Rep 2009; 58:1071–4. [PubMed] [Google Scholar]
- 8.Fleming-Dutra KE, Taylor T, Link-Gelles R, et al. Effect of the 2009 influenza A (H1N1) pandemic on invasive pneumococcal pneumonia. J Infect Dis 2013; 207:1135–43. [DOI] [PubMed] [Google Scholar]
- 9.Klugman KP, Madhi SA. Pneumococcal vaccines and flu preparedness. Science 2007; 316:49–50. [DOI] [PubMed] [Google Scholar]
- 10.Sun K, Metzger DW. Inhibition of pulmonary antibacterial defense by interferon-γ during recovery from influenza infection. Nat Med 2008; 14:558–64. [DOI] [PubMed] [Google Scholar]
- 11.Sun K, Metzger DW. Influenza infection suppresses NADPH oxidase-dependent phagocytic bacterial clearance and enhances susceptibility to secondary methicillin-resistant Staphylococcus aureus infection. J Immunol 2014; 192:3301–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shahangian A, Chow EK, Tian X, et al. Type I IFNs mediate development of postinfluenza bacterial pneumonia in mice. J Clin Invest 2009; 119:1910–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamura S, Davis KM, Weiser JN. Synergistic stimulation of type I interferons during influenza virus coinfection promotes Streptococcus pneumoniae colonization in mice. J Clin Invest 2011; 121:3657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Didierlaurent A, Goulding J, Patel S, et al. Sustained desensitization to bacterial Toll-like receptor ligands after resolution of respiratory influenza infection. J Exp Med 2008; 205:323–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goulding J, Godlee A, Vekaria S, Hilty M, Snelgrove R, Hussell T. Lowering the threshold of lung innate immune cell activation alters susceptibility to secondary bacterial superinfection. J Infect Dis 2011; 204:1086–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghoneim HE, Thomas PG, McCullers JA. Depletion of alveolar macrophages during influenza infection facilitates bacterial super-infections. J Immunol 2013; 191:1250–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kudva A, Scheller EV, Robinson KM, et al. Influenza A inhibits Th17-mediated host defense against bacterial pneumonia in mice. J Immunol 2011; 186:1666–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madhi SA, Klugman KP. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat Med 2004; 10:811–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lynch JM, Briles DE, Metzger DW. Increased protection against pneumococcal disease by mucosal administration of conjugate vaccine plus interleukin-12. Infect Immun 2003; 71:4780–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Who Expert Committee on Biological Standardization. World Health Organ Tech Rep Ser 2005; 927:1–154. [PubMed] [Google Scholar]
- 21.Mina MJ, Klugman KP, McCullers JA. Live attenuated influenza vaccine, but not pneumococcal conjugate vaccine, protects against increased density and duration of pneumococcal carriage after influenza infection in pneumococcal colonized mice. J Infect Dis 2013; 208:1281–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janssen WJ, Barthel L, Muldrow A, et al. Fas determines differential fates of resident and recruited macrophages during resolution of acute lung injury. Am J Respir Crit Care Med 2011; 184:547–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seo SU, Kwon HJ, Ko HJ, et al. Type I interferon signaling regulates Ly6Chi monocytes and neutrophils during acute viral pneumonia in mice. PLOS Pathog 2011; 7:e1001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arredouani M, Yang Z, Ning Y, et al. The scavenger receptor MARCO is required for lung defense against pneumococcal pneumonia and inhaled particles. J Exp Med 2004; 200:267–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breslow-Deckman JM, Mattingly CM, Birket SE, et al. Linezolid decreases susceptibility to secondary bacterial pneumonia postinfluenza infection in mice through its effects on IFN-γ. J Immunol 2013; 191:1792–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huber VC, Peltola V, Iverson AR, McCullers JA. Contribution of vaccine-induced immunity toward either the HA or the NA component of influenza viruses limits secondary bacterial complications. J Virol 2010; 84:4105–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun K, Ye J, Perez DR, Metzger DW. Seasonal FluMist vaccination induces cross-reactive T cell immunity against H1N1 (2009) influenza and secondary bacterial infections. J Immunol 2011; 186:987–93. [DOI] [PubMed] [Google Scholar]
- 28.Haynes L, Szaba FM, Eaton SM, et al. Immunity to the conserved influenza nucleoprotein reduces susceptibility to secondary bacterial infections. J Immunol 2012; 189:4921–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mina MJ, McCullers JA, Klugman KP. Live attenuated influenza vaccine enhances colonization of Streptococcus pneumoniae and Staphylococcus aureus in mice. mBio 2014; 5:e01040–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.