Abstract
Controlled, reductionist approaches are required in order to obtain a more complete understanding of the functional capabilities of the gut microbiota. We recently identified microbial bile salt hydrolase (BSH) activity as a gut microbial activity that has the capacity to profoundly alter both local (gastrointestinal) and systemic (hepatic) host functions. Using both germ free and conventionally-raised mouse models we demonstrated that gastrointestinal expression of BSH results in local bile acid deconjugation with concomitant alterations in lipid and cholesterol metabolism, signaling functions and weight gain. Key mediators of cholesterol homeostasis (Abcg5/8), gut homeostasis (RegIIIγ) and circadian rhythm (Dbp) were influenced by elevated BSH in our study. In this addendum we discuss the implications of this work for the rational development of probiotics with the potential to modulate host weight gain.
Keywords: bile, circadian rhythm, Lactobacillus, obesity, probiotic
The Functional Genetic Analyses of Gut Microbes and Probiotics
Large-scale new generation sequencing projects have improved our understanding of the composition of the gastrointestinal microbiota in health and disease1,2 and have identified potential markers of disease risk.3,4 However there remains a need for controlled, reductionist studies to determine the impact of specific microorganisms (including probiotic microorganisms) or specific microbial functions upon the host.5 Such studies will support the interpretation of microbial community analyses and community shotgun sequencing studies and will indicate the potential for targeted intervention strategies for the amelioration of disease.
Dietary supplementation with probiotics has been shown to potentially enhance or reduce weight gain in humans and/or animals, an effect that is strain-dependent (recently reviewed in6). Weight-enhancing probiotics have applications in growth promotion of farm animals and in counteracting malnutrition or sarcopenia in humans. Weight-reducing probiotics may find an application in weight management in obese humans (in combination with dietary interventions). Understanding the molecular features of probiotic microorganisms which influence host energy metabolism is therefore important in order to select probiotics for safe, effective administration for specified purposes.
Numerous studies have linked the gut microbiota to obesity and weight gain in mice and humans (reviewed in7-9). Studies have demonstrated an association between an elevated Firmicutes-to-Bacteroidetes ratio and enhanced weight gain10-12 and have shown that the phenotype is transmissible between mice via the microbiota13 Gut microbial factors positively linked with elevated weight gain include the increased translocation of lipopolysaccharide (LPS) in mice14 and in humans15 indicating reduced gut barrier function.9 Studies have indicated an inverse correlation between the mucin-degrading bacterium Akkermansia mucinophila and obesity in humans16 and mouse studies have confirmed that this organism is protective against obesity by mediating enhanced gut barrier function.17 In addition to these mechanisms we postulated that microbial metabolism of bile acids may also play a role in regulation of host weight gain given that individual bile acids are regulators of host energy metabolism18,19 (a hypothesis that we investigated in our recent study outlined below20).
Bile Salt Hydrolase Distribution and Potential Function
In our recent work we focused upon a single microbial function (bile salt hydrolase (BSH) activity) to determine its overall impact upon host physiology.20 Microbial BSH activity has previously been proposed to be a regulator of lipid metabolism in the host21-24 but to our knowledge direct, controlled studies of this activity using isogenic mutants are limited.
BSH enzymatic activity functions as a gateway reaction in the metabolism of bile acids (BAs) in the small intestine by deconjugating tauroconjugated or glycoconjugated primary BAs to produce their unconjugated counterparts (see Fig. 1). These unconjugated BAs are further metabolised by the gut microbiota through the activity of 7 α dehydroxylase enzymes to ultimately produce secondary and tertiary BAs (Fig. 1).21
Figure 1.
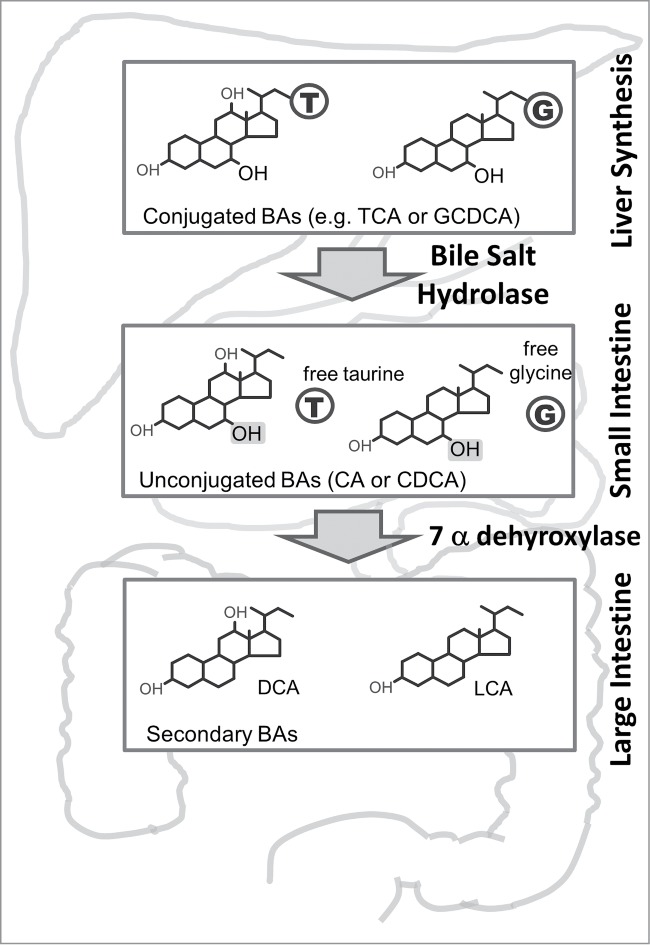
Microbial Metabolism of Bile Acids. Bile acids are synthesized from cholesterol in the liver as moieties that are conjugated either to a glycine (G) or taurine (T) molecule. They are stored in the gallbladder and subsequently released into the duodenum. In the small intestine microbial BSH activity removes the glycine or taurine molecules to produce unconjugated bile acids (BAs). Bile acids are efficiently reabsorbed via the terminal ileum into the enterohepatic portal system but some enter the large intestine where they are further metabolised by microbial 7a dehydroxylase enymes to produce secondary BAs. CA, cholic acid; CDCA, chenodeoxycholic acid; TCA, taurocholic acid; GCDCA, glycochenodeoxycholic acid; DCA, deoxycholic acid; LCA, lithocholic acid. T, taurine; G, glycine, represent free amino acids liberated through BSH activity.
Previous work by our group used a functional metagenomics approach to show that BSH is an abundant enzyme found in all major phyla within the gut microbiota (including the Firmicutes, Bacteroidetes, Actinobacteria and Proteobacteria) and across both Domains of life (gut Bacteria and gut Archaea).25 We determined that active bona fide BSH enzymes are restricted to gut microorganisms, while Penicillin V acylase enzymes that have a similar structure are found both in gut and non-gut organisms. Experimental evidence from our group25 and others21,26 suggests that BSH activity may contribute to microbial bile resistance and colonisation of the gastrointestinal environment. We have demonstrated that expression of cloned BSH in Listeria innocua significantly enhances both in vitro microbial bile tolerance and gut colonisation in mice relative to the BSH negative control strain.25 In the context of the development of a nascent gut microbial community in neonates, BSH may therefore represent an important factor which influences initial survival and colonisation. Furthermore the wide distribution of this function and apparent host-driven evolution of this trait within the gastrointestinal microbial community suggested that BSH may influence host health in ways that permit persistent mutualism.
This hypothesis is strengthened by the dual digestive and signaling roles of bile acids in the host.27 Individual BAs have been shown to specifically interact with host receptors including FXR and the G-coupled protein receptor TGR5.19,28 Analysis of bile acid metabolites in rats revealed their widespread distribution in diverse tissues (including heart, liver, kidney and plasma) indicating that BAs potentially act as mediators of host signaling in multiple tissue compartments.29 In an elegant study the deconjugated BA, cholic acid, was shown to enhance weight loss in mice fed a high fat diet through a mechanism involving TGR5 signaling to increase metabolic rate in brown fat tissue.19
Gut Associated BSH Activity Alters Host Weight Gain and Specific Host Metabolic Pathways
We recently adopted a controlled, reductionist approach to specifically analyze the impact of bacterial BSH activity upon host physiology and metabolism.20 In our model system genes encoding well characterized BSH enzymes26 (designated as BSH1 or BSH2) were cloned into an E. coli strain (MG1655) that colonises the GI tract of mice allowing localized delivery of high levels of enzymatic activity. This permitted the direct comparison of 2 different bsh alleles using an otherwise isogenic system. Mono-colonisation of the GI tract of germ-free mice with E. coli producing either BSH enzyme (clones designated as ECBSH1 or ECBSH2) led to significant BA deconjugation activity (as determined by UPLC-MS analysis of BA profiles) that was evident in the gut and in liver and plasma compartments. The system allowed us to analyze the host response to in situ microbial BSH activity and revealed the regulation of multiple effector pathways that regulate lipid and cholesterol metabolism, peripheral circadian rhythm and immune and epithelial homeostasis. In conventionally raised mice BSH1 activity in particular was capable of reducing weight gain (with an attendant reduction in fat mass), lowering serum cholesterol and reducing liver triglyceride levels.
Potential Effectors in our System
Given the impaired physiological development of the gut in germ free mice it is important to validate host responses in conventionally raised animals.18 We used our global transcriptomic analysis in germ free mice to generate hypotheses which could subsequently be tested in conventionally-raised animals. For instance, others have postulated a role for the gastrointestinal cholesterol efflux system encoded by Abcg5/8 in the lowering of cholesterol by BSH-active probiotics (reviewed in30). In our controlled experimental system we determined that expression of Abcg5/8 is specifically elevated in the GI tract by BSH expression in both germ free and conventionally-raised animals.
We determined that BSH activity in the GI tract of germ free mice promotes expression of a number of systems that play a role in gut homeostasis. Regenerating islet-derived protein 3 gamma (REGIIIγ) is an antibacterial protein produced by the host that modulates local immune homeostasis in the gut by targeting gram positive bacteria.31 We determined that gastrointestinal expression of RegIIIγ is enhanced through re-conventionalisation of germ-free mice and also by BSH1 activity both in germ-free and conventionally raised mice. This suggests that bile acid deconjugation may be an important signal in the regulation of this homeostatic feedback loop. Overall this fits with a concept in which local bile acids may feedback to regulate the microbiota directly32,33 or through regulation of host homeostatic factors which may impact upon the microbiota34,35 (see Fig. 2). We demonstrate the potential to influence this process through an intervention which enhances the local expression of BSH.
Figure 2.
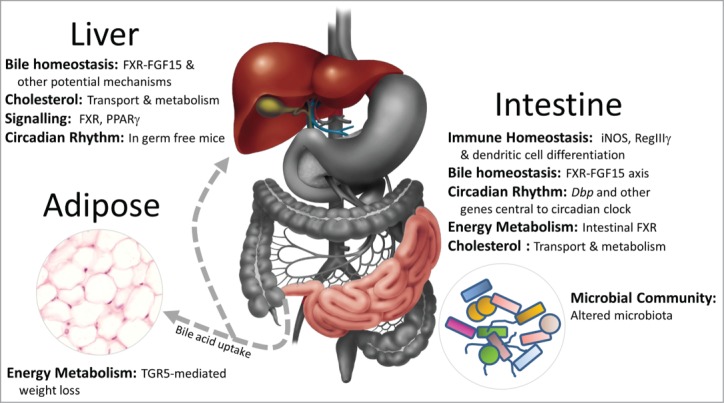
Impact of unconjugated bile acids upon local and systemic physiological processes in the host. Some of the key findings linking predominately unconjugated bile acids with intestinal and systemic responses in the host. In addition to a role in the emulsification of dietary fats in the gut, bile acids affect immune homeostasis, energy metabolism, bile acid homeostasis and potentially peripheral circadian rhythm. Importantly unconjugated bile acids also have a significant impact upon the makeup of the gut microbial community which may have further implications for the host. iNOS,34 RegIIIγ,20 dendritic cell differentiation,35 FXR-FGF15 axis,43 circadian rhythm,20 intestinal FXR,45 cholesterol metabolism,30 microbial community structure,32,33 liver FXR signaling,18,27,29 PPARγ liver,20 energy metabolism in adipose tissue.19
We demonstrated that high-level gastrointestinal BSH activity can reduce weight gain in conventionally raised mice. Unconjugated bile acids are less effective than conjugated bile acids in the emulsification of dietary fats21 and it is therefore likely that local lipid absorption is affected in our model (a phenomenon that we are currently investigating). However work published contemporaneously with our own study indicated that bile acid signaling via FXR also plays a significant role in weight loss (in particular following bariatric surgery36). Indeed we detected increased expression of Fiaf (fasting induced adipose factor) which encodes a lipoprotein lipase inhibitor normally associated with reduced obesity in mice.37 We are also currently examining the influence of BSH upon local and systemic expression of other regulators of lipid metabolism in conventionally raised animals (based upon data generated in our germ free model system). It is likely that the impact of gastrointestinal BSH activity upon weight gain is a reflection of local effects upon lipid absorption and the local and systemic signaling properties of unconjugated bile acids uncovered by other studies19,28,36 (Fig. 2).
Intriguingly BSH1 expression in the gut of germ free mice reversed the expression pattern of genes responsible for regulating circadian rhythm.20 Mice colonised by E. coli expressing BSH1 induced a gene expression profile that reflects conditions of energy utilization (associated with daylight exposure in humans) (elevated Clock and Arntl expression and reduced Per1, Per2 and Per3 expression).38 In conventionally raised animals the diurnally-regulated gene Dbp (encoding the albumin D-box binding protein) was also regulated by BSH1 activity (Fig. 2). The potential influence of bile acid deconjugation upon circadian rhythm locally and systemically is interesting given the links between circadian desynchronizations and altered weight gain in humans.39 Recently it has emerged that interactions between the gut microbiota and toll like receptors on intestinal epithelial cells are influenced by the circadian clock and that deregulation of this interaction can enhance the development of a pre-diabetic syndrome.40 This highlights the importance of circadian rhythm in regulating host microbe interactions which protect against metabolic disease. It is well established that bile acid synthesis is regulated by the circadian clock.41,42 While acknowledging the need for more studies our data suggest that microbial BSH activity represents a potential switch that can further influence peripheral circadian responses both locally and systemically.
Probiotics and Bile Acid Deconjugation
Whether probiotics influence host physiology through in vivo bile acid metabolism is uncertain but merits consideration. It has been demonstrated recently that the mixed probiotic formulation VSL#3 (containing BSH-active Lb. acidophilus and B. infantis strains) is capable of significantly altering bile acid profiles in mice with repression of the FXR-FGF15 axis and increased hepatic bile acid synthesis.43 An increase in unconjugated bile acids in the plasma of human volunteers receiving BSH-active Lb. reuteri NCIMB 30242 has also been noted indicating the potential for probiotics to alter bile acid metabolism in humans.44 Mice and humans differ in their baseline bile acid profiles with murine bile containing a predominance of tauro-conjugated bile acids (including muricholic acids) while human bile contains greater concentrations of glyco-conjugated bile acids21,27 In this regard we have previously noted significant differences between the BSH complement of the murine gut and that of humans, perhaps indicating subtle, species-specific differences in the host-driven selection of this trait.25 This may influence translation of the existing work from mice to humans and should guide the selection of probiotics with appropriate deconjugation activities.
A number of studies have demonstrated a reduction in body weight through administration of probiotics in mice and humans (recently reviewed in6). In many cases these probiotics represent Lactobacillus species that are likely to have bile salt deconjugating activity. However, some species of Lactobacillus promote weight gain in animals and humans6 suggesting that there are multiple potential mechanisms by which probiotics alter weight gain and that effects may be species or even strain dependent. To our knowledge there is limited mechanistic data linking certain probiotics to weight gain and others to weight loss. Based upon our data20 and other recent work36,45 it is likely that BSH activity plays a significant role in this process. Other probiotic factors which may influence the process of weight gain in the host may include an overall influence upon the community structure of the microbiome and a direct or indirect influence upon production of other significant metabolites including short chain fatty acids.5,46
Potential adverse effects on the host from any strategy designed to alter bile acid metabolism need careful consideration. For instance, highly elevated levels of unconjugated bile acids could potentially lead to lipid malabsorption and resultant steatorrhea47 More importantly, the secondary bile acids deoxycholic acid (DCA) and lithocholic acid (LCA) are produced from unconjugated bile acids following specific microbial conversions in the colon (Fig. 1). These secondary bile acids have been linked to an elevated risk of colorectal cancer.48 It is unclear whether elevated BSH activity per se results in an elevation of secondary bile acid metabolism in the host. In the recent study of VSL#3 probiotic administration in mice elevated BSH activity and elevated de novo bile acid synthesis did not increase relative levels of DCA.43 In our study very high levels of BSH1 activity in our model system resulted in a marginal increase in secondary bile acid levels but this was not statistically significant.20 Furthermore, the FXR bile acid receptor, which is preferentially stimulated by unconjugated bile acids, is thought to be protective against colorectal cancer suggesting that bile acid deconjugation may enhance the anti-cancer effects of FXR.49,50 Certainly more work is warranted to examine how alteration of the gut microbial community changes secondary bile acid metabolism and the implications of these changes for host health.
Conclusions
The BSH activity of the gut microbiota influences body weight and lipid metabolism in mice. This raises the potential for novel strategies to control weight gain in the host. First it will be necessary to clarify all of the host effector mechanisms that are triggered through elevated microbial BSH activity and to ensure the safety of any approach which alters host metabolism. Our findings are supportive of studies linking the activity of certain antibiotics to weight gain via an impact upon bile metabolism by the microbiota.8,23,51,52 The work also supports the intriguing concept of specifically targeting intestinal bile salt hydrolase activity to enhance weight gain in animal husbandry.23,24,53
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
The work was funded by grants from Science Foundation Ireland in the form of a center grant (Alimentary Pharmabiotic Center; grant number SFI/12/RC/2273). SAJ, FS and CGMG also acknowledge receipt of funding from the Health Research Board, Ireland (grant number HRA-POR-2013-296).
References
- 1. Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010; 464:59-65; PMID:20203603; http://dx.doi.org/ 10.1038/nature08821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tong M, Li X, Wegener Parfrey L, Roth B, Ippoliti A, Wei B, et al. A modular organization of the human intestinal mucosal microbiota and its association with inflammatory bowel disease. PLoS One 2013; 8:e80702; PMID:24260458; http://dx.doi.org/ 10.1371/journal.pone.0080702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Karlsson FH, Tremaroli V, Nookaew I, Bergstrom G, Behre CJ, Fagerberg B, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 2013; 498:99-103; PMID:23719380; http://dx.doi.org/ 10.1038/nature12198 [DOI] [PubMed] [Google Scholar]
- 4. Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013; 500:541-6; PMID:23985870; http://dx.doi.org/ 10.1038/nature12506 [DOI] [PubMed] [Google Scholar]
- 5. Joyce SA, Gahan CG. The gut microbiota and the metabolic health of the host. Curr Opin Gastroenterol 2014; 30:120-7; PMID:24468803; http://dx.doi.org/ 10.1097/MOG.0000000000000039 [DOI] [PubMed] [Google Scholar]
- 6. Angelakis E, Merhej V, Raoult D. Related actions of probiotics and antibiotics on gut microbiota and weight modification. Lancet Infect Dis 2013; 13:889-99; PMID:24070562; http://dx.doi.org/ 10.1016/S1473-3099(13)70179-8 [DOI] [PubMed] [Google Scholar]
- 7. Clarke SF, Murphy EF, Nilaweera K, Ross PR, Shanahan F, O'Toole PW, et al. The gut microbiota and its relationship to diet and obesity: new insights. Gut Microbes 2012; 3:186-202; PMID:22572830; http://dx.doi.org/ 10.4161/gmic.20168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nieuwdorp M, Gilijamse PW, Pai N, Kaplan LM. Role of the microbiome in energy regulation and metabolism. Gastroenterology 2014; 146:1525-33; PMID:24560870; http://dx.doi.org/ 10.1053/j.gastro.2014.02.008 [DOI] [PubMed] [Google Scholar]
- 9. Everard A, Cani PD. Diabetes, obesity and gut microbiota. Best Pract Res Clin Gastroenterol 2013; 27:73-83; PMID:23768554; http://dx.doi.org/ 10.1016/j.bpg.2013.03.007 [DOI] [PubMed] [Google Scholar]
- 10. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006; 444:1027-31; PMID:17183312; http://dx.doi.org/ 10.1038/nature05414 [DOI] [PubMed] [Google Scholar]
- 11. Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature 2006; 444:1022-3; PMID:17183309; http://dx.doi.org/ 10.1038/4441022a [DOI] [PubMed] [Google Scholar]
- 12. Murphy EF, Cotter PD, Healy S, Marques TM, O'Sullivan O, Fouhy F, et al. Composition and energy harvesting capacity of the gut microbiota: relationship to diet, obesity and time in mouse models. Gut 2010; 59:1635-42; PMID:20926643; http://dx.doi.org/ 10.1136/gut.2010.215665 [DOI] [PubMed] [Google Scholar]
- 13. Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013; 341:1241214; PMID:24009397; http://dx.doi.org/ 10.1126/science.1241214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007; 56:1761-72; PMID:17456850; http://dx.doi.org/ 10.2337/db06-1491 [DOI] [PubMed] [Google Scholar]
- 15. Creely SJ, McTernan PG, Kusminski CM, Fisher fM, Da Silva NF, Khanolkar M, et al. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab 2007; 292:E740-7; PMID:17090751; http://dx.doi.org/ 10.1152/ajpendo.00302.2006 [DOI] [PubMed] [Google Scholar]
- 16. Karlsson CL, Onnerfalt J, Xu J, Molin G, Ahrne S, Thorngren-Jerneck K. The microbiota of the gut in preschool children with normal and excessive body weight. Obesity (Silver Spring) 2012; 20:2257-61; PMID:22546742; http://dx.doi.org/ 10.1038/oby.2012.110 [DOI] [PubMed] [Google Scholar]
- 17. Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A 2013; 110:9066-71; PMID:23671105; http://dx.doi.org/ 10.1073/pnas.1219451110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sayin SI, Wahlstrom A, Felin J, Jantti S, Marschall HU, Bamberg K, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-β-muricholic acid, a naturally occurring FXR antagonist. Cell Metab 2013; 17:225-35; PMID:23395169; http://dx.doi.org/ 10.1016/j.cmet.2013.01.003 [DOI] [PubMed] [Google Scholar]
- 19. Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 2006; 439:484-9; PMID:16400329; http://dx.doi.org/ 10.1038/nature04330 [DOI] [PubMed] [Google Scholar]
- 20. Joyce SA, MacSharry J, Casey PG, Kinsella M, Murphy EF, Shanahan F, et al. Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut. Proc Natl Acad Sci U S A 2014; 111:7421-6; PMID:24799697; http://dx.doi.org/ 10.1073/pnas.1323599111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Begley M, Gahan CG, Hill C. The interaction between bacteria and bile. FEMS Microbiol Rev 2005; 29:625-51; PMID:16102595; http://dx.doi.org/ 10.1016/j.femsre.2004.09.003 [DOI] [PubMed] [Google Scholar]
- 22. Begley M, Hill C, Gahan CG. Bile salt hydrolase activity in probiotics. Appl Environ Microbiol 2006; 72:1729-38; PMID:16517616; http://dx.doi.org/ 10.1128/AEM.72.3.1729-1738.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lin J. Antibiotic growth promoters enhance animal production by targeting intestinal bile salt hydrolase and its producers. Front Microbiol 2014; 5:33; PMID:24575079; . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smith K, Zeng X, Lin J. Discovery of bile salt hydrolase inhibitors using an efficient high-throughput screening system. PLoS One 2014; 9:e85344; PMID:24454844; http://dx.doi.org/ 10.1371/journal.pone.0085344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jones BV, Begley M, Hill C, Gahan CG, Marchesi JR. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc Natl Acad Sci U S A 2008; 105:13580-5; PMID:18757757; http://dx.doi.org/ 10.1073/pnas.0804437105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fang F, Li Y, Bumann M, Raftis EJ, Casey PG, Cooney JC, et al. Allelic variation of bile salt hydrolase genes in Lactobacillus salivarius does not determine bile resistance levels. J Bacteriol 2009; 191:5743-57; PMID:19592587; http://dx.doi.org/ 10.1128/JB.00506-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Aguiar Vallim TQ, Tarling EJ, Edwards PA. Pleiotropic roles of bile acids in metabolism. Cell Metab 2013; 17:657-69; PMID:23602448; http://dx.doi.org/ 10.1016/j.cmet.2013.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Houten SM, Watanabe M, Auwerx J. Endocrine functions of bile acids. EMBO J 2006; 25:1419-25; PMID:16541101; http://dx.doi.org/ 10.1038/sj.emboj.7601049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Swann JR, Want EJ, Geier FM, Spagou K, Wilson ID, Sidaway JE, et al. Systemic gut microbial modulation of bile acid metabolism in host tissue compartments. Proc Natl Acad Sci U S A 2011; 108(Suppl 1):4523-30; PMID:20837534; http://dx.doi.org/ 10.1073/pnas.1006734107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jones ML, Tomaro-Duchesneau C, Martoni CJ, Prakash S. Cholesterol lowering with bile salt hydrolase-active probiotic bacteria, mechanism of action, clinical evidence, and future direction for heart health applications. Expert Opin Biol Ther 2013; 13:631-42; PMID:23350815; http://dx.doi.org/ 10.1517/14712598.2013.758706 [DOI] [PubMed] [Google Scholar]
- 31. Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, et al. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science 2011; 334:255-8; PMID:21998396; http://dx.doi.org/ 10.1126/science.1209791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Islam KB, Fukiya S, Hagio M, Fujii N, Ishizuka S, Ooka T, et al. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology 2011; 141:1773-81; PMID:21839040; http://dx.doi.org/ 10.1053/j.gastro.2011.07.046 [DOI] [PubMed] [Google Scholar]
- 33. Yokota A, Fukiya S, Islam KB, Ooka T, Ogura Y, Hayashi T, et al. Is bile acid a determinant of the gut microbiota on a high-fat diet? Gut Microbes 2012; 3:455-9; PMID:22825495; http://dx.doi.org/ 10.4161/gmic.21216 [DOI] [PubMed] [Google Scholar]
- 34. Inagaki T, Moschetta A, Lee YK, Peng L, Zhao G, Downes M, et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci U S A 2006; 103:3920-5; PMID:16473946; http://dx.doi.org/ 10.1073/pnas.0509592103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ichikawa R, Takayama T, Yoneno K, Kamada N, Kitazume MT, Higuchi H, et al. Bile acids induce monocyte differentiation toward interleukin-12 hypo-producing dendritic cells via a TGR5-dependent pathway. Immunology 2012; 136:153-62; PMID:22236403; http://dx.doi.org/ 10.1111/j.1365-2567.2012.03554.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 2014; 509:183-8; PMID:24670636; http://dx.doi.org/ 10.1038/nature13135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Backhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci U S A 2007; 104:979-84; PMID:17210919; http://dx.doi.org/ 10.1073/pnas.0605374104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bass J. Circadian topology of metabolism. Nature 2012; 491:348-56; PMID:23151577; http://dx.doi.org/ 10.1038/nature11704 [DOI] [PubMed] [Google Scholar]
- 39. Ekmekcioglu C, Touitou Y. Chronobiological aspects of food intake and metabolism and their relevance on energy balance and weight regulation. Obes Rev 2011; 12:14-25; PMID:20122134; http://dx.doi.org/ 10.1111/j.1467-789X.2010.00716.x [DOI] [PubMed] [Google Scholar]
- 40. Mukherji A, Kobiita A, Ye T, Chambon P. Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell 2013; 153:812-27; PMID:23663780; http://dx.doi.org/ 10.1016/j.cell.2013.04.020 [DOI] [PubMed] [Google Scholar]
- 41. Li YC, Wang DP, Chiang JY. Regulation of cholesterol 7 α-hydroxylase in the liver. Cloning, sequencing, and regulation of cholesterol 7 α-hydroxylase mRNA. J Biol Chem 1990; 265:12012-9; PMID:1694852; . [PubMed] [Google Scholar]
- 42. Zhang YK, Guo GL, Klaassen CD. Diurnal variations of mouse plasma and hepatic bile acid concentrations as well as expression of biosynthetic enzymes and transporters. PLoS One 2011; 6:e16683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Degirolamo C, Rainaldi S, Bovenga F, Murzilli S, Moschetta A. Microbiota modification with probiotics induces hepatic bile acid synthesis via downregulation of the Fxr-Fgf15 axis in mice. Cell Rep 2014; 7:12-8; PMID:24656817; http://dx.doi.org/ 10.1016/j.celrep.2014.02.032 [DOI] [PubMed] [Google Scholar]
- 44. Jones ML, Martoni CJ, Prakash S. Cholesterol lowering and inhibition of sterol absorption by Lactobacillus reuteri NCIMB 30242: a randomized controlled trial. Eur J Clin Nutr 2012; 66:1234-41; PMID:22990854; http://dx.doi.org/ 10.1038/ejcn.2012.126 [DOI] [PubMed] [Google Scholar]
- 45. Li F, Jiang C, Krausz KW, Li Y, Albert I, Hao H, et al. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat Commun 2013; 4:2384; PMID:24064762; . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014; 156:84-96; PMID:24412651; http://dx.doi.org/ 10.1016/j.cell.2013.12.016 [DOI] [PubMed] [Google Scholar]
- 47. Choi SB, Lew LC, Yeo SK, Nair Parvathy S, Liong MT. Probiotics and the BSH-related cholesterol lowering mechanism: a Jekyll and Hyde scenario. Crit Rev Biotechnol 2014; PMID:24575869; . [DOI] [PubMed] [Google Scholar]
- 48. Ajouz H, Mukherji D, Shamseddine A. Secondary bile acids: an underrecognized cause of colon cancer. World J Surg Oncol 2014; 12:164; PMID:24884764; http://dx.doi.org/ 10.1186/1477-7819-12-164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Maran RR, Thomas A, Roth M, Sheng Z, Esterly N, Pinson D, et al. Farnesoid X receptor deficiency in mice leads to increased intestinal epithelial cell proliferation and tumor development. J Pharmacol Exp Ther 2009; 328:469-77; PMID:18981289; http://dx.doi.org/ 10.1124/jpet.108.145409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Modica S, Murzilli S, Salvatore L, Schmidt DR, Moschetta A. Nuclear bile acid receptor FXR protects against intestinal tumorigenesis. Cancer Res 2008; 68:9589-94; PMID:19047134; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-1791 [DOI] [PubMed] [Google Scholar]
- 51. Guban J, Korver DR, Allison GE, Tannock GW. Relationship of dietary antimicrobial drug administration with broiler performance, decreased population levels of Lactobacillus salivarius, and reduced bile salt deconjugation in the ileum of broiler chickens. Poult Sci 2006; 85:2186-94; PMID:17135676; http://dx.doi.org/ 10.1093/ps/85.12.2186 [DOI] [PubMed] [Google Scholar]
- 52. Vrieze A, Out C, Fuentes S, Jonker L, Reuling I, Kootte RS, et al. Impact of oral vancomycin on gut microbiota, bile acid metabolism, and insulin sensitivity. J Hepatol 2014; 60:824-31; PMID:24316517; http://dx.doi.org/ 10.1016/j.jhep.2013.11.034 [DOI] [PubMed] [Google Scholar]
- 53. Wang Z, Zeng X, Mo Y, Smith K, Guo Y, Lin J. Identification and characterization of a bile salt hydrolase from Lactobacillus salivarius for development of novel alternatives to antibiotic growth promoters. Appl Environ Microbiol 2012; 78:8795-802; PMID:23064348; http://dx.doi.org/ 10.1128/AEM.02519-12 [DOI] [PMC free article] [PubMed] [Google Scholar]


