Introduction
Percutaneous pulmonary valve implantation (PPVI) is one of the most exciting recent developments in the treatment of structural heart disease and has evolved as an attractive alternative to surgery in patients with dysfunctional right ventricle-pulmonary artery conduits. Although surgical pulmonary valve replacement is associated with low morbidity and mortality rates, there are many instances when operative risks are high or surgery is prohibitive.1 Patients requiring RVOT reconstruction require multiple sternotomies and cardiac surgical procedures throughout their lives due to the limited lifespan of such conduits (∼10 years).2 Patient management strategies have, therefore, been based on delaying surgical intervention for as long as possible, in order to minimize the number of surgical procedures. However, this approach carries the risk of delaying surgery beyond a point of irreversible right ventricular (RV) dysfunction. A balance between the deleterious effects of RVOT dysfunction and the need to minimize the total number of lifetime surgeries for a given patient is a clinically challenging task prompting the need to develop minimally invasive valve therapies. Since the introduction of PPVI in 2000,3 the learning curve with this technology lead to significant improvements in both valve design and procedural approach, with several clinical trials demonstrating the safety and efficacy in restoring valvular competence with excellent short to medium term outcomes;4–6 transforming this technique from its early pioneering nature into routine clinical care at specialized centers. Evolving data from these studies have shown beneficial effects of PPVI in right ventricular volume reduction,7 left ventricular filling properties,8 exercise capacity9 and electrical remodeling.10
This review discusses the evolution, indications, and technical aspects of PPVI using the Melody and Edwards SAPIEN transcatheter heart valve as non-surgical treatment options for dysfunctional RVOT/pulmonary trunk.
Procedural evolution
The first-in-man PPVI was performed by Dr. Bonhoeffer in 2000 on a 12-year-old boy with significant RV-PA conduit dysfunction.3 The bovine jugular valve was placed via the transfemoral approach and successfully implanted into the degenerated valve of the conduit. The pre-existing pulmonary insufficiency was almost eliminated with subsequent reduction in right ventricular dimensions. Two years later, Bonhoeffer et al published further clinical experience in eight patients11 who underwent successful implantation of the valve in the pulmonic position.
Bonhoeffer's valve design was eventually acquired by Medtronic and renamed it Melody Valve (Medtronic Inc., Minneapolis, MN, USA) (Figure 1). The valve's implantation was evaluated in further clinical reports demonstrating high procedural success and effectiveness in improving symptoms and eliminating pulmonary insufficiency.4–6 Nevertheless, limitations of both patients and available valve sizes led to the subsequent use of the Edwards SAPIEN (Edwards Lifesciences INC., Irvine, CA) transcatheter heart valve in the pulmonary position. The SAPIEN valve is available in larger diameters that allow implantation in larger right ventricular outflow tracts compared to the Melody valve. The Edwards SAPIEN THV was initially introduced as a transcatheter alternative to surgical valve replacement in elderly patients with severe aortic stenosis.12 In 2005, Garay and colleagues reported the first successful implantation of the SAPIEN valve in the pulmonic position of a 16-year-old patient with dysfunctional 24 mm homograft.13 This was followed by the launch of the COMPASSION trial, a US multicenter study, to assess the safety and efficacy of the SAPIEN valve for the treatment of dysfunctional conduits demonstrating high procedural success and symptomatic improvement.14
Figure 1.
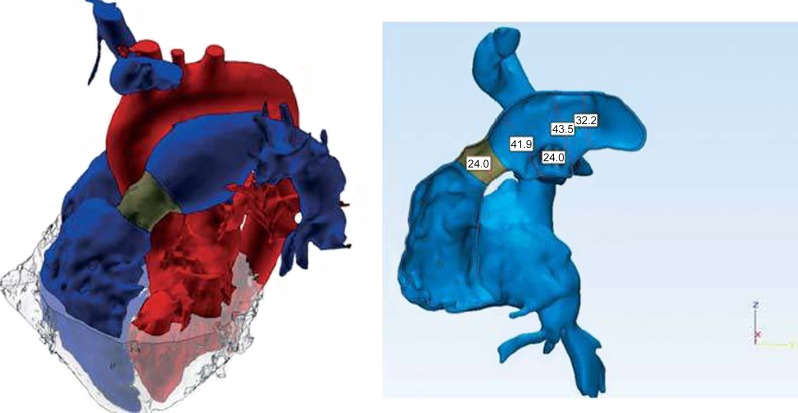
3D anatomical model reconstructed from gadolinium-enhanced MR images (red: systemic circulation, blue: pulmonary circulation with the homograft shown in dark gray, light gray: myocardium).15
PPVI application has been extended further to patients with native right ventricular outflow tracts; however, size restrictions of the currently available valves prevents deployment in the majority of patients in this setting. Newer devices are being developed to accommodate a larger pool of patients that would benefit from this technology.15
Indications and patient selection
To establish clinical indication criteria, all patients undergo a standardized assessment protocol to ensure appropriate patient selection. Echocardiography is usually the initial screening tool to determine the RVOT gradient, RV pressure, RV to LV pressure ratio and to semi-quantitatively assess the severity of pulmonary regurgitation. Recent advances in cardiac MRI imaging allow detailed quantitative assessment of the structure of the right ventricle as well as the flow dynamics in the setting of pulmonary homograft dysfunction (Figures 1 and 2).16 This quantitative assessment aids in the clinical determination of the need and timing of re-intervention in asymptomatic patients.
Figure 2.
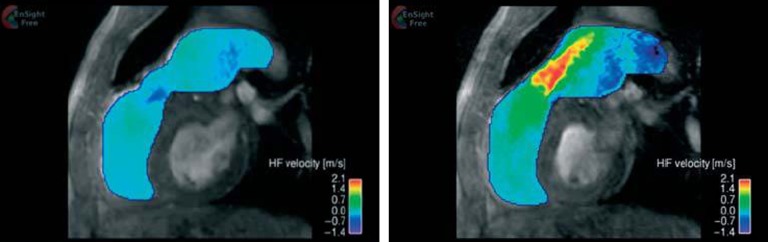
In-plane velocity map based on phase-contrast MR images illustrating the systolic and diastolic jets through the homograft (left: diastole, right: systole).15
Present indications for PPVI have been adapted from those accepted for surgical PVR. Currently, surgical intervention is generally indicated with the following features:
-
•
Presence of symptoms
-
•
Indexed RVEDV >150ml/m2 ± regurgitant fraction > 40%
-
•
RVOT peak instantaneous gradient > 50 mmHg
-
•
RV dysfunction (RVEF < 40%)
-
•
Moderate-severe accompanying tricuspid regurgitation
The scientific statement from AHA on indications for intervention in pediatric cardiac diseases advocated PPVI in a patient with an RV-to-pulmonary artery conduit with associated moderate-to-severe pulmonary regurgitation or stenosis provided the patient meets inclusion/exclusion criteria for the available valve (Class IIa) (Table 1).17
Table 1.
Inclusion criteria for clinical trials with both the Melody® and SAPIEN™ valves.
| Melody® 16 | SAPIEN™14 |
| Age ≥ 5 years/weight ≥ 30 kg | Weight > 35 kg |
| Original Conduit diameter ≥ 16 mm | In situ conduit ≥ 16 mm and ≤ 24 mm |
| Echocardiographic RVOT conduit dysfunction | ≥ +3 PR (TTE) or PRF > 40% (MRI) |
| • Patints classified as NYHA class II, III, or IV: Doppler mean gradient ≥ 35 mmHg or ≥ moderate PR | With or without stenosis |
| • Patients classified as NYHA class I: Doppler mean gradient ≥ 40 mmHg or severe PR associated with TV annulus z-score ≥ 2 or RVEF ≤ 40% |
Abbreviations: PR, pulmonary regurgitation; PRF, pulmonary regurgitation fraction; RVEF, right ventricle ejection fraction; TTE, transthoracic echocardiography; TV, tricuspid valve.
Two other important indications for intervention include the risk for longer-term arrhythmia and progressive left ventricular dysfunction. QRS duration ≥ 180 ms have been associated with 42-fold increased risk of sustained ventricular tachycardia and a 2.2-fold increased risk of sudden cardiac death during a 10-year follow-up study.18 PPVI has been found to significantly reduce QRS duration in those with predominant regurgitation and therefore may affect arrhythmia burden in this patient group.10 Left ventricular dysfunction has been seen in approximately 20% of adult patients with repaired tetralogy of Fallot.19 The exact mechanisms underlying LV dysfunction are not clear, but ventricular diastolic interaction, along with prolonged abnormal electrical remodeling may be involved. The impact of PPVI on LV dynamics has shown small but significant increases in left ventricular ejection fraction.20,21
In addition to clinical indications, several anatomical criteria need to be fulfilled to qualify for PPVI. The ideal anatomy for PPVI is a uniform diameter from RVOT to pulmonary artery with adequate main pulmonary artery length to avoid stenting into the pulmonary artery bifurcation. With the current iterations of the Melody valve, the RVOT, pulmonary valve annulus, and proximal main pulmonary artery must be 22 mm or less to prevent leaflet malcoaptation. Using the 22 mm Ensemble delivery system, the outer diameter of the Melody valve is approximately 24 mm, and therefore any inner diameter of a conduit larger than this would be insufficient to securely anchor the valve. Nevertheless, there is limited experience with mounting the Melody valve on a 24 mm BiB balloon delivered through a 24 French sheath.22 The SAPIEN valve can be deployed in RVOT sizes up to 29mm in diameter.
Furthermore, assessing the course of the proximal coronary arteries in relation to the RVOT is crucial prior to PPVI. The coronary arteries can run in close proximity to the RVOT carrying the risk of coronary artery obstruction due to expansion of the RVOT.23
Current technologies
Melody transcatheter pulmonary valve
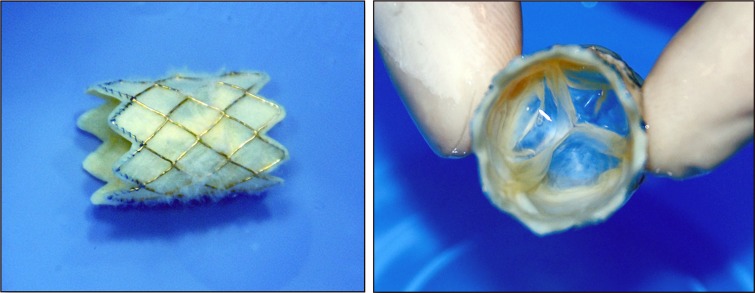
The Melody valve (Medtronic Inc, Minneapolis, MN) has been available in Canada and Europe since 2006 and was approved by the United States FDA in 2010 with a HDE (humanitarian device exemption). However, in 2015, it was fully approved as a PMA (pre-market approval) device. It consists of a harvested valve from a bovine jugular vein that is sutured into a platinum-iridium stent frame that is 28 mm long and 18 mm in diameter (3). The stent is crimped onto the Ensemble delivery balloon system and can be expanded up to 22 mm diameter. The system is a 22 French catheter comprising a balloon-in-balloon deployment design and is available with three outer balloon diameters: 18, 20, and 22 mm. 16mm diameter is also now available. The tip of the system is blue, to correspond with the outflow suture of the stent. There is a retractable sheath that covers the stented valve during delivery and is pulled back just prior to deployment. Contrast can be injected via the retracted sheath from a side port to confirm positioning prior to valve deployment. Proximally there are three ports; one for the guidewire (green), one for inner balloon inflation (indigo) and one for outer balloon inflation (orange).
Figure 3.
The Medtronic Melody pulmonic valve; long axis (left) and short axis views (right).
Edwards SAPIEN transcatheter heart valve
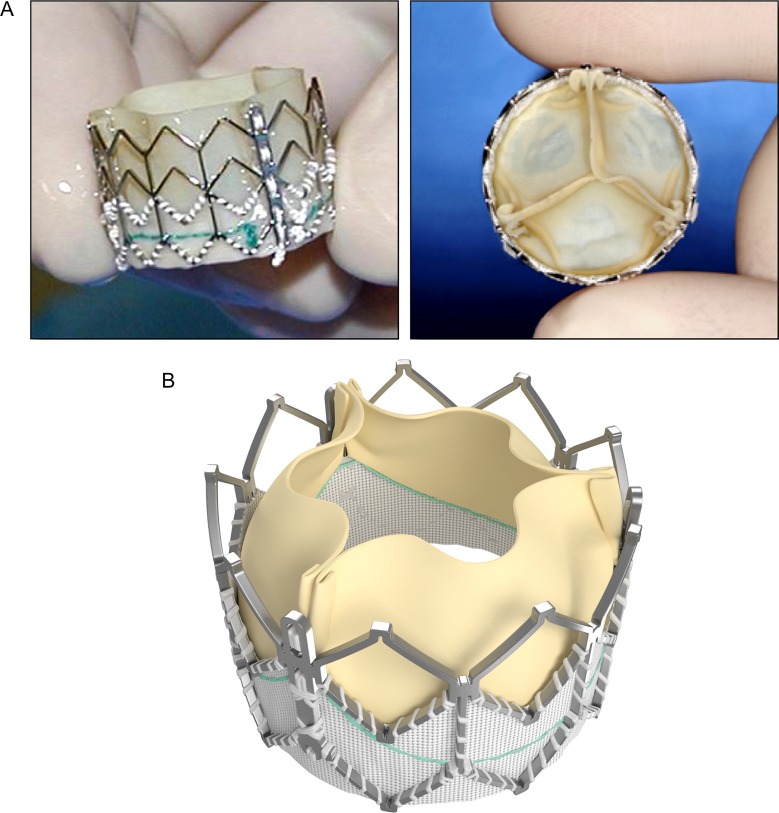
The SAPIEN valve is a trileaflet bioprosthesis made of bovine pericardium that is mounted on a balloon-expandable stainless steel stent (Figure 4A). The stent has a fabric cuff placed in the ventricular side that covers one half of the frame, limiting stent expansion and decreasing perivalvular insufficiency. The valve is available in 23 and 26 mm sizes allowing for implantation in larger RVOTs than the Melody valve. The new generation device, SAPIEN XT valve is made of cobalt-chromium alloy that provides the same radial strength yet reduces the valve profile. This valve is available in 20, 23, 26, and 29 mm sizes.
Figure 4.
A) Edwards SAPIEN valve; long axis (left) and short axis views (right), B) SAPIEN XT valve.
Each size of valve is supplied with a matched delivery system, which incorporates a 30-mm long, appropriately sized, noncompliant high-pressure balloon. The valve is tightly crimped onto the balloon shaft utilizing a specialized manual crimping tool. The original RetroFlex transfemoral delivery systems (generations 1, 2, and 3; Edwards Lifesciences Inc.) incorporated a stiff deflectable catheter that covered the deployment balloon shaft and facilitated steering the balloon-mounted valve around the aortic arch and through the stenotic aortic valve.12 The widely utilized RetroFlex 3 system requires a 22 French sheath for the 23 mm valve and a 24 French sheath for the 26 mm SAPIEN valve.
The current generation NovaFlex+ catheter (Edwards Lifesciences Inc.) is utilized with the newer SAPIEN XT valve (Figure 4B). The low profile valve is crimped onto the balloon catheter shaft. After introduction through the arterial sheath the valve is moved onto, and aligned with, the deployment balloon.24 The NovaFlex system requires a 16 French e-sheath for the 23 mm valve, 18 French e-sheath for the 26 mm valve and a 20 French e-sheath for the 29 mm SAPIEN XT valve.
Table 2 outlines the main differences between the SAPIEN and Melody valve.
Table 2.
Comparison of Melody® and the SAPIEN™ valves.
| Characteristics | Melody® valve | SAPIEN™ valve |
| Stent material | Iridium 10%, Platinum 90% | Stainless steel |
| Valve material | Bovine jugular vein | Bovine pericardium treated with Thermafix |
| Available diameter (mm) | 18–22 | 20, 23, 26, 29 (23, 26 in US*) |
| Stent height (mm) | 34 | 14.5 or 16 |
| Delivery sheath size (Fr) | 22 | 22 or 24 (16, 18 or 20 for XT**) |
| Prestenting | Recommended | Required |
| Stent fracture | 21% | None reported |
The procedure and technical considerations
Transcatheter pulmonary valve replacement is usually performed under general anaesthesia with biplane fluoroscopic guidance. Femoral approach is usually chosen due to the setup of the catheterization lab; alternatively, jugular access can be preferentially selected to provide a more favorable anatomic curvature for device delivery. Pretreatment with intravenous antibiotics and loading with aspirin before valve implantation are recommended. Intra-procedural heparin is required to maintain activated clotting time (ACT) longer than 200s. Right heart catheterization is initially performed to assess pressures and saturations with special attention to any relevant pulmonary branch stenosis. The balloon-tipped catheter is then replaced with a stiff guidewire (0.035 inch Amplatzer extra stiff (AGA Medical, Plymouth, MN) or Lunderquist ultra stiff (Cook, Bloomington, IN)) and positioned into a distal branch pulmonary artery, preferably the left lower branch given its vertical orientation, to provide adequate support to advance the delivery system. Great care is needed for maintaining wire stability during device advancement to avoid the risk of pulmonary artery injury. Biplane RVOT angiography is performed to assess the proposed site for device implantation and quantification of pulmonary regurgitation. Implantation site dimension is then measured using a compliant sizing balloon (St Jude Medical, Plymouth, MN). Simultaneous coronary angiography should be performed during full balloon inflation to assess the course and proximity of the coronary arteries to the RVOT due to the risk of compression with RVOT expansion.23 Typically, lateral and LAO/caudal projections profile the left coronary artery very well in relation to the balloon position. Potential coronary artery compression is not uncommon with 4.4% of the US cohort demonstrating unsuitable anatomy,4,23 and deaths have been reported as a consequence of coronary compression in this setting. Either coronary artery may be at risk, although the left main or proximal left anterior descending arteries are the most often affected. Although non-invasive testing may demonstrate significant distance between RVOT and coronary arteries, all patients should have simultaneous coronary angiogram with balloon inflation of the outflow tract. If coronary compression is documented, the procedure has to be aborted.25
Pre-stenting of the conduit with a bare metal stent has several advantages. It is required in almost all cases (for Edwards valve) to provide a landing zone for the stented valve, to reduce the risk of stent fracture (for Melody valve), to maintain a circular configuration of the valve in the long term, and in case of the SAPIEN valve to allow greater margin of safety when positioning the relatively short valve prosthesis (14–19 mm) which would not cover the whole length of conduit or pulmonary trunk obstruction. The stent is typically deployed on a BiB (balloon-in-balloon) catheter (NuMED Inc., Hokinton, New York) to a diameter of up to 2 mm less than the original conduit size in stenotic conduits or slightly larger in conduits without stenosis. We typically use the balloon-expandable IntraStent (ev3, Plymouth, MN) or the Palmaz XL stents. Covered stents, when available, can be alternatively used if there are concerns of conduit rupture. Post-dilatation of the stent using a non-compliant balloon may be needed to achieve the intended diameter. Pressure measurements after stenting should document no to minimal residual gradient across the outflow tract prior to proceeding with device implantation. It is crucial to recognize the potential deleterious effects of residual stenosis on exercise capacity and valve degeneration with higher RVOT gradients after PPVI; thus, attempts should be made to abolish any residual gradient before proceeding with valve implantation.5
The appropriate device size is then selected based on the diameter of the balloon at full inflation during pre-stenting. For the Melody valve, the choice of Ensemble delivery system depends upon the size of the conduit (16, 18, 20 or 22 mm). As for the SAPIEN valve, prosthesis size is determined based on the degree of conduit stenosis.
After appropriate device selection and preparation, the delivery system is advanced over the guidewire, bringing the valve into the implantation site. A maneuver that can facilitate advancing the delivery system when it is at the entrance of the conduit is looping the system within the right atrium. This generates a forward force aiding passage into the conduit. Once the Melody valve is in the appropriate position, it is unsheathed followed by gradual inflation of the inner then the outer balloons. Slow inflation allows time to adjust the position in the event of device slippage. Similarly, the SAPIEN valve is inflated on a single balloon slowly. Repeat angiography and pressure measurements are made to confirm a positive outcome. Occasionally, the implanted valves may require post dilatation with a non-compliant balloon. Fluoroscopic steps and echocardiographic results of SAPIEN valve deployment are depicted in Figures 5 and 6, respectively.
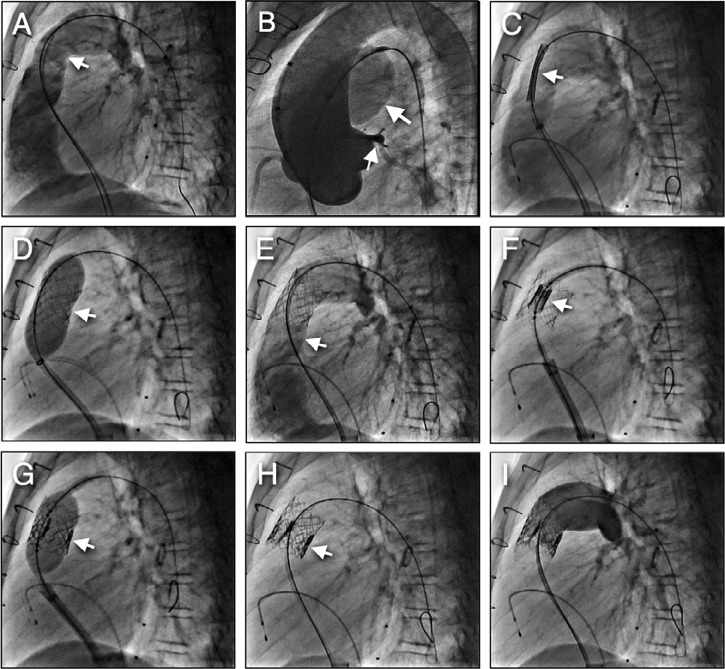
Figure 5.
SAPIEN valve placement steps. A) Pulmonary homograft angiography demonstrates severe pulmonic regurgitation, B) Conduit balloon sizing with simultaneous aortic root angiography (large arrow shows the balloon inflation in the conduit, small arrow demonstrates left coronary artery with an acceptable distance from the conduit, C) Bare metal stent placement in conduit with hand injection angiography to delineate stent position, D) Balloon stent deployment, E) Angiography post stent deployment demonstrating no conduit stenosis with free pulmonary regurgitation, F) SAPIEN valve positioned in the middle of the stent, G) Balloon deployment of the valve, H) Excellent valve position inside the stent, I) Final angiography in conduit demonstrating no significant pulmonary regurgitation.
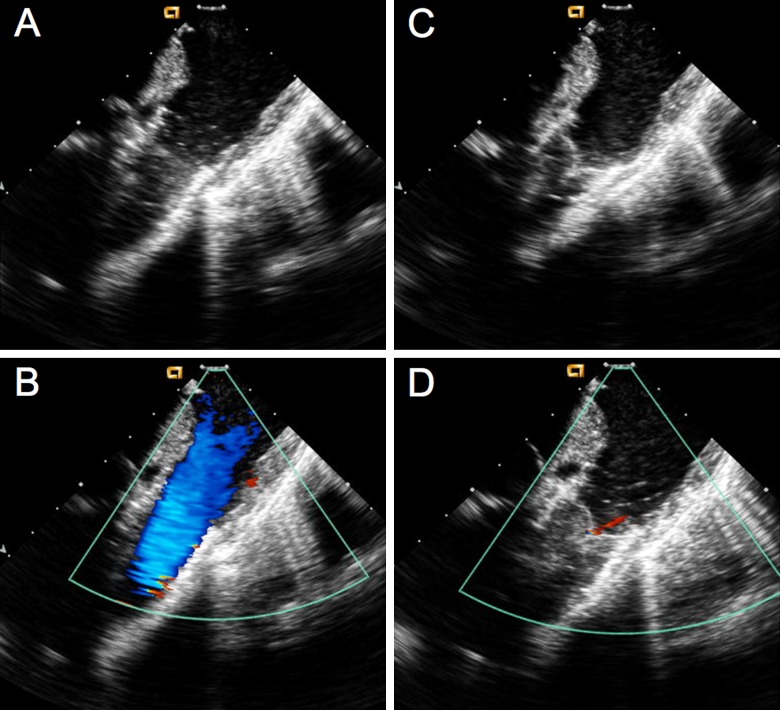
Figure 6.
Intracardiac echocardiography (ICE) images. A) Two-dimensional ICE in systole demonstrating complete opening of SAPIEN valve leaflets. B) Color Doppler in systole demonstrating no narrowing passing SAPIEN valve. C) Two-dimensional ICE in diastole demonstrates complete valve closure. D) Color Doppler in diastole demonstrates trivial pulmonary regurgitation.
Once the procedure is complete, we routinely perform figure-of-eight suture to achieve hemostasis. Some operators prefer preclosing the femoral venous access site with the suture-mediated Proglide device (Abbott Inc, Redwood city, CA). Patients are typically observed overnight in telemetry wards. Empiric IV antibiotics are administered. The following day, ECG, PA/Lateral chest x-ray and transthoracic echocardiogram should be performed. Patients are instructed to take endocarditis prophylaxis before dental procedures. As far as antiplatelet therapy, there is little data supporting specific antiplatelet regimen, and to date no definite reports of thromboembolism have been reported. In our institution, we prescribe life-long low-dose aspirin. Patients are typically discharged the following day with arrangement for routine cardiology follow-up care including regular fluoroscopic evaluation.
Hemodynamic and clinical outcomes
Data from the largest most recently published studies evaluating attempted PPVI (Melody and SAPIEN) in 500 patients are outlined in Table 3. Procedural success is generally high, with mean valve deployment of 95%. The mean procedural major complication rate was just greater than 4%. The overall mean freedom from re-intervention was 86% over a mean follow-up of 26 months. The most common reason for re-intervention with the Melody valve was stent fracture despite pre-stenting (5–16%). Stent fracture has not been reported to date with the SAPIEN valve. The risk of infective endocarditis has been reported consistently between 1% and 4% with the Melody valve.26
Table 3.
Procedural Outcomes from Clinical studies.
| Investigators | N | Success Rate | Procedural Complications | Stent Fracture | Freedom from Reintervention (Follow-up) |
| Lurz et al.5 | 163 | 155 (95%) | 7 (4.5%) | 21% | 70% (70 mo) |
| McElhinney et al.4 | 136 | 124 (91%) | 8 (6%) | 22% | 93.5% (12 mo) |
| Eicken et al.19 | 102 | 100% | 2 (2%) | 5% | 89% (12 mo) |
| Kenny et al.14 | 36 | 33 (92%) | 7 (20.5%) | 0 | 97% (6 mo) |
| Butera et al.30 | 63 | 61 (97%) | 9 (14%) | 16% | 81.4% (30 mo) |
The US Melody Transcatheter Pulmonary valve study was a prospective, non-randomized trial that was designed to assess the safety and efficacy of the valve in patients with dysfunctional RVOT conduits. Implantation was performed in 124 of 136 enrolled subjects (12 patients were excluded due to study contraindications). PPVI resulted in acute reduction of RV pressure to a median of 42 mmHg and a reduction in peak gradient across the RVOT to a median of 12 mmHg. All patients had no or trace pulmonary regurgitation except one with moderate pulmonary regurgitation. Overall periprocedural mortality or other serious complications were low. Freedom from Melody valve dysfunction was 94% at 1 year and 86% at 2 years. The stent fracture rate was approximately 22% at 14 months.4,27
The COMPASSION trial (COngenital Multicenter trial of Pulmonic vAlve regurgitation Studying the SAPIEN interventional) was the first prospective multicenter study to assess the safety and efficacy of the SAPIEN THV for the treatment of dysfunctional RV-PA conduits with moderate to severe pulmonary regurgitation with or without stenosis.14 Among 36 patients, successful valve deployment was achieved in 33 of 34 attempts. Pullback peak-to-peak systolic gradient across the conduit decreased from 27 to 12 mmHg (p < 0.001). At six months follow-up, the number of patients in NYHA functional class I increased from 5 at baseline to 27. Pulmonic regurgitation was moderate or less in 97 percent of patients.
Implantation of Edwards THV in native outflow tract is feasible, however, experience with the Edwards valve in the native pulmonary position remains limited.28 Stent migration appears to be the major potential complication of the procedure due to the fact that the compliance of the RVOT is non-predictable as compared to that of conduits. The availability of the 29 mm SAPIEN XT valve allowed increasing the pool of patients with native RVOT who may benefit from this technology.
Randomized data comparing the outcome of the surgical and percutaneous approaches on the right ventricle are lacking. With the advent of recent advances in cardiac MRI quantitative assessment,16 it would be intriguing to evaluate the structural and dynamic effects of both approaches on the right ventricle.
Complications
Possible device and procedural complications after PPVI are outlined in Table 4. Acute hemodynamic deterioration during the procedure can result from 1) obstruction of pulmonary blood flow caused by valve dislodgement into the pulmonary arteries, 2) coronary ischemia resulting from coronary impingement and 3) hemorrhage resulting from conduit rupture. The rate of serious complications in the US Melody trial was reported at 6%, including death from coronary dissection (n = 1), conduit rupture (n = 1), unstable arrhythmia (n = 1), wire perforation in distal pulmonary artery (n = 2), and femoral vein thrombosis (n = 1). In the COMPASSION trial, the rate of serious complications was 21% (7 patients). Valve or stent migration occurred in 4 patients (three requiring surgical retrieval and one was deployed in the inferior vena cava), unstable arrhythmias in one patient, and self-limited wire perforation in the distal pulmonary arteries in two patients.
Table 4.
Procedural Complications after percutaneous pulmonary valve replacement.
| Death | |
| Stent fracture | |
| Coronary impingement | |
| Device embolization | |
| Perivalvular leak | |
| Hammock effect | |
| Homograft rupture | |
| Venous thrombosis | |
| Infective endocarditis | |
| Thromboembolism | |
| Valve stenosis or regurgitation |
Stent fracture
Despite routine pre-stenting has been implemented as standard of care in most centers, stent fracture remains an important event with the Melody valve (5-16%) and remains the most common reason for re-intervention. The etiology is likely to be multifactorial and depends both on the nature of the stent and the characteristics of the implantation site. Stent fracture classification system has been proposed by Nordmeyer et al based on the initial European experience.29 Type I fracture involves disruption of one strut without loss of stent integrity. These can be seen in up to 40 % of patients but usually not associated with adverse effect. Type II involves fracture with loss of stent integrity and Type III describes fractures associated with separation of fragments. The extent of stent fracture is relevant to clinical outcomes. Type I stent fractures are likely to occur initially, and only 56% of patients are free from Type II fractures at 2 years from initial diagnosis of stent fracture and therefore require careful monitoring.30 Type II and III fractures may require re-intervention either by surgical replacement or repeat PPVI as they are associated with early conduit restenosis and valve failure. Therefore, follow up fluoroscopy at regular intervals is recommended for early detection of stent fractures. A second PPVI, if needed, can be performed similar to the initial implant. It is advisable, however, to implant another stent in all cases of stent fractures prior to valve implantation. Stent fracture has not been reported with SAPIEN valve.
Valve migration/embolization
Embolization or migration of the valve may require surgical explantation. Potentially, retrieval of the valve can be attempted with deployment in the inferior vena cava followed by stenting to flatten the valve leaflets; however this bears the risk of injury to the right ventricle and tricuspid valve. Specific to the SAPIEN valve, if wire position is lost prior to valve deployment, the valve cannot be removed out of the body percutaneously and surgical removal from the venous system may be required. Alternatively, the valve can be deployed in the IVC and stented as described above. On the other hand, the Melody valve can be removed from the femoral vein before deployment if needed.
Conduit rupture
Conduit rupture usually manifests as hemothorax rather than pericardial effusion due to bleeding into the pleura. Opacification of the hemithorax on fluoroscopy is rapid and generally associated with hemodynamic compromise. If this happens, pleurocentesis and autotransfusion should be initiated as soon as possible to stabilize the patient until other measures can be executed. Most of the time, the bleeding either stops or slows down significantly, requiring surgery in minority of patients. This complication may occur with either the Melody or SAPIEN valve. Despite the fact that Melody valve is a covered stent, the stent may not completely oppose against the inner surface of a calcified conduit leading to an ineffective seal.
The future
The aim of PPVI is to prolong the life span of conduits which were surgically placed from the right ventricle to the pulmonary artery. This prolonged conduit life span should reduce the number of multiple open heart operations over the total life span of children and young adults with congenital heart disease and potentially improve life expectancy of these patients.
Although PPVI has been described in patients with native outflow tracts,7 size restriction on the currently available valves prevents deployment in the majority of patients in this setting. One of the major challenges for the future is to expand percutaneous pulmonary valve implantation to a broader population of patients, especially those with native outflow tracts, as size restriction on the currently available valves prevents deployment in the majority of patients in this setting. With significantly dilated RVOTs, hybrid surgical approaches have been described using off-pump RVOT ring placement along with PPVI. New self-expanding valve systems are in development, however clinical data are required before longer term applicability is assessed.15 Furthermore, animal studies have proven feasibility of downsizing the pulmonary trunk and RVOT using modified hourglass-shaped stents that would create a platform that is narrow enough for valve deployment (7).31
Figure 7.
RVOT reducer. Latest version of the RVOT reducer developed by Boudjemline et al. A) Front and B) Oblique view of the device.
Ultimately, future aspirations must include efforts to merge these approaches with tissue engineering technologies to provide living autologous valve replacements with growth potential. The concept of a “living valve” delivered on a bioresorbable scaffold with the potential to grow with the patient is one of the final destinations of this exciting journey, although whether this goal is achievable in the near future is unclear.
Abbreviations
-
•
PPVI: Percutaneous pulmonary valve implantation
-
•
RVOT: Right ventricular outflow tract
-
•
RV: Right ventricle
-
•
LV: Left ventricle
-
•
CPET: Cardiopulmonary exercise testing
-
•
BiB: Balloon-in-balloon
References
- 1.Holst KA, Dearani JA, Burkhart HM, Connolly HM, Warnes CA, Li Z, Schaff HV. Risk factors and early outcomes of multiple reoperations in adults with congenital heart disease. The Annals of thoracic surgery. 2011;92(1):122–8. doi: 10.1016/j.athoracsur.2011.03.102. discussion9-30. Epub 2011/07/02. [DOI] [PubMed] [Google Scholar]
- 2.Boethig D, Thies WR, Hecker H, Breymann T. Mid term course after pediatric right ventricular outflow tract reconstruction: a comparison of homografts, porcine xenografts and Contegras. European journal of cardio-thoracic surgery: official journal of the European Association for Cardio-thoracic Surgery. 2005;27(1):58–66. doi: 10.1016/j.ejcts.2004.09.009. Epub 2004/12/29. [DOI] [PubMed] [Google Scholar]
- 3.Bonhoeffer P, Boudjemline Y, Saliba Z, Merckx J, Aggoun Y, Bonnet D, Acar P, Le Bidois J, Sidi D, Kachaner J. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet. 2000;356(9239):1403–5. doi: 10.1016/S0140-6736(00)02844-0. Epub 2000/10/29. [DOI] [PubMed] [Google Scholar]
- 4.McElhinney DB, Hellenbrand WE, Zahn EM, Jones TK, Cheatham JP, Lock JE, Vincent JA. Short- and medium-term outcomes after transcatheter pulmonary valve placement in the expanded multicenter US melody valve trial. Circulation. 2010;122(5):507–16. doi: 10.1161/CIRCULATIONAHA.109.921692. Epub 2010/07/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lurz P, Coats L, Khambadkone S, Nordmeyer J, Boudjemline Y, Schievano S, Muthurangu V, Lee TY, Parenzan G, Derrick G, Cullen S, Walker F, Tsang V, Deanfield J, Taylor AM, Bonhoeffer P. Percutaneous pulmonary valve implantation: impact of evolving technology and learning curve on clinical outcome. Circulation. 2008;117(15):1964–72. doi: 10.1161/CIRCULATIONAHA.107.735779. Epub 2008/04/09. [DOI] [PubMed] [Google Scholar]
- 6.Asoh K, Walsh M, Hickey E, Nagiub M, Chaturvedi R, Lee KJ, Benson LN. Percutaneous pulmonary valve implantation within bioprosthetic valves. Eur Heart J. 2010;31(11):1404–9. doi: 10.1093/eurheartj/ehq056. Epub 2010/03/17. [DOI] [PubMed] [Google Scholar]
- 7.Khambadkone S, Coats L, Taylor A, Boudjemline Y, Derrick G, Tsang V, Cooper J, Muthurangu V, Hegde SR, Razavi RS, Pellerin D, Deanfield J, Bonhoeffer P. Percutaneous pulmonary valve implantation in humans: results in 59 consecutive patients. Circulation. 2005;112(8):1189–97. doi: 10.1161/CIRCULATIONAHA.104.523266. Epub 2005/08/17. [DOI] [PubMed] [Google Scholar]
- 8.Lurz P, Puranik R, Nordmeyer J, Muthurangu V, Hansen MS, Schievano S, Marek J, Bonhoeffer P, Taylor AM. Improvement in left ventricular filling properties after relief of right ventricle to pulmonary artery conduit obstruction: contribution of septal motion and interventricular mechanical delay. European heart journal. 2009;30(18):2266–74. doi: 10.1093/eurheartj/ehp258. Epub 2009/06/30. [DOI] [PubMed] [Google Scholar]
- 9.Batra AS, McElhinney DB, Wang W, Zakheim R, Garofano RP, Daniels C, Yung D, Cooper DM, Rhodes J. Cardiopulmonary exercise function among patients undergoing transcatheter pulmonary valve implantation in the US Melody valve investigational trial. American heart journal. 2012;163(2):280–7. doi: 10.1016/j.ahj.2011.10.017. Epub 2012/02/07. [DOI] [PubMed] [Google Scholar]
- 10.Plymen CM, Bolger AP, Lurz P, Nordmeyer J, Lee TY, Kabir A, Coats L, Cullen S, Walker F, Deanfield JE, Taylor AM, Bonhoeffer P, Lambiase PD. Electrical remodeling following percutaneous pulmonary valve implantation. Am J Cardiol. 2011;107(2):309–14. doi: 10.1016/j.amjcard.2010.09.017. Epub 2011/01/08. [DOI] [PubMed] [Google Scholar]
- 11.Bonhoeffer P, Boudjemline Y, Qureshi SA, Le Bidois J, Iserin L, Acar P, Merckx J, Kachaner J, Sidi D. Percutaneous insertion of the pulmonary valve. Journal of the American College of Cardiology. 2002;39(10):1664–9. doi: 10.1016/s0735-1097(02)01822-3. Epub 2002/05/22. [DOI] [PubMed] [Google Scholar]
- 12.Webb JG, Chandavimol M, Thompson CR, Ricci DR, Carere RG, Munt BI, Buller CE, Pasupati S, Lichtenstein S. Percutaneous aortic valve implantation retrograde from the femoral artery. Circulation. 2006;113(6):842–50. doi: 10.1161/CIRCULATIONAHA.105.582882. Epub 2006/02/08. [DOI] [PubMed] [Google Scholar]
- 13.Garay F, Webb J, Hijazi ZM. Percutaneous replacement of pulmonary valve using the Edwards-Cribier percutaneous heart valve: first report in a human patient. Catheterization and cardiovascular interventions: official journal of the Society for Cardiac Angiography & Interventions. 2006;67(5):659–62. doi: 10.1002/ccd.20753. Epub 2006/04/06. [DOI] [PubMed] [Google Scholar]
- 14.Kenny D, Hijazi ZM, Kar S, Rhodes J, Mullen M, Makkar R, Shirali G, Fogel M, Fahey J, Heitschmidt MG, Cain C. Percutaneous implantation of the Edwards SAPIEN transcatheter heart valve for conduit failure in the pulmonary position: early phase 1 results from an international multicenter clinical trial. Journal of the American College of Cardiology. 2011;58(21):2248–56. doi: 10.1016/j.jacc.2011.07.040. Epub 2011/11/15. [DOI] [PubMed] [Google Scholar]
- 15.Cao QL, Kenny D, Zhou D, Pan W, Guan L, Ge J, Hijazi ZM: Early clinical expereinece with a novel self-expanding percutaneous stent-valve in the native right ventricular outflow tract. Catheter Cardiovasc Interven. 2014;84:1131–1137. doi: 10.1002/ccd.25544. [DOI] [PubMed] [Google Scholar]
- 16.Chapron JAH, Theodoropoulos S, Kalantzi M, Yacoub M, Torii R. Quantitative assessment of right ventricular structure and flow dynamics in pulmonary homograft obstruction. Global Cardiology Science and Practice. 2014:47. doi: 10.5339/gcsp.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feltes TF, Bacha E, Beekman RH. 3rd. Cheatham JP, Feinstein JA, Gomes AS, Hijazi ZM, Ing FF, de Moor M, Morrow WR, Mullins CE, Taubert KA, Zahn EM Indications for cardiac catheterization and intervention in pediatric cardiac disease: a scientific statement from the American Heart Association. Circulation. 2011;123(22):2607–52. doi: 10.1161/CIR.0b013e31821b1f10. Epub 2011/05/04. [DOI] [PubMed] [Google Scholar]
- 18.Gatzoulis MA, Balaji S, Webber SA, Siu SC, Hokanson JS, Poile C, Rosenthal M, Nakazawa M, Moller JH, Gillette PC, Webb GD, Redington AN. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet. 2000;356(9234):975–81. doi: 10.1016/S0140-6736(00)02714-8. Epub 2000/10/21. [DOI] [PubMed] [Google Scholar]
- 19.Broberg CS, Aboulhosn J, Mongeon FP, Kay J, Valente AM, Khairy P, Earing MG, Opotowsky AR, Lui G, Gersony DR, Cook S, Ting JG, Webb G, Gurvitz MZ. Prevalence of left ventricular systolic dysfunction in adults with repaired tetralogy of fallot. The American journal of cardiology. 2011;107(8):1215–20. doi: 10.1016/j.amjcard.2010.12.026. Epub 2011/02/26. [DOI] [PubMed] [Google Scholar]
- 20.Eicken A, Ewert P, Hager A, Peters B, Fratz S, Kuehne T, Busch R, Hess J, Berger F. Percutaneous pulmonary valve implantation: two-centre experience with more than 100 patients. European heart journal. 2011;32(10):1260–5. doi: 10.1093/eurheartj/ehq520. Epub 2011/01/29. [DOI] [PubMed] [Google Scholar]
- 21.Vezmar M, Chaturvedi R, Lee KJ, Almeida C, Manlhiot C, McCrindle BW, Horlick EM, Benson LN. Percutaneous pulmonary valve implantation in the young 2-year follow-up. JACC Cardiovascular interventions. 2010;3(4):439–48. doi: 10.1016/j.jcin.2010.02.003. Epub 2010/04/20. [DOI] [PubMed] [Google Scholar]
- 22.Cheatham SL, Holzer RJ, Chisolm JL, Cheatham JP. The Medtronic Melody(R) transcatheter pulmonary valve implanted at 24-mm diameter–it works. Catheterization and cardiovascular interventions: official journal of the Society for Cardiac Angiography & Interventions. 2013;82(5):816–23. doi: 10.1002/ccd.24821. Epub 2013/01/30. [DOI] [PubMed] [Google Scholar]
- 23.Morray BH, McElhinney DB, Cheatham JP, Zahn EM, Berman DP, Sullivan PM, Lock JE, Jones TK. doi: 10.1161/CIRCINTERVENTIONS.113.000202. Risk of coronary artery compression among patients referred for transcatheter pulmonary valve implantation: a multicenter experience. [DOI] [PubMed] [Google Scholar]
- 24.Webb JG, Altwegg L, Masson JB. Al Bugami S. Al Ali A, Boone RA. A new transcatheter aortic valve and percutaneous valve delivery system. Journal of the American College of Cardiology. 2009;53(20):1855–8. doi: 10.1016/j.jacc.2008.07.075. Epub 2009/05/16. [DOI] [PubMed] [Google Scholar]
- 25.Sridharan S, Coats L, Khambadkone S, Taylor AM, Bonhoeffer P. Images in cardiovascular medicine. Transcatheter right ventricular outflow tract intervention: the risk to the coronary circulation. Circulation. 2006;113(25):e934–5. doi: 10.1161/CIRCULATIONAHA.105.599514. Epub 2006/06/28. [DOI] [PubMed] [Google Scholar]
- 26. Infective endocarditis after transcatheter pulmonary valve replacement using the Melody valve: combined results of 3 prospective North American and European studies. [DOI] [PubMed]
- 27.Zahn EM, Hellenbrand WE, Lock JE, McElhinney DB. Implantation of the melody transcatheter pulmonary valve in patients with a dysfunctional right ventricular outflow tract conduit early results from the u.s. Clinical trial. Journal of the American College of Cardiology. 2009;54(18):1722–9. doi: 10.1016/j.jacc.2009.06.034. Epub 2009/10/24. [DOI] [PubMed] [Google Scholar]
- 28.Guccione P, Milanesi O, Hijazi ZM, Pongiglione G. Transcatheter pulmonary valve implantation in native pulmonary outflow tract using the Edwards SAPIEN transcatheter heart valve. Eur J Cardiothorac Surg. 2012;41(5):1192–4. doi: 10.1093/ejcts/ezr130. Epub 2012/01/10. [DOI] [PubMed] [Google Scholar]
- 29.Nordmeyer J, Khambadkone S, Coats L, Schievano S, Lurz P, Parenzan G, Taylor AM, Lock JE, Bonhoeffer P. Risk stratification, systematic classification, and anticipatory management strategies for stent fracture after percutaneous pulmonary valve implantation. Circulation. 2007;115(11):1392–7. doi: 10.1161/CIRCULATIONAHA.106.674259. Epub 2007/03/07. [DOI] [PubMed] [Google Scholar]
- 30.McElhinney DB, Cheatham JP, Jones TK, Lock JE, Vincent JA, Zahn EM, Hellenbrand WE. Stent fracture, valve dysfunction, and right ventricular outflow tract reintervention after transcatheter pulmonary valve implantation: patient-related and procedural risk factors in the US Melody Valve Trial. Circulation Cardiovascular interventions. 2011;4(6):602–614. doi: 10.1161/CIRCINTERVENTIONS.111.965616. Epub 2011/11/15. 2014. Epub 2014/05/16. [DOI] [PubMed] [Google Scholar]
- 31.Mollet A, Basquin A, Stos B, Boudjemline Y. Off-pump replacement of the pulmonary valve in large right ventricular outflow tracts: a transcatheter approach using an intravascular infundibulum reducer. Pediatric research. 2007;62(4):428–33. doi: 10.1203/PDR.0b013e318142aa3e. Epub 2007/08/02. [DOI] [PubMed] [Google Scholar]