Abstract
Aim: we aimed to systematically review drugs to treat lower urinary tract symptoms (LUTS) regularly used in older persons to classify appropriate and inappropriate drugs based on efficacy, safety and tolerability by using the Fit fOR The Aged (FORTA) classification.
Methods: to evaluate the efficacy, safety and tolerability of drugs used for treatment of LUTS in older persons, a systematic review was performed. Papers on clinical trials and summaries of individual product characteristics were analysed regarding efficacy and safety in older persons (≥65 years). The most frequently used drugs were selected based on current prescription data. An interdisciplinary international expert panel assessed the drugs in a Delphi process.
Results: for the 16 drugs included here, a total of 896 citations were identified; of those, only 25 reported clinical trials with explicit data on, or solely performed in older people, underlining the lack of evidence in older people for drug treatment of LUTS. No drug was rated at the FORTA-A-level (indispensable). Only three were assigned to FORTA B (beneficial): dutasteride, fesoterodine and finasteride. The majority was rated FORTA C (questionable): darifenacin, mirabegron, extended release oxybutynin, silodosin, solifenacin, tadalafil, tamsulosin, tolterodine and trospium. FORTA D (avoid) was assigned to alfuzosin, doxazosin, immediate release oxybutynin, propiverine and terazosin.
Conclusions: dutasteride, fesoterodine and finasteride were classified as beneficial in older persons or frail elderly people (FORTA B). For most drugs, in particular those from the group of α-blockers and antimuscarinics, use in this group seems questionable (FORTA C) or should be avoided (FORTA D).
Keywords: elderly, lower urinary tract symptoms, antimuscarinics, adrenergic α blockers, 5α-reductase inhibitors, phosphodiesterase 5 inhibitors, adrenergic β-3 agonists, effectiveness, tolerability, older people, systematic review
Introduction
Drugs for the treatment of lower urinary tract symptoms (LUTS) rank among the most frequently prescribed medications for older men and women [1]. While there are several drug classes with proven efficacy available for their treatment (i.e. α-blockers, antimuscarinics, 5α-reductase inhibitors, phosphodiesterase type 5 (PDE5) inhibitors, and β3-agonists), there is no systematic comparative study on the published evidence base for their appropriateness or inappropriateness for this population.
Older people are more heterogeneous than younger individuals; they have more medical problems (multimorbidity) and take more medications as a consequence (polypharmacy). Pharmacotherapy for older persons requires clinicians to consider the alternations in pharmacodynamics and pharmacokinetics associated with ageing and the increased likelihood of drug–drug interactions and adverse events. The efficacy and safety of any proposed treatment may be different from that in younger people. For older persons with multimorbidity and/or geriatric syndromes (e.g. dementia), remaining life expectancy, and caregiver wishes and expectations also play a role in treatment decisions. Multimorbidity is extremely common in today's older people; those aged >80 years have an average of three diagnoses [2] leading to polypharmacy with 44% of men and 57% of women ≥65 years in the USA [3], and one-third in Germany [4] taking five or more drugs.
When used appropriately, there are health benefits associated with multiple appropriate drugs but adverse reactions reported by the Food and Drug Administration tripled between 1995 and 2005. These reactions have been shown to be the fourth leading cause of death in the USA [5]. Choosing the right drug, for the right patient, at the right time, is also critically important in the management of LUTS in older people, because they are highly prevalent and bothersome in both men and women [6].
The FORTA (Fit fOR The Aged) classification was introduced in 2008 with the aim of guiding physicians in their screening process for inappropriate or harmful medications and drug omissions in older patients in an everyday clinical setting [7, 8]. FORTA represents the first classification system in which both negative and positive labelling is combined at the level of individual drug or drug groups. The system aims at the individual indications (implicit listing requiring patient characteristics/diagnoses) and is therefore different from negative lists such as the American Geriatrics Society Beers Criteria list [9], the STOPP (Screening Tool of Older Person's Prescriptions) criteria [10] or the German PRISCUS list [11]. Involving a two-step Delphi process and rating by a total of 25 experts, the FORTA classification has led to a listing (FORTA list) of >200 different drugs/drug groups for over 20 main therapeutic areas with relevance to older people which is continuously expanded and refined [12].
In brief (for details, see ref. 12), the FORTA classification labels, depending on the state of evidence for safety, efficacy and overall age-appropriateness, are assigned for individual drugs as follows:
• Class A (absolutely): indispensable drug, clear-cut benefit in terms of efficacy/safety ratio proven for a given indication in older people.
• Class B (beneficial): drugs with proven or obvious efficacy in older people, but limited extent of effect or safety concerns.
• Class C (careful): drugs with questionable efficacy/safety profiles in older people, to be avoided or omitted in the presence of too many other drugs, lack of benefits or emerging side effects; review/find alternatives.
• Class D (don't): avoid in older people, omit first, review/find alternatives.
For example, relevant antihypertensive drugs are classified as follows: ACE-inhibitors/angiotensin-receptor antagonists and long-acting dihydropyridine calcium antagonists: FORTA A; β-blockers/diuretics: FORTA B; spironolactone/moxonidine: FORTA C; clonidine/verapamil: FORTA D [12]. FORTA does not take the place of individual therapeutic considerations or decisions. Contraindications always take precedence over the FORTA classification and the system allows for exceptions. Application of the FORTA list has led to the first published endpoint effect of a listing approach: the rate of falls, a major geriatric problem, could be reduced by two-thirds and overall medication quality improved compared with standard care [13].
In this article, we present the analysis and rating process of an independent multiprofessional international expert panel for the 16 most commonly prescribed oral drugs for long-term use in patients with LUTS, based on a systematic literature review and a subsequent two-step Delphi approach using the FORTA classification.
Methods
Supplementary data, Appendix S3, available in Age and Ageing online.
Procedure
The present expert rating procedure evolved from a previous conference during which the situation of older people with urinary incontinence was evaluated [14]. The procedure was performed in the following steps:
• Identification of the rater team members: The initiators of the project (M.W. and M.O.) identified raters based on available information on the internet. Experts were eligible if they met the following criteria: geriatric internists/geriatricians, general practitioners or urologists with documented clinical experience in the pharmacotherapy of (multimorbid) older people; high academic status; prominent standing in the leading geriatric/urological medical associations; substantial number, quality and relevance of publications. Accordingly, five raters (K.B., D.C., E.C.K., M.K., A.W.) were identified who met those criteria and could also accept the invitation to participate.
• Selection of drugs to be assessed: In the first step, the initiators M.W. and M.O. selected groups of drugs used orally for long-term treatment of LUTS and listed relevant agents in each group. Urological drugs were chosen if they are typically used as long-term treatment. Given this limitation, antibiotics drugs were not considered. The proposed choice of drugs was refined by the raters who voted for removing non-oral drugs (e.g. oestrogen ointment).
• The relevant drug classes were 5α-reductase inhibitors, α1-blockers, antimuscarinics, β3-agonists and PDE5 inhibitors (Table 1). Medication codes using the WHO Drug Reference List, which employs the Anatomical Therapeutic Chemical (ATC) classification system, were added for completeness and to facilitate subsequent searches.
• Systematic literature review: A literature search in PubMed/Medline and the Cochrane library was performed in March 2014 using the search terms [drug name] in the INN terminology plus the standard filters [clinical trials] [full text available] [age 65+ years], and [age 80+ years]. The aim of the search was to identify appropriate clinical trials to examine the efficacy, safety and tolerability of drugs used for the treatment of LUTS in older people. A total of 835 abstracts were retrieved and reviewed by M.W. and M.O. for appropriateness, in particular whether the article explicitly reported results in the age groups ≥65 years, ≥70 years, ≥75 years, ≥80 years, ≥85 years. In a second step, [drug name] and the terms [elderly] or [older] in the title were searched. In total, 62 additional abstracts were retrieved and checked for appropriateness. A duplicate article was removed. Analysis of studies as full texts: A total of 34 potentially appropriate articles were identified. They were reviewed as full texts and key information extracted by M.W. and M.O. into an extensive Microsoft Excel file with particular focus on the presence of information on particular side effects (see Supplementary data, Appendix S1, available in Age and Ageing online). No attempt was made to contact authors to acquire additional data or unpublished data.
• Analysis of summary of product characteristics: The most recent summary of product characteristics (SmPC) was downloaded for all drugs from the EMA website, or if not available, from other reliable sources (e.g. www.fachinfo.de). For generic drugs, the most frequently used brand was selected based on the prescription volume in Germany [1]. The texts were thoroughly analysed by M.W. and M.O. using the same template as for the full texts above. From this material, the initiators, M.W. and M.O. derived a proposal for initial FORTA labels. The proposal together with the spreadsheet and full texts/abstracts were forwarded to the rater team for review and addition of further articles that were felt to be relevant.
• Two-step Delphi process: The initiators and members of the rater group convened at a meeting at the annual EAU congress in Stockholm on 15 April 2014 and were instructed by M.W. and M.O. about the process with particular focus on the FORTA procedure.
After the meeting, raters reviewed the literature and classified each of the listed drugs according to FORTA, together with optional comments. The related survey is deposited in the Supplementary data, Appendix S2, available in Age and Ageing online; 16 drugs and 17 items were finally rated (low dose/extended release and standard dose/immediate release oxybutynin separately rated).
Table 1.
Selected drugs for the long-term treatment of lower urinary tract symptoms in older people
| Drug class (drugs in alphabetical order) | Agent | FORTA classa | Number of ratersb | Consensus coefficient, Round 1 (cut-off 0.800) | Expert ratings on a numerical scale: A = 1, B = 2, C = 3, D = 4 Round 1 (R1) Round 2 (R2) Mean (Mode) |
|---|---|---|---|---|---|
| 5α-reductase inhibitors | Dutasteride | B | 5 | 1.000 | 2.0; 2 |
| Finasteride | B | 5 | 0.900 | 2.2; 2 | |
| α1-blockers | Alfuzosin | D | 5 | 0.900 | 3.8; 4 |
| Doxazosin | D | 5 | 0.900 | 3.8; 4 | |
| Silodosin | C | 5 | 1.000 | 3.0; 3 | |
| Tamsulosin | C | 5 | 1.000 | 3.0; 3 | |
| Terazosin | D | 5 | 0.800 | R1: 3.6; 4 R2: 3.8; 4 |
|
| Antimuscarinics | Darifenacin | C | 5 | 1.000 | 3.0; 3 |
| Fesoterodine | B | 5 | 0.900 | 2.2; 2 | |
| Oxybutynin standard dose/ immediate release | D | 5 | 0.900 | 3.8; 4 | |
| Oxybutynin low dose/extended release | C | 4 | 1.000 | 3.0; 3 | |
| Propiverine | D | 5 | 0.700 | R1: 3.4; 3 R2: 3.8; 4 |
|
| Solifenacin | C | 5 | 1.000 | 3.0; 3 | |
| Tolterodine | C | 5 | 1.000 | 3.0; 3 | |
| Trospium | C (B) | 5 | 0.800 | R 1: 2.4; 2 R 2: 2.6; 3 |
|
| β3-agonist | Mirabegron | C | 5 | 1.000 | 3.0; 3 |
| PDE5 inhibitor | Tadalafil | C | 5 | 0.900 | 2.8; 3 |
LUTS, lower urinary tract symptoms.
aOriginal FORTA class in parentheses if different from consensus results.
bNo changes between Rounds 1 and 2.
Statistics
Details of the Delphi method (all experts rate independently without knowing their peers' ratings, knowing only the reached consensus) and the corresponding statistical analysis have been described in detail previously [12]. In brief, the aggregated list of rater labels was statistically analysed (see below), the aggregate findings were sent out to the reviewers for a second rating round if the consensus coefficient was <0.800.
For this statistics, raters' FORTA labels were converted into numerical values A→1, B→2, C→3 and D→4, respectively; the mean (m) and mode were calculated for each item, reconverted to FORTA labels and compared with the original author-based labels. The range for each label was defined as:
if 1 ≤ m < 1.5 → FORTA Class A
if 1.5 ≤ m < 2.5 → FORTA Class B
if 2.5 ≤ m < 3.5 → FORTA Class C
if m ≥ 3.5 → FORTA Class AD
where m = mean based on the raters' Grades 1–4.
Consensus parameters were generated by calculating the percentage of experts' FORTA ratings (minus abstentions) in line with the original FORTA values, both overall and for each item separately. The coefficients were then corrected (cons_corr) to weight the degree of deviation between the experts' individual FORTA ratings, expressed in terms of range class, from 0 to 3 as defined:
Range = 0: unanimity among all experts (no deviation);
Range = 1: greatest range only from A to B or B to C, or C to D (neighbouring classes), ½ weight;
Range = 2: greatest distance from A to C or B to D, 2/3 weight;
Range = 3: greatest distance from A to D, full weight.
In the second round, as in the first procedure, values were converted and medians and means calculated. The arithmetic mean provided the basis for back conversion to FORTA labels that were compiled for all drugs in a separate, annotated list. After the second round, the Delphi process led to unequivocal results and was finished.
Evidence synthesis
Literature search
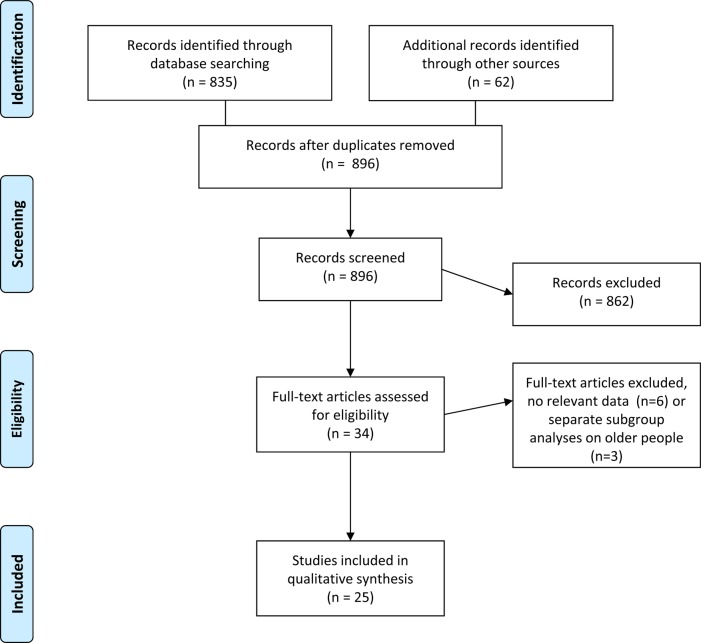
In total, 897 abstracts were potentially relevant based on the search in PubMed/Medline. As shown in Figure 1 (PRISMA flow chart, [s16]), 34 papers were identified from those abstracts that seemingly met the inclusion criteria and were further checked as full text; only 25 papers contained results on clinical trials on older patients or explicitly reported data from subgroups of older patients aged ≥65 years (which is the most commonly used, but unauthorised definition of ‘elderly’) for the 16 drugs of interest. Explicit results on clinical trials for older patients were reported for the 5α-reductase inhibitors dutasteride [s15, s17, s18] and finasteride [s15, s19], the α1-blockers alfuzosin [s20], doxazosin [21] and tamsulosin [s18, s22], the antimuscarinic drugs darifenacin [s23, s24], fesoterodine [s25-s30], oral oxybutynin [s31-s33], solifenacin [s33, s34], tolterodine [s26, s35, s36], and trospium [s37], the β3-agonist mirabegron [s38] and the PDE5 inhibitor tadalafil [s39, s40]. As seen in Table 2, the number of studies reporting data on older patients to support drug efficacy and safety was one for alfuzosin, doxazosin, trospium, and mirabegron, two for darifenacin, solifenacin, tadalafil and tamsulosin, three for oxybutynin (both formulations) and for tolterodine, and six for fesoterodine. Several studies were small (<100 patients) with the SOFIA trial, exposing 392 elderly patient to fesoterodine in the double-blind phase, being one of the largest RCTs dedicated to older patients [s30]. Only 18 of the 25 papers reported on randomised, controlled clinical trials (RCTs).
Figure 1.
Flow diagram for the systematic review according to the PRISMA statement [s16].
Table 2.
Drug names by FORTA classification, basic data from studies included and rationale for classification
| Drug | Total number of studies on efficacy, safety and tolerability in older patients | Number of studies thereof presenting subclass analyses in older patients | Total number of older people treated | Number of studies showing efficacy | Approximate rate of side effects (%) | Description of properties relevant for FORTA classification |
|---|---|---|---|---|---|---|
| FORTA A (indispensable) | ||||||
| No drugs identified | ||||||
| FORTA B (beneficial) | ||||||
| Dutasteride | 3 | 2 | 4,430 | 3 | 12 (not assessed in two trials) | Efficacious in elderly, no geriatrically relevant side effects (mental deterioration, fall, cardiovascular), impotence or breast problems not seen as major drawbacks |
| Fesoterodine | 6 | 3 | 2,511 | 6 | 60 | The best studied antimuscarinic drug in older people (large patient numbers that render lack of impact on cognitive functions, favourable cognitive profile, clear-cut efficacy in older people) |
| Finasteride | 2 | 1 | 3,283 | 2 | 12 (not assessed in one trial) | Efficacious in elderly, comparably well studied, no geriatrically relevant side effects (mental deterioration, fall, cardiovascular), impotence or breast problems not seen as major drawbacks |
| FORTA C (caution) | ||||||
| Darifenacin | 2 | 2 | 421 | 2 | 58 (not systematically reported in [23], added from text) | Associated with cardiovascular notes of caution, significant anticholinergic reactions, with constipation as typical geriatric problem being frequent, mental deterioration not unequivocally shown, but used as argument of caution |
| Mirabegron | 1 | 1 | 1,183 | 1 | 56 | Relatively new drug, efficacy also shown for older people; almost all data are from regulatory documents which only state qualitatively that elderly do react differently from younger patients. No specific data on major problems in older people: cognitive effects not properly studied. The cardiovascular side effects are of major concern in older people and may contribute to increased risk even in normotensive patients; atrial fibrillation is seen as serious potential side effect. Rating may change to B if proper data on elderly are provided in the future |
| Oxybutynin (low dose/extended release) | 1 | 1 | 111 | 1 | 65 | Acceptable rate of anticholinergic side effects if used in low doses (extended release); however, a paucity of data in older people exists |
| Silodosin | – | – | – | – | No data on efficacy and safety in older people available, hypotensive reactions as contraindication or matter of caution. Superiority in efficacy or safety over other α-blockers has not been substantiated in studies in older people. High concomitant incidence of arterial hypertension which under any treatment may render patients hypotensive; even low α-blocking activity may be detrimental in this situation. Discrepancy of reporting low incidence of hypotensive reactions as summary of studies in the experts' information and its rating as being frequent in the side effect table | |
| Solifenacin | 2 | 2 | 1,159 | 2 | 36 | Associated with cardiovascular notes of caution, significant anticholinergic adverse events, with constipation as typical geriatric problem being frequent, mental deterioration not unequivocally shown, but used as argument of caution and post-marketing reports |
| Tadalafil | 2 | 2 | 558 | 1 | 26 | Cardiovascular contraindications. No primary efficacy studies in elderly patients but pooled analysis shows efficacy in patients aged 65 years or older |
| Tamsulosin | 2 | 2 | 1,121 | – | 37, not specifically assessed in [s18] | No data on efficacy in older people, small cardiovascular study, hypotensive reactions as contraindication or matter of caution, especially upon treatment initiation, indicating increased risks for falls and fractures. Though hypotensive reactions may be less than with other α-blockers, this has not been substantiated in studies in older people. High concomitant incidence of arterial hypertension which under any treatment may render patients hypotensive; even low α-blocking activity may be detrimental in this situation |
| Tolterodine | 3 | 1 | 643 | 3 | 48 | Associated with cardiovascular notes of caution, significant anticholinergic reactions, with constipation as typical geriatric problem being frequent, mental deterioration shown in two studies, used as argument of caution |
| Trospium | 1 | 1 | 178 | 1 | 47 | Understudied but plausible results on mental safety in conjunction with measurements in CSF. Peripheral anticholinergic action of no less concern than for other antimuscarinics, thought QTc effects do not seem to occur |
| FORTA D (avoid) | ||||||
| Alfuzosin | 1 | 1 | 2,121 | 1 | 6 | Data from an open study on efficacy and safety in older people, vasodilatory effects including hypotensive reactions as contraindication or matter of caution frequent. High concomitant incidence of arterial hypertension which under any treatment may render patients hypotensive; cardiac arrhythmias and even syncope may be precipitated |
| Doxazosin | 1 | 1 | 341 | – | 42 | Only old data on efficacy and safety in older people available, hypotensive reactions as contraindication or matter of caution frequent. High concomitant incidence of arterial hypertension which under any treatment may render patients hypotensive; even low α-blocking activity may be detrimental in this situation. Cardiac arrhythmias, myocardial infarction and stroke may be precipitated. May only be used to primarily treat arterial hypertension concomitantly (FORTA C) if properly indicated |
| Oxybutynin (standard dose/immediate release) | 2 | 1 | 60 | 1 | 73 (dry mouth 86) | High rate of anticholinergic side effects, cardiovascular risk profile, proven mental/cognitive side effects including falls, even for extended release preparations, paucity of clear data in older people (note low dose extended release: FORTA C) |
| Propiverine | – | – | – | – | High rate of anticholinergic side effects, cardiovascular risk profile, unclear cognitive side effects, lack of clear data in older people | |
| Terazosin | – | – | – | – | No data on efficacy and safety in older people, hypotensive reactions as contraindication or matter of caution. High concomitant incidence of arterial hypertension which under any treatment may render patients hypotensive; even low α-blocking activity may be detrimental in this situation. Cardiac arrhythmias. May only be used to primarily treat arterial hypertension concomitantly (FORTA C) if properly indicated | |
The total number of study papers for each drug, the number of subclass analyses for older people contained therein (out of the database of 25 papers), the numbers of older patients treated in those studies, the number of studies showing efficacy in older people and the approximate rate of adverse events (AE, mean value) are shown from left to right (AEs were difficult to compare as the overall rate of AEs was not explicitly stated in several reports from which they had to be added from the individual items not taking into account multiple AEs in the same patients. The number of patients was added from separate analyses including those in which open label studies have been conducted on the same patients as in the preceding clinical trials.) The integral assessment of all data including those from the SmPCs is summarised in the last column to the right.
Safety data from these trials were heterogeneous, in several cases not detailed, and a clear and comparable overview was difficult to achieve. Therefore, the integral and consensual assessment of all data including those from the SmPCs is additionally given as summary in Table 2 (right column).
No specific reports in older patients were available for the α1-blockers silodosin, or terazosin, respectively, or the antimuscarinic drug propiverine.
Analysis of summary of product characteristics
No package inserts, with the exception of that for oxybutynin, explicitly mentioned the elderly population. A summary of the published evidence is provided as Supplementary data, Appendix S1, available in Age and Ageing online. The amount of information available on side effects and contraindications of particular interest in the elderly population was highly variable. For example, for many agents, study results are available on typical geriatric problems such as dementia (or cognition overall), falls, anticholinergic effects or constipation, while other side effects of specific relevance such as the serotonin syndrome were not mentioned in any of the reviewed SmPC.
Delphi process leading to the final FORTA classification
Final ratings after round 2 are shown in Table 1. Proposed ratings were confirmed in 94% of cases (deviation for 1/17 items). Only 3 of the 16 studied drugs were re-rated in the second survey (Table 1). Ratings changed for propiverine (C → D) and trospium (B → C). Table 2 summarises the rationales (key points) behind the categorisation of the individual drugs.
The ratings showed little variance with a corrected consensus coefficient of >0.8 in all but four cases which had to be re-rated in the second round. Trospium was finally assigned a C rating, but with only a one vote majority (2 B, 3 C).
Eventually, no drug was labelled with FORTA A. Out of the 17 items for 16 drugs, only 3 were assigned the FORTA B (beneficial) classification: dutasteride, fesoterodine and finasteride. The majority of agents were rated with FORTA C (careful) (in alphabetical order): darifenacin, mirabegron, oral oxybutynin (low dose/extended release), silodosin, solifenacin, tadalafil, tamsulosin, tolterodine and trospium, labelling them as potentially harmful if not properly monitored for effects and adverse events; this does not entirely preclude their use, as in Category D but mandates close clinical surveillance and individualisation of use. Furthermore, the following agents were assigned a FORTA D classification (avoid): alfuzosin, doxazosin, oral oxybutynin (immediate release), propiverine and terazosin.
Discussion
On the basis of our systematic literature search and analysis as well as subsequent rating process for frequently used drugs for the treatment of LUTS in older patients, only three were labelled FORTA B (beneficial), dutasteride, fesoterodine and finasteride. The majority of drugs should only be used with caution in older persons, for specific indications, and with safety monitoring (FORTA C), while alfuzosin, doxazosin, oxybutynin in standard dose/immediate release formulations, propiverine and terazosin should be avoided (FORTA D).
Key findings on urological drug appropriateness
The unique FORTA process makes clear that, within a given drug class, the appropriateness of individual drugs may substantially vary; such differences can be based on real differences in efficacy and safety (e.g. newer drugs may have optimised profiles), but also on the quality of the trial, and the specific patient population studied. Strict and citable evidence, as typically derived from RCTs, is an exception rather than the rule for the older population, although important if available. Likewise, data from studies in older people may reflect specific outcomes of interest, for example, cognition, rather than efficacy or tolerability and, therefore, may have not been given due emphasis. For example, those studies specifically examining the short-/medium-term cognitive safety of antimuscarinics in elderly people are rare.
Recognition of older patients as important recipients of pharmacological treatments for LUTS has increased over recent years, leading to a focus on the need for data specific to that population. Studies had previously only reported on older people taking part in registration trials of medications where those >65 years of age comprised approximately one-third of the total sample. Older people included in such trials were often unrepresentative of the general elderly, being healthier and with relatively fewer co-morbid conditions. Trials specifically in community-dwelling elderly [s29, 41] and in the more medically complex elderly [s25] are relatively recent developments that should become a standard approach to testing of novel agents in therapeutic areas which predominantly affect older people. Likewise, older trials often fail to identify adverse events that may predominantly affect older people such as cognition [42].
The antimuscarinic agent fesoterodine has been the subject of considerable testing in people aged ≥65 years, with purposive recruitment of patients aged ≥75 years [s25,s29,s30]. Fesoterodine has been found to be efficacious in the treatment of urinary urgency and urgency incontinence in older patients with beneficial outcomes in terms of health-related quality of life and in those with considerable co-morbidity and polypharmacy, with few adverse cognitive events over the period of the clinical trials. Darifenacin, in its single pre-planned study of efficacy in patients aged ≥65 years failed to reach statistical significance on its primary outcome but did note that the proportion of people who derived benefit from active treatment were greater than those who received placebo treatment [43]. The cognitive safety of darifenacin has also been extensively tested in a series of chronic dosing studies in cognitively intact older people [44, 45]. Data on solifenacin, tolterodine and alternative preparations of oral oxybutynin are derived from post hoc pooled analyses of older people participating in registration trials [s34,s36] and as such, conclusions from such data are limited by their nature. Data on the efficacy of trospium, often touted as elder friendly due to its propensity not being able to cross the blood–brain barrier [46] and its lack of drug–drug interactions [47] are notably lacking; this may have hampered its FORTA rating. In the updated Beers list [9], all antimuscarinics are classified as ‘anticholinergics’ to be avoided in people with constipation.
The FORTA classification presented here underlines clinically relevant differences between drugs in this class ranging from D to B ratings which are not adequately reflected in a pure negative list (‘drugs to avoid’) as opposed to the combined positive/negative approach of FORTA.
Mirabegron has only recently been introduced into clinical practice and data supporting its use in older patients [s38] naturally lag behind those drugs that have been on the market longer. Therefore, any update of this list in the future may result in different FORTA classifications.
Whereas much of the available data consider the use of these drugs in the community-dwelling, largely robust elderly, there has been scant attention paid to the needs of the frail elderly, a point clearly made in the 5th International Consultation on Incontinence guidelines for the frail elderly [48].
Other drug delivery systems, such as transdermal or transvaginal formulations of oxybutynin or local injections of onabotulinum toxin [49] for the treatment of urgency or urgency incontinence, may be alternatives for orally administered drugs in older people. However, we have limited our literature search and analyses to oral medications as they represent the majority of prescriptions [1]. Additionally, transdermal, transvaginal or local application of drugs has not been subject of thorough investigations in older people, and therefore, scientific data for the age groups of interest are lacking. These facts were discussed by the rater group who explicitly recommended refraining from categorising these preparations to limit heterogeneity and maintain feasibility of the process. Nevertheless, these treatment options could be of interest in the future to avoid first pass effects, improve tolerability and lower/avoid adverse events particularly in the group of elderly or frail older patients. Transdermal oxybutynin could be seen, for example, as a reasonable, but understudied and not widely utilised alternative.
Methodological considerations on literature analysis
To obtain a robust basis for the rating, the rater group applied a standard literature search that focused on the older population (age ≥65 years). Despite the fact that urological drugs are mainly used in this population, including the very old and frail elderly patient, there was a lack of published data for this population. In a recent hearing of the FDA as part of the Safety and Innovation Act of 2012, it was noted that the geriatric population is clearly under-represented in cardiovascular trials (only 9% of all patients included). We demonstrated in our literature search and analyses that the situation is not substantially different for drugs used to treat LUTS [50].
To detect and include available information on studies in older people that may not have been published in PubMed/Medline or the Cochrane library, we also reviewed current summaries of product characteristics. Information about the safety of drugs has to be gathered by the respective drug manufacturers and—usually every 6 months—submitted to regulatory authorities (periodic safety update reports, PSUR) [51]. Based on the European Union legislation 2309/93, articles 21 and 22, and the EU directive 75/319/EEC, articles 29c and 29d, and the ICH Guideline CPMP/ICH/377/95 [52], such information consists of spontaneous reports about adverse drug events, results of clinical studies and systematic literature searches on case reports and other sources. Given this background and in view of the paucity of published studies of relevance for our review, we also used this document as source for our assessment.
While, in principle, all manufacturers of a generic drug have to submit PSUR to the regulatory authorities, the prescribing information for the same drug marketed under different brands may differ due to varying update intervals but also due to differences in drugs with respect to auxiliary ingredients such as salts. Thus, if a drug was marketed under several brands, we chose the most frequently prescribed one as listed in the drug-prescribing report [1]; it is based on all prescriptions paid by the statutory health insurance in Germany and updated on an annual basis.
The rating process followed the Delphi procedure [53]. The consensus process was driven by an evidence-based approach, i.e. experts made their decision based on the results of the literature search, while their personal experience was secondary.
Limitations of the study
It is possible that some inconsistencies were related to the multidisciplinary nature of this Delphi exercise. For instance, not all of the respondents had equal practical experience with the various drugs (e.g. mirabegron was launched in June 2014 in Germany and Spain but is still not available in France). Furthermore, the group was small and a larger set of experts (also from additional countries) might come to slightly different conclusions. However, the degree of consensus was notable given the fact that experts with different specialisations voted without knowing their colleagues' opinions. This is in line with the degree of rating consensus for the first round of the Delphi process for the published FORTA list [12] which was almost the same (92%) for a much larger group of raters (20 from different countries).
As the raters were collectively instructed about FORTA at the inaugural meeting, anonymity could not be warranted; however, communication of any voting opinion was suppressed at this meeting, and independence was ensured by formal agreement on not communicating the individual votes during the Delphi rounds.
Consensus aspects cannot be excluded from drug assessments in older people as limited evidence is the rule rather than the exception, and integration of all data including SmPC undoubtedly relies on personal assessment. This is underlined by the fact that even in the fesoterodine studies of older patients, those with significant cognitive impairment at baseline were excluded from participation, illustrating a typical gap of clinical evidence.
In some larger studies, which included patients with a mean age of 60–65 years with a typical age distribution, a substantial proportion of elderly/geriatric patients will be present but not reported separately. If the subgroups were not explicitly reported, these data remained unnoticed in our systematic review. For urological drugs, a limited amount of scientific evidence is currently available for the evaluation of active substances, potential therapeutic alternatives and indicated monitoring procedures [11].
Although SmPC were thought to yield unpublished information, some valuable information from trials may not be detected by the screening procedure. The experiences from uncontrolled trials or even case reports are lost in such an approach, but they may add relevant information. The gold standard would have been a qualified meta-analysis which, however, would have failed in most instances or not added information as in most instances only singular or heterogeneous studies could have been included.
FORTA does not specifically address drug–drug interactions or contraindications that still need to be checked individually. Thus, (in)appropriateness in general terms does not necessarily imply the same in individual patients.
Conclusions
Drugs commonly used in urology for the treatment of LUTS differ widely in terms of appropriateness for the older population. From this analysis, based on yet sparse clinical evidence, it appears that beneficial drugs are only available in the classes 5α-reductase inhibitors (dutasteride, finasteride) and antimuscarinics (fesoterodine), whereas the use of the majority of drugs for LUTS may be problematic or should be avoided in older people.
Key points.
Using a systematic approach including a literature search and subsequent Delphi process, an interdisciplinary expert group rated the appropriateness of the most frequently used drugs for long-term treatment of LUTS in older people with regard to efficacy, tolerability and safety.
In older people, the majority of these drugs, in particular those from the group of α-blockers and antimuscarinics, should either be used with caution or be avoided.
The evidence base for the use of these drugs in older people is limited.
Supplementary Material
Acknowledgements
Brigitte Ulrich and Daniela Böhm, Pfizer Pharma GmbH, Berlin, organised the kick-off meeting of the rater group in Stockholm on 15 April 2014. Pfizer Pharma GmbH funded the meeting and the conduct of the systematic literature analysis including assistance for writing of the manuscript, but had no influence on the contents. Systematic literature search and medical writing support were provided by 3P Consulting, Seefeld, Germany. The statistical analysis was performed by Dr Christel Weiss, Department for Medical Statistics, Biomathematics and Information Processing Mannheim, University of Heidelberg, Germany. All authors were responsible for critical revisions of the manuscript and for important intellectual content. They approved the final contents of the manuscript.
Conflicts of interest
M.O. has received lecturing or consulting fees, participated in drug trial or received funds from: Allergan, Apogepha, Astellas, Bayer, Ferring, GlaxoSmithKline, Lilly, Mundipharma, Pfizer and Recordati. K.F.B. has received lecturing and consulting fees from Astellas, Pfizer and MSD. D.C.-D. has been consultant, lecturer or investigator for Allergan, AMS, Astellas, Medtronic and Pfizer. E.C.-K. received lecturing and/or consulting fees from Astellas, Allergan, AMS, Coloplast, Lilly, Medtronic, Pfizer, Zambon; participated clinical trials with AB Science, Allergan, Astellas, Coloplast, Ipsen Biotech and Medtronic. M.K. received lecturing and consulting fees from Astellas, GSK, Lilly, MSD and Pfizer. A.W. received funds from Astellas Pharma, Pfizer Inc., for research, speaker honoraria and consulting, SCA for speaker honoraria and consulting, Merus Labs for speaker honoraria. M.W. was employed by AstraZeneca R&D, Mölndal, as director of discovery medicine (=translational medicine) from 2004 to 2006, while on sabbatical leave from his professorship at the University of Heidelberg. After return to this position in January 2007, he received lecturing and consulting fees from Bristol Myers, LEO, Mundipharma, Novartis, Pfizer, Roche, Sanofi-Aventis, Shire and Takeda.
Supplementary data
References
(The very long list of references supporting this review has meant that only the most important are listed here and are represented by bold type throughout the text. The full list of references is given in Supplementary data, available in Age and Ageing online.)
- 1. Schwabe U, Paffrath D. Arzneiverordnungs-Report 2013: Aktuelle Daten, Kosten, Trends und Kommentare [in German]. Heidelberg: Springer Verlang, 2013. [Google Scholar]
- 2. van den Akker M, Buntinx F, Metsemakers JF, Roos S, Knottnerus JA. Multimorbidity in general practice: prevalence, incidence, and determinants of co-occurring chronic and recurrent diseases. J Clin Epidemiol 1998; 51: 367–75. [DOI] [PubMed] [Google Scholar]
- 3. Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. JAMA 2002; 287: 337–44. [DOI] [PubMed] [Google Scholar]
- 4. Barmer Insurance. Barmer GEK Arzneimittelverordnungsreport 2013 [in German]. www.khbrisch.de/files/barmer_gek_arzneimittelreport_2013.pdf (27 July 2014, date last accessed).
- 5. Light D. Bearing the risks of prescription drugs. In: Light DW, ed. The Risks of Prescription Drugs. New York: Columbia University Press, 2010; 1–39. [Google Scholar]
- 6. Coyne KS, Sexton CC, Thompson CL, et al. The prevalence of lower urinary tract symptoms (LUTS) in the USA, the UK and Sweden: results from the Epidemiology of LUTS (EpiLUTS) study. BJU Int 2009; 104: 352–60. [DOI] [PubMed] [Google Scholar]
- 7. Wehling M. Multimorbidity and polypharmacy: how to reduce the harmful drug load and yet add needed drugs in the elderly? Proposal of a new drug classification: fit for the aged. J Am Geriatr Soc 2009; 57: 560–1. [DOI] [PubMed] [Google Scholar]
- 8. Wehling M, ed. Drug Therapy for the Elderly. Vienna, Austria: Springer Publisher, 2013. ISBN 978-3-7091-0911-3. [Google Scholar]
- 9. American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2012; 60: 616–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gallagher P, Ryan C, Byrne S, Kennedy J, O'Mahony D. STOPP (Screening Tool of Older Person's Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther 2008; 46: 72–83. [DOI] [PubMed] [Google Scholar]
- 11. Holt S, Schmiedl S, Thurmann PA. Potentially inappropriate medications in the elderly: the PRISCUS list. Dtsch Arztebl Int 2010; 107: 543–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuhn-Thiel A, Weiss C, Wehling M. Consensus validation of the FORTA (Fit fOR The Aged) list: a clinical tool for increasing the appropriateness of pharmacotherapy in the elderly. Drugs Aging 2014; 31: 131–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Michalek C, Wehling M, Schlitzer J, Frohnhofen H. Effects of “Fit fOR The Aged” (FORTA) on pharmacotherapy and clinical endpoints-a pilot randomized controlled study. Eur J Clin Pharmacol 2014; 70: 1261–7. [DOI] [PubMed] [Google Scholar]
- 14. Becher K, Oelke M, Grass-Kapanke B, et al. Improving the health care of geriatric patients: management of urinary incontinence: a position paper. Z Gerontol Geriatr 2013; 46: 456–64. [DOI] [PubMed] [Google Scholar]
- 41. Chapple C, Steers W, Norton P, et al. A pooled analysis of three phase III studies to investigate the efficacy, tolerability and safety of darifenacin, a muscarinic M3 selective receptor antagonist, in the treatment of overactive bladder. BJU Int 2005; 95: 993–1001. [DOI] [PubMed] [Google Scholar]
- 42. Paquette A, Gou P, Tannenbaum C. Systematic review and meta-analysis: do clinical trials testing antimuscarinic agents for overactive bladder adequately measure central nervous system adverse events? J Am Geriatr Soc 2011; 59: 1332–9. [DOI] [PubMed] [Google Scholar]
- 43. Chapple C, DuBeau C, Ebinger U, Rekeda L, Viegas A. Darifenacin treatment of patients >or= 65 years with overactive bladder: results of a randomized, controlled, 12-week trial. Curr Med Res Opin 2007; 23: 2347–58. [DOI] [PubMed] [Google Scholar]
- 44. Lipton RB, Kolodner K, Wesnes K. Assessment of cognitive function of the elderly population: effects of darifenacin. J Urol 2005; 173: 493–8. [DOI] [PubMed] [Google Scholar]
- 45. Kay G, Crook T, Rekeda L, et al. Differential effects of the antimuscarinic agents darifenacin and oxybutynin ER on memory in older subjects. Eur Urol 2006; 50: 317–26. [DOI] [PubMed] [Google Scholar]
- 46. Staskin D, Kay G, Tannenbaum C, et al. Trospium chloride is undetectable in the older human central nervous system. J Am Geriatr Soc 2010; 58: 1618–9. [DOI] [PubMed] [Google Scholar]
- 47. Doroshyenko O, Jetter A, Odenthal KP, Fuhr U. Clinical pharmacokinetics of trospium chloride. Clin Pharmacokinet 2005; 44: 701–20. [DOI] [PubMed] [Google Scholar]
- 48. Wagg A, Gibson W, Ostaszkiewicz J, et al. Urinary incontinence in frail elderly persons: Report from the 5th International Consultation on Incontinence. Neurourol Urodyn 2015; 34: 398–406. [DOI] [PubMed] [Google Scholar]
- 49. Santos-Silva A, da Silva CM, Cruz F. Botulinum toxin treatment for bladder dysfunction. Int J Urol 2013; 20: 956–62. [DOI] [PubMed] [Google Scholar]
- 50. Food and Drug Administration. FDA Report. Collection, Analysis, and Availability of Demographic Subgroup Data for FDA-Approved Medical Products. Rockville, August 2013 http://www.fda.gov/downloads/…/fdasia/ucm365544.pdf (9 July 2014, date last accessed).
- 51. Klepper MJ. The periodic safety update report as a pharmacovigilance tool. Drug Saf 2004; 27: 569–78. [DOI] [PubMed] [Google Scholar]
- 52. Committee for Medicinal Products for Human Use (CHMP). CPMP/ICH/377/95. ICH Topic E 2 A. Clinical Safety Data Management: Definitions and Standards for Expedited Reporting. Step 5. www.ema.europa.eu/docs/…/WC500002749.pdf (9 July 2014, date last accessed).
- 53. Roth R, Wood W. A Delphi approach to acquiring knowledge from single and multiple experts. In: Trends and Direction in Expert Systems Proceedings of the 1990 ACM SIGBDP Conference on Trends and Directions in Expert Systems, Orlando, FL, 1990, pp. 301–24. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.