Abstract
As various new sibling species within the Scedosporium spp. have been described recently, this study was conducted to investigate distribution and antifungal susceptibility profiles of the different species of Scedosporium spp. in China. Twenty-one clinical strains of Scedosporium from China and two strains from Japan were reidentified by MLSA. The analysis included BT2, CAL, RPB, SOD, and ACT and the combination of the five loci. Pseudallescheria boydii complex (17 strains) and S. apiospermum (6 strains) were identified. P. boydii complex included four closely related subgroups: P. boydii (9 strains), P. ellipsoidea (6 strains), P. fusoidea (1 strain), and P. angusta (1 strain). There were no significant differences in MICs for neither VOR, POS, nor AMB over all the five species in study. For itraconazole, intraspecific diversity was evident.
1. Introduction
Scedosporium spp. is one of the species of the opportunistic pathogenic fungi that can always be found in environment, especially in sewerage. It infects immunocompromised patients and drowning men, involving lungs, sinuses, bones, joints, eyes, and brain [1].
In recent years, members of the genus Scedosporium are increasingly recognized as opportunistic agents of disease, for example, in transplant recipients. Scedosporium species have been identified as the second most prevalent mold after Aspergillus colonizing the lungs of patients with cystic fibrosis [2]. Scedosporium infections occur worldwide. In European countries, USA and Australia, Scedosporium species were always found in patients with chronic lung diseases, cystic fibrosis (CF), lung or allogenic bone-marrow transplantation, and hematologic malignancies [1, 3, 4]. Accordingly, the common types were pulmonary, nasal sinuses, skin/soft tissues, CNS, and disseminated infection [1, 3, 4].
It is traditionally recognized that Pseudallescheria boydii is the sexual stage of the S. apiospermum. However, recent molecular studies [5–7] showed that Scedosporium is a species complex comprising at least five distinct groups: S. aurantiacum, P. minutispora, S. dehoogii, S. apiospermum, and P. boydii, the last group consisting of four closely related subgroups called P. boydii, P. angusta, P. ellipsoidea, and P. fusoidea. Furthermore, S. prolificans is now renamed as Lomentospora prolificans [8]. It is important to identify Scedosporium spp. to species level because their virulence, metabolic trait, and in vitro susceptibility may be various based on their different species [3–9].
2. Materials and Methods
2.1. Strains
From 1990 to 2014, twenty-one Scedosporium strains isolated from patients were reserved in Research Center for Medical Mycology at Peking University. The clinical samples were collected from 14 Chinese hospitals which located mainly in central and south of China. All the isolates were identified as S. apiospermum or P. boydii by morphology. A total of 23 isolates (including two strains from Japan) as shown in Table 1 were investigated in this study. Furthermore, in vitro susceptibility was performed on the same set strains.
Table 1.
Origin, sequence data, and species identification of studied isolates.
∗Strains from Japan.
2.2. Molecular Studies
The isolates were cultured on PDA at 28°C for 7 days. For fungal DNA extraction, glass beads method previously described by van Burik et al. was followed [10]. Adapted from earlier genotyping studies [11–14], PCR amplification with different primer pairs was attempted for Scedosporium species for the following genes: β-tubulin (BT2, exons 2–4) [11], calmodulin (CAL, exons 3–4) [12], the second largest subunit of RNA polymerase II (RPB) [13], superoxide dismutase (SOD), and actin (ACT) [14].
The PCR assay (25 μL) included 2 μL of fungal DNA extract, 1 μM of each gene-specific primer, 2.5 mmol dNTP Mix 1 μL, 10x PCR buffer 2.5 μL, and LA Taq polymerase 0.25 μL (Fermentas, St. Leon-Rot, Germany). The amplification for all targeted genes was performed in a Eppendorf PCR machine (AG22331) as follows: 5 min of initial denaturation at 95°C, followed by 35 cycles at 95°C for 30 s, gene-specific annealing temperature for 30 s, and 72°C for 1 min (for RPB2 the annealing time was 2.5 min). The PCR products were visualized by electrophoresis on a 1% (w/v) agarose gel. Both strands of the PCR fragments were sequenced using the above-mentioned primers. The consensus sequences were obtained using SeqMan (DNAStar-Lasergene, Madison, WI, USA) software. Newly obtained sequences were deposited in GenBank under accession numbers KP 981107 to KP 981221 (Table 1). They were used to conduct alignment analysis for preliminary species identification in the NCBI genomic database (http://blast.ncbi.nlm.nih.gov/) and CBS database (http://www.cbs.knaw.nl/). The sequences were aligned using MUSCLE. For the maximum likelihood analysis, the distances between sequences were calculated using the best parameter model found by MEGA 6.0 6 (http://www.megasoftware.net/). A bootstrap analysis was conducted with 1000 replications.
2.3. Susceptibility Test
The in vitro susceptibility of the 23 Scedosporium isolates against four antifungal agents was evaluated by using the Clinical and Laboratory Standards Institute (CLSI) M38-A2 broth microdilution method [15]. The inocula suspensions were prepared in new sterile tubes and adjusted to 0.4−5 × 106 colony-forming units per milliliter (CFU/mL) by counting spores in a hemocytometer and subsequently verifying them through quantitative colony counts on PDA plates. The nongerminated spore suspensions were diluted 1 : 100 in an RPMI 1640 to achieve a final inoculum concentration of 0.4–5 × 104 CFU/mL. The following antifungal agents were used: voriconazole (VOR; Shouguang Fukang Pharmaceutical Co., Ltd., China), posaconazole (POS; Merck, Rahway, NJ, USA), itraconazole (ITR; Shouguang Pharm), and amphotericin B (AMB; Sigma-Aldrich Co., St. Louis, USA). They were all diluted in 100% dimethyl sulphoxide as a stock solution with a concentration of 1.600 mg/L. Final drug concentrations ranged from 16 to 0.03 mg/L for all the four drugs. The minimal inhibitory concentrations (MIC) endpoints were defined as the lowest concentration at which there was a complete inhibition of growth. Aspergillus flavus ATCC 204304 served as a quality control strain. The microtiter panels were incubated at 35°C and the results were read after 72 h. All tests were performed in triplicate on three different days.
3. Results
3.1. Molecular Phylogeny
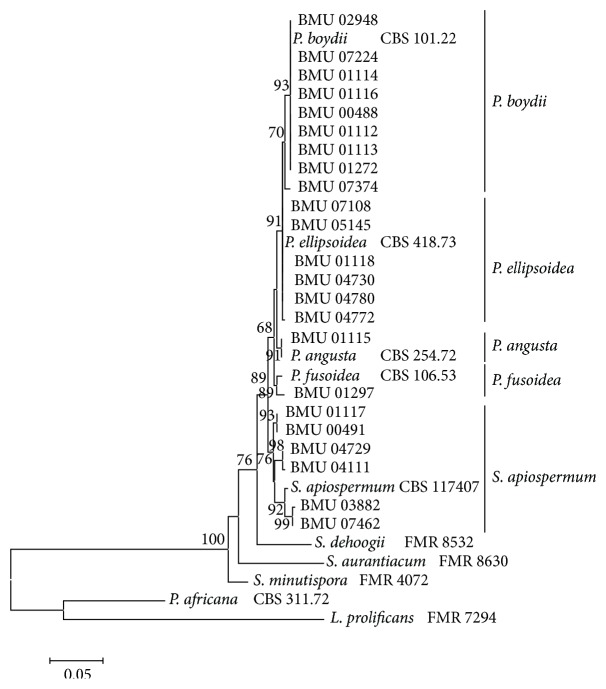
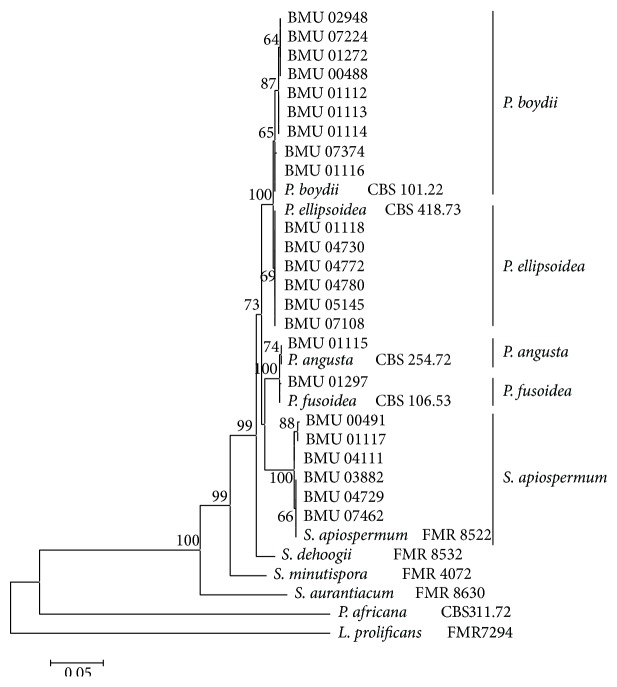
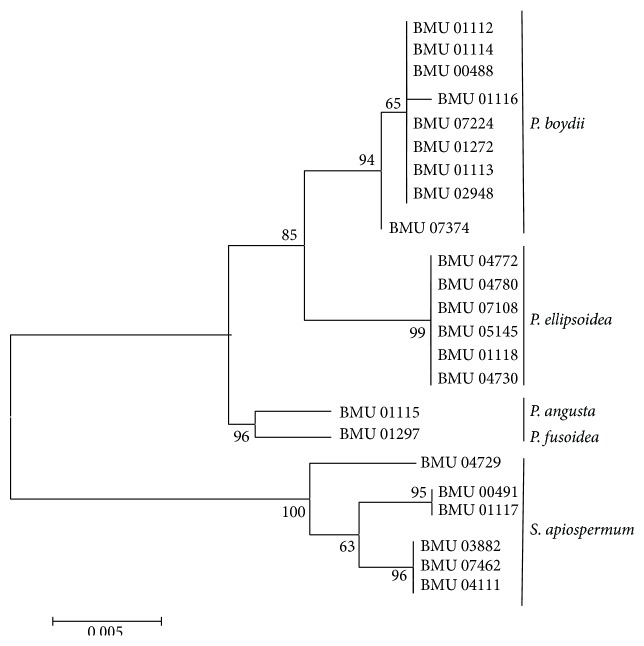
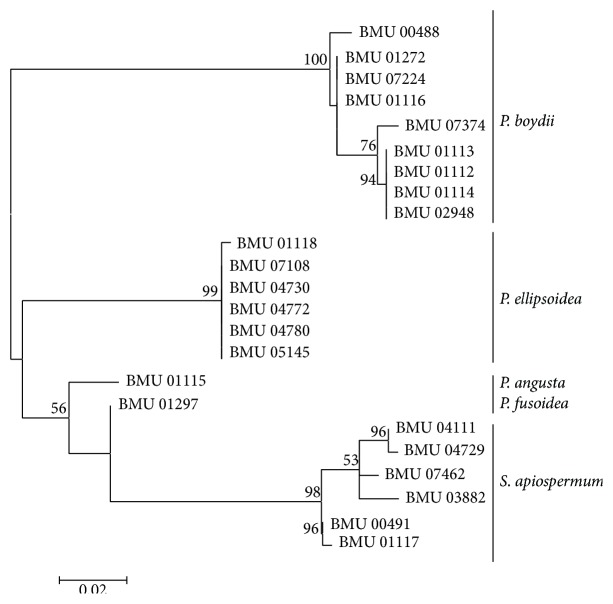
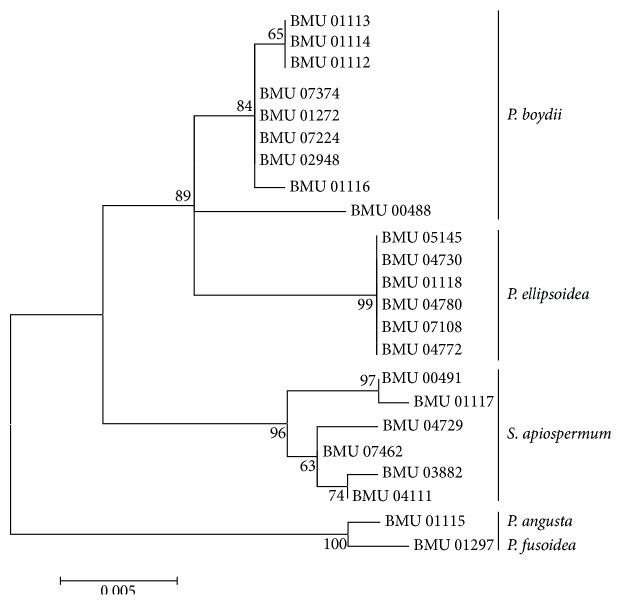
We were able to amplify and sequence 470 bp, 645 bp, 935 bp, 775 bp, and 396 bp of the BT2, CAL, RPB, ACT, and SOD loci, respectively. Of the 3221 nucleotides sequenced, 209 (6.5%) were informative for parsimony in the different Scedosporium isolates. For identification, reference sequence of Scedosporium species available in public database were used including BT2 and CAL, but no sequences for RPB, SOD, and ACT were available. The sequences for BT2, CAL, RPB, SOD, and ACT yielded phylogenetic trees with the same topology (Figures 1 –5). In the combination phylogenetic trees based on BT2 and CAL (Figure 6), 23 strains in our study were reidentified to the species level according to the reference strains, which were analyzed in the Gilgado literature. P. boydii (9/23) and its closely related subtypes P. ellipsoidea (6/23), P. fusoidea (1/23), and P. angusta (1/23) were the most common, and the other 6 of 23 strains were identified as S. apiospermum.
Figure 1.
Maximum likelihood tree based on BT2 sequences. Bootstrap values of >50% are indicated ion branches. The bar indicates the number of substitutions per site.
Figure 2.
Maximum likelihood tree based on CAL sequences. Bootstrap values of >50% are indicated ion branches. The bar indicates the number of substitutions per site.
Figure 3.
Maximum likelihood tree based on RPB. Bootstrap values of >50% are indicated ion branches. The bar indicates the number of substitutions per site.
Figure 4.
Maximum likelihood tree based on SOD sequences. Bootstrap values of >50% are indicated ion branches. The bar indicates the number of substitutions per site.
Figure 5.
Maximum likelihood tree based on ACT sequences. Bootstrap values of >50% are indicated ion branches. The bar indicates the number of substitutions per site.
Figure 6.
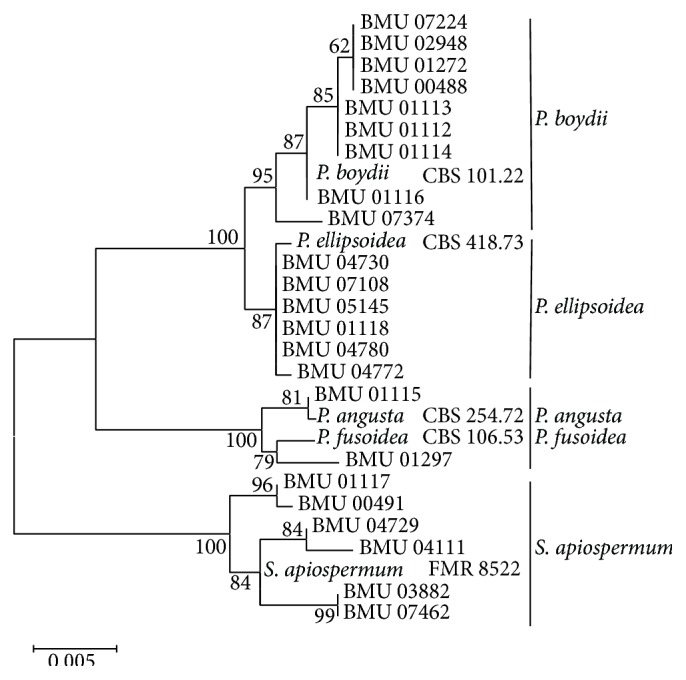
Maximum likelihood tree based on the analysis produced from the combined BT2 and CAL data. Bootstrap values of >50% are indicated ion branches. The bar indicates the number of substitutions per site.
The topology of the combined tree (Figure 7) of all five loci was similar to those observed in the trees of individual locus and the combined tree of BT2 and CAL. Four principal clades were obtained. The four clades were the P. boydii clade, P. ellipsoidea clade, P. fusoidea/P. angusta clade, and S. apiospermum clade. P. fusoidea and P. angusta always assemble together, and P. boydii and P. ellipsoidea were very closely related. S. apiospermum has the highest intraspecies variability (genetic distance = 0.008), which is comparable to the interspecies variability between P. fusoidea and P. angusta (genetic distance = 0.008).
Figure 7.
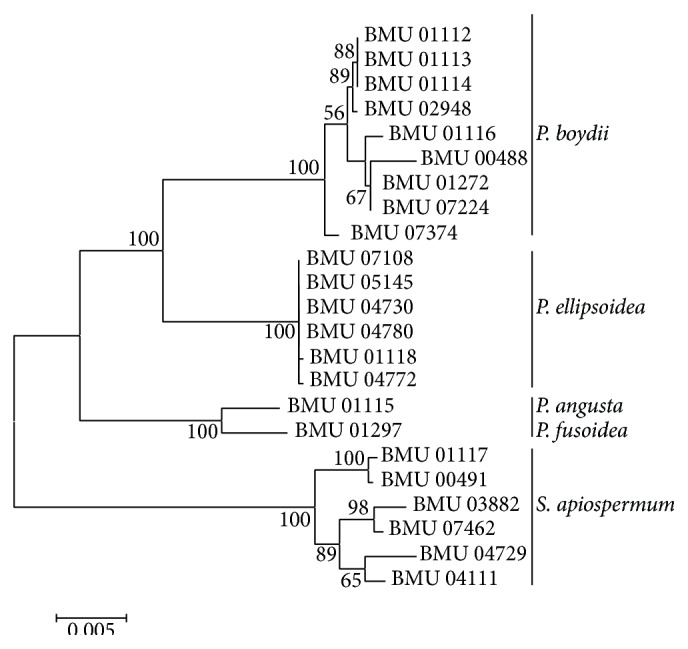
Maximum likelihood tree based on the analysis produced from the combined all five markers data. Bootstrap values of >50% are indicated ion branches. The bar indicates the number of substitutions per site.
3.2. In Vitro Susceptibility Test
The MIC values for the four antifungal agents examined by the CLSI M-38A2 microdilution method against the 23 strains are presented in Table 2. VOR was the most active agent against all 23 strains with a MIC range from 0.25 to 1 μg/mL and a 0.46 μg/mL GM. POS was the second most active agent with MIC values of 2 or 4 μg/mL. For ITR, the intraspecific diversity was obvious as most strains had a MIC between 2 and 4 μg/mL, but five strains were higher at a MIC of 32 μg/mL. AMB is the least effective with high MIC values ranging from 4 to 32 μg/mL in vitro.
Table 2.
In vitro susceptibility of 23 Scedosporium strains studied in this paper.
| Strain species | Strain ID number | MIC (μg/mL) | |||
|---|---|---|---|---|---|
| VOR | POS | ITR | AMB | ||
| P. boydii | BMU 00488 | 0.25 | 2 | 4 | 4 |
| BMU 01112 | 0.25 | 2 | 2 | 32 | |
| BMU 01113 | 0.25 | 4 | 4 | 32 | |
| BMU 01114 | 0.5 | 2 | 4 | 8 | |
| BMU 01116 | 0.5 | 2 | 4 | 4 | |
| BMU 01272 | 0.5 | 2 | 2 | 8 | |
| BMU 02948 | 0.5 | 2 | 2 | 4 | |
| BMU 07224 | 0.25 | 2 | 4 | 16 | |
| BMU 07374 | 0.25 | 2 | 4 | 4 | |
|
| |||||
| P. ellipsoidea | BMU 01118 | 1 | 4 | 32 | 4 |
| BMU 04730 | 0.5 | 2 | 2 | 4 | |
| BMU 04772 | 0.5 | 4 | 32 | 32 | |
| BMU 04780 | 0.5 | 4 | 4 | 16 | |
| BMU 05145 | 0.5 | 4 | 32 | 8 | |
| BMU 07108 | 0.25 | 4 | 4 | 8 | |
|
| |||||
| P. angusta | BMU 01115 | 0.5 | 2 | 4 | 8 |
|
| |||||
| P. fusoidea | BMU 01297 | 0.5 | 2 | 2 | 32 |
|
| |||||
| S. apiospermum | BMU 00491 | 0.5 | 4 | 4 | 4 |
| BMU 01117 | 0.5 | 4 | 4 | 8 | |
| BMU 03882 | 0.5 | 2 | 4 | 8 | |
| BMU 04111 | 1 | 4 | 32 | 16 | |
| BMU 04729 | 0.5 | 2 | 2 | 8 | |
| BMU 07462 | 1 | 2 | 32 | 8 | |
The MIC50 and MIC90 for the four antifungal agents against P. boydii, P. ellipsoidea, and S. apiospermum are presented in Table 3. There were similar MIC50 and MIC90 for VOR, POS, or AMB of the three species above. For ITR, the MIC90 of P. boydii (4 μg/mL) was lower than that of P. ellipsoidea (32 μg/mL) and S. apiospermum (32 μg/mL).
Table 3.
The MIC50 and MIC90 for the four antifungal agents against P. boydii, P. ellipsoidea, and S. apiospermum.
| Species (number of isolates) | Drug concentration (μg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| VOR | POS | ITR | AMB | |||||
| MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | |
| P. boydii (9) | 0.25 | 0.5 | 2 | 4 | 4 | 4 | 8 | 32 |
| P. ellipsoidea (6) | 0.5 | 1 | 4 | 4 | 4 | 32 | 8 | 32 |
| S. apiospermum (6) | 0.5 | 1 | 2 | 4 | 4 | 32 | 8 | 16 |
4. Discussions and Literature Review
Scedosporiosis is a rare infection in China. Sporadic cases of Scedosporium infection have been reported since 1990, but most cases were identified in recent years. We reviewed 39 cases reported in China from 1990 to 2013 [16–31]. We found that infection in the nasal sinuses (16 cases) was the most frequently involved location, followed by eye (13), CNS (5), lung (4), skin/soft tissues (2), arthritis or osteomyelitis (1), and disseminated infection (1). The risk factors included trauma, drowning, and a compromised immune system (bone marrow transplant or stem cell transplantation). Trauma is the most common risk factor for scedosporiosis in Chinese patients, as nearly half of these patients have trauma history. Because cystic fibrosis is extremely rare in the Chinese population, no scedosporiosis in CF was reported in China.
Based on molecular identification, we found that P. boydii complex and S. apiospermum were the only two species in our study. Actually, P. boydii and S. apiospermum demonstrated a closely phylogenetic relationship. This is also reflected by their undifferentiated morphological characteristics, assimilation of different sugars, and growth temperature (data not shown). In P. boydii complex, four closely related subgroups, P. boydii (9 strains), P. ellipsoidea (6 strains), P. fusoidea (1 strain), and P. angusta (1 strain), were present in the study.
In a set of clinical and environmental strains from Austria, Germany, and Netherlands, S. apiospermum was the most prevalent, followed by P. boydii [32, 33]. In Australia, S. apiospermum and S. aurantiacum were the Scedosporium species with a high incidence except for L. prolificans [4]. Based on our study, we found that in China the P. boydii complex represents the most prevalent species (16/21) followed by S. apiospermum (5/21), which is the same as in Northern Spain and France [34, 35]. In the Chinese strains, we could not find S. aurantiacum, which had a high incidence in Australia and a relatively low incidence in Europe.
Antifungal susceptibility profiles for clinical breakpoint of Scedosporium species are still under study. Of the four antifungal tested in this study, VOR was found to be the most active agent against Scedosporium species; POS was the second, and AMB had limited antifungal activity. For ITR, the intraspecific diversity was obvious as the range of MIC from 2 to 32 μg/mL and P. boydii had a lower MIC90 than that of P. ellipsoidea and S. apiospermum. This in vitro susceptibility data is consistent with previous reports [32, 34, 36–38]. In China, most cases caused by Scedosporium were treated with either ITR or VOR. Although Scedosporium strains have relatively high MIC values for ITR (GM = 5.25) in vitro, studies have verified that they are clinically effective [7].
5. Conclusion
The P. boydii complex and S. apiospermum were the main Scedosporium species in clinical samples in China. Scedosporium species can be distinguished unambiguously by all the five loci in this study. VOR is the most active agent in vitro against the set of Scedosporium species in this study, followed by POS and ITR.
Acknowledgment
This work was supported by the grants from the National Natural Science Foundation of China (31570015 and 81401713).
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Cortez K. J., Roilides E., Quiroz-Telles F., et al. Infections caused by Scedosporium spp . Clinical Microbiology Reviews. 2008;21(1):157–197. doi: 10.1128/cmr.00039-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borman A. M., Palmer M. D., Delhaes L., et al. Lack of standardization in the procedures for mycological examination of sputum samples from CF patients: a possible cause for variations in the prevalence of filamentous fungi. Medical Mycology. 2010;48(supplement 1):S88–S97. doi: 10.3109/13693786.2010.511287. [DOI] [PubMed] [Google Scholar]
- 3.Guarro J., Kantarcioglu A. S., Horré R., et al. Scedosporium apiospermum: changing clinical spectrum of a therapy-refractory opportunist. Medical Mycology. 2006;44(4):295–327. doi: 10.1080/13693780600752507. [DOI] [PubMed] [Google Scholar]
- 4.Heath C. H., Slavin M. A., Sorrell T. C., et al. Population-based surveillance for scedosporiosis in Australia: epidemiology, disease manifestations and emergence of Scedosporium aurantiacum infection. Clinical Microbiology and Infection. 2009;15(7):689–693. doi: 10.1111/j.1469-0691.2009.02802.x. [DOI] [PubMed] [Google Scholar]
- 5.Cuenca-Estrella M., Gomez-Lopez A., Mellado E., Garcia-Effron G., Monzon A., Rodriguez-Tudela J. L. In vitro activity of ravuconazole against 923 clinical isolates of nondermatophyte filamentous fungi. Antimicrobial Agents and Chemotherapy. 2005;49(12):5136–5138. doi: 10.1128/aac.49.12.5136-5138.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lackner M., Hagen F., Meis J. F., et al. Susceptibility and diversity in the therapy-refractory genus Scedosporium . Antimicrobial Agents and Chemotherapy. 2014;58(10):5877–5885. doi: 10.1128/aac.03211-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saracli M. A., Erdem U., Gonlum A., Yildiran S. T. Scedosporium apiospermum keratitis treated with itraconazole. Medical Mycology. 2003;41(2):111–114. doi: 10.1080/mmy.41.2.111.114. [DOI] [PubMed] [Google Scholar]
- 8.Lackner M., de Hoog G. S., Yang L. Y., et al. Proposed nomenclature for Pseudallescheria, Scedosporium and related genera. Fungal Diversity. 2014;67(1):1–10. doi: 10.1007/s13225-014-0295-4. [DOI] [Google Scholar]
- 9.Gilgado F., Cano J., Gené J., Serena C., Guarro J. Different virulence of the species of the Pseudallescheria boydii complex. Medical Mycology. 2009;47(4):371–374. doi: 10.1080/13693780802256539. [DOI] [PubMed] [Google Scholar]
- 10.van Burik J.-A. H., Schreckhise R. W., White T. C., Bowden R. A. Comparison of six extraction techniques for isolation of DNA from filamentous fungi. Journal of Medical and Veterinary Mycology. 1998;36(5):299–303. doi: 10.1080/02681219880000471. [DOI] [PubMed] [Google Scholar]
- 11.Glass N. L., Donaldson G. C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology. 1995;61(4):1323–1330. doi: 10.1128/aem.61.4.1323-1330.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernhardt A., Sedlacek L., Wagner S., Schwarz C., Würstl B., Tintelnot K. Multilocus sequence typing of Scedosporium apiospermum and Pseudallescheria boydii isolates from cystic fibrosis patients. Journal of Cystic Fibrosis. 2013;12(6):592–598. doi: 10.1016/j.jcf.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y. J., Whelen S., Hall B. D. Phylogenetic relationships among ascomycetes: evidence from an RNA polymerse II subunit. Molecular Biology and Evolution. 1999;16(12):1799–1808. doi: 10.1093/oxfordjournals.molbev.a026092. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann K., Discher S., Voigt K. Revision of the genus Absidia (Mucorales, Zygomycetes) based on physiological, phylogenetic, and morphological characters; thermotolerant Absidia spp. form a coherent group, Mycocladiaceae fam. nov. Mycological Research. 2007;111(10):1169–1183. doi: 10.1016/j.mycres.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi. 2nd. Wayne, Pa, USA: Clinical and Laboratory Standards Institute; 2008. (Approved Standard. CLSI Document M38-A2). [Google Scholar]
- 16.Yin X. Y., Chen J. K., Yu Z. Y., et al. Scedosporium apiospermum in patient with acute leukemia and bacterial multiple infections: experimental diagnosis and treatment. Chinese Journal of Nosocomiology. 2010;20(23):3833–3835. [Google Scholar]
- 17.Wang P., Xu Y. C., Dou H. T., et al. Invasive Scedosporium apiospermum and Scedosporium prolificans infections: case report and review. Chinese Journal of Mycology. 2007;2(4):210–213. [Google Scholar]
- 18.Zhou S.-S., Cheng S.-H., Liu B., Zhang L.-L., Zhao J.-S., Gao X.-L. Invasive Scedosporium apiospermum infection: one case report. Chinese Journal of Infection and Chemotherapy. 2009;9(6):466–468. [Google Scholar]
- 19.Wang Y. L., Sun J. M., Zhou X. Z., et al. Fungal rhinosinusitis casused by Scedosporium apiospermum: a case report and literature review. Chinese Journal of Mycology. 2011;6(5):285–289. [Google Scholar]
- 20.Dong F. A case report of Scedosporium apiospermum infection. Journal of Jining Medical College. 2013;36(1):p. 76. [Google Scholar]
- 21.Zhou X. Y. A case report of Scedosporium apiospermum infection in bronchial mucosa. Journal of Harbin Medical University. 2011;45(4):p. 395. [Google Scholar]
- 22.Zheng Y. C., Zeng J. S., Yue J. X., et al. A case report of Pseudallescheria boydii brain abscess. National Medical Journal of China. 2007;87(14):p. 1007. [Google Scholar]
- 23.Lin H. H., Xu L. H., Dong Y. S., et al. The first case of Pseudallescheria boydii brain abscess in China. Journal of Tongji University (Medical Science) 1990;19(6):p. 399. [Google Scholar]
- 24.Paride A., Takashi Y., Luo D. M., et al. A case report of sinusitis due to Scedosporium prolificans: identification of causative agent and antifungal susceptibility testing in vitro . Chinese Journal of Mycology. 2010;5(1):1–4. [Google Scholar]
- 25.Xu Y., Hao F., Zhong B. Y., et al. A case report of aspiration pneumonitis caused by Scedosporium apiospermum . Chinese Journal of Mycology. 2008;3(5):292–294. [Google Scholar]
- 26.Li J. J., Li H., Hua Z. L. Endophthalmitis caused by Scedosporium apiospemum after eye injury. Chinese Journal of Ocular Trauma and Occupational Eye Disease. 2011;33(3):161–162. [Google Scholar]
- 27.Zhang L., Zhao W., Jin M. X. Pathogens Nasal sinus of fungal infection. Chinese Journal of Nosocomiology. 2008;1(10):1492–1493. [Google Scholar]
- 28.Wang Y. X., Liu M., Liu H. C. Ten cases of nasal sinus of fungal infection. Chinese Journal of Medicine. 1997;32(6):42–44. [Google Scholar]
- 29.Yang X. M., Wang Y. X., Liu M., et al. Analysis of pathogenic agents in 100 cases with mycotogenic sinusitis. Chinese Journal of Otorhinolaryngology. 2000;7(1):9–12. [Google Scholar]
- 30.Wang Y. X., Liu M., Liu H. C., et al. Analysis of pathogenic agents from 34 cases of mycotic infection in masosinuses. Chinese Journal of Laboratory Medicine. 1996;19(5):265–266. [Google Scholar]
- 31.Xi L. Y., Lu C. M., Liu A. M., et al. A case of pseudallescheriasis in brain. Chinese Journal of Laboratory Medicine. 2000;23(1):p. 25. [Google Scholar]
- 32.Lackner M., de Hoog G. S., Verweij P. E., et al. Species-specific antifungal susceptibility patterns of Scedosporium and Pseudallescheria species. Antimicrobial Agents and Chemotherapy. 2012;56(5):2635–2642. doi: 10.1128/aac.05910-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tintelnot K., Just-Nübling G., Horré R., et al. A review of German Scedosporium prolificans cases from 1993 to 2007. Medical Mycology. 2009;47(4):351–358. doi: 10.1080/13693780802627440. [DOI] [PubMed] [Google Scholar]
- 34.Lackner M., Rezusta A., Villuendas M. C., Palacian M. P., Meis J. F., Klaassen C. H. Infection and colonisation due to Scedosporium in Northern Spain, an in vitro antifungal susceptibility and molecular epidemiology study of 60 isolates. Mycoses. 2011;54(supplement 3):12–21. doi: 10.1111/j.1439-0507.2011.02110.x. [DOI] [PubMed] [Google Scholar]
- 35.Zouhair R., Rougeron A., Razafimandimby B., Kobi A., Bouchara J.-P., Giraud S. Distribution of the different species of the Pseudallescheria boydii/Scedosporium apiospermum complex in French patients with cystic fibrosis. Medical Mycology. 2013;51(6):603–613. doi: 10.3109/13693786.2013.770606. [DOI] [PubMed] [Google Scholar]
- 36.Alastruey-Izquierdo A., Cuenca-Estrella M., Monzón A., Rodriguez-Tudela J. L. Prevalence and susceptibility testing of new species of Pseudallescheria and Scedosporium in a collection of clinical mold isolates. Antimicrobial Agents and Chemotherapy. 2007;51(2):748–751. doi: 10.1128/aac.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cuenca-Estrella M., Alastruey-Izquierdo A., Alcazar-Fuoli L., et al. In vitro activities of 35 double combinations of antifungal agents against Scedosporium apiospermum and Scedosporium prolificans . Antimicrobial Agents and Chemotherapy. 2008;52(3):1136–1139. doi: 10.1128/aac.01160-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carrillo A. J., Guarro J. In vitro activities of four novel triazoles against Scedosporium spp . Antimicrobial Agents and Chemotherapy. 2001;45(7):2151–2153. doi: 10.1128/aac.45.7.2151-2153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]