Abstract
From birth to adulthood, the gut microbiota matures from a simple community dominated by a few major bacterial groups into a highly diverse ecosystem that provides both benefits and challenges to the host. Currently there is great interest in identifying environmental and host factors that shape the development of our gut microbiota. Breast milk is a rich source of maternal antibodies, which provide the first source of adaptive immunity in the newborn's intestinal tract. In this addendum, we summarize our recent data demonstrating that maternal antibodies in breast milk promote long-term intestinal homeostasis in suckling mice by regulating the gut microbiota and host gene expression. We also discuss important unanswered questions, future directions for research in this field, and implications for human health and disease.
Keywords: breastfeeding, gut microbiota, gene expression, intestinal epithelial cells, inflammatory bowel disease, polymeric immunoglobulin receptor, secretory IgA
Introduction
As children we are often told, “Listen to your mother!” and (usually much later) we appreciate the wisdom of her advice. It turns out that in addition to regulating our behavior, our mothers play a key role in regulating our gut microbiota and our own responses to that diverse microbial community. During parturition, our previously sterile gastrointestinal tracts receive their first inoculation with microbes from our mothers. Interestingly, the composition of our initial gut microbiota is influenced substantially by the mode of delivery. A recent study of mothers and newborns revealed that the early microbiota of vaginally delivered infants resembled their mother's vaginal microbiota, whereas the microbiota of infants delivered by Caesarian section was more similar to that found on the skin surface.1 Over time, our gut microbiota matures from a simple community dominated by a few major bacterial groups into a highly diverse adult microbiota (for a review, see ref. 2). The development of our gut microbiota is regulated by environmental factors, including diet, and by host factors associated with innate and adaptive immunity. There is a growing body of epidemiological evidence that the composition of the gut microbiota differs significantly between breast-fed and formula-fed infants.3-10 For breastfed infants, maternal antibodies provide the first source of antigen-specific immunity in the intestinal tract (for a review see ref. 11). The predominant type of antibody in breast milk is secretory (S)IgA, which is transported across mammary gland epithelial cells by the polymeric immunoglobulin receptor (pIgR).12 At the apical surface, proteolytic cleavage releases SIgA, in which IgA is covalently attached to secretory component (SC), the extracellular domain of pIgR. The SC moiety of SIgA protects SIgA from degradation by host and microbial proteases and provides additional innate immune functions. The mucosal epithelial chemokine CCL28 is up-regulated in the mammary gland during lactation, and plays a key role in attracting IgA+CCR10+ antibody-secreting cells that were primed in mucosal lymphoid tissues of the intestinal and respiratory tracts.13 Thus, a significant proportion of the SIgA antibodies in breast milk are targeted against microbes in the mother's gastrointestinal tract, which are the same microbes that will seed the gut microbiota of the infant (for a review see ref. 14). However, the specific mechanisms through which breast milk-derived SIgA may regulate the gut microbiota of suckling infants have not systematically been investigated. Furthermore, little is known about the long-term effects of breastfeeding on the composition of the microbiota of older children and adults. Our recently published work15 employed a mouse model to investigate the role of maternal SIgA on short- and long-term regulation of the gut microbiota.
Breast Milk-Derived SIgA vs. Endogenous SIgA
Breast milk contains many bioactive molecules, including SIgA, which could potentially regulate the composition of the gut microbiota and the host response to these microbes.16,17 As the intestinal immune system develops in the offspring, there is a slow transition to endogenous production of SIgA via pIgR-mediated transport of locally synthesized IgA into the gut lumen. In human infants the timing of endogenous SIgA production varies widely, influenced by environmental factors such as microbial load in the intestine.18 Whereas endogenous production of SIgA has been demonstrated in infants in developing countries, it may take several years before the concentrations of intestinal SIgA achieve adult levels in developed countries where exposure to environmental and dietary microbes is lower. To develop a mouse model in which we could separate the effects of breast milk-derived and endogenous SIgA, while holding other factors in breast milk constant, we utilized mice with a targeted deletion in the Pigr gene (Fig. 1). Pigr−/− mice cannot transport SIgA into external secretions, including milk and intestinal fluids.19 By breeding Pigr+/− females with Pigr−/− males, and vice versa, we generated offspring that were or were not exposed to maternal SIgA in breast milk. The genotypes of the offspring of both crosses were equally distributed between Pigr+/− and Pigr−/−, the former of which could produce endogenous SIgA by transport across intestinal epithelial cells, and the latter of which could not. Surprisingly, we found high levels of IgA in the milk from both Pigr+/− and Pigr−/− dams, suggesting that IgA could enter the milk stream by paracellular leakage in the absence of pIgR-mediated transport. While this SC-devoid IgA was found in the stomachs of suckling newborns, it did not survive transport through the protease-rich environment of the intestinal tract. We found that the colonic lumens of newborn offspring of Pigr−/− dams were totally devoid of IgA, whereas the offspring of Pigr+/− mice had abundant IgA in their colonic lumens and in their feces. At weaning, fecal IgA levels dropped to low levels in all mice, reflecting the loss of maternal SIgA. Fecal IgA levels began to increase at about 4 weeks of age in Pigr+/− offspring, regardless of maternal Pigr genotype, reflecting endogenous pIgR-mediated transport of IgA across intestinal epithelial cells. By contrast, fecal IgA was undetectable in Pigr−/− offspring for the rest of their lives. Thus our model system allowed us to study 4 distinct groups of mice that differed only in the presence of maternal and/or endogenous SIgA.
Figure 1.
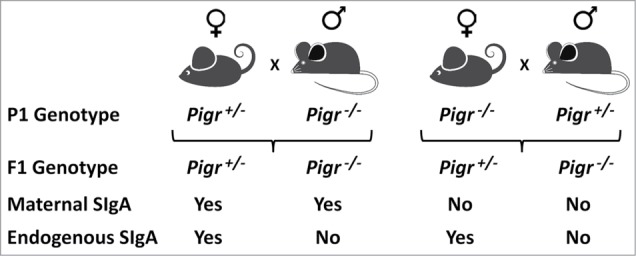
Breeding scheme for generating offspring mice exposed to breast milk-derived maternal SIgA and/or endogenous SIgA derived from intestinal epithelial transport of locally synthesized IgA.
Breast Milk-Derived SIgA Strengthens the Intestinal Barrier
Epidemiological studies have provided support for the concept that SIgA antibodies in breast milk provide protection against a wide range of bacterial, viral and parasitic infections in suckling infants (for a review, see ref. 11). There is also evidence that early exposure to breast milk-derived SIgA may reduce the incidence of allergic diseases later in life. However, the benefits of breast milk-derived SIgA remain controversial, largely due to the lack of standardization of epidemiological studies with regard to duration of breastfeeding, concentrations of SIgA and other anti-inflammatory factors in breast milk, and effects of other variables such as diet and sanitation. While the predominant protective mechanism of SIgA in breast milk likely involves immune exclusion of pathogens and allergens at mucosal surfaces of the suckling infant, other protective mechanisms could include innate enhancement of the intestinal barrier and promotion of a healthy commensal microbiota that limits the growth of potential pathogens. We hypothesized that early exposure to maternal SIgA antibodies in breast milk would foster a mutualistic relationship with the developing microbiota, and could enhance intestinal barrier function. In our mouse model, we found that failure to receive SIgA in breast milk resulted in increased translocation of aerobic bacteria from the neonatal gut into draining mesenteric lymph nodes, regardless of the Pigr genotype of the offspring. A prominent species among the bacteria that breached the epithelial barrier was Ochrobactrum anthropi, which has been identified as an opportunistic pathogen in preterm infants and immunocompromised individuals.20-22 Although significant numbers of bacteria were able to penetrate the intestinal barrier in the absence of breast milk-derived SIgA, there was no evidence of systemic infection. This finding was not surprising, given that the systemic immune systems of the neonatal mice were intact. However, it should be noted that our mice were maintained under specific pathogen-free conditions, housed in barrier cages with sterile bedding, and fed autoclaved food and water. Future experiments in which mice deprived of maternal SIgA are challenged with known pathogens could shed light on the protective effects of SIgA antibodies in breast milk. It will be particularly interesting to compare responses to pathogen challenges in the early post-weaning period vs. later in life.
SIgA Antibodies in Breast Milk Regulate the Commensal Gut Microbiota
There is substantial evidence from studies in humans and mice that intestinal SIgA regulates the composition and activity of the commensal microbiota (for a review, see ref. 11). Less well understood are the effects of breast milk-derived SIgA on the developing gut microbiota, and whether these effects persist into later life. To analyze the short- and long-term effects of maternal SIgA, while controlling for endogenous SIgA, we analyzed the composition of the fecal microbiota in Pigr+/− offspring of Pigr+/− and Pigr−/− dams at weaning (3 weeks of age), and in the same mice when they reached adulthood (10 weeks of age). Not only did early exposure to breast milk-derived SIgA result in a significantly different microbiota at weaning, but these differences were magnified when the mice reached adulthood. Some bacterial taxa varied in abundance due to lack of exposure to maternal SIgA, whereas other taxa were uniquely present or absent in the gut microbiota. Gram-negative Proteobacteria of the family Pasteurellaceae and Gram-positive Firmicutes of the family Lachnospiraceae dominated the taxa that were upregulated in the absence of passive SIgA. By contrast, the majority of unique taxa that were present in mice deprived of maternal SIgA were members of the family Comamonadaceae (phylum Proteobacteria). Interestingly, increased abundance of Pasteurellaceae and Lachnospiraceae has been observed in the gut microbiota of pediatric patients with inflammatory bowel disease (IBD),23 and increased abundance of Comamonadaceae has been observed in the gut microbiota of adult IBD patients with chronic pouchitis.24 Although caution must be exercised when comparing results from controlled animal experiments to human diseases, our findings are consistent with the concept that early exposure to SIgA in breast milk promotes the development of a healthy microbiota that may provide long-term protection against inflammatory diseases.
Early Exposure to SIgA in Breast Milk Affects Intestinal Epithelial Gene Expression Later in Life
Microbial-host mutualism in the gut is promoted by extensive cross-talk between the gut microbiota and epithelial cells at the luminal interface, largely mediated by host receptors that recognize microbe associated-molecular patterns (for a review see ref. 25). Having observed that early exposure to breast milk-derived SIgA caused long-term alterations in the composition of the gut microbiota, we hypothesized that the epithelial cell response to this distinctive microbiota would be altered as well. To test this hypothesis, we analyzed global gene expression in epithelial cells isolated from the colons of adult mice in the 4 groups comprising our model system. We observed a unique (and presumably optimal) pattern of gene expression in mice that had received both maternal and endogenous SIgA, with specific changes resulting from the loss of either source of SIgA. When we challenged these mice in vivo with the epithelial-disrupting agent dextran sulfate sodium, we observed that altered patterns of epithelial gene expression were correlated with increased susceptibility to colonic inflammation. It can be postulated that signals from the epithelium (including but not limited to SIgA) regulate the composition and activity of the microbiota in such a manner as to discourage mutually destructive inflammatory responses. In this regard it was significant that some of the genes whose expression was regulated by SIgA were murine orthologs of genes that have been associated with IBD and other inflammatory diseases in humans, including FUT2, IRF1, PLA2G2A, SLC26A3, VDR and ZMIZ1. In summary, our recent findings suggest that host-microbial mutualism is promoted by a lifelong relationship between SIgA and gut microbes, beginning at birth with maternal SIgA antibodies provided in breast milk, and continuing throughout life with endogenous SIgA antibodies provided by pIgR-mediated transport across intestinal epithelial cells. A key aspect of this relationship, which deserves further investigation, is the specificity and affinity of SIgA antibodies directed against gut microbes.
Unanswered Questions, Future Directions for Research, and Therapeutic Opportunities
While we believe that our recent findings provide compelling support for the benefits of breast milk-derived SIgA in promoting lifelong intestinal homeostasis, there remain many unanswered questions and exciting directions for future research. In order for this field to progress, it will be essential to elucidate the mechanisms through which SIgA regulates the gut microbiota and in turn, gene expression by host cells. Fig. 2 illustrates theoretical scenarios for the development of neonatal intestinal immunity in the presence and absence of breast milk-derived maternal SIgA. Our findings suggest that maternal SIgA regulates the composition of the gut microbiota in suckling infants, and that some microbes can penetrate the epithelial barrier in the absence of maternal SIgA. However, it is not known whether SIgA associates preferentially with selected members of the gut microbiota. If so, does SIgA promote or inhibit the proliferation of bacteria with which it is associated? We recently reported that SIgA is concentrated with gut microbes in the loose outer layer of colonic mucus in mice and humans, whereas the dense inner layer of colonic mucus, rich in antimicrobial peptides, is relatively devoid of both SIgA and bacteria.26 One could envision mechanisms through which coating of the bacterial surface with SIgA could facilitate (or inhibit) the association of bacteria with colonic mucus, thus promoting (or blocking) biofilm formation. Methods for fluorescence-activated cell sorting of IgA-bound fecal bacteria have been developed,27 and this approach could be coupled with deep sequence analysis of the SIgA-bound and -unbound bacterial cohorts. Similar approaches could be used to investigate how bound SIgA affects gene expression and metabolic activity of gut microbes. Understanding the transition from maternal to endogenous SIgA will require longitudinal studies in experimental animals and humans. A key question to be resolved is, how does early exposure to breast milk-derived SIgA exert long-term effects on host-microbial mutualism? There are a number of potential answers, which are not mutually exclusive and can be addressed with current technologies. Key among these involves the role of SIgA in the transition from the simple gut microbiota of newborn mammals to the complex microbiota of adults. An interesting, and testable, hypothesis is that early exposure to breast milk-derived SIgA shapes the subsequent endogenous IgA response to gut microbes. SIgA has been shown to play a role the natural sensing of commensal bacteria by mouse Peyer's patch dendritic cells,28 likely involving both antigen-specific and glycan-mediated recognition of microbes by SIgA. Thus maternal SIgA could “prime” the neonatal immune system for recognizing specific types of gut microbes, which could lead to the generation of microbe-specific memory IgA+ B cells as a lifelong source of endogenous SIgA. We know that, once established, the gut microbiota is resilient in the face of a wide range of environmental challenges, such as infections and antibiotics.2 What is the role of SIgA in this resilience? Perhaps SIgA-bound microbes are more resistant to perturbation and/or SIgA promotes the recovery of selected microbial communities. What is the role of host genetics in SIgA-epithelial-microbial mutualism? This question could readily be addressed by crossing pIgR-sufficient and -deficient mice with mice bearing other genetic mutations known to impact intestinal homeostasis. Given the technology to generate cell type-specific genetic mutations in mice, there are limitless opportunities to explore the host-microbial interface. For example, we recently reported that mice with an intestinal epithelial-specific deletion of the Myd88 gene, encoding a cytoplasmic receptor that interacts with microbial pattern recognition receptors, had reduced expression of pIgR, impaired transport of SIgA, an altered composition of the gut microbiota, and increased susceptibility to chemically-induced intestinal inflammation.29 Recent advances in genome-wide studies of polymorphisms in humans associated with increased risk for IBD and other inflammatory diseases should provide many opportunities for studying the impact of human genetics on SIgA expression and function.
Figure 2.
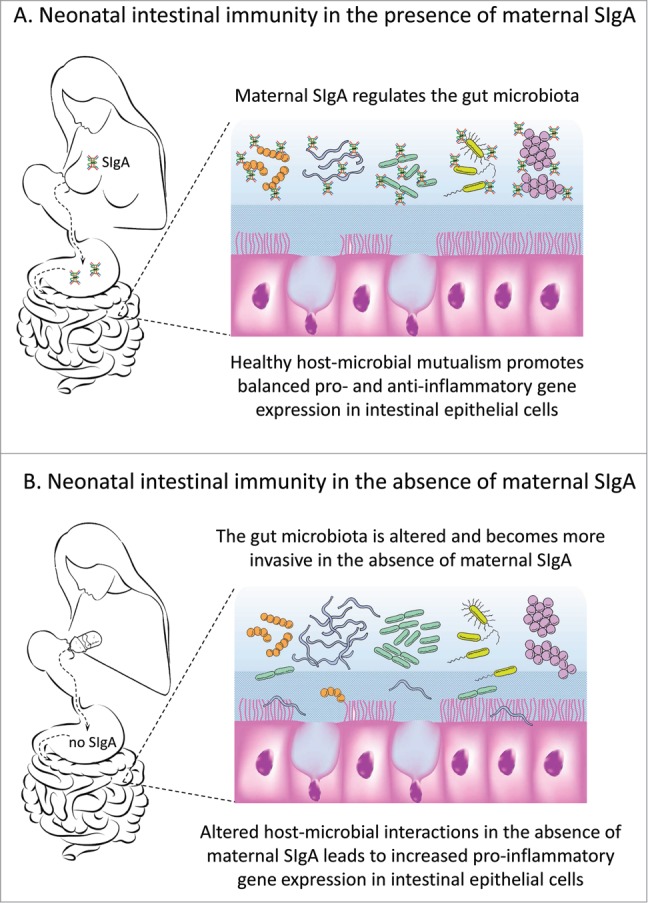
Hypothetical model of the role of breast milk-derived secretory (S)IgA in the development of intestinal immunity. (A) In the presence of maternal SIgA, gut microbes are concentrated in the loose outer layer of colonic mucus (shown here in light blue), while the dense inner layer of mucus (shown here in darker blue) is relatively devoid of both SIgA and microbes. Microbial products stimulate intestinal epithelial cells and promote a healthy balance between pro- and anti-inflammatory gene expression. (B) In the absence of maternal SIgA, the composition of the gut microbiota is altered, and some microbes penetrate the mucus and epithelial barriers to invade draining mesenteric lymph nodes. Intestinal dysbiosis can persist into adult life and result in imbalanced pro-inflammatory gene expression in intestinal epithelial cells.
In conjunction with mechanistic studies, there are many avenues through which the potential benefits of SIgA in infectious, allergic and inflammatory diseases could be explored. The protective role of SIgA as a constituent of breast milk in humans needs to be better defined, beginning with epidemiological studies that link the concentration and activity of SIgA in breast milk to its beneficial effects. An example of this approach is the PASTURE project (Protection against Allergy: STUdy in Rural Environments), a large prospective birth cohort study conducted in Austria, Finland, France, Germany and Switzerland.30 In this study, levels of SIgA were analyzed in 610 breast milk samples collected 2 months after delivery. Multivariate logistic regression analysis revealed a significant inverse association between the total amount of SIgA that was ingested via breast milk during the first year of life and the development of atopic dermatitis.31 While this type of epidemiological study represents a step in the right direction for assessing the potential benefits of breast milk-derived SIgA, many questions remain to be answered. It will be important to assess the relationship between early exposure to SIgA and subsequent allergic (and inflammatory) diseases in other geographic settings (including urban areas) and in other demographic groups. Approaches could be developed to improve the quantity and quality of SIgA in breast milk, for example, nutritional supplementation and vaccination of pregnant women. These studies should be coupled with analyses of the gut microbiota at birth and throughout life to illuminate the role of host-microbial interactions in immune-mediated diseases. Advances in epidemiological studies should pave the way for therapeutic applications of human SIgA. An obvious starting point would be to analyze the concentration and activity of SIgA in donated human milk used to supplement the diets of newborn infants faced with challenges such as prematurity and infectious diseases. For example, the methods used for pasteurization of donor milk can dramatically affect its content of SIgA and other bioactive molecules (for a review, see ref. 32). SIgA purified from human colostrum and/or mature milk could represent a safe, effective and standardized therapy for infectious, allergic and inflammatory diseases in infants and possibly in children and adults. Development of SIgA-based therapeutic approaches should be informed by findings from mechanistic studies regarding the optimal timing and dosage of SIgA for specific diseases in targeted populations.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by an NIH grant AI069027 (and an associated American Recovery and Reinvestment Act supplement), a Senior Research Award from the Crohn's and Colitis Foundation of America (CCFA), and a grant from the Kentucky Bioinformatics Research Infrastructure Network to CSK; a Senior Research Award from the CCFA to DAC; and NIH grants NCATS UL1TR000117, NCRR 5P20RR016481-12 and NIGMS 8 P20 GM103436-12 to AJS.
References
- 1. Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A 2010; 107:11971-5; PMID:20566857; http://dx.doi.org/ 10.1073/pnas.1002601107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature 2012; 489:220-30; PMID:22972295; http://dx.doi.org/ 10.1038/nature11550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roger LC, Costabile A, Holland DT, Hoyles L, McCartney AL. Examination of faecal Bifidobacterium populations in breast- and formula-fed infants during the first 18 months of life. Microbiology 2010; 156:3329-41; PMID:20864478; http://dx.doi.org/ 10.1099/mic.0.043224-0 [DOI] [PubMed] [Google Scholar]
- 4. Fallani M, Amarri S, Uusijarvi A, Adam R, Khanna S, Aguilera M, Gil A, Vieites JM, Norin E, Young D, et al. Determinants of the human infant intestinal microbiota after the introduction of first complementary foods in infant samples from five European centres. Microbiology 2011; 157:1385-92; PMID:21330436; http://dx.doi.org/ 10.1099/mic.0.042143-0 [DOI] [PubMed] [Google Scholar]
- 5. Tsuji H, Oozeer R, Matsuda K, Matsuki T, Ohta T, Nomoto K, Tanaka R, Kawashima M, Kawashima K, Nagata S, et al. Molecular monitoring of the development of intestinal microbiota in Japanese infants. Benef Microbes 2012; 3:113-25; PMID:22683836; http://dx.doi.org/ 10.3920/BM2011.0038 [DOI] [PubMed] [Google Scholar]
- 6. Turroni F, Peano C, Pass DA, Foroni E, Severgnini M, Claesson MJ, Kerr C, Hourihane J, Murray D, Fuligni F, et al. Diversity of bifidobacteria within the infant gut microbiota. PLoS ONE 2012; 7:e36957; PMID:22606315; http://dx.doi.org/ 10.1371/journal.pone.0036957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Azad MB, Konya T, Maughan H, Guttman DS, Field CJ, Chari RS, Sears MR, Becker AB, Scott JA, Kozyrskyj AL. Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. Can Med Assoc J 2013; 185:385-94; PMID:23401405; http://dx.doi.org/ 10.1503/cmaj.121189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fan W, Huo G, Li X, Yang L, Duan C, Wang T, Chen J. Diversity of the intestinal microbiota in different patterns of feeding infants by Illumina high-throughput sequencing. World J Microbiol Biotechnol 2013; 29:2365-72; PMID:23793940; http://dx.doi.org/ 10.1007/s11274-013-1404-3 [DOI] [PubMed] [Google Scholar]
- 9. Gomez-Llorente C, Plaza-Diaz J, Aguilera M, Munoz-Quezada S, Bermudez-Brito M, Peso-Echarri P, Martinez-Silla R, Vasallo-Morillas MI, Campana-Martin L, Vives-Pinera I, et al. Three main factors define changes in fecal microbiota associated with feeding modality in infants. J Pediatr Gastroenterol Nutr 2013; 57:461-6; PMID:23752082; http://dx.doi.org/ 10.1097/MPG.0b013e31829d519a [DOI] [PubMed] [Google Scholar]
- 10. Bergstrom A, Skov TH, Bahl MI, Roager HM, Christensen LB, Ejlerskov KT, Molgaard C, Michaelsen KF, Licht TR. Establishment of intestinal microbiota during early life: a longitudinal, explorative study of a large cohort of Danish infants. Appl Environ Microbiol 2014; 80:2889-900; PMID:24584251; http://dx.doi.org/ 10.1128/AEM.00342-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kaetzel CS. Cooperativity among secretory IgA, the polymeric immunoglobulin receptor, and the gut microbiota promotes host-microbial mutualism. Immunol Lett 2014;10; S0165-2478:00100-X; PMID:24877874; http://dx.doi.org/ 10.1016/j.imlet.2014.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaetzel CS. The polymeric immunoglobulin receptor. eLS 2013; http://dx.doi.org/ 10.1002/9780470015902.a0024237 [DOI] [Google Scholar]
- 13. Wilson E, Butcher EC. CCL28 controls immunoglobulin (Ig)A plasma cell accumulation in the lactating mammary gland and IgA antibody transfer to the neonate. J Exp Med 2004; 200:805-9; PMID:15381732; http://dx.doi.org/ 10.1084/jem.20041069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brandtzaeg P. The mucosal immune system and its integration with the mammary glands. J Pediatr 2010; 156:S8-15; PMID:20105666; http://dx.doi.org/ 10.1016/j.jpeds.2009.11.014 [DOI] [PubMed] [Google Scholar]
- 15. Rogier EW, Frantz AL, Bruno MEC, Wedlund L, Cohen DA, Stromberg AJ, Kaetzel CS. Secretory antibodies in breast milk promote long-term intestinal homeostasis by regulating the gut microbiota and host gene expression. Proc Natl Acad Sci USA 2014; 111:3074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lonnerdal B. Bioactive proteins in human milk: mechanisms of action. J Pediatr 2010; 156:S26-30; PMID:20105661; http://dx.doi.org/ 10.1016/j.jpeds.2009.11.017 [DOI] [PubMed] [Google Scholar]
- 17. Hettinga K, van VH, de VS, Boeren S, van HT, van AJ, Vervoort J. The host defense proteome of human and bovine milk. PLoS ONE 2011; 6:e19433; PMID:21556375; http://dx.doi.org/ 10.1371/journal.pone.0019433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Renz H, Brandtzaeg P, Hornef M. The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nat Rev Immunol 2012; 12:9-23; PMID:22158411; http://dx.doi.org/ 10.1038/nri3112 [DOI] [PubMed] [Google Scholar]
- 19. Johansen FE, Pekna M, Norderhaug IN, Haneberg B, Hietala MA, Krajci P, Betsholtz C, Brandtzaeg P. Absence of epithelial immunoglobulin A transport, with increased mucosal leakiness, in polymeric immunoglobulin receptorsecretory component-deficient mice. J Exp Med 1999; 190:915-22; PMID:10510081; http://dx.doi.org/ 10.1084/jem.190.7.915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stiakaki E, Galanakis E, Samonis G, Christidou A, Maraka S, Tselentis Y, Kalmanti M. Ochrobactrum anthropi bacteremia in pediatric oncology patients. Pediatr Infect Dis J 2002; 21:72-4; PMID:11791106; http://dx.doi.org/ 10.1097/00006454-200201000-00018 [DOI] [PubMed] [Google Scholar]
- 21. Duran R, Vatansever U, Acunas B, Basaran UN. Ochrobactrum anthropi bacteremia in a preterm infant with meconium peritonitis. Int J Infect Dis 2009; 13:e61-3; PMID:18842433; http://dx.doi.org/ 10.1016/j.ijid.2008.06.027 [DOI] [PubMed] [Google Scholar]
- 22. Naik C, Kulkarni H, Darabi A, Bhanot N. Ochrobactrum anthropi: a rare cause of pneumonia. J Infect Chemother 2013; 19:162-5; PMID:22669505; http://dx.doi.org/ 10.1007/s10156-012-0436-1 [DOI] [PubMed] [Google Scholar]
- 23. Saulnier DM, Riehle K, Mistretta TA, Diaz MA, Mandal D, Raza S, Weidler EM, Qin X, Coarfa C, Milosavljevic A, et al. Gastrointestinal microbiome signatures of pediatric patients with irritable bowel syndrome. Gastroenterology 2011; 141:1782-91; PMID:21741921; http://dx.doi.org/ 10.1053/j.gastro.2011.06.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tannock GW, Lawley B, Munro K, Lay C, Taylor C, Daynes C, Baladjay L, Mcleod R, Thompson-Fawcett M. Comprehensive analysis of the bacterial content of stool from patients with chronic pouchitis, normal pouches, or familial adenomatous polyposis pouches. Inflamm Bowel Dis 2012; 18:925-34; PMID:22114001; http://dx.doi.org/ 10.1002/ibd.21936 [DOI] [PubMed] [Google Scholar]
- 25. Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol 2010; 10:131-44; PMID:20098461; http://dx.doi.org/ 10.1038/nri2707 [DOI] [PubMed] [Google Scholar]
- 26. Rogier EW, Frantz AL, Bruno MEC, Kaetzel CS. Secretory IgA is concentrated in the outer layer of intestinal mucus along with gut bacteria. Pathogens 2014; 3:390-403; PMID:24710095; http://dx.doi.org/ 10.3390/pathogens3020390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van der Waaij LA, Limburg PC, Mesander G, van der WD. In vivo IgA coating of anaerobic bacteria in human faeces. Gut 1996; 38:348-54; PMID:8675085; http://dx.doi.org/ 10.1136/gut.38.3.348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rol N, Favre L, Benyacoub J, Corthesy B. The role of secretory immunoglobulin A in the natural sensing of commensal bacteria by mouse Peyer's patch dendritic cells. J Biol Chem 2012; 287:40074-82; PMID:23027876; http://dx.doi.org/ 10.1074/jbc.M112.405001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Frantz AL, Rogier EW, Weber CR, Shen L, Cohen DA, Fenton LA, Bruno ME, Kaetzel CS. Targeted deletion of MyD88 in intestinal epithelial cells results in compromised antibacterial immunity associated with downregulation of polymeric immunoglobulin receptor, mucin-2, and antibacterial peptides. Mucosal Immunol 2012; 5:501-12; PMID:22491177; http://dx.doi.org/ 10.1038/mi.2012.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. von Mutius E., Schmid S. The PASTURE project: EU support for the improvement of knowledge about risk factors and preventive factors for atopy in Europe. Allergy 2006; 61:407-13; PMID:16512801; http://dx.doi.org/ 10.1111/j.1398-9995.2006.01009.x [DOI] [PubMed] [Google Scholar]
- 31. Orivuori L, Loss G, Roduit C, Dalphin JC, Depner M, Genuneit J, Lauener R, Pekkanen J, Pfefferle P, Riedler J, et al. Soluble immunoglobulin A in breast milk is inversely associated with atopic dermatitis at early age: the PASTURE cohort study. Clin Exp Allergy 2014; 44:102-12; PMID:24102779; http://dx.doi.org/ 10.1111/cea.12199 [DOI] [PubMed] [Google Scholar]
- 32. Colaizy TT. Donor human milk for preterm infants: what it is, what it can do, and what still needs to be learned. Clin Perinatol 2014; 41:437-50; PMID:24873842; http://dx.doi.org/ 10.1016/j.clp.2014.02.003 [DOI] [PubMed] [Google Scholar]


