Abstract
In 2011, a Shiga toxin (Stx) type 2a-producing enteroaggregative E. coli (EAEC) strain of serotype O104:H4 caused a large lethal outbreak in Northern Europe. Until recently, the pathogenic mechanisms explaining the high virulence of the strain have remained unclear. Our laboratories have shown that EAEC genes encoded on the pAA virulence plasmid, particularly the AggR-regulated AAF/I fimbriae, enhance inflammation and enable the outbreak strain to both adhere to epithelial cells and translocate Stx2a across the intestinal epithelium, possibly explaining the high incidence of the life threatening post-diarrheal sequelae of hemolytic uremic syndrome. Epidemiologic evidence supports a model of EAEC pathogenesis comprising the concerted action of multiple virulence factors along with induction of inflammation. Here, we suggest a model for the pathogenesis of the O104:H4 outbreak strain that includes contributions from EAEC alone, but incorporating additional injury induced by Stx2a.
Keywords: diarrhea, enteroaggregative Escherichia coli (EAEC), Mechanisms of pathogenesis of gut microbes, O104:H4, Shiga toxin, virulence
Shiga Toxin (Stx)-producing Enteroaggregative E. coli (Stx-EAEC)
Infectious diarrhea is one of the most frequent causes of human illness. The greatest burden of diarrhea is in children under 5 years of age in developing areas. In fact, childhood diarrhea accounts for almost one million directly attributable deaths per year globally.1 Additionally, morbidity associated with repeated episodes of childhood diarrhea can be long-lasting.2 Collectively, the diarrheagenic Escherichia coli (DEC) represent the most common bacterial pathogens worldwide.3-5 Some of the DEC pathotypes represent a major cause of morbidity and mortality in developing and low-income countries; these pathotypes include the enteropathogenic E. coli (EPEC), enterotoxogenic E. coli (ETEC), enteroinvasive E. coli (EIEC) and enteroaggregative E. coli (EAEC).3,6 In contrast, the Shiga toxin (Stx)-producing E. coli (STEC), a DEC which can cause bloody diarrhea, constitute a challenge for public health systems in industrialized countries. The epidemiology of infections caused by DEC ranges from sporadic endemic diarrhea to small or large outbreaks of food- or water-borne diarrhea. Some of the most severe and well-known DEC outbreaks have been due to STEC, particularly the O157:H7 serotype.7,8 O157:H7, referred to by some authors as enterohemorrhagic E. coli (EHEC), are known to cause sporadic cases and outbreaks of hemorrhagic colitis and a potentially lethal sequelae: hemolytic uremic syndrome (HUS).9,10 HUS is defined clinically by the triad of hemolytic anemia, thrombocytopenia, and renal failure.
HUS is the most frequent cause of renal failure in children,11 and Shiga toxin is the critical STEC virulence factor associated with the development of HUS.12 Shiga toxins are classified as belonging to one of 2 types, Stx1 or Stx2, and within the 2 types there exist several subtypes.13 However, O157:H7 E. coli strains that produce Stx2a are more likely to be associated with HUS.
From May through June 2011, an unprecedented outbreak of gastroenteritis occurred in Germany, with 4,137 known affected individuals and 54 deaths.14-16 The responsible organism was an unusual Stx2a-producing strain of serotype O104:H4. In addition to the Stx2a-encoding phage, the O104:H4 isolate carried the extended-spectrum β-lactamase (ESBL) antibiotic resistance plasmid.16 The consumption of fenugreek-sprouted seeds imported from Egypt was identified as the most likely source of infection for primary outbreak cases.17 Implication of O104:H4 in a massive outbreak was remarkable, but equally remarkable was the high proportion of cases developing HUS: 22% of the recognized O104:H4 cases were diagnosed with this complication contemporaneously. In contrast, historical rates of HUS after O157:H7 infection typically range from 6%-15%.15,18,19 Although passive clinical surveillance cannot accurately ascertain the rate of any complication, it is indisputable that the outbreak strain was unusually virulent for a non-O157:H7 strain.
Interestingly, whereas the O104:H4 strain carried a Stx2a phage characteristic of HUS associated STEC strains, it did not possess other O157:H7 virulence traits, most notably the locus of enterocyte effacement (LEE) pathogenicity island,16 which promotes the distinctive attaching and effacing epithelial lesion associated with LEE positive STEC.20,21 Instead, the O104:H4 outbreak isolate harbored genes characteristic of a completely different DEC pathotype, EAEC. Such an unusual combination of Stx with virulence factors typically found in EAEC has been documented prior to the 2011 German outbreak. This include reports of sporadic small outbreaks of HUS attributed to Stx-producing EAEC of the O111:H2, O111:H21 and, yes, O104:H4 serotypes. Nevertheless, these cases or outbreaks were localized to small populations in France, Ireland, and the Republic of Georgia and were not spread throughout Europe, as seen with the German outbreak strain.22-24
No standard nomenclature has been put forward for Stx-producing EAEC strains. We will argue that they are not in fact “hybrid strains,” but are rather EAEC that have been infected with Stx-encoding phage and should be designated Stx-EAEC.
An Unusual Combination of Virulence Genes
EAEC is a cause of acute and persistent diarrhea in multiple settings25-31 and EAEC strains express a heterogeneous array of putative virulence factors32-36 encoded on the bacterial chromosome or on the EAEC-specific pAA plasmid. EAEC strains often harbor a transcriptional activator of the AraC/XylS class called AggR,37 which controls expression of genes on both the plasmid and the chromosome. Genes under AggR control include those that encode the aggregative adherence fimbriae (AAF), of which at least 5 variants exist (AAF/I-V).38-43 AAF adhesins are essential for EAEC adherence to human intestinal explants, and for both cytokine release and opening of epithelial tight junctions in a polarized epithelial model.44,45 AggR is also required for expression of genes encoding dispersin (the aap gene), the Aat dispersin translocator46 and a chromosomal cluster termed Aai, encoding a type VI secretion system.47 Recently, Santiago et al. described a novel EAEC regulator called Aar (AggR-activated regulator), which is a member of a previously unrecognized large class of regulators in pathogenic Gram negative bacteria.48 The identified aar gene is activated by AggR, however when aar is deleted, aggR and the AggR regulon remain persistently activated. Thus, Aar could act directly or indirectly as a virulence suppressor as it down-regulates the expression of the positive regulator AggR. However, Aar is present in the German O104:H4 outbreak strain, so the exact contribution of Aar to EAEC pathogenesis remains enigmatic. Additionally, EAEC strains also often harbor a variable number of serine protease autotransporters of Enterobacteriaceae (SPATEs) that are implicated in immune evasion, mucosal damage, secretogenicity, and colonization.49
Limited knowledge exists on EAEC of the O104:H4 serotype. In a recent study of children's diarrhea in Mali, we identified Stx-negative EAEC O104:H4 in 6 children with and without diarrhea.32 Whole-genome sequencing of the Stx2a+ O104:H4 outbreak strain, Stx-negative O104:H4 Malian EAEC isolates and other O104:H4 isolates confirmed that the outbreak strain was indeed genetically EAEC.50 Genomic forensics suggested that the O104:H4 outbreak strain was an EAEC that had acquired the Stx2a phage because the outbreak strain was phylogenetically clustered with typical EAEC of O104:H4 and other serotypes. In addition, the genome analysis demonstrated that, other than Stx2a, the outbreak strain contained no virulence factors not already described in EAEC strains, including the AAF variant I (AAF/I), Aap/dispersin, the Aat translocator, the Aai type VI secretion system, the AggR regulator, and 3 SPATE proteases (Pic, SigA and SepA).50 That said, SPATE proteases are present in pathogenic E. coli strains and Shigella spp;51 however, the confluence of Pic, SigA, and SepA is a combination typically found among Shigella flexneri 2a strains,35 a finding that suggests that the German outbreak strain shares characteristics with that highly invasive and inflammatory diarrheagenic pathogen.
The Pathogeneses of the O104:H4 Outbreak Strain
So why was the O104:H4 EAEC outbreak strain so virulent? And which genes or combinations of genes could explain the high HUS incidence rate in patients? Answering these questions is challenging, given that EAEC are human-specific pathogens and that few animal infection models mimic human disease. However, Zangari et al. recently investigated the virulence of the German O104:H4 outbreak strain in a mouse model and showed that Stx2a from the outbreak strain was associated with weight loss, renal impairment, and death.52 By contrast, those factors associated specifically with “typical” EAEC virulence are less clear because EAEC is a highly heterogeneous group of pathogens. EAEC as currently defined most likely encompasses both pathogenic and non-pathogenic E. coli strains.53 Nonetheless, EAEC has unmistakably been associated with diarrhea in some individuals (e.g. in volunteers and outbreak patients),15,54-58 subclinical colonization in endemic areas is common, urinary tract infections and sepsis, and recently with poor growth in children.59-61
The essential differences between pathogenic and non-pathogenic strains are mostly unknown, but pathogenesis both ex vivo and in vivo suggest that the current pathogenic scheme of EAEC is comprised of these 3 stages: (1) after ingestion and passage through the stomach, EAEC adheres to the intestinal mucosa within a mucus-containing biofilm by virtue of AAF and possibly other adherence factors such as Pic mucinase and dispersin62,63; (2) stimulation of mucus production following biofilm formation at the surface of the mucosa; and (3) inflammation of the mucosa, manifested by cytokine release, cell exfoliation, and intestinal secretion.45,60,64,65 The final pathogenic step described previously is mediated as follows: both AAF and flagellin induce an inflammatory response in intestinal epithelial cells,45,66-68 manifested by increased cytokine production, as well as fecal lactoferrin in stools of patients with EAEC-induced diarrhea. SPATE toxins (such as Pet, SigA, Sat) cause cytotoxicity due to alteration of cytoskeletal elements.51,69
Based on the above outline of the stages of EAEC-mediated gastroenteritis, we hypothesized that the plasmid-borne virulence factors of EAEC contributed to the high pathogenicity of the German outbreak strain by promoting strong adherence to the epithelium and/or by opening epithelial tight junctions and facilitating Stx2a translocation. We used isolate C227-11 from the German outbreak, and investigated the potential contribution of pAA with mutants of C227-11, either cured of the pAA plasmid or deleted individually for known pAA-encoded virulence-associated genes aggR, aggA, or sepA.
As expected, lack of the pAA plasmid abolished the capacity of C227-11 to adhere to viable colonic tissue harvested from the cynomolous monkey Macaca fascicularis.68 In contrast, C227-11 adheres in an aggregative manner and forms heavy biofilms with a thick mucus layer upon interaction with the monkey colonic tissue (Fig. 1A) compared to commensal strain HS and uninfected control (Fig. 1B and C). Adherence is followed by mucosal toxicity, including crypt dilation, microvillous vesiculation, and epithelial cell extrusion. We also investigated the role of AAF/I in biofilm formation and epithelial cell adherence for C227-11, and found that like variants AAF/II, IV and V, expression of the AggA fimbrial adhesin was necessary and sufficient for adherence and biofilm formation (not shown68). Fig. 2 shows an electron micrograph of C227-11 in which AAF/I can be visualized.
Figure 1.
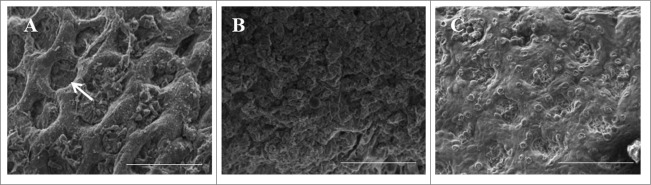
Adherence to Macaca fascicularis intestinal explants. Sections of proximal colon from freshly euthanized cynomolgus monkeys were incubated with strain C227-11 (A), with HS (B) or with media alone for 3 hours (C) and then visualized by Scanning Electron Microscopy (SEM).68 In panel (A), the arrow indicates dilation of the crypts. Scale bar is 100 µm.
Figure 2.

Scanning Electron Microscopy of C227-11 and biofilm formation after 18 hours on glass cover slips. Arrow indicates aggregating bacteria and AAF/I fimbriae.
We next interrogated cytoskeletal rearrangement in cells infected with C227-11, because such rearrangement has been demonstrated for other EAEC and has been linked to the presence of AAF fimbriae.44 In addition, disruption of the epithelial barrier coincides with perturbation of the actin cytoskeleton for STEC strains that carry the LEE island.70,71 However, as mentioned previously, C227-11 does not harbor the LEE island. We found that disruption of the actin cytoskeleton and reductions in transepithelial resistance in T84 cells infected with C227-11 were dependent on AggR and AggA but not on the Stx2a phage. Thus, the contribution of AggR in altering actin arrangement (and reducing adherence) is likely due to its role in the expression of AAF/I. We also found that a prototype O157:H7 failed to decrease transepithelial resistance to the same extent as EAEC, supporting the notion that it is AAF or AAF-dependent factors rather than Stx2a that are responsible for epithelial barrier loss in this model. These observations were confirmed by expressing the AAF/I fimbrial adhesion in an E. coli K12 background, which proved sufficient to enhance translocation of Stx2a across the epithelial monolayer.68 STEC strains associated with HUS possess several T3SS-associated effector proteins that can disrupt the integrity of the epithelial junctions and possibly contribute to Stx penetration. However, we suggest that AAF-associated tight junction disruption caused by the German outbreak strain more plausibly explains the increased rate of Stx-related complications induced by this strain.
We also observed that incubation of C227-11 with infected cell monolayers released the chemokine interleukin 8 (IL-8), and that cytokine release was significantly lower when the cells were infected with the strain cured of the Stx2a phage (C227-11Φcu). In addition, deletion of aggA, aggR, or sepA significantly decreased secretion of IL-8 into the basolateral compartment. Furthermore, when expressed in K12, the agg-fimbrial cluster from C227-11was sufficient to induce a pro-inflammatory response, as previously reported for the EAEC AAF/I prototype strain, JM221.67 Taken together these results suggest that induction of an inflammatory response by C227-11 is multifactorial and depends on both Stx2a and EAEC factors.
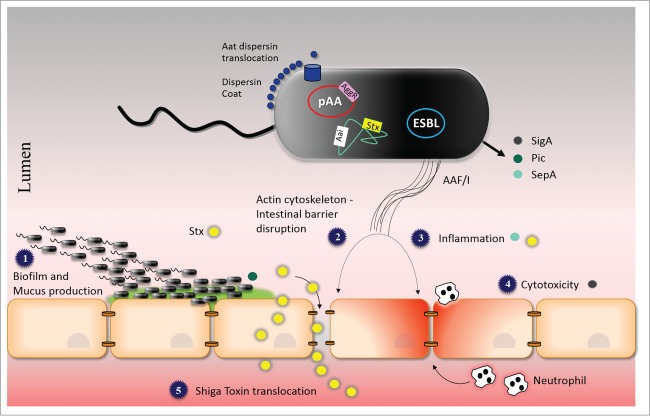
We suggest a model for the pathogenesis of the O104:H4 outbreak strain that include steps listed above for EAEC alone, but incorporates additional features explained by the expression of Stx2a (Fig. 3). (1) O104:H4 adheres to the intestinal mucosa within a mucus-containing biofilm, by virtue of AAF and possibly other adherence promoting factors such as the Pic mucinase and dispersin. (2) Interactions between AAF/I and the epithelial monolayer causes disruption of the epithelial barrier and actin cytoskeletal rearrangement. (3) AAF/I, SepA and Stx2a triggers release of inflammatory cytokines and recruitment of neutrophils.67,68,72 Such transepithelial migration of neutrophils may also lead to further Stx2a penetration.73 (4) SigA causes cytotoxicity possibly due to alteration of cytoskeletal elements.51 (5) AAF/I-mediated disruption of the epithelial barrier in addition to para-cellular passage of neutrophils facilitates the delivery of Stx2a to the lamina propria, with increased access to intestinal vasculature and, ultimately, to the target organs that express high levels of the Stx2a globotriaosylceramide receptor, Gb3 (particularly, kidneys and brain).
Figure 3.
Model of the Pathogenesis of the O104:H4 outbreak strain. See text for explanation. Abbreviations: AAF/I; aggregative adherence fimbriae type I, pAA; virulence plasmid of C227-11, ESBL; extended-spectrum β-lactamase antibiotic resistance plasmid, AggR; AraC/XylS family activator, Aai; Type VI secretion system, Aat; dispersin translocator, Stx; Shiga Toxin, SigA; IgA protease-like homolog, Pic; Serine protease precursor, and SepA; Shigella extracellular protease.
Our findings thus suggest a mechanism whereby the O104:H4 outbreak was associated with unusually high rates of HUS. Our studies demonstrate the importance of the pAA plasmid in the pathogenesis of the O104:H4 outbreak strain, both in ways typical of EAEC (adherence and inflammation), and in a unique and sinister new synergy (barrier dysfunction that augments Stx2a translocation). Consistent with our results is the observation that a proportion of O104:H4 isolates during the outbreak had lost the pAA plasmid, and patients whose initial isolate expressed the plasmid were significantly more likely to develop HUS than those infected with a pAA-negative strain.74
Given the virulence of the Stx-EAEC O104:H4 strain it would be relevant for the health community to consider, if the acquisition of the Stx2 phage by an EAEC is an uncommon event or something likely to occur more frequently. As mentioned, the German outbreak was not the first clinically linked instance of a Stx-EAEC, but it is an example of such a strain causing a large- scale outbreak of disease. However, whether the German outbreak was due to a particularly virulent Stx-EAEC, a rare epidemiologic opportunity, or both is unclear.
Concluding, the emerging of Stx-EAEC strains possesses a major challenge for the public health, which needs to implement diagnostic procedures that identify the most virulent clones and this is particular challenging with this heterogeneous pathotype.
Funding
The work described here was supported by AI-033096 to JPN, R37 AI020148 and R0731977 to ADO and DFF1333-00156 to NBO.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, Rudan I, Campbell H, Cibulskis R, Li M, et al.. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet [Internet] 2012. [cited 2014July10]; 379:2151-61. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22579125; PMID:22579125; http://dx.doi.org/ 10.1016/S0140-6736(12)60560-1 [DOI] [PubMed] [Google Scholar]
- 2.Petri WA, Miller M, Binder HJ, Levine MM, Dillingham R, Guerrant RL. Enteric infections, diarrhea, and their impact on function and development. J Clin Invest [Internet] 2008. [cited 2015February15]; 118:1277-90. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2276781&tool=pmcentrez&rendertype=abstract; PMID:18382740; http://dx.doi.org/ 10.1172/JCI34005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, et al.. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet [Internet] 2013. [cited 2014April29]; 382:209-22. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23680352; PMID:23680352; http://dx.doi.org/ 10.1016/S0140-6736(13)60844-2 [DOI] [PubMed] [Google Scholar]
- 4.Farthing MJ. Diarrhoea: a significant worldwide problem. Int J Antimicrob Agents [Internet] 2000. [cited 2015February15]; 14:65-9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10717503; PMID:10717503; http://dx.doi.org/ 10.1016/S0924-8579(99)00149-1 [DOI] [PubMed] [Google Scholar]
- 5.Wanke CA. To know Escherichia coli is to know bacterial diarrheal disease. Clin Infect Dis [Internet] 2001. [cited 2015February15]; 32:1710-2. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11360212; PMID:11360212; http://dx.doi.org/ 10.1086/320763 [DOI] [PubMed] [Google Scholar]
- 6.Bryce J, Boschi-Pinto C, Shibuya K, Black RE. WHO estimates of the causes of death in children. Lancet [Internet] 2005; [cited 2015February3]; 365:1147-52. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15794969; PMID:15794969; http://dx.doi.org/ 10.1016/S0140-6736(05)71877-8 [DOI] [PubMed] [Google Scholar]
- 7.Riley LW. The epidemiologic, clinical, and microbiologic features of hemorrhagic colitis. Annu Rev Microbiol [Internet] 1987. [cited 2015February15]; 41:383-407. Available from: http://www.ncbi.nlm.nih.gov/pubmed/3318674; PMID:3318674; http://dx.doi.org/ 10.1146/annurev.mi.41.100187.002123 [DOI] [PubMed] [Google Scholar]
- 8.Watanabe H, Wada A, Inagaki Y, Itoh K, Tamura K. Outbreaks of enterohaemorrhagic Escherichia coli O157:H7 infection by two different genotype strains in Japan, 1996. Lancet [Internet] 1996. [cited 2015February15]; 348:831-2. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8814014; PMID:8814014; http://dx.doi.org/ 10.1016/S0140-6736(05)65257-9 [DOI] [PubMed] [Google Scholar]
- 9.Karmali MA, Steele BT, Petric M, Lim C. Sporadic cases of haemolytic-uraemic syndrome associated with faecal cytotoxin and cytotoxin-producing Escherichia coli in stools. Lancet [Internet] 1983. [cited 2015February9]; 1:619-20. Available from: http://www.ncbi.nlm.nih.gov/pubmed/6131302; PMID:6131302; http://dx.doi.org/ 10.1016/S0140-6736(83)91795-6 [DOI] [PubMed] [Google Scholar]
- 10.O'Brien AO, Lively TA, Chen ME, Rothman SW, Formal SB. Escherichia coli O157:H7 strains associated with haemorrhagic colitis in the United States produce a Shigella dysenteriae 1 (SHIGA) like cytotoxin. Lancet [Internet] 1983. [cited 2015February15]; 1:702 Available from: http://www.ncbi.nlm.nih.gov/pubmed/6132054; http://dx.doi.org/ 10.1016/S0140-6736(83)91987-6 [DOI] [PubMed] [Google Scholar]
- 11.Siegler RL. Postdiarrheal Shiga toxin-mediated hemolytic uremic syndrome. JAMA [Internet] 2003. [cited 2015March29]; 290:1379-81. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12966132; PMID:12966132; http://dx.doi.org/ 10.1001/jama.290.10.1379 [DOI] [PubMed] [Google Scholar]
- 12.Paton JC, Paton AW. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin Microbiol Rev [Internet] 1998. [cited 2015March29]; 11:450-79. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=88891&tool=pmcentrez&rendertype=abstract; PMID:9665978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheutz F, Teel LD, Beutin L, Piérard D, Buvens G, Karch H, Mellmann A, Caprioli A, Tozzoli R, Morabito S, et al.. Multicenter evaluation of a sequence-based protocol for subtyping Shiga toxins and standardizing Stx nomenclature. J Clin Microbiol [Internet] 2012. [cited 2015March29]; 50:2951-63. Available from: http://jcm.asm.org/content/50/9/2951.long; PMID:22760050; http://dx.doi.org/ 10.1128/JCM.00860-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bielaszewska M, Mellmann A, Zhang W, Köck R, Fruth A, Bauwens A, Peters G, Karch H. Characterisation of the Escherichia coli strain associated with an outbreak of haemolytic uraemic syndrome in Germany, 2011: a microbiological study. Lancet Infect Dis [Internet] 2011. [cited 2014May8]; 11:671-6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21703928; PMID:21703928; http://dx.doi.org/ 10.1016/S1473-3099(11)70165-7 [DOI] [PubMed] [Google Scholar]
- 15.Frank C, Werber D, Cramer JP, Askar M, Faber M, an der Heiden M, Bernard H, Fruth A, Prager R, Spode A, et al.. Epidemic profile of Shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. N Engl J Med [Internet] 2011. [cited 2014May13]; 365:1771-80. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21696328; PMID:21696328; http://dx.doi.org/ 10.1056/NEJMoa1106483 [DOI] [PubMed] [Google Scholar]
- 16.Scheutz F, Nielsen EM, Frimodt-Møller J, Boisen N, Morabito S, Tozzoli R, Nataro JP, Caprioli A. Characteristics of the enteroaggregative Shiga toxin/verotoxin-producing Escherichia coli O104:H4 strain causing the outbreak of haemolytic uraemic syndrome in Germany, May to June 2011. Euro Surveill [Internet] 2011. [cited 2014May13]; 16; pii: 19889. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21699770; PMID:21699770 [DOI] [PubMed] [Google Scholar]
- 17.Buchholz U, Bernard H, Werber D, Böhmer MM, Remschmidt C, Wilking H, Deleré Y, an der Heiden M, Adlhoch C, Dreesman J, et al.. German outbreak of Escherichia coli O104:H4 associated with sprouts. N Engl J Med [Internet] 2011. [cited 2014May2]; 365:1763-70. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22029753; PMID:22029753; http://dx.doi.org/ 10.1056/NEJMoa1106482 [DOI] [PubMed] [Google Scholar]
- 18.Gould LH, Demma L, Jones TF, Hurd S, Vugia DJ, Smith K, Shiferaw B, Segler S, Palmer A, Zansky S, et al.. Hemolytic uremic syndrome and death in persons with Escherichia coli O157:H7 infection, foodborne diseases active surveillance network sites, 2000–2006. Clin Infect Dis [Internet] 2009. 2009[cited 2015 Feb 15]; 49:1480-5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19827953; PMID:19827953; http://dx.doi.org/ 10.1086/644621 [DOI] [PubMed] [Google Scholar]
- 19.Alpers K, Werber D, Frank C, Koch J, Friedrich AW, Karch H, An DER Heiden M, Prager R, Fruth A, Bielaszewska M, et al.. Sorbitol-fermenting enterohaemorrhagic Escherichia coli O157:H- causes another outbreak of haemolytic uraemic syndrome in children. Epidemiol Infect [Internet] 2009. [cited 2015February15]; 137:389-95. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19021923; PMID:19021923; http://dx.doi.org/ 10.1017/S0950268808001465 [DOI] [PubMed] [Google Scholar]
- 20.Tzipori S, Wachsmuth IK, Chapman C, Birden R, Brittingham J, Jackson C, Hogg J. The pathogenesis of hemorrhagic colitis caused by Escherichia coli O157:H7 in gnotobiotic piglets. J Infect Dis [Internet] 1986. [cited 2015February15]; 154:712-6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/3528323; PMID:3528323; http://dx.doi.org/ 10.1093/infdis/154.4.712 [DOI] [PubMed] [Google Scholar]
- 21.McDaniel TK, Jarvis KG, Donnenberg MS, Kaper JB. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc Natl Acad Sci U S A [Internet] 1995. [cited 2015February2]; 92:1664-8. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=42580&tool=pmcentrez&rendertype=abstract; PMID:7878036; http://dx.doi.org/ 10.1073/pnas.92.5.1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dallman T, Smith GP, O'Brien B, Chattaway MA, Finlay D, Grant KA, Jenkins C. Characterization of a verocytotoxin-producing enteroaggregative Escherichia coli serogroup O111:H21 strain associated with a household outbreak in Northern Ireland. J Clin Microbiol [Internet] 2012. [cited 2014May13]; 50:4116-9. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3502983&tool=pmcentrez&rendertype=abstract; PMID:23035193; http://dx.doi.org/ 10.1128/JCM.02047-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vashakidze E, Megrelishvili T, Pachkoria E, Tevzadze L, Lashkarashvili M. Enterohemorrhagic E. coli and hemolytic uremic syndrome in Georgia. Georgian Med News 2010; 38-41. [PubMed] [Google Scholar]
- 24.Morabito S, Karch H, Mariani-Kurkdjian P, Schmidt H, Minelli F, Bingen E, Caprioli A. Enteroaggregative, Shiga toxin-producing Escherichia coli O111:H2 associated with an outbreak of hemolytic-uremic syndrome. J Clin Microbiol [Internet] 1998. [cited 2015February15]; 36:840-2. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=104641&tool=pmcentrez&rendertype=abstract; PMID:9508328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adachi JA, Jiang ZD, Mathewson JJ, Verenkar MP, Thompson S, Martinez-Sandoval F, Steffen R, Ericsson CD, DuPont HL. Enteroaggregative Escherichia coli as a major etiologic agent in traveler's diarrhea in 3 regions of the world. Clin Infect Dis [Internet] 2001. [cited 2014May13]; 32:1706-9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11360211; PMID:11360211; http://dx.doi.org/ 10.1086/320756 [DOI] [PubMed] [Google Scholar]
- 26.Okeke IN, Lamikanra A, Steinrück H, Kaper JB. Characterization of Escherichia coli strains from cases of childhood diarrhea in provincial southwestern Nigeria. J Clin Microbiol [Internet] 2000. [cited 2014May13]; 38:7-12. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=86005&tool=pmcentrez&rendertype=abstract; PMID:10618054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fang GD, Lima AA, Martins CV, Nataro JP, Guerrant RL. Etiology and epidemiology of persistent diarrhea in northeastern Brazil: a hospital-based, prospective, case-control study. J Pediatr Gastroenterol Nutr [Internet] 1995. [cited 2014May13]; 21:137-44. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7472898; PMID:7472898; http://dx.doi.org/ 10.1097/00005176-199508000-00003 [DOI] [PubMed] [Google Scholar]
- 28.Bhan MK, Raj P, Levine MM, Kaper JB, Bhandari N, Srivastava R, Kumar R, Sazawal S. Enteroaggregative Escherichia coli associated with persistent diarrhea in a cohort of rural children in India. J Infect Dis [Internet] 1989. [cited 2014May6]; 159:1061-4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/2656875; PMID:2656875; http://dx.doi.org/ 10.1093/infdis/159.6.1061 [DOI] [PubMed] [Google Scholar]
- 29.Wanke CA, Mayer H, Weber R, Zbinden R, Watson DA, Acheson D. Enteroaggregative Escherichia coli as a potential cause of diarrheal disease in adults infected with human immunodeficiency virus. J Infect Dis [Internet] 1998. [cited 2014May13]; 178:185-90. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9652439; PMID:9652439; http://dx.doi.org/ 10.1086/515595 [DOI] [PubMed] [Google Scholar]
- 30.Sahl JW, Steinsland H, Redman JC, Angiuoli SV, Nataro JP, Sommerfelt H, Rasko DA. A comparative genomic analysis of diverse clonal types of enterotoxigenic Escherichia coli reveals pathovar-specific conservation. Infect Immun [Internet] 2011. [cited 2014May9]; 79:950-60. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3028850&tool=pmcentrez&rendertype=abstract; PMID:21078854; http://dx.doi.org/ 10.1128/IAI.00932-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valentiner-Branth P, Steinsland H, Fischer TK, Perch M, Scheutz F, Dias F, Aaby P, Mølbak K, Sommerfelt H. Cohort study of Guinean children: incidence, pathogenicity, conferred protection, and attributable risk for enteropathogens during the first 2 years of life. J Clin Microbiol [Internet] 2003. [cited 2014May13]; 41:4238-45. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=193811&tool=pmcentrez&rendertype=abstract; PMID:12958251; http://dx.doi.org/ 10.1128/JCM.41.9.4238-4245.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boisen N, Scheutz F, Rasko D a, Redman JC, Persson S, Simon J, Kotloff KL, Levine MM, Sow S, Tamboura B, et al.. Genomic characterization of enteroaggregative Escherichia coli from children in Mali. J Infect Dis [Internet] 2012; 205:431-44. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3256949&tool=pmcentrez&rendertype=abstract; http://dx.doi.org/ 10.1093/infdis/jir757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lima IFN, Boisen N, Quetz J, da S, Havt A, de Carvalho EB, Soares AM, Lima NL, Mota RMS, Nataro JP, Guerrant RL, et al.. Prevalence of enteroaggregative Escherichia coli and its virulence-related genes in a case-control study among children from north-eastern Brazil. J Med Microbiol [Internet] 2013. [cited 2014May9]; 62:683-93. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3709657&tool=pmcentrez&rendertype=abstract; PMID:23429698; http://dx.doi.org/ 10.1099/jmm.0.054262-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okeke IN, Lamikanra A, Czeczulin J, Dubovsky F, Kaper JB, Nataro JP. Heterogeneous virulence of enteroaggregative Escherichia coli strains isolated from children in Southwest Nigeria. J Infect Dis [Internet] 2000. [cited 2014May13]; 181:252-60. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10608774; PMID:10608774; http://dx.doi.org/ 10.1086/315204 [DOI] [PubMed] [Google Scholar]
- 35.Boisen N, Ruiz-Perez F, Scheutz F, Krogfelt KA, Nataro JP. Short report: high prevalence of serine protease autotransporter cytotoxins among strains of enteroaggregative Escherichia coli. Am J Trop Med Hyg [Internet] 2009. [cited 2014May13]; 80:294-301. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2660206&tool=pmcentrez&rendertype=abstract; PMID:19190229 [PMC free article] [PubMed] [Google Scholar]
- 36.Jenkins C, van Ijperen C, Dudley EG, Chart H, Willshaw GA, Cheasty T, Smith HR, Nataro JP. Use of a microarray to assess the distribution of plasmid and chromosomal virulence genes in strains of enteroaggregative Escherichia coli. FEMS Microbiol Lett [Internet] 2005. [cited 2014May13]; 253:119-24. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16243450; PMID:16243450; http://dx.doi.org/ 10.1016/j.femsle.2005.09.040 [DOI] [PubMed] [Google Scholar]
- 37.Nataro JP, Yikang D, Yingkang D, Walker K. AggR, a transcriptional activator of aggregative adherence fimbria I expression in enteroaggregative Escherichia coli. J Bacteriol [Internet] 1994. [cited 2014May13]; 176:4691-9. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=196291&tool=pmcentrez&rendertype=abstract; PMID:7913930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernier C, Gounon P, Le Bouguénec C. Identification of an aggregative adhesion fimbria (AAF) type III-encoding operon in enteroaggregative Escherichia coli as a sensitive probe for detecting the AAF-encoding operon family. Infect Immun [Internet] 2002. [cited 2014May13]; 70:4302-11. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=128174&tool=pmcentrez&rendertype=abstract; PMID:12117939; http://dx.doi.org/ 10.1128/IAI.70.8.4302-4311.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boisen N, Struve C, Scheutz F, Krogfelt KA, Nataro JP. New adhesin of enteroaggregative Escherichia coli related to the Afa/Dr/AAF family. Infect Immun [Internet] 2008. [cited 2014May13]; 76:3281-92. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2446688&tool=pmcentrez&rendertype=abstract; PMID:18443096; http://dx.doi.org/ 10.1128/IAI.01646-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Czeczulin JR, Balepur S, Hicks S, Phillips A, Hall R, Kothary MH, Navarro-Garcia F, Nataro JP. Aggregative adherence fimbria II, a second fimbrial antigen mediating aggregative adherence in enteroaggregative Escherichia coli. Infect Immun [Internet] 1997. [cited 2014May13]; 65:4135-45. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=175595&tool=pmcentrez&rendertype=abstract; PMID:9317019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henderson IR, Hicks S, Navarro-Garcia F, Elias WP, Philips AD, Nataro JP. Involvement of the enteroaggregative Escherichia coli plasmid-encoded toxin in causing human intestinal damage. Infect Immun [Internet] 1999. [cited 2014May13]; 67:5338-44. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=96889&tool=pmcentrez&rendertype=abstract; PMID:10496914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nataro JP, Yikang D, Giron JA, Savarino SJ, Kothary MH, Hall R. Aggregative adherence fimbria I expression in enteroaggregative Escherichia coli requires two unlinked plasmid regions. Infect Immun [Internet] 1993. [cited 2014May13]; 61:1126-31. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=302849&tool=pmcentrez&rendertype=abstract; PMID:8094379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jønsson R, Struve C, Boisen N, Mateiu RV, Santiago AE, Jenssen H, Nataro JP, Krogfelt KA. A novel Aggregative Adherence Fimbriae (AAF/V) of Enteroaggregative Escherichia coli (EAEC). Infect Immun [Internet] 2015. [cited 2015February15]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/25624357; PMID:25624357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strauman MC, Harper JM, Harrington SM, Boll EJ, Nataro JP. Enteroaggregative Escherichia coli disrupts epithelial cell tight junctions. Infect Immun [Internet] 2010. [cited 2014May8]; 78:4958-64. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2976312&tool=pmcentrez&rendertype=abstract; PMID:20823198; http://dx.doi.org/ 10.1128/IAI.00580-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harrington SM, Strauman MC, Abe CM, Nataro JP. Aggregative adherence fimbriae contribute to the inflammatory response of epithelial cells infected with enteroaggregative Escherichia coli. Cell Microbiol [Internet] 2005. [cited 2014May13]; 7:1565-78. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16207244; PMID:16207244; http://dx.doi.org/ 10.1111/j.1462-5822.2005.00588.x [DOI] [PubMed] [Google Scholar]
- 46.Sheikh J, Czeczulin JR, Harrington S, Hicks S, Henderson IR, Le Bouguénec C, Gounon P, Phillips A, Nataro JP. A novel dispersin protein in enteroaggregative Escherichia coli. J Clin Invest [Internet] 2002. [cited 2014May13]; 110:1329-37. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=151617&tool=pmcentrez&rendertype=abstract; PMID:12417572; http://dx.doi.org/ 10.1172/JCI16172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dudley EG, Thomson NR, Parkhill J, Morin NP, Nataro JP. Proteomic and microarray characterization of the AggR regulon identifies a pheU pathogenicity island in enteroaggregative Escherichia coli. Mol Microbiol [Internet] 2006. [cited 2014May13]; 61:1267-82. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16925558; PMID:16925558; http://dx.doi.org/ 10.1111/j.1365-2958.2006.05281.x [DOI] [PubMed] [Google Scholar]
- 48.Santiago AE, Ruiz-Perez F, Jo NY, Vijayakumar V, Gong MQ, Nataro JP. A large family of antivirulence regulators modulates the effects of transcriptional activators in Gram-negative pathogenic bacteria. PLoS Pathog [Internet] 2014. [cited 2015February15]; 10:e1004153. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4038620&tool=pmcentrez&rendertype=abstract; PMID:24875828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dutta PR, Cappello R, Navarro-García F, Nataro JP. Functional comparison of serine protease autotransporters of enterobacteriaceae. Infect Immun [Internet] 2002. [cited 2014May13]; 70:7105-13. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=133081&tool=pmcentrez&rendertype=abstract; PMID:12438392; http://dx.doi.org/ 10.1128/IAI.70.12.7105-7113.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rasko DA, Webster DR, Sahl JW, Bashir A, Boisen N, Scheutz F, Paxinos EE, Sebra R, Chin C-S, Iliopoulos D, et al.. Origins of the E. coli strain causing an outbreak of hemolytic-uremic syndrome in Germany. N Engl J Med [Internet] 2011. [cited 2014May13]; 365:709-17. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3168948&tool=pmcentrez&rendertype=abstract; PMID:21793740; http://dx.doi.org/ 10.1056/NEJMoa1106920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Henderson IR, Navarro-Garcia F, Nataro JP. The great escape: structure and function of the autotransporter proteins. Trends Microbiol [Internet] 1998. [cited 2014May13]; 6:370-8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9778731; PMID:9778731; http://dx.doi.org/ 10.1016/S0966-842X(98)01318-3 [DOI] [PubMed] [Google Scholar]
- 52.Zangari T, Melton-Celsa AR, Panda A, Boisen N, Smith MA, Tatarov I, De Tolla LJ, Nataro JP, O'Brien AD. Virulence of the Shiga toxin type 2-expressing Escherichia coli O104:H4 German outbreak isolate in two animal models. Infect Immun [Internet] 2013. [cited 2014May13]; 81:1562-74. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23439303; PMID:23439303; http://dx.doi.org/ 10.1128/IAI.01310-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nat Rev Microbiol [Internet] 2004. [cited 2014April28]; 2:123-40. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15040260; PMID:15040260; http://dx.doi.org/ 10.1038/nrmicro818 [DOI] [PubMed] [Google Scholar]
- 54.Nataro JP, Deng Y, Cookson S, Cravioto A, Savarino SJ, Guers LD, Levine MM, Tacket CO. Heterogeneity of enteroaggregative Escherichia coli virulence demonstrated in volunteers. J Infect Dis [Internet] 1995. [cited 2014May13]; 171:465-8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7844392; PMID:7844392; http://dx.doi.org/ 10.1093/infdis/171.2.465 [DOI] [PubMed] [Google Scholar]
- 55.Itoh Y, Nagano I, Kunishima M, Ezaki T. Laboratory investigation of enteroaggregative Escherichia coli O untypeable:H10 associated with a massive outbreak of gastrointestinal illness. J Clin Microbiol [Internet] 1997. [cited 2015February15]; 35:2546-50. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=230008&tool=pmcentrez&rendertype=abstract; PMID:9316905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cobeljić M, Miljković-Selimović B, Paunović-Todosijević D, Velicković Z, Lepsanović Z, Zec N, Savić D, Ilić R, Konstantinović S, Jovanović B, et al.. Enteroaggregative Escherichia coli associated with an outbreak of diarrhoea in a neonatal nursery ward. Epidemiol Infect [Internet] 1996. [cited 2015February15]; 117:11-6. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2271665&tool=pmcentrez&rendertype=abstract; PMID:8760945; http://dx.doi.org/ 10.1017/S0950268800001072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith HR, Cheasty T, Rowe B. Enteroaggregative Escherichia coll and outbreaks of gastroenteritis in UK. Lancet [Internet] 1997. [cited 2015February15]; 350:814-5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9298029; PMID:9298029; http://dx.doi.org/ 10.1016/S0140-6736(05)62611-6 [DOI] [PubMed] [Google Scholar]
- 58.Scavia G, Staffolani M, Fisichella S, Striano G, Colletta S, Ferri G, Escher M, Minelli F, Caprioli A. Enteroaggregative Escherichia coli associated with a foodborne outbreak of gastroenteritis. J Med Microbiol [Internet] 2008. [cited 2015February15]; 57:1141-6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18719185; PMID:18719185; http://dx.doi.org/ 10.1099/jmm.0.2008/001362-0 [DOI] [PubMed] [Google Scholar]
- 59.Roche JK, Cabel A, Sevilleja J, Nataro J, Guerrant RL. Enteroaggregative Escherichia coli (EAEC) impairs growth while malnutrition worsens EAEC infection: a novel murine model of the infection malnutrition cycle. J Infect Dis [Internet] 2010. [cited 2015February15]; 202:506-14. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2919845&tool=pmcentrez&rendertype=abstract; PMID:20594107; http://dx.doi.org/ 10.1086/654894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Steiner TS, Lima AA, Nataro JP, Guerrant RL. Enteroaggregative Escherichia coli produce intestinal inflammation and growth impairment and cause interleukin-8 release from intestinal epithelial cells. J Infect Dis [Internet] 1998. [cited 2014May13]; 177:88-96. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9419174; PMID:9419174; http://dx.doi.org/ 10.1086/513809 [DOI] [PubMed] [Google Scholar]
- 61.Olesen B, Scheutz F, Andersen RL, Menard M, Boisen N, Johnston B, Hansen DS, Krogfelt KA, Nataro JP, Johnson JR. Enteroaggregative Escherichia coli O78:H10, the Cause of an Outbreak of Urinary Tract Infection. J Clin Microbiol [Internet] 2012. [cited 2014May13]; 50:3703-11. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3486220&tool=pmcentrez&rendertype=abstract; PMID:22972830; http://dx.doi.org/ 10.1128/JCM.01909-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hicks S, Candy DC, Phillips AD. Adhesion of enteroaggregative Escherichia coli to pediatric intestinal mucosa in vitro. Infect Immun [Internet] 1996. [cited 2015February15]; 64:4751-60. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=174442&tool=pmcentrez&rendertype=abstract; PMID:8890236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tzipori S, Montanaro J, Robins-Browne RM, Vial P, Gibson R, Levine MM. Studies with enteroaggregative Escherichia coli in the gnotobiotic piglet gastroenteritis model. Infect Immun [Internet] 1992. [cited 2015February15]; 60:5302-6. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=258311&tool=pmcentrez&rendertype=abstract; PMID:1452364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jiang Z-D, Greenberg D, Nataro JP, Steffen R, DuPont HL. Rate of occurrence and pathogenic effect of enteroaggregative Escherichia coli virulence factors in international travelers. J Clin Microbiol [Internet] 2002. [cited 2014May13]; 40:4185-90. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=139678&tool=pmcentrez&rendertype=abstract; PMID:12409395; http://dx.doi.org/ 10.1128/JCM.40.11.4185-4190.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bouckenooghe AR, Dupont HL, Jiang ZD, Adachi J, Mathewson JJ, Verenkar MP, Rodrigues S, Steffen R. Markers of enteric inflammation in enteroaggregative Escherichia coli diarrhea in travelers. Am J Trop Med Hyg [Internet] 2000. [cited 2015February15]; 62:711-3. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11304060; PMID:11304060 [DOI] [PubMed] [Google Scholar]
- 66.Steiner TS, Nataro JP, Poteet-Smith CE, Smith JA, Guerrant RL. Enteroaggregative Escherichia coli expresses a novel flagellin that causes IL-8 release from intestinal epithelial cells. J Clin Invest [Internet] 2000. [cited 2014May13]; 105:1769-77. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=378507&tool=pmcentrez&rendertype=abstract; PMID:10862792; http://dx.doi.org/ 10.1172/JCI8892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boll EJ, Struve C, Sander A, Demma Z, Nataro JP, McCormick BA, Krogfelt KA. The fimbriae of enteroaggregative Escherichia coli induce epithelial inflammation in vitro and in a human intestinal xenograft model. J Infect Dis [Internet] 2012. [cited 2014May3]; 206:714-22. Available from: http://jid.oxfordjournals.org/content/early/2012/06/21/infdis.jis417; PMID:22723643; http://dx.doi.org/ 10.1093/infdis/jis417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boisen N, Hansen A-M, Melton-Celsa AR, Zangari T, Mortensen NP, Kaper JB, O'Brien AD, Nataro JP. The Presence of the pAA Plasmid in the German O104:H4 Shiga Toxin Type 2a (Stx2a)-Producing Enteroaggregative Escherichia coli Strain Promotes the Translocation of Stx2a Across an Epithelial Cell Monolayer. J Infect Dis [Internet] 2014. [cited 2014November15]; 210(12):1909-19; Available from: http://www.ncbi.nlm.nih.gov/pubmed/25038258; PMID:25038258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Navarro-García F, Sears C, Eslava C, Cravioto A, Nataro JP. Cytoskeletal effects induced by pet, the serine protease enterotoxin of enteroaggregative Escherichia coli. Infect Immun [Internet] 1999. [cited 2014May13]; 67:2184-92. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=115956&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Frankel G, Phillips AD. Attaching effacing Escherichia coli and paradigms of Tir-triggered actin polymerization: getting off the pedestal. Cell Microbiol [Internet] 2008. [cited 2014May13]; 10:549-56. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18053003; PMID:18053003; http://dx.doi.org/ 10.1111/j.1462-5822.2007.01103.x [DOI] [PubMed] [Google Scholar]
- 71.DeVinney R, Puente JL, Gauthier A, Goosney D, Finlay BB. Enterohaemorrhagic and enteropathogenic Escherichia coli use a different Tir-based mechanism for pedestal formation. Mol Microbiol [Internet] 2001. [cited 2014May13]; 41:1445-58. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11580847; PMID:11580847; http://dx.doi.org/ 10.1046/j.1365-2958.2001.02617.x [DOI] [PubMed] [Google Scholar]
- 72.Thorpe CM, Hurley BP, Lincicome LL, Jacewicz MS, Keusch GT, Acheson DW. Shiga toxins stimulate secretion of interleukin-8 from intestinal epithelial cells. Infect Immun [Internet] 1999. [cited 2014May13]; 67:5985-93. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=96984&tool=pmcentrez&rendertype=abstract; PMID:10531258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hurley BP, Thorpe CM, Acheson DW. Shiga toxin translocation across intestinal epithelial cells is enhanced by neutrophil transmigration. Infect Immun [Internet] 2001. [cited 2014May13]; 69:6148-55. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=98745&tool=pmcentrez&rendertype=abstract; PMID:11553554; http://dx.doi.org/ 10.1128/IAI.69.10.6148-6155.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang W, Bielaszewska M, Kunsmann L, Mellmann A, Bauwens A, Köck R, Kossow A, Anders A, Gatermann S, Karch H. Lability of the pAA virulence plasmid in escherichia coli O104:H4: implications for virulence in humans. PLoS One [Internet] 2013. [cited 2014May13]; 8:e66717. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3689698&tool=pmcentrez&rendertype=abstract; PMID:23805269; http://dx.doi.org/ 10.1371/journal.pone.0066717 [DOI] [PMC free article] [PubMed] [Google Scholar]