Abstract
Objective: Atomoxetine is the most widely used nonstimulant for the treatment of attention-deficit/hyperactivity disorder (ADHD). It selectively acts on the norepinephrine (NE) system. Dopamine beta hydroxylase (DBH) regulates the synthesis of NE. This study aimed to investigate whether variants in the DBH gene have an effect on the differential response to atomoxetine.
Methods: Children and adolescents with ADHD were enrolled in a prospective, open-label study of atomoxetine for 8–12 weeks. The dose was titrated to 1.2–1.4 mg/kg per day and maintained for at least 4 weeks. The primary efficacy measure was the investigator-rated ADHD Rating Scale-IV (ADHD-RS-IV). Three categorical evaluations of treatment effects (defined as response, robust response, and remission) were used. We used a candidate gene approach. Eight single nucleotide polymorphisms (SNPs) in DBH were selected and genotyped based on the functional annotation in literature. Their association with response or remission status was analyzed.
Results: Four SNPs were found nominally associated with response status (rs1076150, p = 0.0484; rs2873804, p = 0.0348; rs1548364, p = 0.0383; and rs2519154, p = 0.0097), two were associated with robust response (rs1076150, p = 0.0349; and rs2519154, p = 0.0047), and one was associated with remission (rs2519154, p = 0.0479). The association between rs2519154 and robust response was significant after correction of multiple comparison (p = 0.0384). Two haplotypes of linkage disequilibrium (LD) block1 (constituted by rs1108580, rs2873804, rs1548364, and rs2519154) were nominally associated with response and robust response status (CTAC: p = 0.0301 for response, p = 0.0374 for robust response; TCGT: p = 0.0317 for response, p = 0.021 for robust response), whereas one haplotype (GC) of LD block2 (constituted by rs2073837 and rs129882) was associated with robust response and remission status (p = 0.0377 for robust response; p = 0.0321 for remission), although none achieved significant threshold after multiple comparison.
Conclusions: Variants in DBH genes were associated with atomoxetine response in the treatment of ADHD. Further replication in larger samples would be warranted.
Introduction
Atomoxetine is the first approved nonstimulant for the treatment of attention-deficit/hyperactivity disorder (ADHD). It was marketed in 2002 in the United States and in 2007 in China, providing another potent medication for ADHD. In the clinical trials before and after marketing, atomoxetine showed superior effect on ADHD symptoms compared with placebo control (Kelsey et al. 2004; Michelson et al. 2002), with moderate effect size estimated to be 0.71.
Following studies showed that the effect of atomoxetine varied among patients. The response rate (defined as ≥40% reduction from baseline ADHD Rating Scale [ADHD-RS] scores) was reported to be 45% at the end of 6 weeks treatment (Newcorn et al. 2008). This figure was very close to that continuing treatment (48.4%) after clinical trials (Wilens et al. 2006), as one of the most common reason of discontinuation was lack of effectiveness.
Some other studies reported similar adherent rate of atomoxetine. In the COMPLY observational study performed in Germany, only 48.8% patients who took atomoxetine continued treatment after 12 months. Given the slow onset of the effect, and relatively low response rate, it was necessary to find predicting markers that could early identified patients who would benefit from atomoxetine treatment.
The first pharmacogenetic study of ADHD was published in 1999 (Winsberg et al. 1999). After this initiation, most of the following studies focused on methylphenidate (MPH). The candidate genes were selected from the pharmacodynamic, pharmacokinetic, and etiological pathways of ADHD. Possible associated genes involved were DAT1, DRD4, NET1, ADRA2A, CES1, HTT, and GRM7 (Seeger et al. 2001; Hamarman et al. 2004; Cheon et al. 2005; Faraone et al. 2005; Cheon et al. 2007; Polanczyk et al. 2007, da Silva, et al. 2008; Kooij et al. 2008; Mick et al. 2008; Purper-Ouakil et al. 2008; Stein and McGough 2008; McGough et al. 2009; Nemoda et al. 2009; Froehlich et al. 2010; Genro et al. 2010; Kieling et al. 2010; Kim et al. 2010; Polanczyk et al. 2010; Froehlich et al. 2011; Park et al. 2012a,b). Only two articles investigated atomoxetine response, which consistently reported association of genes in the NE system; that is, NET1 and ADRA2A (Ramoz et al. 2009; Yang et al. 2013).
Atomoxetine is a high selective inhibitor of the NE transporter, which may exert its therapeutic effect through change in the NE concentration in the synapses. The effectiveness of atomoxetine in ADHD patients led to the hypothesis that ADHD might be a noradrenergic disorder (Biederman and Spencer 1999). This hypothesis comes, not only from pharmacological evidence, but also from the fact that the noradrenergic system regulates many higher cognitive functions including attention (Solanto et al. 1998). Low levels of NE reduce motivation and performance in learning tasks (Viggiano et al. 2004). NE interacts with dopamine (DA) to regulate motor activity, as decreased DA reduces motor activity, whereas increased DA promotes activity.
Dopamine beta hydroxylase (DBH) is a synthetic enzyme for NE. Knocking out the DBH gene led to decreased NE levels in central neural system (Cryan et al. 2001), which suggested the important role of this enzyme in the maintenance of normal NE functions. Some antidepressants that act on NE system, such as reboxetine, have no effect in DBH knockout (KO) mice (Cryan et al. 2001), which made us speculate that any functional DNA variants in DBH genes, changing the activity of the enzyme, might modulate the response to atomoxetine in the treatment of ADHD. Discovery of such variants would make it possible to predict the treatment effect before it started. Up until now, there have been no studies investigating the association of the DBH gene with atomoxetine response in ADHD children.
We selected 8 single nucleotide polymorphisms (SNPs) in the DBH gene (rs1076150, rs1611115, rs1108580, rs2873804, rs1548364, rs2519154, rs2073837, and rs129882) to analyze their association with categorical assessments of atomoxetine response.
Methods
Participants
Children and adolescents who met the ADHD criteria of the Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV) were recruited from the Child and Adolescent Psychiatric Outpatient Department of Beijing University Sixth Hospital (American Psychiatric Association 1994). The diagnosis was first made by a child psychiatrist, and then validated by a semistructured interview with the parents and the child, using Barkley's Clinical Diagnostic Interview Scale (Barkley 1998). This scale was based on DSM-IV criteria. It included questions about the 18 items regarding ADHD symptoms, onset of age, impairment of function, and exclusion criteria. We had used this scale in our previous pharmacogenetic study (Yang et al. 2004, 2013). All patients were required to meet the following symptom severity thresholds: A total score of investigator-rated ADHD-RS-IV no less than 25 for boys or 22 for girls, or a subtype corresponding subscale score ≥12 (DuPaul et al. 1998; Wang et al. 2007). The subjects were unmedicated, or had been medicated with MPH preparations or atomoxetine but had stopped for at least 1 or 4 weeks, respectively. All patients and their parents were Han Chinese. The exclusion criteria were: 1) Allergy to atomoxetine, 2) combined treatment with other psychotropic drugs or non-drug intervention for ADHD; 3) non-compliance with the blood draw. The study was approved by the Beijing University Sixth Hospital Institutional Review Board. Parents signed written informed consent. For younger adolescents, oral assent was acquired.
Clinical trial
The subjects received open-label treatment with atomoxetine for 8–12 weeks. The dose was titrated from 0.5 mg/kg/day in the 1st week, to 0.8 mg/kg per day in the 2nd week, and to 1.2 mg/kg per day in the 3rd–4th weeks. If necessary, the dose could be increased to 1.4mg/kg per day in the 5th week. Then the dose was maintained for at least 4 weeks. Those with side effects at any stage of titration could be maintained at the dose for 1–2 weeks. The total course of treatment was no more than 12 weeks. Treatment response was assessed at baseline, and at the end of the 1st, 2nd, and 4th weeks, and at the 8th week or at the end of the trial. Medication compliance was assessed by directly asking the parent at every visit. Patients who missed the whole or a partial dose for 3 consecutive days or for 10 total days were defined as noncompliant and were withdrawn from the trial.
Treatment response assessment
The primary efficacy measure was the investigator rated ADHD-RS-IV, (DuPaul 1998). It was rated based on both parent and teacher reports. The ADHD-RS-IV consists of 18 items corresponding to DSM-IV criteria for ADHD. The total symptom score as well as the inattention and hyperactivity-impulsivity subscales scores were used to evaluate the core symptoms of ADHD. This scale had been translated into Chinese. The validity and reliability of the Chinese version were demonstrated by Su et al. (2006).
In this study, we used categorical definitions of treatment response. A decrease of at least 25% on the ADHD-RS-IV total score from baseline to the end of the trial was defined as “response” (Swanson et al. 2001; Steele et al. 2006; Ramoz et al. 2009; Dickson et al. 2011). A decrease of 40% or more was defined as “robust response” (Newcorn et al. 2008). An average ADHDRS-IV item score ≤1 at the end of the treatment was defined as “remission” (Stein et al. 2003; Steele et al. 2006).
Genotyping
Eight SNPs in the DBH gene were selected via the ABI SNPbrowser™ (Table 1). These SNPs were either associated with ADHD in previous studies, or were tag SNPs selected by ABI SNPbrowser. We preferentially selected potential functional SNPs that located at coding regions, 5′ or 3′ untranslated regions, the boundary of exon and intron, and the 5′ regulatory region, including the promoter. Although a this was a bioinformatic analysis, all the eight SNPs were regulatory SNPs (http://rsnp.psych.ac.cn/).
Table 1.
List of 8 SNPs Across the DBH Gene Investigated in this Study
| NCBI SNP reference | dbSNP allele | Public location | Location on gene region | SNP type | Residue change | MAF (HapMap-CHB) |
|---|---|---|---|---|---|---|
| rs1076150 | C/T | chr.9-136498761 | Flanking_5′UTR | Regulatory | – | 0.195 |
| rs1611115 | C/T | chr.9-136500515 | Flanking_5′UTR | Regulatory | NA = > NA | 0.207 |
| rs1108580 | C/T | chr.9-136505114 | Exon 2–intron2 splice junction |
Silent mutation Regulatory |
E [Glu] = > E [Glu] |
0.183 |
| rs2873804 | C/T | chr.9-136505644 | Intron 2 | Regulatory | – | 0.244 |
| rs1548364 | A/G | chr.9-136507742 | Intron 3 | Regulatory | – | – |
| rs2519154 | C/T | chr.9-136512275 | Intron 5 | Regulatory | – | 0.125 |
| rs2073837 | A/G | chr.9-136522928 | Intron 11 | Regulatory | – | – |
| rs129882 | C/T | chr.9-136523669 | UTR 3′ | Regulatory | NA = > NA | 0.366 |
SNP, single nucleotide polymorphism; DBH, dopamine beta hydroxylase; NCBI, National Center for Biotechnology Information; MAF, minor allele frequency; UTR, untranslated region.
The SNP was genotyped using TaqMan allelic discrimination assays (Livak 1999) on an ABI 7900HT instrument (Applied Biosystems, Foster City, CA), using predesigned and validated TaqMan assay reagent kits. The polymerase chain reaction (PCR) was performed following a standard protocol with 5 ng DNA in 5 mL reaction volumes for each sample. Thermal cycle included 95°C for 10 minutes, followed by 92°C for 15 seconds and 60°C for 1 minute for 40–45 cycles. SDS version 2.3 software (Applied Biosystems) was used for genotype identification. For quality control, 10% of the samples were genotyped as duplicates. Call rates for SNPs were 99.14%. Two to four negative test controls were set in every plate.
Statistical analysis
The SNPs were tested for Hardy–Weinberg equilibrium (HWE) by calculating the probability that the deviation from HWE could be explained by chance. None of the eight SNPs significantly deviated from HWE (p > 0.05). To evaluate the relationship of the SNPs, we used HaploView Program (http://www.broad.mit.edu/mpg/haploview) to calculate the pairwise value of linkage disequilibrium, D, D', and r2. Blocks were defined using the algorithm of confidence interval (CI) by Gabriel et al. (2002). Two linkage disequilibrium (LD) blocks were detected (Fig. 1). Block 1 included four SNPs: rs1108580, rs2873804, rs1548364, and rs2519154, whereas Block 2 consisted of two SNPs: rs2073837 and rs129882. Baseline demographic and clinical features between differential response groups were compared using the SPSS 19.0 software, with categorical variables assessed by χ2 test, and continuous variables by t test. The association among alleles, haplotypes, and treatment response to atomoxetine were evaluated using the χ2 test by Haploview 4.0. The level of significance was 0.05 for all analyses. Five thousand permutation tests were used to control multiple comparisons. The odds ratio (OR) was calculated as the measure of effect size.
FIG. 1.
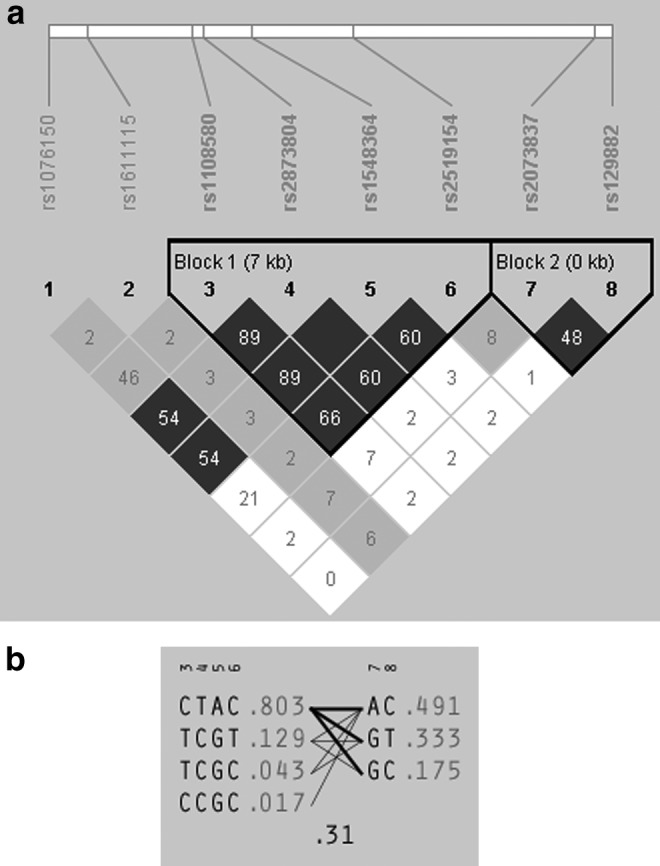
(a) Linkage disequilibrium (LD) blocks of the dopamine beta hydroxylase (DBH) gene. The numbers marked in the cells were pairwise r2. Dark gray represents “stong evidence of LD,” light gray represents “uninformative,” and white represents “stong evidence of recombination.” (b) Haplotypes and estimated frequency of the two LD blocks of the DBH gene.
Results
Eighty seven subjects completed 8–12 weeks of treatment and provided both baseline and end-point assessments. The demographic and clinical features are presented in Table 2.
Table 2.
Demographic and Clinical Features of the Subjects According to Differential Response Status
| Responsea | Robust responseb | Remissionc | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Features | Yes (n = 64) | No (n = 23) | χ2/t | p value | Yes (n = 45) | No (n = 42) | χ2/t | P value | Yes (n = 49) | No (n = 38) | χ2/t | p value |
| Male, n (%) | 53 (82.8) | 19 (82.6) | 0.000 | 1.000 | 38 (84.4) | 34 (81.0) | 0.186 | 0.667 | 41 (83.7) | 31 (81.6) | 0.066 | 0.798 |
| Age, mean (SD) | 9.1 ± 2.3 | 8.5 ± 2.0 | 0.785 | 0.438 | 9.3 ± 2.6 | 8.6 ± 1.8 | 1.046 | 0.302 | 9.4 ± 2.6 | 8.6 ± 1.8 | 1.121 | 0.269 |
| IQ, mean (SD) | 107.3 ± 16.1 | 97.7 ± 11.5 | 1.855 | 0.072 | 108.6 ± 16.2 | 100.3 ± 13.8 | 1.735 | 0.091 | 108.7 ± 16.0 | 100.2 ± 13.9 | 1.781 | 0.083 |
| ADHD-Rating Scale, mean (SD)d | ||||||||||||
| Total | 31.1 ± 8.2 | 33.1 ± 9.5 | 0.968 | 0.336 | 31.0 ± 8.4 | 32.4 ± 8.7 | 0.791 | 0.431 | 28.5 ± 7.9 | 35.7 ± 7.6 | 4.252 | 0.000 |
| Inattention | 17.9 ± 3.6 | 18.7 ± 4.2 | 0.790 | 0.432 | 18.1 ± 3.4 | 18.2 ± 4.1 | 0.097 | 0.923 | 17.5 ± 3.2 | 19.0 ± 4.2 | 1.778 | 0.080 |
| Hyperactive-impulsive | 13.2 ± 6.5 | 14.5 ± 6.8 | 0.806 | 0.422 | 12.9 ± 6.3 | 14.2 ± 6.9 | 0.972 | 0.334 | 11.0 ± 6.3 | 16.7 ± 5.5 | 4.421 | 0.000 |
Response was defined as a decrease of at least 25% on the ADHD-RS-IV total score from baseline to the end of the trial.
Robust response was defined as the decrease of ≥40% on the ADHD-RS-IV total score from baseline to the end of the trial.
Remission was defined as the average ADHDRS-IV item score ≤1 at the end of the treatment.
This indicated the baseline ratings.
IQ, intelligence quotient.
Single variant association
Using an ADHD-RS score decrease of ≥25% as the response criterion, 64 patients were responders and 23 were nonresponders. Among the eight SNPs used in the analysis, four showed nominal significant association with responder status, and one showed trend association (rs1076150, p = 0.0484; rs2873804, p = 0.0348; rs1548364, p = 0.0383; rs2519154, p = 0.0097; and rs1108580, p = 0.0736). rs2519154 kept a trend association after 5000 permutations performed for multiple test correction (p = 0.0926). Using the “robust response” criteria, 45 patients were robust responders and 42 were nonresponders. The abovementioned five SNPs also showed nominal significance or trend association (rs1076150, p = 0.0349; rs2873804, p = 0.0665; rs1548364, p = 0.0564; rs2519154, p = 0.0047; and rs1108580, p = 0.0718), with t rs2519154 still significant after 5000 permutations (p = 0.0384). The C allele was associated with being a nonresponder (93.6% vs. 77.6%, OR: 4.207, 95% CI: 1.465–12.076). Using remission criteria, 41 patients achieved remission, and 36 did not. rs2519154 and rs1076150 showed nominal significant or trend association with remission status (rs2519154, p = 0.0479; rs1076150, p = 0.0969), but none survived the 5000 permutation of multiple test correction (p > 0.05) (Table 3).
Table 3.
Association of 8 SNPs in DBH Gene with Atomoxetine Response
| Responsea | Robust responseb | Remissionc | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SNPs | Alleles | Yes (n = ) | No | χ2 | p value | Permutation p value | Yes | No | χ2 | p value | Permutation p value | Yes | No | χ2 | p value | Permutation p value |
| rs1076150 | T | 108 (84.4%) | 44 (95.7%) | 3.896 | 0.0484 | 0.295 | 74 (82.2%) | 78 (92.9%) | 4.449 | 0.0349 | 0.2188 | 82 (83.7%) | 70 (92.1%) | 2.755 | 0.0969 | 0.5548 |
| C | 20 (15.6%) | 2 (4.3%) | (16 (17.8%) | 6 (7.1%) | 16 (16.3%) | 6 (7.9%) | ||||||||||
| rs1611115 | C | 15 (11.7%) | 6 (13.0%) | 0.056 | 0.813 | 1 | 79 (87.8%) | 74 (88.1%) | 0.004 | 0.9488 | 1 | 84 (85.7%) | 69 (90.8%) | 1.039 | 0.3081 | 0.9536 |
| T | 113 (88.3%) | 40 (87.0%) | (11 (12.2%) | 10 (11.9%) | 14 (14.3%) | 7 (9.2%) | ||||||||||
| rs1108580 | C | 102 (79.7%) | 42 (91.3%) | 3.2 | 0.0736 | 0.4256 | 70 (77.8%) | 74 (88.1%) | 3.241 | 0.0718 | 0.4522 | 78 (79.6%) | 66 (86.8%) | 1.577 | 0.2092 | 0.8442 |
| T | 26 (20.3%) | 4 (8.7%) | 20 (22.2%) | 10 (11.9%) | 20 (20.4%) | 10 (13.2%) | ||||||||||
| rs2873804 | T | 97 (77.0%) | 42 (91.3%) | 4.457 | 0.0348 | 0.2324 | 68 (75.6%) | 71 (86.6%) | 3.367 | 0.0665 | 0.3796 | 76 (77.6%) | 63 (85.1%) | 1.564 | 0.2111 | 0.8448 |
| C | 29 (23.0%) | 4 (8.7%) | 22 (22.4%) | 11 (14.9%) | (22 (24.4%) | 11 (13.4%) | ||||||||||
| rs1548364 | A | 99 (77.3%) | 42 (91.3%) | 4.291 | 0.0383 | 0.2348 | 68 (75.6%) | 73 (86.9%) | 3.641 | 0.0564 | 0.3608 | 76 (77.6%) | 65 (85.5%) | 1.772 | 0.1832 | 0.7982 |
| G | 29 (22.7%) | 4 (8.7%) | 22 (24.4%) | 11 (13.1%) | 22 (22.4%) | 11 (14.5%) | ||||||||||
| rs2519154 | C | 91 (81.2%) | 41 (97.6%) | 6.684 | 0.0097 | 0.0926 | 59 (77.6%) | 73 (93.6%) | 8.006 | 0.0047 | 0.0384 | 66 (80.5%) | 66 (91.7%) | 3.913 | 0.0479 | 0.3578 |
| T | 21 (18.8%) | 1 (2.4%) | 17 (22.4%) | 5 (6.4%) | 16 (19.5%) | 6 (8.3%) | ||||||||||
| rs2073837 | A | 63 (50.0%) | 23 (52.3%) | 0.067 | 0.7952 | 1 | 41 (46.6%) | 43 (52.4%) | 0.581 | 0.446 | 1 | 45 (47.9%) | 39 (51.3%) | 0.199 | 0.6553 | 1 |
| G | 63 (50.0%) | 21 (47.7%) | 47 (53.4%) | 39 (47.6%) | 49 (52.1%) | 37 (48.7%) | ||||||||||
| rs129882 | T | 39 (30.5%) | 19 (41.3%) | 1.788 | 0.1812 | 0.837 | 28 (31.1%) | 30 (35.7%) | 0.414 | 0.5198 | 1 | 29 (29.6%) | 29 (38.2%) | 1.413 | 0.2345 | 0.8728 |
| C | 89 (69.5%) | 27 (58.7%) | (62 (68.9%) | 54 (64.3%) | 69 (70.4%) | 47 (61.8%) | ||||||||||
Response was defined as a decrease of at least 25% on the Attention-Deficit/Hyperactivity Disorder Rating Scale-IV (ADHD-RS-IV) total score from baseline to the end of the trial.
Robust response was defined as the decrease of ≥40% on the ADHD-RS-IV total score from baseline to the end of the trial.
Remission was defined as the average ADHDRS-IV item score ≤1 at the end of the treatment.
SNP, single nucleotide polymorphism; DBH, dopamine beta hydroxylase.
Haplotype association
Based on the current sample, we calculated blocks in which SNPs were in strong LD and might transfer to the next generation together. SNPs in a block could present the same trend of association with a phenotype as the causal variant. In this sample, we obtained two blocks. The LD plot and haplotype are illustrated in Figure 1a and b. Haplotypes of block 1 were nominally associated with responder status, with haplotype CTAC more prevalent in nonresponders (haplotype frequency: responders 76.4%, nonresponders 91.2%, p = 0.0301), whereas haplotype TCGT was more prevalent in responders (16.2% vs. 3.8%, p = 0.0317). These two haplotypes were also nominally associated with robust response (CTAC: 74.3% vs. 86.8%, p = 0.0374; TCGT: 18.6% vs. 6.8%, p = 0.021). One haplotype of block 2, GC, was nominally associated with robust response (23.3% vs. 11.3%, p = 0.0377). This haplotype was also nominally associated with remission (23.0% vs. 10.5%, p = 0.0321). But none achieved significance after a permutation test (p > 0.05) (Table 4).
Table 4.
Association of Haplotypes in DBH Gene with Atomoxetine Response
| Responsea | Robust responseb | Remissionc | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Haplotypes | Haplotype frequency | Yes | No | χ2 | p value | Permutation p value | Yes | No | χ2 | p value | Permutation p value | Yes | No | χ2 | p value | Permutation p value |
| Block 1 | ||||||||||||||||
| CTAC | 0.803 | 97.8 (76.4%) | 42.0 (91.2%) | 4.702 | 0.0301 | 0.209 | 66.9 (74.3%) | 72.9 (86.8%) | 4.334 | 0.0374 | 0.2296 | 65.0 (85.5%) | 74.8 (76.4%) | 2.244 | 0.1341 | 0.7026 |
| TCGT | 0.129 | 20.7 (16.2%) | 1.7 (3.8%) | 4.613 | 0.0317 | 0.228 | 16.7 (18.6%) | 5.7 (6.8%) | 5.329 | 0.021 | 0.1406 | 6.7 (8.9%) | 15.7 (16.0%) | 1.962 | 0.1613 | 0.7702 |
| TCGC | 0.043 | 5.3 (4.1%) | 2.3 (4.9%), | 0.047 | 0.8287 | 1 | 3.3 (3.7%) | 4.3 (5.1%) | 0.211 | 0.6459 | 1 | 3.3 (4.3%) | 4.3 (4.4%) | 0.001 | 0.9807 | 1 |
| CCGC | 0.017 | 3.0 (2.3%) | 0.0 (0.0%), | 1.097 | 0.2949 | 0.9606 | 2.0 (2.2%) | 1.0 (1.2%) | 0.273 | 0.6014 | 1 | 1.0 (1.3%) | 2.0 (2.0%) | 0.133 | 0.7156 | 1 |
| Block 2 | ||||||||||||||||
| AC | 0.491 | 63.0 (49.2%) | 22.5 (48.9%) | 0.002 | 0.9663 | 1 | 41.0 (45.6%) | 44.5 (52.9%) | 0.949 | 0.3299 | 0.9524 | 39.0 (51.3%), | 46.5 (47.4%) | 0.26 | 0.6104 | 1 |
| GT | 0.333 | 39.0 (30.5%) | 19.0 (41.3%), | 1.788 | 0.1812 | 0.837 | 28.0 (31.1%) | 30.0 (35.7%), | 0.414 | 0.5198 | 1 | 29.0 (38.2%) | 29.0 (29.6%) | 1.413 | 0.2345 | 0.8728 |
| GC | 0.175 | 4.5 (9.8%) | 26.0 (20.3%) | 2.566 | 0.1092 | 0.5726 | 21.0 (23.3%) | 9.5 (11.3%) | 4.32 | 0.0377 | 0.2356 | 8.0 (10.5%) | 22.5 (23.0%) | 4.594 | 0.0321 | 0.2124 |
Response was defined as a decrease of at least 25% on the Attention-Deficit/Hyperactivity Disorder Rating Scale-IV (ADHD-RS-IV) total score from baseline to the end of the trial.
Robust response was defined as the decrease of ≥40% on the ADHD-RS-IV total score from baseline to the end of the trial.
Remission was defined as the average ADHDRS-IV item score ≤1 at the end of the treatment.
DBH, dopamine beta hydroxylase.
Discussion
This study found SNPs and haplotypes of the DBH gene in association with atomoxetine response. Of the four associated SNPs (rs1076150, rs2873804, rs1548364, and rs2519154), rs2519154 survived the multiple test correction size. Variants in LD with a causal variant show elevated test statistics in association analysis. The trend association of the other SNPs and haplotypes in LD with rs2519154 suggested it to be a true association rather than inflation (Bulik-Sullivan et al. 2015).
ADHD was suggested to be an NE disorder (Biederman and Spencer 1999). NE neurons mainly originated from the locus coeruleus (LC) and projected to forebrain, cerebellum, and spinal cord (Cerbone and Sadile 1994). It has been widely acknowledged that ADHD children have functional alteration in the prefrontal cortex and cerebellum. LC cells have effects on the regulation of locomotor activity, attention and arousal, and information storage, as well as fear and anxiety (Mason 1981; Cerbone and Sadile 1994; Sadile 1996; Robbins et al. 1997). Low levels of NE reduce motivation and performance in learning tasks (Kobayashi et al. 2000).
DBH was the key enzyme in the biosynthesis process of NE; therefore, it was considered to be the candidate gene for ADHD susceptibility. Daly et al. (1999) first reported that the TaqI polymorphism in the fifth intron of DBH was associated with ADHD. Roman et al. (2002) and Smith et al. (2003) replicated this association. Hawi et al. (2003) performed haplotype analysis, and reported a haplotype containing that the A2 allele of the TaqI polymorphism was associated with ADHD. The meta-analysis of all the candidate genes for ADHD by Faraone et al. (2005) identified DBH to be one of the significant associated genes (OR = 1.33, 95% CI = 1.11–1.59).
One study investigated the DBH gene in association with MPH response in an adult sample (Contini et al. 2012), but no significant result was reported. They investigated seven genes; however, none got a significant result, even though some had been reported to be associated in previous studies. Because MPH had its effect mainly on the dopamine system, whereas atomoxetine played a major role on the adrenergic system, the response to atomoxetine might be more sensitive to the variability of DBH activity.
As a nonstimulant, the efficacy of atomoxetine has been well documented. It is a selective NE reuptake inhibitor. Although atomoxetine were considered to be effective and safe, there is considerable interindividual variability of the medication response among patients (Greenhill et al. 1996; Vaughan and Kratochvil 2006). Clinical treatment often used a trial and error approach, and gradual titration to the optimal dosage. We searched the literature and found only two studies investigating the genetic association of atomoxetine response. Ramoz et al. (2009) first investigated the SLC6A2 and CYP2D6 genes. The genomic regions across exon 2 and exon 4–9 of SLC6A2 were significantly associated with atomoxetine response in two independent samples. No association was found for the CYP2D6 gene. Another study was performed by our group. We found that rs3785143 in SLC6A2 had significant association with responder status, whereas rs2279805 was associated with remission status (Yang et al. 2012). The former SNP was located in the sixth intron of SLC6A2. It was among the region of exons 4–9. Therefore, our result was consistent with Ramoz's. In the Yang et al. (2012) study, we also reported a haplotype with two SNPs at ADRA2A (rs1800544 and rs553668) in association with nonremission of ADHD symptoms after atomoxetine treatment.
There was no previous study investigating the association between DBH and atomoxetine response. Given the results in animals, namely that some effective antidepressants for ADHD via the NE system had no effect in DBH KO mice (Cryan et al. 2001), it is reasonable that functional variants in DBH gene might interfere with the response in human beings. The significant associated SNP rs2519154 of this study was located in the intron of the DBH gene (http://www.ncbi.nlm.nih.gov/projects/SNP/snp_ref.cgi?rs=2519154). Although it does not change the structure of the DBH enzyme, it is a regulatory SNP, which locates in the protein binding sites of encoded RNA, and has a distal regulation effect (http://rsnp.psych.ac.cn/quickSearch.do). The relationship of this SNP and the activity of the DBH enzyme need further research.
The limitations of this study included that the sample size was small; therefore many nominally associated SNPs and haplotypes of the DBH gene had not achieved significance after permutation correction. Validation of this result in large samples appears to be necessary.
Conclusions
Variants in the DBH gene, especially rs2519154, were associated with atomoxetine response in the treatment of ADHD. Given the small sample size, we still could not exclude a random association, and further replication in larger samples would be warranted.
Clinical Significance
This study suggested that DBH rs2519154 polymorphism was associated with the treatment response to atomoxetine in children and adolescents with ADHD. This SNP might be used as a predictor of atomoxetine response. Patients with the C allele were more likely to be nonresponders. The mechanism of how variants of the DBH gene moderate the atomoxetine response needs further research.
Disclosures
No competing financial interests exist.
Reference
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- Barkley R, Murphy K: Attention-Deficit Hyperactivity Disorder: Clinical Workbook, 2nd ed. New York: Guilford Publications; 1998 [Google Scholar]
- Biederman J, Spencer T: Attention-deficit/hyperactivity disorder (ADHD) as a noradrenergic disorder. Biol Psychiatry 46:1234–1242, 1999 [DOI] [PubMed] [Google Scholar]
- Bulik-Sullivan BK, Loh P-R, Finucane H, Ripke S, Yang J, Schizophrenia Working Group of the Psychiatric Genomics Consortium, Patterson N, Daly MJ, Price AL, Neale BM: LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet 47:291–295, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerbone A, Sadile AG: Behavioral habituation to spatial novelty: Interference and noninterference studies. Neurosci Biobehav Rev 18:497–518, 1994 [DOI] [PubMed] [Google Scholar]
- Cheon KA, Kim BN, Cho SC: Association of 4-repeat allele of the dopamine D4 receptor gene exon III polymorphism and response to methylphenidate treatment in Korean ADHD children. Neuropsychopharmacology 32:1377–1383, 2007 [DOI] [PubMed] [Google Scholar]
- Cheon KA, Ryu YH, Kim JW, Cho DY: The homozygosity for 10-repeat allele at dopamine transporter gene and dopamine transporter density in Korean children with attention deficit hyperactivity disorder: Relating to treatment response to methylphenidate. Eur Neuropsychopharmacol 15:95–101, 2005 [DOI] [PubMed] [Google Scholar]
- Contini V, Victor MM, Bertuzzi GP, Salgado CA, Picon FA, Grevet EH, Rohde LA, Belmonte-de-Abreu P, Bau CH: No significant association between genetic variants in 7 candidate genes and response to methylphenidate treatment in adult patients with ADHD. J Clin Psychopharmacol 32:820–823, 2012 [DOI] [PubMed] [Google Scholar]
- Cryan JF, Dalvi A, Jin SH, Hirsch BR, Lucki I, Thomas SA: Use of dopamine-betahydroxylase-deficient mice to determine the role of norepinephrine in the mechanism of action of antidepressant drugs. J Pharmacol Exp Ther 298:651–657, 2001 [PubMed] [Google Scholar]
- da Silva TL1, Pianca TG, Roman T, Hutz MH, Faraone SV, Schmitz M, Rohde LA: Adrenergic alpha2A receptor gene and response to methylphenidate in attention-deficit/hyperactivity disorder-predominantly inattentive type. J Neural Transm 115:341–345, 2008 [DOI] [PubMed] [Google Scholar]
- Daly G, Hawi Z, Fitzgerald M, Gill M: Mapping susceptibility loci in attention deficit hyperactivity disorder: preferential transmission of parental alleles at DAT1, DBH and DRD5 to affected children. Mol Psychiatry 4:192–196, 1999 [DOI] [PubMed] [Google Scholar]
- Dickson RA, Maki E, Gibbins C, Gutkin SW, Turgay A, Weiss MD: Time courses of improvement and symptom remission in children treated with atomoxetine for attention-deficit/hyperactivity disorder: analysis of Canadian open-label studies. Child Adolesc Psychiatry Ment Health 5:14, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R: ADHD Rating Scale-IV: Checklists, Norms, and Clinical Interpretations. New York: Guilford Press; 1998 [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, Sklar P: Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry 57:1313–1323, 2005 [DOI] [PubMed] [Google Scholar]
- Froehlich TE, Epstein JN, Nick TG, Melguizo Castro MS, Stein MA, Brinkman WB, Graham AJ, Langberg JM, Kahn RS: Pharmacogenetic predictors of methylphenidate dose-response in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 50:1129–1139, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich TE, McGough JJ, Stein MA: Progress and promise of attention-deficit hyperactivity disorder pharmacogenetics. CNS Drugs 24:99–117, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genro JP, Kieling C, Rohde LA, Hutz MH: Attention-deficit/hyperactivity disorder and the dopaminergic hypotheses. Expert Rev Neurother 10:587–601, 2010 [DOI] [PubMed] [Google Scholar]
- Greenhill LL, Abikoff HB, Arnold LE, Cantwell DP, Conners CK, Elliott G, Hechtman L, Hinshaw SP, Hoza B, Jensen PS, March JS, Newcorn J, Pelham WE, Severe JB, Swanson JM, Vitiello B, Wells K: Medication treatment strategies in the MTA Study: Relevance to clinicians and researchers. J Am Acad Child Adolesc Psychiatry 35:1304–1313, 1996 [DOI] [PubMed] [Google Scholar]
- Hamarman S1, Fossella J, Ulger C, Brimacombe M, Dermody J: Dopamine receptor 4 (DRD4) 7-repeat allele predicts methylphenidate dose response in children with attention deficit hyperactivity disorder: A pharmacogenetic study. J Child Adolesc Psychopharmacol 14:564–574, 2004 [DOI] [PubMed] [Google Scholar]
- Hawi Z, Lowe N, Kirley A, Gruenhage F, Nothen M, Greenwood T, Kelsoe J, Fitzgerald M, Gill M: Linkage disequilibrium mapping at DAT1, DRD5 and DBH narrows the search for ADHD susceptibility alleles at these loci. Mol Psychiatry 8:299–308, 2003 [DOI] [PubMed] [Google Scholar]
- Kelsey DK, Sumner CR, Casat CD, Coury DL, Quintana H, Saylor KE, Sutton VK, Gonzales J, Malcolm SK, Schuh KJ, Allen AJ: Once-daily atomoxetine treatment for children with attention-deficit/hyperactivity disorder, including an assessment of evening and morning behavior: A double-blind, placebo-controlled trial. Pediatrics 114:e1–e8, 2004 [DOI] [PubMed] [Google Scholar]
- Kieling C, Genro JP, Hutz MH, Rohde LA: A current update on ADHD pharmacogenomics. Pharmacogenomics 11:407–419, 2010 [DOI] [PubMed] [Google Scholar]
- Kim BN, Kim JW, Hong SB, Cho SC, Shin MS, Yoo HJ. Possible association of norepinephrine transporter −3081(A/T) polymorphism with methylphenidate response in attention deficit hyperactivity disorder. Behav Brain Funct 6:57, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Noda Y, Matsushita N, Nishii K, Sawada H, Nagatsu T, Nakahara D, Fukabori R, Yasoshima Y, Yamamoto T, Miura M, Kano M, Mamiya T, Miyamoto Y, Nabeshima T: Modest neuropsychological deficits caused by reduced noradrenaline metabolism in mice heterozygous for a mutated tyrosine hydroxylase gene. J Neurosci 20:2418–2426, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooij JS, Boonstra AM, Vermeulen SH, Heister AG, Burger H, Buitelaar JK, Franke B: Response to methylphenidate in adults with ADHD is associated with a polymorphism in SLC6A3 (DAT1). Am J Med Genet B Neuropsychiatr Genet 147B:201–208, 2008 [DOI] [PubMed] [Google Scholar]
- Livak KJ: Allelic discrimination using fluorogenic probes and the 5′nuclease assay. Genet Anal 14:143–149, 1999 [DOI] [PubMed] [Google Scholar]
- Mason ST: Noradrenaline in the brain: Progress in theories of behavioral function. Prog Neurobiol 16:263–303, 1981 [DOI] [PubMed] [Google Scholar]
- McGough JJ, McCracken JT, Loo SK, Manganiello M, Leung MC, Tietjens JR, Trinh T, Baweja S, Suddath R, Smalley SL, Hellemann G, Sugar CA: A candidate gene analysis of methylphenidate response in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 48:1155–1164, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson D, Allen AJ, Busner J, Casat C, Dunn D, Kratochvil C, Newcorn J, Sallee FR, Sangal RB, Saylor K, West S, Kelsey D, Wernicke J, Trapp NJ, Harder D: Once-daily atomoxetine treatment for children and adolescents with attention deficit hyperactivity disorder: a randomized, placebo-controlled study. Am J Psychiatry 159:1896–1901, 2002 [DOI] [PubMed] [Google Scholar]
- Mick E, Neale B, Middleton FA, McGough JJ, Faraone SV: Genome-wide association study of response to methylphenidate in 187 children with attention-deficit/hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet 147B:1412–1418, 2008 [DOI] [PubMed] [Google Scholar]
- Nemoda Z1, Angyal N, Tarnok Z, Gadoros J, Sasvari–Szekely M: Carboxylesterase 1 gene polymorphism and methylphenidate response in ADHD. Neuropharmacology 57:731–733, 2009 [DOI] [PubMed] [Google Scholar]
- Newcorn JH, Kratochvil CJ, Allen AJ, Casat CD, Ruff DD, Moore RJ, Michelson D: Atomoxetine and osmotically released methylphenidate for the treatment of attention deficit hyperactivity disorder: Acute comparison and differential response. Am J Psychiatry 165:721–730, 2008 [DOI] [PubMed] [Google Scholar]
- Park S, Kim JW, Yang YH, Hong SB, Park MH, Kim BN, Shin MS, Yoo HJ, Cho SC: Possible effect of norepinephrine transporter polymorphisms on methylphenidate-induced changes in neuropsychological function in attention-deficit hyperactivity disorder. Behav Brain Funct 8:22, 2012a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MH, Kim JW, Yang YH, Hong SB, Park S, Kang H, Kim BN, Shin MS, Yoo HJ, Cho SC: Regional brain perfusion before and after treatment with methylphenidate may be associated with the G1287A polymorphism of the norepinephrine transporter gene in children with attention-deficit/hyperactivity disorder. Neurosci Lett 514:159–163, 2012b [DOI] [PubMed] [Google Scholar]
- Polanczyk G, Bigarella MP, Hutz MH, Rohde LA: Pharmacogenetic approach for a better drug treatment in children. Curr Pharm Des 16:2462–2673, 2010 [DOI] [PubMed] [Google Scholar]
- Polanczyk G, Zeni C, Genro JP, Guimarães AP, Roman T, Hutz MH, Rohde LA: Association of the adrenergic alpha2A receptor gene with methylphenidate improvement of inattentive symptoms in children and adolescents with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 64:218–224, 2007 [DOI] [PubMed] [Google Scholar]
- Purper–Ouakil D, Wohl M, Orejarena S, Cortese S, Boni C, Asch M, Mouren MC, Gorwood P: Pharmacogenetics of methylphenidate response in attention deficit/hyperactivity disorder: Association with the dopamine transporter gene (SLC6A3). Am J Med Genet B Neuropsychiatr Genet 147B:1425–1430, 2008 [DOI] [PubMed] [Google Scholar]
- Ramoz N, Boni C, Downing AM, Close SL, Peters SL, Prokop AM, Allen AJ, Hamon M, Purper–Ouakil D, Gorwood P: A haplotype of the norepinephrine transporter (Net) gene Slc6a2 is associated with clinical response to atomoxetine in attention-deficit hyperactivity disorder (ADHD). Neuropsychopharmacology 34:2135–2142, 2009 [DOI] [PubMed] [Google Scholar]
- Robbins TW: Arousal systems and attentional processes. Biol Psychol 45:57–71, 1997 [DOI] [PubMed] [Google Scholar]
- Roman T, Schmitz M, Polanczyk GV, Eizirik M, Rohde LA, Hutz MH: Further evidence for the association between attention–deficit/hyperactivity disorder and the dopamine-beta-hydroxylase gene. Am J Med Genet 114:154–158, 2002 [DOI] [PubMed] [Google Scholar]
- Sadile AG: Long-term habituation of theta related activity components of albino rats in the Ltt-maze. In: Motor Activity and Movement Disorders: Measurement and Analysis. Edited by Sanberg P.R., Ossenkopp K.P., Kavaliers M. New York: Humana Press; 1996; pp. 1–54 [Google Scholar]
- Seeger G1, Schloss P, Schmidt MH. Marker gene polymorphisms in hyperkinetic disorder—predictors of clinical response to treatment with methylphenidate? Neurosci Lett 313:45–48, 2001 [DOI] [PubMed] [Google Scholar]
- Smith KM, Daly M, Fischer M, Yiannoutsos CT, Bauer L, Barkley R, Navia BA: Association of the dopamine beta hydroxylase gene with attention deficit hyperactivity disorder: Genetic analysis of the Milwaukee longitudinal study. Am J Med Genet B Neuropsychiatr Genet 119B:77–85, 2003 [DOI] [PubMed] [Google Scholar]
- Solanto MV: Neuropsychopharmacological mechanisms of stimulant drug action in attention-deficit hyperactivity disorder: A review and integration. Behav Brain Res 94:127–152, 1998 [DOI] [PubMed] [Google Scholar]
- Steele M, Jensen PS, Quinn DM: Remission versus response as the goal of therapy in ADHD: A new standard for the field? Clin Ther 28:1892–1908, 2006 [DOI] [PubMed] [Google Scholar]
- Stein MA, McGough JJ: The pharmacogenomic era: Promise for personalizing attention deficit hyperactivity disorder therapy. Child Adolesc Psychiatr Clin N Am 17:475–490, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MA, Sarampote CS, Waldman ID, Robb AS, Conlon C, Pearl PL, Black DO, Seymour KE, Newcorn JH: A dose-response study of OROS methylphenidate in children with attention-deficit/hyperactivity disorder. Pediatrics 112:e404, 2003 [DOI] [PubMed] [Google Scholar]
- Su LY, Geng YG, Wang H, Du YS: Norm of ADHD rating scale – parent version in Chinese urban children [in Chinese]. Zhongguo Shiyong Erke Zazhi 21:833–836, 2006 [Google Scholar]
- Swanson JM, Kraemer HC, Hinshaw SP, Arnold LE, Conners CK, Abikoff HB, Clevenger W, Davies M, Elliott GR, Greenhill LL, Hechtman L, Hoza B, Jensen PS, March JS, Newcorn JH, Owens EB, Pelham WE, Schiller E, Severe JB, Simpson S, Vitiello B, Wells K, Wigal T, Wu M: Clinical relevance of the primary findings of the MTA: Success rates based on severity of ADHD and ODD symptoms at the end of treatment. J Am Acad Child Adolesc Psychiatry 40:168–179, 2001 [DOI] [PubMed] [Google Scholar]
- Viggiano D, Ruocco LA, Arcieri S, Sadile AG: Involvement of norepinephrine in the control of activity and attentive processes in animal models of attention deficit hyperactivity disorder. Neural Plast 11:133–149, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan BS, Kratochvil CJ: Pharmacotherapy of ADHD in young children. Psychiatry (Edgmont) 3:36–45, 2006 [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zheng Y, Du Y, Song DH, Shin YJ, Cho SC, Kim BN, Ahn DH, Marquez–Caraveo ME, Gao H, Williams DW, Levine LR: Atomoxetine versus methylphenidate in paediatric outpatients with attention deficit hyperactivity disorder: A randomized, double-blind comparison trial. Aust N Z J Psychiatry 41:222–230, 2007 [DOI] [PubMed] [Google Scholar]
- Winsberg BG, Comings DE: Association of the dopamine transporter gene (DAT1) with poor methylphenidate response. J Am Acad Child Adolesc Psychiatry 38:1474–1477, 1999 [DOI] [PubMed] [Google Scholar]
- Yang L, Cao Q, Shuai L, Li H, Chan RC, Wang YF: Comparative study of OROS-MPH and atomoxetine on executive function improvement in ADHD: A randomized controlled trial. Int J Neuropsychopharmacol 15:15–26, 2012 [DOI] [PubMed] [Google Scholar]
- Yang L, Qian QJ, Liu L, Li H, Faraone SV, Wang YF: Adrenergic neurotransmitter system transporter and receptor genes associated with atomoxetine response in attention-deficit hyperactivity disorder children. J Neural Transm 120:1127–1133, 2013 [DOI] [PubMed] [Google Scholar]
- Yang L, Wang YF, Li J, Faraone SV: Association of norepinephrine transporter gene with methylphenidate response. J Am Acad Child Adolesc Psychiatry 43:1154–1158, 2004 [DOI] [PubMed] [Google Scholar]


